Abstract
For over a century it has been known that tumor hypoxia, regions of a tumor with low levels of oxygenation, are important contributors to tumor resistance to radiation therapy and failure of radiation treatment of cancer. Recently, using novel pulse electron paramagnetic resonance (EPR) oxygen imaging, near absolute images of the partial pressure of oxygen (pO2) in tumors of living animals have been obtained. We discuss here the means by which EPR signals can be obtained in living tissues and tumors. We review development of EPR methods to image the pO2 in tumors and the potential for the pO2 image acquisition in human subjects.
Introduction: The importance of imaging molecular oxygen in cancer therapy
A near universal characteristic of solid human tumors is hypoxia. Hypoxia is defined as clinically significant regions of a malignant tumor with low levels of molecular oxygen, or low values of pO2. Resistance of human solid tumors to radiation induced by such regions of hypoxia has been recognized for over a century.1 Similar resistance has more recently been noted for chemotherapy.2,3 Human trials investigating hyperbaric oxygen and hypoxic sensitizers to overcome tumor hypoxia have shown promise but limited success.4,5 However, the extensive variability of tumor oxygenation, both in overall extent and in the location of hypoxic regions, may confound such attempts. The results demonstrate the importance of, assessment not only of overall tumor oxygenation, but of locating, through imaging, significant regions of hypoxia in the tumor. We will briefly review this data, and then discuss various alternative methods of measuring tissue and imaging tumor oxygenation.
Imaging Physiology in Living Animals
Short of transillumination, the first images of deep structures in living animals began with Wilhelm Roentgen’s discovery of the X-ray in 18956 Since then imaging modalities have evolved sensitivity to varied soft tissue states of animals and humans.7,8 In parallel, spectroscopies have evolved, revealing the physical aspects of solid and liquid states of matter. These states are the environment in which biologic processes evolve.9 However, living samples are heterogeneous. The full definition of physiologic states, thus, requires spectroscopic imaging. Various forms of spectroscopic imaging have evolved in the past three decades. This makes available from the spectroscopic process information that localized within a subvolume of a living sample.10,11
The Nature of Molecular Oxygen
The oxygen molecule, O2, is a diradical with two unpaired electrons in the triplet state that define its interaction with other molecules bearing unpaired electron and nuclear spins and with electromagnetic radiation. It is a rapidly tumbling diatomic molecule with its two unpaired spins rapidly relaxing each other. Thus, the oxygen molecule in solution at room temperature has a nearly unmeasurably fast electron relaxation rate. When interacting with another molecule bearing a single highly stable unpaired electron spin-- a spin probe-- oxygen increases the relaxation rates of the probe, mainly via Heisenberg exchange12,13. The solution interaction rates between the spin probe and O2 are described by the Smoluchowski diffusion equation, which predicts a linear relation between pO2 and spin probe relaxation rate, validated for multiple free radicals14.
Measurement of Molecular Oxygen
A number of techniques for measurement of molecular oxygen have been developed. The chemical and physical properties of oxygen enable a variety of methods, each with their own applicability and advantages. The “gold standard” of oxymetry in cells and live animal tissues is the platinum electrode.15 A recent enhancement of the standard platinum electrode is the Eppendorf electrode. This is inserted into tissues of living animals and human subjects with a highly regular advance and retreat pattern for measurement consistency. It has a 200–300 µm diameter tip that is inserted into the tissue, which measures oxygen along a series of tracks16. The OxyLite™ probe (Oxford Optronix, Oxford, UK) utilizes fluorescence quenching of fluorophore by oxygen17. OxyLite™ can be used for repetitive measurements in the same location or along tracks, although the 200 µm glass fiber will wander from a straight line sampling. The electrode and Oxylite provide highly local samples, and do not provide an overall inventory of the pO2 distributions in tissues.
There are a number of noninvasive qualitative oxymetries available. These include near-infrared spectroscopy, typically of blood saturation, fluoramisonidozole retention PET18, and Blood Oxygenation Level Dependent (BOLD) Magnetic Resonance Imaging (MRI)19,20. These methods are subject to confounding variation that frustrates quantitative measurement, and, particularly if the animal is stressed or otherwise changes its state, prevents reliable repeated measurement. Phosphorescence quenching21, 19F22,23 proton–electron double resonance imaging (PEDRI)19,24 and Electron Paramagnetic Resonance (EPR) are more quantitative pO2 imaging modalities. Despite their quantitative imaging capabilities, phosphorescence quenching, 19F MRI20 and PEDRI are still subject to confounding variation, most significantly from the effect of the concentration of the sensing molecule (the phosphorescent compound, the 19F for MRI, or the self relaxation of the electron bearing spin probe and the relaxation time of the water proton in PEDRI) on the relaxation rate-dependence on O2. Solutions to this problem have included the use of EPR with particulates or application of longitudinal or spin lattice relaxation rate EPR imaging of soluble spin probes, that provide near absolute measurements of local pO2, with order-of-magnitude reduction in confounding variation.
Principles of Electron Paramagnetic Resonance
The Electron Paramagnetic Resonance technique detects molecular species with one or more unpaired electrons: paramagnetic complexes, radicals, lattice defects, etc. Principles of magnetic resonance are covered in a number of books25–28. Here, we discuss essential relevant aspects of the technique for understanding EPR imaging and oxymetry.
An unpaired electron possesses an intrinsic magnetic moment not associated with its orbital angular momentum. The spin moment is where g is the electron g-factor equal to 2.0023 for a free electron, μB is the Bohr electron magneton and ℏ is Planck’s constant divided by 2π.
A crucial difference between the technique of electron paramagnetic resonance and that of nuclear magnetic resonance and their respective imaging techniques is the difference in the magnitude of the magnetic moments. This three order of magnitude difference completely transforms the respective methods. Relativistic quantum mechanics predicts the spin magnetic moment μB to be, μB = ℏe/2πmc29, where e is the magnitude of the charge of the electron or proton, c is the speed of light and m is the mass of either the electron or, in the case of a hydrogen nucleus, the mass of the proton. The magnetic moment of the electron is 1836 times larger than that of the proton. Due to the anomalous magnetic moment of the proton, which is 2.79 times larger than the pure relativistic prediction because of the finite size or non-point charge distribution of the proton, the actual magnetic moment of the electron is 658 times or still nearly three orders of magnitude larger than that of the proton.
In the presence of a static magnetic field (B0), the energy (E) of a magnetic moment depends on its orientation relative to B0. For a spin 1/2 particle (S=1/2), quantum mechanics counterintuitively requires orientations to be parallel (−) or anti-parallel (+) to the imposed magnetic field B0.
| [1] |
The difference between these energy levels (ΔE) can be described in terms of angular frequency (ω0) via Planck’s energy-frequency relationship.
| [2] |
Combining these two equations gives the relation between frequency and applied field as:
| [3] |
where γe = g μB/ℏ is the electron gyromagnetic ratio. This is equal to 1.76×1011 s−1T−1 for the free electron. The three order of magnitude smaller proton gyromagnetic ratio γp is 2.68 ×108 s−1T−1. ω0 is known as the Larmor frequency, the Zeeman frequency or the basic resonance frequency.
The major instrumental consequences of these results are
The magnetic field at which an electron magnetic absorbs significantly more energy from the oscillating electromagnetic field -- resonance -- is approximately 658 times smaller (=γp/γe) than that for a proton at a given excitation frequency, ω0. The “approximately” is due to the inevitable modification of the local environment of the resonating electron by the magnetic fields of nearby nuclei or, rarely, unpaired electrons. This means that at high field MRI frequencies, EPR imagers use much lower magnetic fields.
The rates at which excited electron spins relax are proportional to γ2.30 Electrons relax ~ (658(=γe/γp))2 or nearly six orders of magnitude faster than water protons. Electrons relax in times of nanoseconds to microseconds. Water protons and common nuclei relax in tens of milliseconds to many tens of seconds. Excited 13C enriched pyruvate, for example in human subjects, relaxes in ~ 1 minute.
The numbers of unpaired spins in common samples measured with magnetic resonance, either electron spins or nuclear spins, are very large, from a billion billion to a trillion trillion spins, which are thermodynamic numbers. These are subject to thermodynamic energy distributions, the Boltzmann distribution, with smaller numbers of spins in the higher energy orientation than in the lower energy orientation. The magnitude of the equilibrium magnetization is proportional to the imposed magnetic field, B0. The overall magnetic moment summed over all the magnetic moments of the spins is referred to as the magnetization of the spin system. The magnetization in a sample can be manipulated by addition of a second, oscillating magnetic field (B1). To be effective for S=1/2 states, B1 is oriented orthogonal to B0 and oscillates at frequencies close to the Larmor frequency. The time varying magnetic field B1 can add energy to the magnetization system. It accomplishes this by inverting the orientations of individual electronic spins and changing the direction of the overall sum of the unpaired spins, the magnetization. The phenomenological Bloch equation describes the time evolution of magnetization orientation in the presence of both B0 and B1 magnetic fields25. In a coordinate system rotating at the spectrometer operating frequency where B1 is not changing, the return of transverse (MT) and longitudinal (MZ) components of magnetization to equilibrium in the absence of B1 is described by25:
| [4] |
| [5] |
Here, Ω is the difference between the Larmor frequency of the electron and the EPR spectrometer’s operating frequency. The longitudinal relaxtion time, T1, and the transverse relaxation time, T2, correspond to the relaxation of MT and MZ, respectively. T1 and T2 are the inverses of the respective longitudinal relaxation rate, R1, and transverse relaxation rate, R2. The conventional EPR time domain signal, s(t), is proportional to MT. M describes an initial state of longitudinal magnetization. The longitudinal magnetization can be encoded into the EPR signal by using special pulse sequences. The transverse magnetization relaxation rate, R2, is commonly referred to as the phase memory relaxation rate. This is the rate at which spins that have been aligned by a very short duration pulse of oscillating magnetic field, which prepares them in a particular magnetization state, lose their coherent alignment in the time after the pulse.
The first EPR experiments were continuous wave (CW) EPR experiments. Here the B1 excitation is applied continuously during an experiment or image. In a CW spectrometer, the EPR signal is produced by applying B1 at a fixed frequency ω0 and sweeping B0 through the resonance condition of Equation [3].28 In principle, the field B0 could be fixed and the frequency swept through the resonance condition of Equation [3], but the former approach simplifies the electronics and allows a simpler amplification of signal. An alternate strategy applied often to simpler, more robust systems, involves subjecting the unpaired electron spin-bearing sample, prepared in a magnetic field, to a high power pulse of radiation, of duration shorter than T2. This pulse aligns the magnetization in the magnetic field. Detecting the rate of decay of the magnetization signal is the basis of this latter experiment. This is referred to as a time domain experiment. It is important to note that the CW magnetic field domain spectrum is related to the time domain signal through a Fourier transformation.
| [6] |
Note that the factor γe converts magnetic field units into inverse time, or rate.
Oxymetry with EPR
Injectable, water soluble spin label or spin probe EPR oxymetry is a minimally invasive method that can report absolute pO2 deep in tissues31–33. In the 1980’s, EPR detection of oxygen using the broadening of the width of the EPR spectrum from a nitroxide spin probe was first reported by Backer et al.34 and Popp et al.35 and later extensively investigated by Swartz and coworkers14,33,36, by using various classes of spin probes. In the nineties, a few groups pioneered multi-dimensional CW imaging on rodents in vivo and ex vivo, enabling repeated measurements of oxygen concentrations in living tissues37–39.
The underlying mechanism of interaction between spin probe and oxygen is predominantly Heisenberg spin exchange.29 In this mechanism, the electrons from the rapidly relaxing oxygen environment, during the encounter with a spin probe, are not distinguishable from the electrons of the spin probe. The spin probe electrons thereby share their environment with that of oxygen during which time they relax more rapidly. The increased rate loss of both phase and energy of the spin probe electron is proportional to the rate of encounter with oxygen. This is directly proportional to the oxygen concentration and the oxygen partial pressure.
EPR techniques like CW or time domain free induction decay (FID) pulse imaging, measure total EPR decay rates or line widths (LW) instead of the transverse or longitudinal relaxation rates. The total EPR decay rate/line width of free radicals is due to two major components: 1) homogeneous broadening due to transverse relaxation and 2) inhomogeneous broadening due to the interaction of the electron with neighboring paramagnetic nuclei26,27. In the case of inhomogeneous broadening, the electron experiences the magnetic fields of multiple neighboring nuclei, which variably shifts the resonance frequency, creating hyperfine (HF) spectral structure. A more precise method for obtaining spin packet line width, that is used in continuous wave oxymetry, explicitly fits the EPR line to spectral models that include hyperfine structure, and accounts for the effect of the magnetic field modulation40–42.
In addition to partial pressure of molecular oxygen, details of EPR lineshape provide an insite into the thiol-disulfide balance or redox state, the related local bioreduction capability, the acidity (pH), the temperature, the presence of specific oxygen centered free radicals, and many others.43
Characterizing the spin relaxation by relaxation rates is the inverse of characterizing it by relaxation times. Relaxation rates are related to relaxation times, T: R=1/T. Relaxation rates can be multiplied by (γe)−1, the inverse of the gyromagnetic ratio, to allow expression of the rates directly in magnetic field units (µT). Thus, line broadening is directly related to relaxation rate increase. Relaxation rates naturally demonstrate the relationship between increasing numbers of relaxation mechanisms and increasing relaxation rates, or linewidths. This process also provides a conceptual link between parameters determined by CW and pulse methods. For Lorentzian line shapes, the half width at half maximum (HWHM) is equal to 1/(γeT2)=(1/γe)R2. For EPR lines with multiple broadening mechanisms, (1/γe)R2 describes the homogeneous broadening of the EPR line (spin packet line width). In early CW oxymetry, the spin packet line width allowed the determination of pO2.
Transverse relaxation-based oxymetry is more susceptible to variation of other physical parameters beside oxygen partial pressure. Parameters such as temperature, viscosity and salinity are tightly controlled in the body of a living animal44. This allows a quantitative enumeration of their effects on relaxation, allowing correction and calibration of oxygen measurements. However, spin probe concentration may vary and affect the accuracy of line width/transverse relaxation rate-based oxymetry.
Spin Probe Sensitivity to Molecular Oxygen (O2 or pO2) and Other Environmental Factors
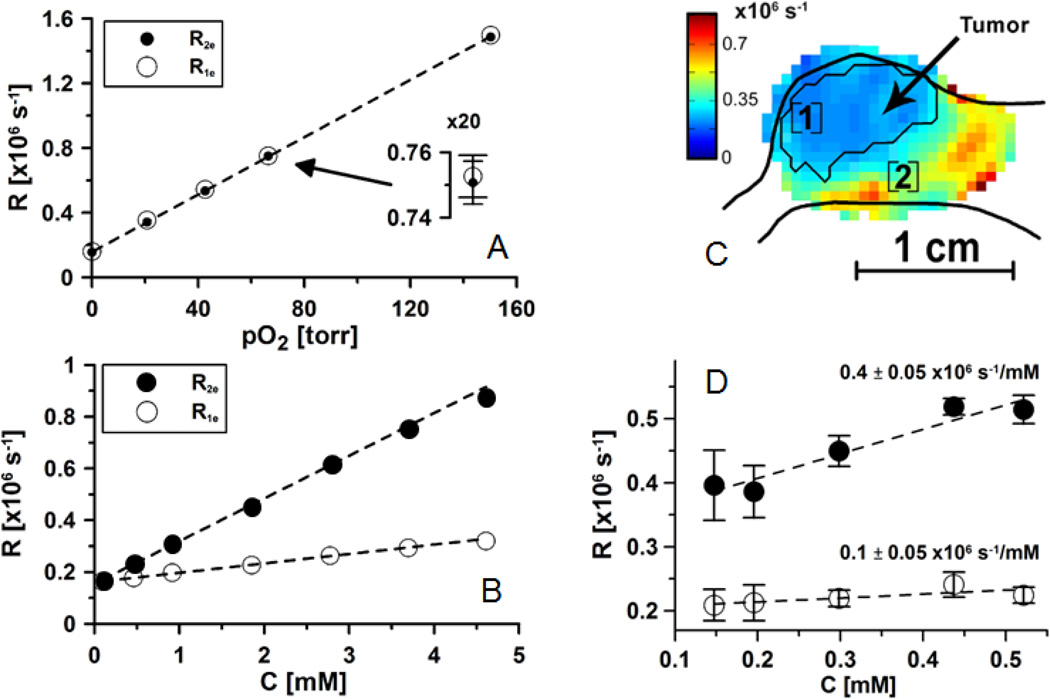
Heisenberg spin exchange between a spin probe and oxygen acts on the spin probe’s longitudinal relaxation in a manner nearly identical to action on transverse relaxation13,45. Importantly, other relaxation processes affect transverse and longitudinal relaxation differently. Electron spin exchange between two trityl spin probe molecules increases R213. The effect of the spin exchange between spin probes on R2 is dependent on the number of collisions per unit time, and, therefore, on the concentration of spin probe. R2 cannot distinguish between the dephasing effect of an interaction of a spin probe with oxygen and a dephasing effect of an interaction of a spin probe with another spin probe. The exchange of energies between two spin probes, however, does not alter the total energy of the interacting spin pair, and therefore does not affect the spin probe longitudinal magnetization component. Thus, R1 is much less susceptible to self-broadening. R1 is nearly an absolute measure of oxygen concentration, or pO2. Fig. 2 shows the effect of O2 concentration, or pO2, on the relaxation rates, R1 and R2, to have identical slopes, but there is nearly an order of magnitude reduction in the dependence of R1 on spin probe concentration relative to R2. R1 is a near absolute measure of pO2 with precision exceeding 1 torr. This increase in probe specificity is a distinguishing feature of EPR oxymetry, in comparison with other noninvasive methods32.
Figure 2.
A and B - relaxation rates of OX063 dissolved in saline at 37°C. The range of the measured spin probe concentrations is considerably larger than the one observed in vivo. C. Relaxation rate image. D. Relaxation rates in position marked as [1] in Fig. C. Rates are measured as a function of spin probe concentration while spin probe is infused at different rates, from low to high. The slopes of concentration dependence are given in the plot.
The ability of an EPR image to quantify molecular oxygen dissolved in the life supporting solvents of a living animal and eventually a human is among the most important of its abilities. The absence of oxygen in the heart, brain, and limbs affected by peripheral vascular disease or diabetes, ischemic bowel, and portions of cancers of human patients rendered resistant to therapy by the absence of oxygen, is responsible for the death of greater than half of our species. The evaluation of treatments and pharmaceutical agents to ameliorate the hypoxic state promises to prolong the useful and involved lives of all of us.46
Radio frequency Magnetization Excitation is Necessary for In Vivo EPR Imaging of Large Animals and Humans
EPR imaging needs to be done under unusual conditions for eventual human application. Conventional EPR spectroscopy is usually carried out at frequencies of gigahertz to hundreds of gigahertz. Lower frequencies are required to penetrate deeply into the body of a human composed two thirds by weigh of salt water. The non-resonant loss of signal amplitude caused by conductive loss at low frequency, or dipolar loss at higher frequency, requires magnetization excitation frequencies of the order of hundreds of MHz for large human-size animals. This is the excitation frequency of a high field, whole body MRI. For small animals, frequencies of up to approximately 1 GHz can be used.47,48 As we mention above, unlike MRI, which requires multi-Tesla superconducting magnets, at 250 MHz, the B0 for EPR images is 9 milliTesla. These stationary magnetic fields can be generated by simple copper air core magnets. Small animal experiments to rapidly and efficiently evaluate treatment and pharmaceutical effectiveness, may use higher frequencies near 1 GHz (L-band), that will give higher signals and will not suffer paralyzing loss that would make large animal experiments difficult. Because relaxation rates of even the most slowly relaxing aqueous spin probes are five to six orders of magnitude faster than those of a water hydrogen nucleus, fixed stepped gradients, tomographic image reconstruction and modest power requirements make the technique relatively inexpensive.
Dissolved Spin Probe Oxymetry
In general, quantification of aspects of the tissue microenvironment requires an environmental reporter and a readout technique. The reporter can be endogenous, such as water protons or sodium ions in MRI, or exogenous, such as implanted particulates or injected soluble spin probes. Endogenous reporters typically have much higher concentrations than exogenous ones and therefore, are easier to detect, but their localization cannot be controlled. Consequently, they are non-specific and sometimes provide an overwhelming background signal that mask a signal of interest. On the contrary, exogenous spin probes can be specifically targeted to the areas of interest.
Endogenous paramagnetic species found in mammalian bodies include hemoglobin, metalloenzymes, but extremely low concentrations of unbound diffusible species except molecular oxygen. Metal centers and oxygen at animal body temperature have very short relaxation times, broad lines and thus are not easily and directly measurable at low EPR frequencies. Moreover, unbound diffusible paramagnetic metal species, can interact with and transform covalent carbon-carbon and carbon-hydrogen molecular bonds as they do as enzymatic reaction centers. Therefore, living systems have developed arrays of binding proteins to maintain endogenous diffusible concentrations below 1 nanomole because of this threat to covalent bonds of the molecules of living systems. At present, exogenous spin probes are really the only practical reporters, and appropriate spin probes are the key to successful imaging. The line width and relaxation times of the probe, and their sensitivity to oxygen, largely define sensitivity of methodology and pO2 accuracy. Probes with narrower line widths and longer relaxation times allow higher resolution imaging. High fractional sensitivity of line width to oxygen ensures better imaging accuracy. Finally, the spin probes should be minimally toxic and metabolically stable.
Summarizing the above, EPR oxymetric imaging requires a spin probe to sample the fluid environment and report the oxygen partial pressure through increase in its spin packet line width or, equivalently, its relaxation rates. Measurement of line broadening of nitroxide spin probes has, until recently, been the principle means of dissolved spin probe oxymetry. Hypoxic nitroxide relaxation rates, 1–2 µs−1, are still too rapid for imaging at several hundred MHz excitation frequencies. Tri-aryl methyl radicals, specifically, partially-deuterated methyl-tris[8-carboxy-2,2,6,6-tetrakis[2-hydroxyethyl]benzo[1,2-d:4,5-d’]bis[1,3]dithiol-4-yl]-trisodium salt, OX063, have an order of magnitude smaller hypoxic R1 value of 1/6 µs−1(T1.=6 µs) These relaxation rates enable pulse measurement and imaging at 250 MHz.49 R1, measured with inversion recovery pulse sequences, increases signal to noise by nearly a factor of two relative to electron spin echo imaging, which measures R2, and has reduced the confounding sensitivity of spin probe to the self-relaxation or broadening described above, by nearly an order of magnitude. This makes the spin probe measurement accurate to within 1–2 torr, an absolute measurement or image for animal application (vide infra).50
At present, two large classes of spin probes are used for in vivo oxymetry: Soluble free radicals and insoluble paramagnetic particles (particulates)51,52. They are introduced into animals in different ways and require different imaging methods.
Soluble spin probes
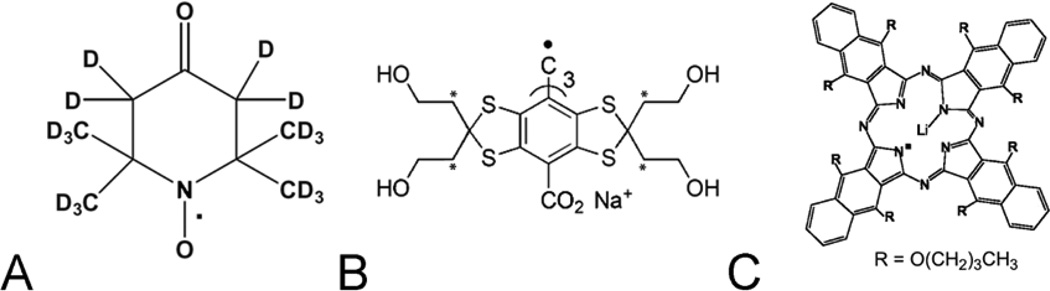
Physiologic EPR imaging has been enabled by the synthesis of free radical reporter molecules. These molecules distribute in specific physiologic compartments. Desirable characteristics for these probes include water solubility, kinetic and metabolic stability, a single narrow line resonance, line widths or relaxation rates directly related to pO2, persistence of signal from tumors longer than imaging times, and low toxicity32. Historically, the first probes applied for oxymetry were nitroxides (Figure 1A)53,54,55,56.
Figure 1.
Chemical structures of typical spin probes : A. water soluble fully deuterated 6-member ring nitroxide; B. water soluble OX071 trityl, the deuterated methylene groups are marked with *; C. particulate LiNc-BuO spin probe.
Trityls
The recent success of spin probe oxymetry was enabled by triarylmethyl radicals (TAM radicals), which are also referred to as trityls (Figure 1B). Trityl compounds were developed by Nycomed innovation (later acquired by GE Healthcare; Little Chalfont, Buckinghamshire, United Kingdom). Trityls have extremely narrow, single EPR lines55. Those which are used for in vivo imaging are OX063 (16 µT peak-to-peak, p-p) and its partially deuterated form Ox63d24 (8 µT p-p) (Figure 1B). The trityl R1 and R2 is linearly dependent on pO255 (Figure 2). These molecules are triacid, charge 3- anionic and distribute in the extracellular fluid compartment32,57. In the blood stream of a mouse, the clearance halftime of these probes is 9 – 10 min, while in tumors they remain and provide strong signals for 40 – 50 min58. The dose at which 50% of animals die, the LD50, of OX063 is large (8 mmol/kg), which allows high dose injections59. Typically, 0.5 ml of 80 to 100 mM solutions at neutral pH are continuously injected intravenously (IV) into 20–25g animals to give tumor average concentrations of several hundred µM.
Particulates
A number of different solid, crystalline particulates have been used for EPR oxymetry: Activated charcoal, and lithium phthalocyanine60 and its derivatives with higher oxygen sensitivity, such as octa-n-butoxy-naphthalocyanine56 (Figure 1C). These insoluble particulate spin probes can be inserted surgically as several tens or hundreds of micron large polycrystals; injected in a form of slurry of finely ground powder; fed to an animal61; or implanted with tumor cells during inoculation in mice62,63. One of their major advantages is that the oxygen sensing is physically decoupled from the local environment. O2 must diffuse into a pore or channel where it interacts with an individual phthalocyanine molecule, which is part of a helical stack. The interaction locally breaks the symmetry of the exchange-narrowed, electron cloud of the stack of phthalocyanine molecules lining the channel, increasing its relaxation rate or linewidth.
Limited mobility of particulates enables repeated measurements of oxygen concentration for an extended time. The higher concentration of unpaired electrons or spin density in comparison with a soluble probe produces higher signal and local sensitivity, although spectroscopy in the absence of imaging gives reduced knowledge of the location of the signal. Spin probe migration and degradation leads to loss of EPR signal intensity. There remain potential biocompatibility concerns with prolonged exposure of particulates to tissue, unless the spin probe is excised. Biocompatible coating of the crystalline probes may overcome some of these problems and enhance their clinical applicability64.
Techniques for EPR Imaging pO2
For imaging, the location in space of a paramagnetic species is encoded using magnetic fields that vary linearly in space, or magnetic field gradients, denoted by a vector along the gradient direction, G. Gradients are designed to alter only local amplitude and not direction of the constant magnetic field. The additional magnetic field experienced by a species at position x in the sample is then ΔB=G·x. Here x is a three-dimensional spatial coordinate in vector form. Since, from Eq. [3], ω0 = γeB0, k = Δω0 = γeΔB this is referred to as frequency encoding. The space of frequencies is referred to as k space.49,65,66 Each measurement represents a trajectory in k space. The trajectory for a static gradient is a radial line. In the process of obtaining a CW spectroscopic image, B0 is swept. Fourier transform of the resulting spectra lies along the radial line. This is referred to as a projection, whose angles are defined by the directional part of G. Alternately, a very short pulse of radiofrequency is applied to the sample. This broadband pulse in the presence of a gradient excites all of the spins in the sample. The time trace of the pulse experiment can be mapped to k-space directly, as k = γGT. It is, thus, a very efficient imaging acquisition strategy. This strategy is referred to as radial imaging and requires filtered backprojection tomographic image reconstruction to recover the images. For oxygen imaging, pulse techniques are used with different detection times after a magnetization inversion to determine the spin-lattice relaxation rate.
A second method for encoding spatial position origin of signal is referred to as phase encoding. Because the relaxation of electrons is very rapid, a variation of the pulse phase encoding technology used in solid state MRI is used. This involves measuring a signal at a the single time point, tp, after a pulse that rotates the magnetization into a plane perpendicular to the magnetic field. Very rapid gradient vector angle and magnitude stepping to cover a 3D Cartesian grid is necessary. Coverage of k-space is produced by selection of gradient kijk = γGijk tp, one for each k-space point. The technique is referred to as single point imaging67. It produces images on a Cartesian grid with a much larger number of acquisitions than tomographic imaging.68 As a result, it is less efficient than filtered back projection, but freer from artifact imposed by radial k-space coverage.
Oxygen Imaging as Longitudinal Relaxation (R1) Parametric Imaging
Oxygen images are derived from three dimensional images of the average longitudinal relaxation rate of the trityl spin probe in each voxel or sub volume that is resolved in the image. This relaxation rate is related to the oxygen concentration or partial pressure through a relevant calibration.
| [7] |
where i=1 for longitudinal relaxation and i=2 for transverse relaxation. As shown in Fig. 2, the constant, b is far less sensitive to the concentration of spin probe for R1 than for R2. One of the main advantages of pulse methods, where multiple spatial images are obtained, each at a different delay time relative to a magnetization orientation pulse, is their ability to determine the relaxation rates directly. The 3D spatial images are reconstructed separately. Then in each image the voxel in the same location is selected. Pulse sequences that have a relaxation sensitive component (for example the delay) are used for imaging. This forms a time dependence of the signal for each voxel. Finally, this time dependence is fitted to exponential decay or recovery, and relaxation rate is extracted. The information obtained to reconstruct each image is obtained from different delay times between the inversion pulse for R1 images, and from the echo time for R2 images. These times are so thoroughly interleaved (separated by a microsecond or less), that the reconstructed voxel locations are remarkably stable and reproducible. One of the advantages of the use of highly water soluble trityl spin probes is that their distribution volume is extracellular. Use of R1-sensitive pulse sequences virtually eliminates confounding variation from spin probe concentration-dependent effects.
EPR imaging in Cancer Biology
Several laboratories report various spin probe EPR oxygen measurement techniques, including spectral-spatial continuous wave imaging and localized spectroscopies and pulse-based imaging.69–74 Here we describe work in our laboratory focusing on images of tumors in animals – mice, although we have demonstrated both pulse and continuous wave EPR images from rats and rabbits.75,76 We will also describe our ideas for the progression of the technique of spin lattice relaxation (SLR) -based imaging of oxygen tumors to human subjects.
Validation of the Oxymetry in Animals
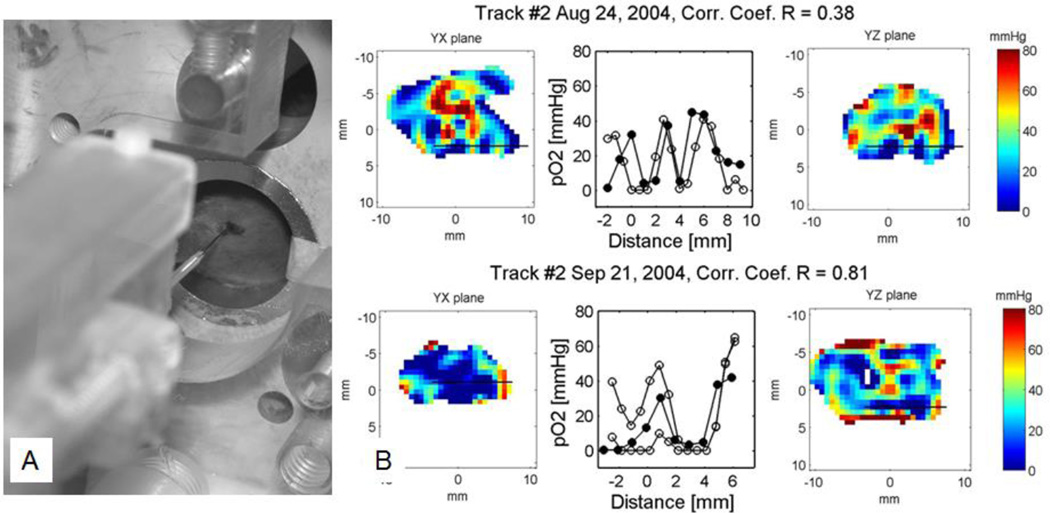
Validation of the biologic relevance of oxygen images in animals has required three sets of experiments. We first compared the pO2 from image voxels (volume elements) with point fluorescence quenching measurements using an Oxylite fiberoptic probe77. We used a stereotactic needle insertion device to locate the end of the 200 µm diameter optical fiber in a FSa mouse fibrosarcoma tumor, and registered it with the EPR oxygen image of the tumor. The Oxylite fiber tip was inserted just after tumor pO2 imaging, inside the imaging resonator/sample holder without disturbing the tumor location. The tumor born by the mouse leg in our resonator with the stereotactically registered fiberoptic probe is shown in Figure 3A. Figure 3B shows examples of 0.7 mm thick pO2 image slices of a mouse leg bearing a tumor. Two orthogonal planes are shown, with a thick line indicating the path of the fiberoptic Oxylite probe, assuming the fiber to pass from the truly registered entrance straight along the axis of the resonator. The pO2 values measured in the Oxylite tracks as the probe was withdrawn from the tumor and the corresponding EPR pO2 values from voxels attributed to the track location are shown between the images.
Figure 3.
A. Leg born mouse tumor in an EPR pO2 imager with an Oxylite fiberoptic pO2 probe being launched into a tumor from a stereotactic frame. B. Two examples of the correlation between EPR and fiberoptic pO2 data. Left and right panels: Sagittal and coronal slices showing tumor pO2. The assumed probe track is shown as a black line in the images. Middle panel: Plot of the oxygen values from the Oxylite™ track (filled circles) and pO2 image (open circles). The remarkable correlation between these values was observed.
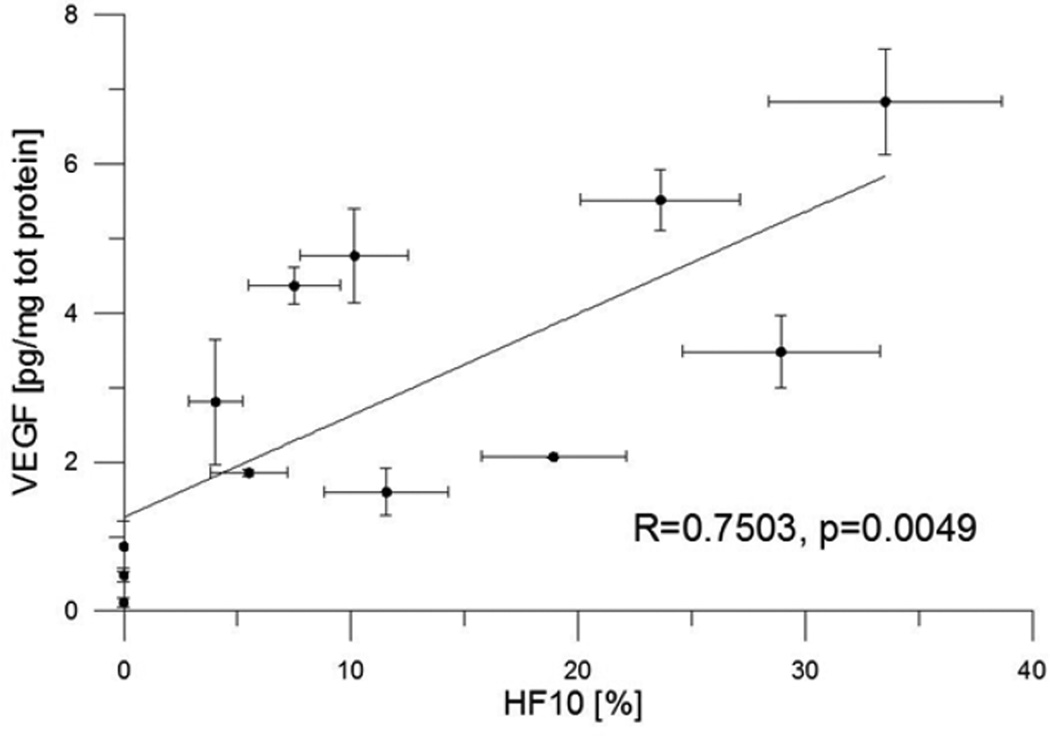
For the second validation, we measured voxel pO2 values within regions of tumors, that were subsequently sampled by a number 12 breast biopsy needle, and compared them with the average levels of hypoxia induced proteins produced by the tumor cells. pO2 images of the tumor were registered with quantitative enzyme-linked immunosorbent assay (ELISA) determinations of the concentration of the hypoxia induced protein Vascular Endothelial Growth Factor from stereotactically localized biopsies from FSa fibrosarcomas. Fig. 4 presents a scatter plot of 17 biopsies, showing typical biological scatter and high statistical significance. About 2/3 of the variation in the protein concentration is associated with the mean pO2, which arises from the image of the volume sampled. Approximately 100 image voxels were localized to each biopsy volume.78
Figure 4.
Correlation between hypoxia and VEGF content. Fraction of volumes in the biopsy with pO2 less than 10 torr, HF10 from the EPR oxygen image vs VEGF concentration in picograms per microgram of total protein.
Locally administered Tumor Necrosis Factor (TNF), produced by viral vectors carrying genes to promote the cellular synthesis of TNF (referred to as TNFerade) injected directly into tumors, has shown dramatic sensitization of tumors to radiation therapy in early human trials. Although this method of treatment of tumors failed a phase 3 trial, there were a number of remarkable cures in phase 2 trials.79 This is contradictory to a history of tumor biology showing that hypoxia in tumors creates resistance to radiation therapy.1 This prompts inquiry as to the contradictory obliteration of tumor vasculature, which, in principle, would increase tumor hypoxia and increase tumor sensitivity to radiation. EPR pO2 images showed80 that tumor pO2 increased after TNF administration. These findings were consistent with the difficulty of chaotic, dysfunctional tumor vasculature to convey the 51 kD TNF protein away from the injection site. Increasing vascular obliteration of intact microvessels, on the other hand, can rid the tumor of TNF. Thus, TNFerade pruned the tumor of chaotic vessels as predicted by Jain et al.81 Consistently, tumor sensitization with the anti-angiogenic drug, Sunitinib, which also interferes with tumor angiogenesis, has been shown to be associated with a similarly paradoxical increase in EPR image-based oxygenation from this anti-blood vessel agent.82 EPR pO2 images provide in situ information defining the molecular biologic response to the microenvironment pO2. EPR imaging is a powerful tool in defining graded molecular biologic response to microenvironment.
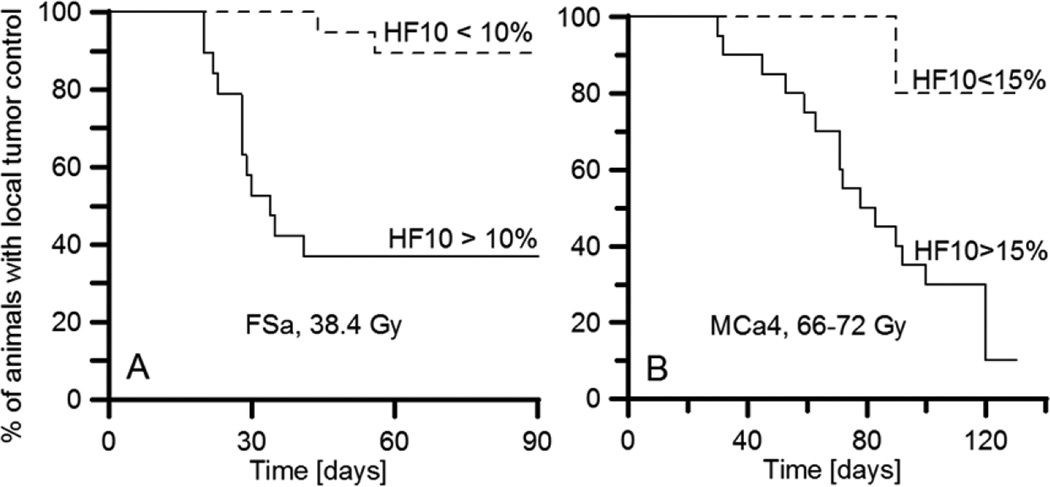
The pO2 images that have been obtained with longitudinal relaxation rate pulsed technology have spatial resolution of approximately 1 mm. A major question to be answered is the biological relevance of this resolution. This third validation asks whether or not these images can show, based on the fractions of tumor voxels with pO2, less than a particular threshold, and also, that they can predict outcomes of radiation treatment of tumors of a given size to a dose sufficient to cure 50% of the tumors. Fig. 5 shows the results of treating two different tumor types, a syngeneic mouse mammary tumor, MCa4, and a syngeneic fibrosarcoma, FSa, grown in the legs of C3H mice. The data demonstrate that a threshold of 10% of voxels with pO2 less than 10 torr for FSa tumors, and 15% for MCa4 tumors, separates the tumor cure probability. Figure 5 dramatically shows that, for both tumor types, the probability for tumor control was significantly better, a factor of two or more better, for the hypoxic fraction less than the threshold than for the hypoxic fraction larger than the threshold. Therefore, the biomedical relevance of images with the 1 mm spatial resolution appears strong.83
Figure 5.
The outcome of a single X-ray dose treatment. Kaplan-Meier plots for two groups of animals separated according to their tumor hypoxic fraction (HF10), a percent of voxels in the pO2 image with pO2 below 10 torr. A. FSA tumors treated with 33.8 Gy. Wilcoxon test shows that HF10 >10% threshold is a significant predictor of tumor failure (p=0.0138). B. MCa4 tumors treated with a single dose in the range of 66–72 Gy. Wilcoxon test shows that HF10 >15% threshold is a significant predictor of tumor failure (p=0.0193).
Transient hypoxia can be imaged with EPR pO2 images
If tumor hypoxic regions are chronic and unchanging with time, then direction of local therapy such as radiation to such regions would be crucially important. However, if tumor pO2 distributions fluctuate wildly, such images become irrelevant. There is data showing fluctuations in tumor blood flow. This can influence oxygen concentrations.84 This may be linked to therapeutic resistance. Dynamic, rapidly acquired images of pO2 are necessary to establish this. Yasui et al. showed that pulsed EPR can monitor fluctuations in oxygen concentrations in mouse models85. Oxygen images acquired every 3 minutes for a total of 30 minutes revealed large fluctuations in pO2 in some tumor regions. Redler et al. demonstrated that peripheral regions of tumors with intermediate levels of hypoxia, and which presumably have more intact vasculature, have larger spontaneous pO2 variations then more central regions of FSa fibrosarcomas.86 This EPR imaging technique, registered with tumor locating MRI, may offer a powerful clinical tool to noninvasively detect variable oxygenation in tumors.
Effectiveness of localizing radiation to regions of the tumor with high hypoxic fraction using EPR pO2 images needs to be tested
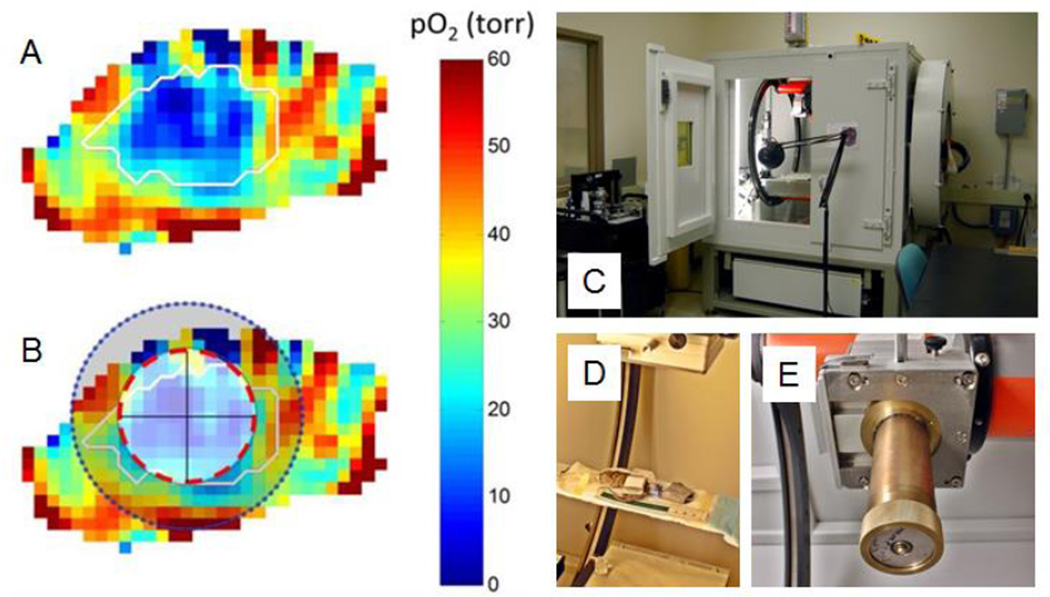
The success noted above in identification of regions within a tumor whose voxels are hypoxic, and showing that large fractions of these voxels induce resistance to a 50% tumor control dose, leads to the question: Does treating only these regions of tumors with extra ‘boost’ dose increase the tumor cure? This needs to be demonstrated in animal models prior to human applications. Figs. 6A and 6B show an hypoxic region defined in a mouse MCa4 breast tumor, and a spherical volume for radiation boost treatment. This boost would be added to a 50% control dose given to the entire tumor. Figs. 6C–E show an XRAD225Cx system to precisely deliver the boost dose to the indicated region. A trial comparing the tumor control using such a boost with treatment to a well oxygenated shell of similar volume (an “anti-boost”) is underway.87
Figure 6.
EPR oxygen image of the leg tumor. A. Oxygen map with tumor contour transferred from the registered MRI image. B. Boost (red line) and anti-boost (shaded area) as determined by the boost planning software. C. D. and E. XRad225Cx small animal cone beam CT imager and irradiator. D. Animal bed. E. Collimator.
Summary
We have brought the technology for the EPR imaging of tumor pO2 to a point where quantitative images of oxygen in the tissues and tumors of living animals, with 1–2 torr pO2 resolution and 1 mm spatial resolution, can be obtained in 10 minutes or faster. We have shown the biologic relevance of the oxygen images and demonstrated that they predict cure from radiation therapy. Rapid acquisition of EPR pO2 images will validate the relevance of transient hypoxia. We are in the process of validation of the potential use of EPR oxygen images in guiding radiation to resistant portions of animal tumors. A path to human applications may be possible.
Acknowledgments
This work was supported by NIH grants P41 EB002034 and R01 CA098575
References
- 1.Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Radiol. 1955;9:539–563. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy KA, Teicher BA, Rockwell S, Sartorelli AC. The hypoxic tumor cell: a target for selective cancer chemotherapy. Biochem Pharmacol. 1980;29:1–8. doi: 10.1016/0006-2952(80)90235-x. [DOI] [PubMed] [Google Scholar]
- 3.Teicher BA. Hypoxia and drug resistance. Cancer Metastasis Rev. 1994;13:139–168. doi: 10.1007/BF00689633. [DOI] [PubMed] [Google Scholar]
- 4.Henk JM, Kunkler PB, Smith CW. Radiotherapy and hyperbaric oxygen in head and neck cancer. Final report of first controlled clinical trial. Lancet. 1977;2:101–103. doi: 10.1016/s0140-6736(77)90116-7. [DOI] [PubMed] [Google Scholar]
- 5.Henk JM, Smith CW. Radiotherapy and hyperbaric oxygen in head and neck cancer. Interim report of second clinical trial. Lancet. 1977;2:104–105. doi: 10.1016/s0140-6736(77)90117-9. [DOI] [PubMed] [Google Scholar]
- 6.Rontgen WC. On a New Kind of Rays. Science. 1896;3:227–231. doi: 10.1126/science.3.59.227. [DOI] [PubMed] [Google Scholar]
- 7.Cormack AM. Representation of a Function by Its Line Integrals with Some Radiological Applications. J Appl Phys. 1963;34:2722-&. [Google Scholar]
- 8.Lauterbur PC. Image Formation by Induced Local Interactions - Examples Employing Nuclear Magnetic-Resonance. Nature. 1973;242:190–191. [PubMed] [Google Scholar]
- 9.Condon EU, Shortley GH. The Theory of Atomic Spectra. Cambridge University Press; 1935. [Google Scholar]
- 10.Lauterbur PC, Levin DN, Marr RB. Theory and simulation of NMR spectroscopic imaging and field plotting by projection reconstruction involving an intrinsic frequency dimension. J Magn Reson. 1984;59:536–541. [Google Scholar]
- 11.Maltempo MM. Differentiation of spectral and spatial components in EPR imaging using 2-D image reconstruction algorithms. J Magn Reson. 1986;69:156–161. [Google Scholar]
- 12.Eastman PE, Kooser RG, Pas MR, Freed JH. Studies of Heisenberg spin exchange in ESR spectra I. Linewidth and saturation effects. J Chem Phys. 1969;54:2690. [Google Scholar]
- 13.Molin YN, Salikhov KM, Zamaraev KI. Spin Exchange: Principles and Applications in Chemistry and Biology. Berlin: Springer-Verlag; 1980. [Google Scholar]
- 14.Swartz HM, Glockner JF. Measurements of Oxygen by EPRI and EPRS. Boca Raton, FL: CRC Press, Inc.; 1991. [Google Scholar]
- 15.Whalen WJ, Riley J, Nair P. A microelectrode for measuring intracellular pO2. J Appl Physiol. 1967;23:798–801. doi: 10.1152/jappl.1967.23.5.798. [DOI] [PubMed] [Google Scholar]
- 16.Harrison DK. Physiological oxygen measurements using oxygen electrodes. In: Wilson DFESYBJPA, editor. Oxygen Transport to Tissue Volume Xxiii: Oxygen Measurements in the 21st Century: Basic Techniques and Clinical Relevance. 2003. pp. 163–167. [Google Scholar]
- 17.Griffiths JR, Robinson SP. The OxyLite: a fibre-optic oxygen sensor. British Journal of Radiology. 1999;72:627–630. doi: 10.1259/bjr.72.859.10624317. [DOI] [PubMed] [Google Scholar]
- 18.Krause BJ, Beck R, Souvatzoglou M, Piert M. PET and PET/CT studies of tumor tissue oxygenation. Quarterly Journal of Nuclear Medicine and Molecular Imaging. 2006;50:28–43. [PubMed] [Google Scholar]
- 19.Ogawa S, Lee TM. MAGNETIC-RESONANCE-IMAGING OF BLOOD-VESSELS AT HIGH FIELDS - INVIVO AND INVITRO MEASUREMENTS AND IMAGE SIMULATION. Magnet Reson Med. 1990;16:9–18. doi: 10.1002/mrm.1910160103. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa S, Lee TM, Kay AR, Tank DW. BRAIN MAGNETIC-RESONANCE-IMAGING WITH CONTRAST DEPENDENT ON BLOOD OXYGENATION. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rumsey WL, Vanderkooi JM, Wilson DF. Imaging of Phosphorescence - a Novel Method for Measuring Oxygen Distribution in Perfused Tissue. Science. 1988;241:1649–1651. doi: 10.1126/science.241.4873.1649. [DOI] [PubMed] [Google Scholar]
- 22.Busse LJ, Pratt RG, Thomas SR. DECONVOLUTION OF CHEMICAL-SHIFT SPECTRA IN TWO-DIMENSIONAL OR 3-DIMENSIONAL F-19 MR IMAGING. Journal of Computer Assisted Tomography. 1988;12:824–835. doi: 10.1097/00004728-198809010-00020. [DOI] [PubMed] [Google Scholar]
- 23.Hunjan S, Zhao D, Constantinescu A, Hahn EW, Antich PP, Mason RP. Tumor oximetry: demonstration of an enhanced dynamic mapping procedure using fluorine-19 echo planar magnetic resonance imaging in the Dunning prostate R3327-AT1 rat tumor. Int J Radiat Oncol Biol Phys. 2001;49:1097–1108. doi: 10.1016/s0360-3016(00)01460-7. [DOI] [PubMed] [Google Scholar]
- 24.Efimova OV, Caia GL, Sun ZQ, et al. Standard-based method for proton-electron double resonance imaging of oxygen. J Magn Reson. 2011;212:197–203. doi: 10.1016/j.jmr.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schweiger A, Jeschke G. Principles of Pulse Electron Paramagnetic Resonance. Oxford University Press; 2001. [Google Scholar]
- 26.Slichter CP. Principles of Magnetic Resonance. New York: Springer-Verlag; 1996. [Google Scholar]
- 27.Abragam A, Bleaney B. EPR of Transition Ions. Oxford: Clarendon; 1970. [Google Scholar]
- 28.Atherton NM. Principles of Electron Spin Resonance. Chichester: Ellis Horwood Ltd; 1993. [Google Scholar]
- 29.Dirac PAM. Principles of Quantum Mechanics. 4th (paperback) Oxford University Press; 1982. [Google Scholar]
- 30.Abragam A. Principles of Nuclear Magnetism. Oxford: Oxford University; 1961. [Google Scholar]
- 31.Ahmad R, Khan M, Vikram D, Bratasz A, Kuppusamy P. EPR Oximetry: Method and Application. In: Das D, editor. Methods in Redox Signaling. Mary Ann Liebert, Inc.; 2010. [Google Scholar]
- 32.Tatum JL, Kelloff GJ, Gillies RJ, et al. Hypoxia: Importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 2006;82:699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]
- 33.Khan N, Williams BB, Hou H, Li H, Swartz HM. Repetitive tissue pO(2) measurements by electron paramagnetic resonance oximetry: Current status and future potential for experimental and clinical studies. Antioxidants & Redox Signaling. 2007;9:1169–1182. doi: 10.1089/ars.2007.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Backer JM, Budker VG, Eremenko SI, Molin YN. Detection of the kinetics of biochemical reactions with oxygen using exchange broadening in the ESR spectra of nitroxide radicals. Biochim Biophys Acta. 1977;460:152–156. doi: 10.1016/0005-2728(77)90161-x. [DOI] [PubMed] [Google Scholar]
- 35.Popp CA, Hyde JS. Effects of Oxygen on Electron-Paramagnetic-Resonance of Nitroxide Spin-Label Probes of Model Membranes. J Magn Reson. 1981;43:249–258. [Google Scholar]
- 36.Swartz HM, Clarkson RB. The measurement of oxygen in vivo using EPR techniques. Physics in Medicine and Biology. 1998;43:1957–1975. doi: 10.1088/0031-9155/43/7/017. [DOI] [PubMed] [Google Scholar]
- 37.Halpern HJ, Yu C, Peric M, Barth E, Grdina DJ, Teicher BA. Oxymetry Deep in Tissues with Low-Frequency Electron-Paramagnetic-Resonance. Proc Natl Acad Sci USA. 1994;91:13047–13051. doi: 10.1073/pnas.91.26.13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zweier JL, Thompsongorman S, Kuppusamy P. Measurement of Oxygen Concentrations in the Intact Beating Heart Using Electron-Paramagnetic Resonance Spectroscopy - a Technique for Measuring Oxygen Concentrations Insitu. J Bioenerg Biomembr. 1991;23:855–871. doi: 10.1007/BF00786005. [DOI] [PubMed] [Google Scholar]
- 39.Kuppusamy P, Chzhan M, Vij K, et al. 3-Dimensional Spectral Spatial EPR Imaging of Free-Radicals in the Heart - a Technique for Imaging Tissue Metabolism and Oxygenation. Proc Natl Acad Sci USA. 1994;91:3388–3392. doi: 10.1073/pnas.91.8.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mailer C, Robinson BH, Williams BB, Halpern HJ. Spectral fitting: The extraction of crucial information from a spectrum and a spectral image. Magnet Reson Med. 2003;49:1175–1180. doi: 10.1002/mrm.10474. [DOI] [PubMed] [Google Scholar]
- 41.Robinson BH, Mailer C, Reese AW. Linewidth analysis of spin labels in liquids - I. Theory and data analysis. J Magn Reson. 1999;138:199–209. doi: 10.1006/jmre.1999.1737. [DOI] [PubMed] [Google Scholar]
- 42.Robinson BH, Mailer C, Reese AW. Linewidth analysis of spin labels in liquids - I. Experimental. J Magn Reson. 1999;138:210–219. doi: 10.1006/jmre.1999.1738. [DOI] [PubMed] [Google Scholar]
- 43.Swartz HM, Khan N, Buckey J, et al. Clinical applications of EPR: overview and perspectives. NMR Biomed. 2004;17:335–351. doi: 10.1002/nbm.911. [DOI] [PubMed] [Google Scholar]
- 44.Wilson JD, Braunwald E, Isselbacher KJ, et al. Harrison's Principles of Internal Medicine. 12. New York: McGraw-Hill; 1991. [Google Scholar]
- 45.Eaton SS, Eaton GR. Biological Magnetic Resonance. New York: Kluwer Academic/Plenum Publishers; 2000. Relaxation Times of Organic Radicals and Transition Metal Ions. [Google Scholar]
- 46.Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors. Harrison's Principles of Internal Medicine. 18. New York, NY: McGraw Hill; 2011. [Google Scholar]
- 47.Halpern HJ, Spencer DP, Vanpolen J, et al. Imaging Radio-Frequency Electron-Spin-Resonance Spectrometer with High-Resolution and Sensitivity for Invivo Measurements. Rev Sci Instrum. 1989;60:1040–1050. [Google Scholar]
- 48.Bottomley PA, Andrew ER. RF magnetic field penetration, phase shift and power dissipation in biological tissue: implications for NMR imaging. Phys Med Biol. 1978;23:630–643. doi: 10.1088/0031-9155/23/4/006. [DOI] [PubMed] [Google Scholar]
- 49.Mailer C, Sundramoorthy SV, Pelizzari CA, Halpern HJ. Spin echo spectroscopic electron paramagnetic resonance imaging. Magn Reson Med. 2006;55:904–912. doi: 10.1002/mrm.20849. [DOI] [PubMed] [Google Scholar]
- 50.Epel B, Bowman MK, Mailer C, Halpern HJ. Absolute oxygen R1 imaging in vivo with pulse electron paramagnetic resonance. Magn Reson Med. 2014;72:362–368. doi: 10.1002/mrm.24926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dunn JW, Swartz HM, editors. Oxygen Transport to Tissue XXIV. New York: Kluwer Academic/Plenum Publishers; 2003. [Google Scholar]
- 52.Berliner LJ, editor. In Vivo EPR (ESR): Theory & Applications. New York: Kluwer Academic; 2003. [Google Scholar]
- 53.Berliner LJ, Fujii H. Magnetic-Resonance Imaging of Biological Specimens by Electron-Paramagnetic Resonance of Nitroxide Spin Labels. Science. 1985;227:517–519. doi: 10.1126/science.2981437. [DOI] [PubMed] [Google Scholar]
- 54.Biller JR, Meyer V, Elajaili H, et al. Relaxation times and line widths of isotopically-substituted nitroxides in aqueous solution at X-band. J Magn Reson. 2011;212:370–377. doi: 10.1016/j.jmr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ardenkjaer-Larsen JH, Laursen I, Leunbach I, et al. EPR and DNP properties of certain novel single electron contrast agents intended for oximetric imaging. J Magn Reson. 1998;133:1–12. doi: 10.1006/jmre.1998.1438. [DOI] [PubMed] [Google Scholar]
- 56.Pandian RP, Parinandi NL, Ilangovan G, Zweier JL, Kuppusamy P. Novel particulate spin probe for targeted determination of oxygen in cells and tissues. Free Radical Biology and Medicine. 2003;35:1138–1148. doi: 10.1016/s0891-5849(03)00496-9. [DOI] [PubMed] [Google Scholar]
- 57.Williams BB, al Hallaq H, Chandramouli GV, et al. Imaging spin probe distribution in the tumor of a living mouse with 250 MHz EPR: correlation with BOLD MRI. Magn Reson Med. 2002;47:634–638. doi: 10.1002/mrm.10089. [DOI] [PubMed] [Google Scholar]
- 58.Matsumoto K, English S, Yoo J, et al. Pharmacokinetics of a triarylmethyl-type paramagnetic spin probe used in EPR oximetry. Magnet Reson Med. 2004;52:885–892. doi: 10.1002/mrm.20222. [DOI] [PubMed] [Google Scholar]
- 59.Krishna MC, English S, Yamada K, et al. Overhauser enhanced magnetic resonance imaging for tumor oximetry: Coregistration of tumor anatomy and tissue oxygen concentration. Proc Natl Acad Sci USA. 2002;99:2216–2221. doi: 10.1073/pnas.042671399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu KJ, Gast P, Moussavi M, et al. Lithium Phthalocyanine - a Probe for Electron-Paramagnetic-Resonance Oximetry in Viable Biological-Systems. Proc Natl Acad Sci USA. 1993;90:5438–5442. doi: 10.1073/pnas.90.12.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He GL, Shankar RA, Chzhan M, Samouilov A, Kuppusamy P, Zweier JL. Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proc Natl Acad Sci USA. 1999;96:4586–4591. doi: 10.1073/pnas.96.8.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ilangovan G, Bratasz A, Kuppusamy P. Non-invasive measurement of tumor oxygenation using embedded microparticulate EPR spin probe. Oxygen Transport to Tissue Xxvi. 2005;566:67–73. doi: 10.1007/0-387-26206-7_10. [DOI] [PubMed] [Google Scholar]
- 63.Ilangovan G, Bratasz A, Li H, Schmalbrock P, Zweier JL, Kuppusamy P. In vivo measurement and imaging of tumor oxygenation using coembedded paramagnetic particulates. Magn Reson Med. 2004;52:650–657. doi: 10.1002/mrm.20188. [DOI] [PubMed] [Google Scholar]
- 64.Meenakshisundaram G, Pandian RP, Eteshola E, Lee SC, Kuppusamy P. A paramagnetic implant containing lithium naphthalocyanine microcrystals for high-resolution biological oximetry. J Magn Reson. 2010;203:185–189. doi: 10.1016/j.jmr.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Epel B, Halpern HJ. Electron paramagnetic resonance oxygen imaging in vivo. In: Gilbert BC, Chechik V, Murphy DM, editors. Electron Paramagnetic Resonance. Cambridge, UK: RSC Publishing; 2012. [Google Scholar]
- 66.Epel B, Sundramoorthy SV, Mailer C, Halpern HJ. A versatile high speed 250-MHz pulse imager for biomedical applications. Concepts in Magnetic Resonance Part B: Magnetic Resonance Engineering. 2008;33B:163–176. doi: 10.1002/cmr.b.20119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Subramanian S, Devasahayam N, Murugesan R, et al. Single-point (constant-time) imaging in radiofrequency Fourier transform electron paramagnetic resonance. Magn Reson Med. 2002;48:370–379. doi: 10.1002/mrm.10199. [DOI] [PubMed] [Google Scholar]
- 68.Epel B, Halpern HJ. Comparison of pulse sequences for R-based electron paramagnetic resonance oxygen imaging. J Magn Reson. 2015;254:56–61. doi: 10.1016/j.jmr.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khan N, Hou H, Hein P, et al. Black Magic and EPR Oxymetry: From Lab to Initial Clinical Trials. New York: Plenum Publishers; 2005. [DOI] [PubMed] [Google Scholar]
- 70.Salikhov I, Walczak T, Lesniewski P, et al. EPR spectrometer for clinical applications. Magn Reson Med. 2005;54:1317–1320. doi: 10.1002/mrm.20689. [DOI] [PubMed] [Google Scholar]
- 71.Khan N, Williams BB, Hou H, Li H, Swartz HM. Repetitive tissue pO2 measurements by electron paramagnetic resonance oximetry: current status and future potential for experimental and clinical studies. Antioxid Redox Signal. 2007;9:1169–1182. doi: 10.1089/ars.2007.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Williams BB, Khan N, Zaki B, Hartford A, Ernstoff MS, Swartz HM. Clinical electron paramagnetic resonance (EPR) oximetry using India ink. Adv Exp Med Biol. 2010;662:149–156. doi: 10.1007/978-1-4419-1241-1_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ellis SJ, Velayutham M, Velan SS, et al. EPR oxygen mapping (EPROM) of engineered cartilage grown in a hollow-fiber bioreactor. Magn Reson Med. 2001;46:819–826. doi: 10.1002/mrm.1262. [DOI] [PubMed] [Google Scholar]
- 74.Zweier JL, Chzhan M, Wang PH, Kuppusamy P. Spatial and Spectral-Spatial EPR Imaging Of Free Radicals and Oxygen In the Heart. Research On Chemical Intermediates. 1996;22:615–624. [Google Scholar]
- 75.Epel B, Haney CR, Hleihel D, Wardrip C, Barth ED, Halpern HJ. Electron paramagnetic resonance oxygen imaging of a rabbit tumor using localized spin probe delivery. Med Phys. 2010;37:2553–2559. doi: 10.1118/1.3425787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Epel B, Sundramoorthy SV, Barth ED, Mailer C, Halpern HJ. Comparison of 250 MHz electron spin echo and continuous wave oxygen EPR imaging methods for in vivo applications. Med Phys. 2011;38:2045–2052. doi: 10.1118/1.3555297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elas M. Electron Paramagnetic Resonance Oxygen Images Correlate Spatially and Quantitatively with Oxylite Oxygen Measurements. Clinical Cancer Research. 2006;12:4209–4217. doi: 10.1158/1078-0432.CCR-05-0446. [DOI] [PubMed] [Google Scholar]
- 78.Elas M, Hleihel D, Barth ED, et al. Where it's at really matters: in situ in vivo vascular endothelial growth factor spatially correlates with electron paramagnetic resonance pO2 images in tumors of living mice. Mol Imaging Biol. 2011;13:1107–1113. doi: 10.1007/s11307-010-0436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mundt AJ, Vijayakumar S, Nemunaitis J, et al. A Phase I trial of TNFerade biologic in patients with soft tissue sarcoma in the extremities. Clin Cancer Res. 2004;10:5747–5753. doi: 10.1158/1078-0432.CCR-04-0296. [DOI] [PubMed] [Google Scholar]
- 80.Haney CR, Parasca AD, Fan X, et al. Characterization of response to radiation mediated gene therapy by means of multimodality imaging. Magn Reson Med. 2009;62:348–356. doi: 10.1002/mrm.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsumoto S, Batra S, Saito K, et al. Antiangiogenic agent sunitinib transiently increases tumor oxygenation and suppresses cycling hypoxia. Cancer Res. 2011;71:6350–6359. doi: 10.1158/0008-5472.CAN-11-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elas M, Magwood JM, Butler B, et al. EPR oxygen images predict tumor control by a 50% tumor control radiation dose. Cancer Res. 2013;73:5328–5335. doi: 10.1158/0008-5472.CAN-13-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown JM. Evidence for Acutely Hypoxic Cells in Mouse-Tumors, and a Possible Mechanism of Re-Oxygenation. Br J Radiol. 1979;52:650–656. doi: 10.1259/0007-1285-52-620-650. [DOI] [PubMed] [Google Scholar]
- 85.Yasui H, Matsumoto S, Devasahayam N, et al. Low-Field Magnetic Resonance Imaging to Visualize Chronic and Cycling Hypoxia in Tumor-Bearing Mice. Cancer Res. 2010;70:6427–6436. doi: 10.1158/0008-5472.CAN-10-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Redler G, Epel B, Halpern HJ. EPR image based oxygen movies for transient hypoxia. Adv Exp Med Biol. 2014;812:127–133. doi: 10.1007/978-1-4939-0620-8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Epel B, Redler G, Pelizzari C, Tormyshev VM, Halpern HJ. Approaching oxygen-guided intensity-modulated radiation therapy. Adv Exp Med Biol. 2015 doi: 10.1007/978-1-4939-3023-4_23. in Press:in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]