Abstract
Non-functioning pituitary adenomas (NFPAs) may be locally invasive. Surgery is a treatment option, but unlike the case for functional pituitary adenomas, there are almost no drug treatments available for NFPAs. Markers of invasiveness are needed to guide therapeutic decision-making and identify potential adjuvant drugs. Owing to the highly heterogeneous nature of NFPAs, little is known regarding the subtype-specific gene expression profiles associated with invasiveness. To identify important biomarkers of invasiveness, we selected 23 null cell adenomas and 20 oncocytomas. These tumors were classified as invasive or non-invasive adenomas based on magnetic resonance imaging, pathology slides and surgical findings. Firstly, we observed that there were significant differences in expression between invasive (n = 3) and non-invasive (n = 4) adenomas by gene expression microarray. A total of 1,188 genes were differentially expressed in the invasive and non-invasive adenomas. Among these 1,188 genes, 578 were upregulated and 610 were downregulated in invasive adenomas. Secondly, the expression of ENC1, which displayed the significant alterations, was further confirmed by qRT-PCR and Western blot analysis in all 43 tumor samples and three normal pituitary glands. Low levels of ENC1 were found in tumor samples, while high levels were detected in normal pituitary glands. Interestingly, the ENC1 expression level was low in invasive null cell adenomas compared with non-invasive adenomas, but this relationship was not observed in invasive oncocytomas. Immunohistochemistry also demonstrated that the staining of ENC1 was different between invasive and non-invasive null cell adenomas. In addition, bioinformatics studies, including gene ontology and protein interaction analyses, were also performed to better understand the critical role of ENC1 in the development and progression of null cell adenomas and oncocytomas. Consequently, ENC1 may be an important biomarker for null cell adenomas and oncocytomas, and it is specific to invasive null cell adenomas.
Keywords: Invasiveness, ENC1, NFPAs, Null cell adenomas, Oncocytomas
Introduction
Pituitary adenomas are being recognized and diagnosed with increasing frequency. One of the most common forms of pituitary lesions is the clinically non-functioning pituitary adenomas (NFPAs). NFPAs represent approximately one-third of pituitary adenomas [1]. Although NFPAs have a benign histological appearance, most of them exhibit characteristics of local invasion, spreading into surrounding structures. This capacity for invasive local growth not only precludes complete surgical excision but also imposes a significant neurological morbidity, including headaches, impaired vision, visual field deficiency, double vision and ptosis. To date, few drugs have been used for therapy, but the effect is controversial. Surgery is the predominant treatment option. Therefore, an alternative treatment capable of managing invasive NFPAs is needed. In the search to develop a logical treatment strategy, several investigators have attempted to understand the pathophysiology underlying the invasive behavior of NFPAs.
Recent advances in the field of molecular pathology have enabled scientists to gain deeper insight into numerous genetic alterations underlying the development of pituitary adenomas, but few data are available on the molecular factors underlying the invasiveness of NFPAs. Several biological markers have been investigated and appear to correlate with the invasive behavior of pituitary adenomas. For example, a role for fibroblast growth factors (FGFs) and their receptors (FGFRs) has been suggested. High expression of FGF mRNA is associated with invasive pituitary tumors [2]. Expression of FGFR4 induces invasive growth of pituitary tumor cells in vivo [3, 4]. Matrix metalloproteases (MMPs), particularly MMP-9, are expressed at significantly higher levels in various types of invasive pituitary adenomas compared with non-invasive pituitary adenomas [2, 5]. Overexpression of the pituitary tumor transforming gene (PTTG) has been related to pituitary adenoma invasion [2]. It was also reported that a higher PTTG/Ki-67 score predicted biologically invasive behavior of pituitary adenomas [6]. TP53 expression has been linked to invasive tumor behavior in pituitary tumors [2]. Additionally, abnormal expression of miRNAs, which are small endogenous noncoding RNAs, has been linked to neoplasia in the pituitary gland [2]. Reduced expression of miR-15a and miR-16-1 has been linked to tumor size in pituitary adenomas [2, 7]. Recent studies also demonstrated higher levels of invasiveness in benign tumors with chromosomal instabilities, regardless of their histological grade.
Non-functioning pituitary adenomas are highly heterogeneous and could be classically subdivided into null cell adenomas, oncocytomas, and gonadotrophic adenomas. Divisions are based on ultrastructural and immunohistochemical characteristics. Subtypes of null cell adenomas and oncocytomas were selected for further study based on negative hormonal pathological immunostaining in the present study. The lack of hormone expression in the two tumor subtypes suggests that they are non-functional adenomas as well as clinically non-functional adenomas. Oncocytomas are a variant of the null cell adenoma with a WHO classification of null cell adenoma, oncocytic variant. These tumors have similar characteristics, including low levels of cytoplasmic organelles, involvement in hormone synthesis and release, and sparse and small secretory granules. However, oncocytomas contain many mitochondria, unlike null cell adenomas [8].
To date, the genetic factors associated with invasiveness in null cell adenomas and oncocytomas are still unclear. Additionally, it is unknown whether genetic biomarkers are different between these two tumor types. There are currently no gene expression microarray studies that have investigated the genetic factors of invasive null cell adenomas and invasive oncocytomas. In the current study, we used gene expression microarrays to identify biomarkers that could play a key role in the invasive behavior of null cell adenomas and oncocytomas to better guide therapeutic decision-making and identify potentially beneficial adjuvant drugs.
Subjects and methods
Subjects
Forty-three NFPAs tumor tissues were obtained after transsphenoidal surgery at Beijing Tiantan Hospital. The clinical and pathological data are shown in Table 1 and Supplementary Table 1 and include age, gender, clinical hormonal findings, pathological findings, electron microscope findings, preoperative imaging, and intraoperative studies. The set of pituitary tumors that were analyzed included 23 null cell adenomas and 20 oncocytomas. Seven of the 43 samples were used for gene expression microarray analysis. All 43 NFPAs were used to validate the set of genes differentially expressed in the microarray analysis.
Table 1.
Clinical characteristics of patients with NFPAs
| Clinical data | NFPAs (n = 43) | Null cell adenomas (n = 23) | Oncocytomas (n = 20) | p value |
|---|---|---|---|---|
| Age (yr) | 51.54 ± 10.50 | 50.95 ± 11.31 | 51.85 ± 9.89 | 0.786 |
| Invasion | 53.54 ± 9.38 | 54.09 ± 8.62 | 53.10 ± 8.40 | 0.132 |
| Non-invasion | 48.63± 11.56 | 49.11 ± 11.14 | 50.60 ± 11.51 | |
| Gender (M/F) | 25/19 | 7/16 | 17/3 | 0.0003 |
| Headache | 39.5 % | 43.5 % | 35 % | 0.571 |
| Visual deficits | 44.2 % | 39.1 % | 50 % | 0.474 |
| Visual field defects | 30.2 % | 21.7 % | 40 % | 0.193 |
| Ptosis | 2.3 % | 4.3 % | 0 | 1.000 |
| Abnormal prolactin | 25.6 % | 30.4 % | 20 % | 0.252 |
| Hypothyroidism | 44.2 % | 34.8 % | 50 % | 0.738 |
| Hypogonadism | 46.5 % | 39.1 % | 60 % | 0.105 |
| Cystic change | 27.9 % | 30.4 % | 25 % | 0.692 |
| Invasive tumors | 55.8 % | 60.9 % | 50 % | 0.474 |
| Hardy grade IV | 30.2 % | 39.1 % | 20 % | 0.457 |
| Suprasellar C grade | 34.9 % | 26.1 % | 45 % | 0.113 |
| Knosp grade IV | 27.9 % | 34.8 % | 20 % | 0.035 |
| Anterior skullbase invasion | 9.3 % | 8.7 % | 10 % | 0.883 |
| Clivus invasion | 16.3 % | 21.7 % | 10 % | 0.298 |
| Brainstem invasion | 9.3 % | 4.3 % | 15 % | 0.230 |
| Sphenoid sinus invasion | 34.9 % | 43.5 % | 25 % | 0.205 |
| Recurrence | 16.2 % | 13 % | 20 % | 0.538 |
Portions of the surgical specimens not used for histology were frozen at −80 ºC in isopentane and stored in liquid nitrogen. Three healthy pituitary glands were used as controls and were obtained from three adult males with no evidence of any endocrine abnormality within 12 h of their deaths. All three of these normal donors died from accidents, and their pituitary glands had not been damaged.
Tumor invasiveness was defined based on preoperative magnetic resonance imaging (MRI), intra-operative records and pathology slides. The tumors were considered invasive adenomas if they met any of the following criteria: (1) Grade 4 according to the Knosp grading system [9], (2) Grade 2/3 according to the Knosp grading system combined with any type of extrasellar extension (eroding bone tissue of sellar floor/clivus, or invading into unilateral/bilateral cavernous sinus) on intra-operative records, and/or with dural invasion on the pathology slides. There were a total of 10 invasive and 10 non-invasive oncocytomas, as well as 14 invasive and 9 non-invasive null cell adenomas, which are presented in Table 1.
The research ethics committee of Tiantan Hospital approved this study. Informed consent was obtained from all enrolled subjects, and the study was carried out in full compliance with all principles of the Helsinki Declaration.
Total RNA extraction
Total RNA was extracted and purified using the mir- Vana™ miRNA Isolation Kit (Cat#AM1561, Ambion, Austin, TX, US) following the manufacturer’s instructions. An Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, US) was used to obtain a RIN number and to inspect RNA integration. Only those samples that showed no degradation (2100 RIN ≥ 7.0 and 28S/18S ≥ 0.7) were used to generate labeled targets.
Microarray hybridization
Total RNA was amplified and labeled using the Low Input Quick Amp Labeling Kit, One-Color (Cat#5190–2305, Agilent Technologies, Santa Clara, CA, US), following the manufacturer’s instructions. Labeled cRNAwas purifiedwith the RNeasy mini kit (Cat#74106, QIAGEN, GmBH, Germany). Each slide was hybridized with 1.65 μg of Cy3- labeled cRNA using the Gene Expression Hybridization Kit (Cat#5188–5242, Agilent Technologies) in a hybridization oven (Cat#G2545A, Agilent Technologies), according to the manufacturer’s instructions. After 17 h of hybridization, slides were washed in staining dishes (Cat#121, Thermo Shandon, Waltham, MA, US) with the Gene Expression Wash Buffer Kit (Cat#5188–5327, Agilent Technologies), following the manufacturer’s instructions. Slides were scanned with an Agilent Microarray Scanner (Cat#G2565CA, Agilent Technologies) using default settings (dye channel: green, scan resolution: 5 μm, PMT: 100, 10 %, 16 bit). Data were extracted with the Feature Extraction software 10.7 (Agilent Technologies). Raw data were normalized by the Quantile algorithm using Gene Spring Software 11.0 (Agilent Technologies).
Reverse transcription and quantitative real-time PCR analysis
Reverse transcription was performed with the RevertAid First Strand cDNA Synthesis Kit (Cat#1621, Thermo Shandon, Waltham, MA, US) based on the kit’s protocol. Ectodermal-neural cortex 1(ENC1) was chosen for testing by quantitative real-time PCR (qPCR). The GAPDH housekeeping gene was used as an endogenous control to normalize the expression level of target genes. qPCR was performed with an ABI 7500 Fast Real-Time PCR System using Platinum® SYBR® Green QPCR SuperMix-UDG (Cat#11744–100, Invitrogen, Grand Island, NY, US).
Protein preparation and Western blot analysis
Protein extraction from pituitary adenomas and normal pituitary gland tissues was performed using a total protein extraction kit (Cat#2140, Millipore, Billerica, MA, US). Protein concentrations were measured using the BCA protein assay kit (Cat#23225, Pierce, Rockford, IL, US). Soluble proteins (30 μg) were separated by electrophoresis in 10 % sodium dodecyl sulfate polyacrylamide gels, transferred to nitrocellulose membranes, and incubated with blocking buffer (5 % non-fat milk) in Tris-buffered saline/Tween-20 (TBST) for 1 h at room temperature. Membranes were then probed with the corresponding primary antibody (Cat#ab124902, Rabbit Anti-ENC1 antibody, Abcam, Cambridge, UK and Cat#G8795, mouse Anti-GAPDH antibody, Sigma, MO, US) at a dilution of 1:1,000 overnight at 4 ºC, followed by three 10-min washes with TBST. Subsequently, membranes were incubated with secondary antibodies conjugated with horseradish peroxidase at room temperature for 1 h. Enhanced chemiluminescence was performed according to the manufacturer’s instructions (Amersham Pharmacia Biotech, Piscataway, NJ, US) to reveal positive bands that were visualized following exposure on a transparent medical X-ray film. The final data were subjected to grayscale scanning and semi-quantitative analysis using the Quantity One software (Bio-Rad, Hercules, CA, US).
Immunohistochemical staining
Formalin-fixed, paraffin-embedded tissues were studied by immunohistochemistry (IHC). A routine immunohistochemical method was followed using appropriate positive and negative controls for each antibody. IHC was performed using specific antibodies against ENC1, Ki-67, and mitochondria. The following antibodies were used at a 1:500 dilution: anti-Ki-67 antibody (Cat#sc-15402, Santa Cruz, Texas, US), anti-ENC1 antibody, and anti-mitochondria antibody (AMA) (Cat#ab12 4902 and Cat#ab92824, Abcam, Cambridge, UK). The incubation period was usually overnight at 4 ºC.
Statistical and clustering analyses
All statistical analyses were conducted using the SPSS Statistics Version 22 (IBM Corporation, Armonk, New- York, US) and SBC Analysis System 2.9 software packages (Shanghai Biotechnology Corporation, Shanghai, China). An unpaired Student’s t test and a Chi square (Fisher’s exact) test were used to compare quantitative and qualitative data. A logistic regression was performed to assess the correlation of the Ki-67 index with invasion and ENC1 gene expression level. A threshold of 3 % was used for the Ki-67 index of risk. Student’s t-test and SAM were performed to calculate the differential expression and false discovery rate (FDR) between tumor samples and normal pituitary glands as well as between invasive tumor samples and non-invasive tumor samples. Filtering was performed to identify genes overexpressed or underexpressed by at least 2.0-fold and to determine q<5 % in tumor samples compared with normal pituitary glands as well as in invasive tumor samples compared with non-invasive tumor samples. Similar to the p value, the q value indicates the probability that the expression differences (FDR) between the two sets of data were due to chance. Hierarchical cluster analysis was performed to create a heat map of the differentially expressed genes using the R programming language of SBC Analysis System software packages. We utilized functional annotation databases as well as the Biological Process, Molecular Function and Cellular Component classifications from Gene Ontology (GO) (available at www.geneontology.org). Protein interaction analyses were performed by STRING Interaction Network. A p value<0.05 was considered statistically significant.
Results
Clinical characteristics of patients
Of the 43 patients included in the study, 23 were diagnosed with null cell adenomas (7 men and 16 women), and 20 were diagnosed with oncocytomas (17 men and 3 women). The Chi square test showed that there were significantly more female and male patients with null cell adenomas and oncocytomas, respectively (p = 0.0003). The age of the patients at the time of surgery was 50.95 ± 11.31 years (range, 31–74 years) in the null cell adenoma group and 51.85 ± 9.89 years (range, 30–66 years) in the oncocytoma group. Student’s t-test showed that patients with noninvasive tumors were younger than those with invasive tumors, but this difference was not statistically significant (Table 1). All tumors were sporadic, and the most common clinical symptom in patients with NFPAs was a visual deficit (44.2 %), followed by headache (39.5 %), visual field defect (30.2 %), and ptosis (2.3 %). However, there was no significantly different clinical symptom distribution frequency between null cell adenoma and oncocytoma patients. The frequencies of hormonal abnormality at the time of diagnosis were as follows: hypogonadism, 46.5 %; hypothyroidism, 44.2 %; and abnormal prolactin, 25.6 %. The frequencies of invasive tumors in null cell adenomas and oncocytomas were 60.9 % and 55 %, respectively. The median follow-up was 34 months (range, 25–48). During follow-up, 5 of the 25 (20 %) patients with invasive tumors had recurrences.
Expression of invasion-related genes
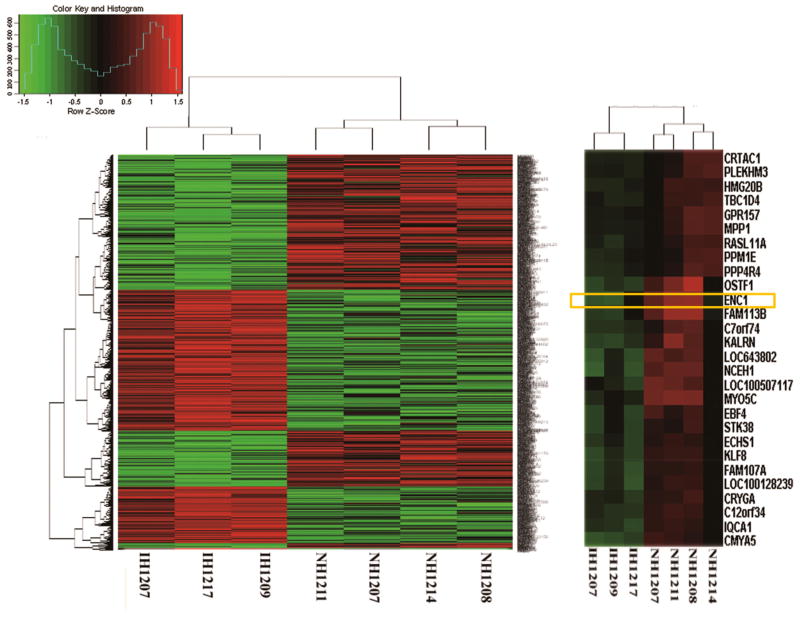
Firstly, we observed that there were significant differences in expression between invasive (n = 3) and non-invasive (n = 4) tumor samples by gene expression microarray. A-total of 1,188 genes were differentially expressed in the invasive and non-invasive tumor samples (p.value and FDR both<0.05, with a fold change>2). Among these 1,188 genes, 578 were upregulated and 610 were downregulated in invasive tumor samples. A heat map with two-dimensional hierarchical clustering (Fig. 1) illustrated that the genes analyzed clearly segregated patients into two groups, namely, those with invasive and non-invasive tumor samples. A total of 773 genes that were found to significantly differentiate between invasive and non-invasive tumor samples were entered into the GO database. In the GO molecular function database, most of the genes (557) were related to binding, as shown in Supplementary Fig. 1. Additionally, differential expression of 1,917 and 2,270 genes was observed when comparing non-invasive tumor samples (n = 4) and normal pituitary glands (n = 3) and invasive tumor samples (n = 3) and normal pituitary glands (n = 3), respectively. The heatmaps are shown in Supplementary Fig. 2. The microarray data has been deposited in the GEO database (http://www.ncbi.nlm.nih.gov/geo/). To identify invasion-related genes, we selected for those genes that were differentially expressed between two of the three groups, namely, invasive tumor samples, non-invasive tumor samples, and normal pituitary glands. This filtering revealed that 35 genes were more highly expressed and that 21 had reduced expression in invasive tumor samples compared with non-invasive tumor samples and normal pituitary glands (Supplementary Table 3). The ENC1 gene was one of the genes identified as having significant decreased expression in tumor samples compared with normal pituitary glands as well as in invasive compared with non-invasive tumor samples. STRING Interaction Network showed the ENC1 protein was strongly associated with RB, which is a key regulator of entry into cell division that acts as a tumor suppressor.
Fig. 1.
A heat map illustrated that the 1,188 genes clearly segregate patients into invasive and non-invasive NFPAs. The horizontal axis is the sample number. The vertical coordinates are the gene names. The ENC-1 gene was selected for validation. In regard to the color key and histogram, the horizontal axis is the z-score (z-score = (gene expression value-mean)/square deviation). The vertical coordinates are the probe set counts
ENC1 gene expression analyses by qRT-PCR
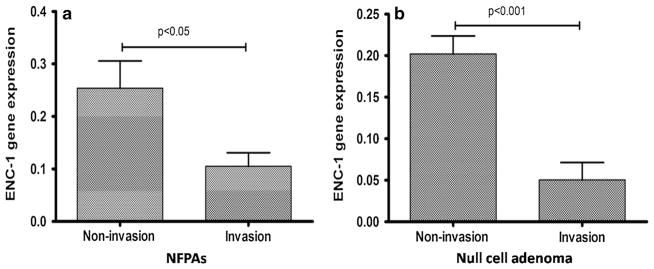
The ENC1 gene was selected for qRT-PCR in a larger series of sporadic NFPAs, including 23 null cell adenomas, 20 oncocytomas (Supplementary Table 1), and three normal pituitary glands (N1–N3). The median ENC1 mRNA expression levels (ENC1/GAPDH) were 0.105 and 0.253 in invasive tumors and non-invasive tumors, respectively. ENC1 mRNA expression was significantly lower in invasive tumors than in non-invasive tumors (p = 0.010; Fig. 2). Furthermore, ENC1 was found to be associated with invasion in null cell adenomas but not in oncocytomas. Lower ENC1 mRNA levels were confirmed in invasive null cell adenomas compared with non-invasive null cell adenomas (p = 8.75 × 10−5; Fig. 2). A correlation of the Ki-67 index with invasion and ENC1 expression level was not observed in this study (Supplementary Table 5).
Fig. 2.
a ENC-1 mRNA expression was significantly lower in invasive NFPAs than in non-invasive NFPAs (p = 0.010). b Significant lower mRNA levels of ENC-1 were shown in invasive null cell adenomas compared to noninvasive null cell adenomas (p = 8.75 × 10−5)
Protein analysis of ENC1 by Western blot and IHC
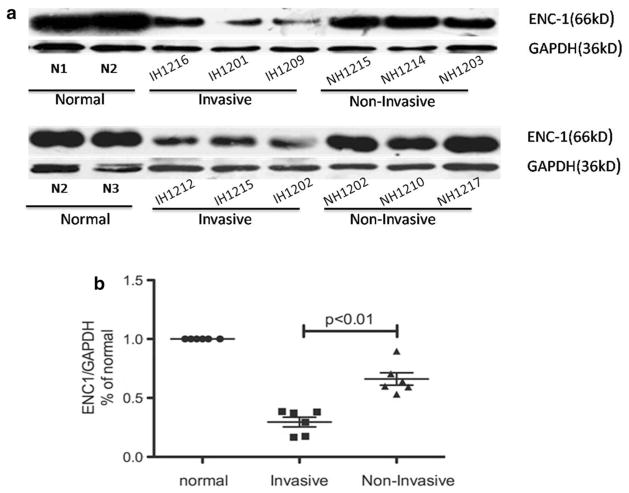
Western blot analyses showed that ENC1 expression was significantly lower in invasive null cell adenomas compared with non-invasive null cell adenomas and normal pituitary glands (p = 0.0003; Fig. 3). However, no significant difference in ENC1 gene expression was identified in oncocytomas (p = 0.370; Fig. 3), indicating that ENC1 may be associated with the invasive phenotype of null cell adenomas.
Fig. 3.
a and b ENC-1 protein expression among invasive null cell adenomas, non-invasive null cell adenomas and normal pituitary glands by Western blot methods. Percentage of normal means that ENC1 was assessed by immunoblotting with an ENC1-specific antibody in relation to GAPDH and normalized to the ENC1/GAPDH levels of normal pituitary tissue
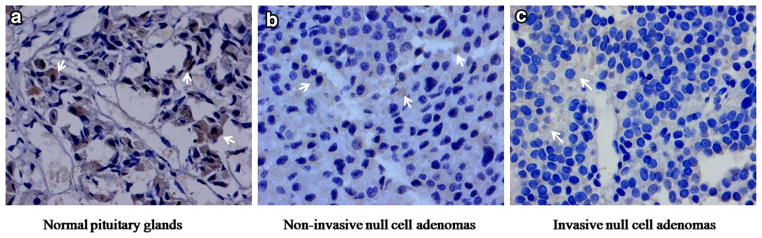
In three normal pituitary samples, ENC1 was strongly expressed in the cytoplasm. In NFPAs, positive immunostaining of ENC1 also showed cytoplasmic localization. The immunostaining results are provided in Fig. 4. The ENC1 staining was lighter in invasive null cell adenomas than in non-invasive null cell adenomas, and it was darker in normal pituitary tissues.
Fig. 4.
Positive products of ENC1 immunostaining showed a fine granular or cloudy distribution in the cytoplasm (indicated by the arrows). The staining was lighter in invasive null cell adenomas compared to non-invasive null cell adenomas and normal pituitary glands
The AMA was found to have stained 20 oncocytomas and 23 null cell adenomas, which was consistent with results that were observed by electron microscopy; these data are shown in Supplementary Fig. 4.
Discussion
In the present study, the study strategy was as follows: (1) to identify differentially expressed genes between NFPAs (null cell adenoma and oncocytoma) and normal pituitary glands and between invasive and non-invasive NFPAs (null cell adenoma and oncocytoma) by gene expression microarray; (2) to validate the association of the identified differentially expressed gene (ENC1) with invasion; and (3) to observe whether the ENC1 expression level was different between invasive null cell adenomas and invasive oncocytomas. The goal of our experiments was to identify differentially expressed genes in null cell adenoma and oncocytoma compared with normal pituitary glands and in invasive null cell adenoma and oncocytoma compared with non-invasive adenomas. Furthermore, it was important to attempt to identify novel genes that may be important in the pathogenesis of these poorly understood invasive tumors. Invasiveness is regarded as one of the most salient determinants of surgical curability because it can limit surgical resection and lead to tumor regrowth. Although mechanisms regulating pituitary tumor development and progression are still unclear, the biology of these tumors has been partially elucidated by recent studies, which demonstrated that changes in gene expression have an important role in pituitary tumorigenesis [10, 11]. Detecting these molecular changes may aid in the identification of new molecular subgroups for the development of novel adjuvant treatments and enhancement of the outcome of patients with invasive NFPAs.
Gene expression profiling has been used extensively in biological research and has resulted in significant advances in the understanding of the molecular mechanisms of complex disorders, including tumors [12]. In the gene expression profile study, an adequate sample size is necessary to achieve sufficient power to demonstrate significance of findings. Determination of an adequate sample size is not only affected by biological and technical factors but also in part by methods of analysis [12, 13]. For example, when microarrays are used for the identification of genes that predict a sample’s class membership, the prediction accuracy for internal validity of the study needs to be validated by an independent separate test sample [12]. In the present study, although the sample size for gene expression array analysis was very small, validation of results was performed by three different methods (qRT-PCR, Western blot and IHC). All the three methods provide the support that ENC-1 gene was related to invasiveness in null cell adenoma, but not in pituitary oncocytomas.
Although NFPAs are highly heterogeneous and the number of samples studied was limited, several significant results regarding NFPAs were obtained in our study. Interestingly, we observed that ENC1 genes may play an important role in the development of null cell adenomas. In particular, we also determined that ENC1 was significantly related to tumor invasion in null cell adenomas. These results have not been reported in previous studies. ENC1 encodes an actin-binding protein [14] that plays important roles in various biological processes, such as central nervous system development [15, 16] and neuronal outgrowth [17, 18], as well as in contributing to brain tumors [17, 19], hairy cell leukemia [20], breast cancer [21], and colorectal carcinogenesis [22]. In our study, ENC1 expression was observed in null cell adenomas, oncocytomas, and normal pituitary glands, and we found that expression of ENC1 was reduced in null cell adenomas and oncocytomas compared with normal pituitary glands. Furthermore, a low level of ENC1 expression was found in invasive null cell adenomas compared to non-invasive tumors.
There are a variety of possible explanations for the relationship between ENC1 expression and the development and progression of null cell adenomas and oncocytomas. First, it has been demonstrated that the ENC1 protein is functionally important in regulating differentiation processes. The expression of ENC1 is most abundant in fetal organs, and expression is significantly reduced or diminished in adult organs. It is possible that low ENC1 expression was observed in tumors that were undifferentiated or dedifferentiated during their development and progression. Low levels of ENC1 were found in neuroblastoma and medulloblastoma tumors, but a high level of ENC1 expression was detected in normal tissues from which these tumors are derived [15]. The null cell adenomas and oncocytomas are a group of tumors that do not resemble normal adenohypophyseal cell types. The histogenesis of null cell adenomas and oncocytomas is not known. It has been postulated that these tumors may be derived from undifferentiated progenitors of mature adenohypophyseal cells or mature adenohypophyseal cells that dedifferentiate during neoplastic proliferation and lose their capacity to produce hormones [23]. Thus, the high levels of ENC1 in the normal tissues from which null cell adenomas and oncocytomas are derived suggest the possibility that ENC1 expression is downregulated in these tumors. Second, it has been shown that the levels of several actin-binding proteins are decreased in neoplastic cells [24]. Actin-binding proteins are the main elements of the cytoskeleton. It has been suggested that changes in cytoskeletal structures and protein composition may be related to the growth and differentiation of cells. Thus, changes in ENC1 expression level may be used to predict or detect neoplastic transformation.
However, ENC1 may be a specific biomarker for invasiveness of null cell adenomas. Few studies have uncovered a gene expression difference in invasiveness between null cell adenomas and oncocytomas. Although the two tumors have some of the same pathological characteristics, oncocytomas contain abundant mitochondria. In the mean time, we found that there are distinctly different gender distributions among patients with null cell adenomas and oncocytomas, suggesting a preponderance of female patients with null cell adenomas and male patients with oncocytomas. Thus, functional studies will be needed to further determine the different role of ENC1 in invasiveness between null cell adenomas and oncocytomas.
In addition, Ki-67 is a nuclear antigen expressed by proliferating cells. As slowly growing neoplasms, pituitary adenomas in general show rather low Ki-67 indices, and the value of Ki-67 labeling as a prognostic factor in NFPA is controversial. Some studies have associated it with invasiveness [25–27], but many other studies have not [28, 29]. The Ki-67 index cut off that best predicts the occurrence of such behavior is also controversial. Our study reveals that a Ki-67 index above 3 % is not significantly associated with tumor invasiveness and ENC1 expression.
Acknowledgments
The authors thank the subjects for donating DNA samples. The study is supported by National Scientific Foundation of China (31000498, 81072075) grants to Chuzhong, Li and Yazhuo, Zhang.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11060-014-1479-1) contains supplementary material, which is available to authorized users.
Conflict of interest None of the authors has a conflict of interest.
Contributor Information
Jie Feng, Email: fi79330@gmail.com, Beijing Neurosurgical Institute, Capital Medical University, Beijing 100050, China.
Lichuan Hong, Email: homerhong1017@163.com, Beijing Neurosurgical Institute, Capital Medical University, Beijing 100050, China, Medical Center, Tsinghua University, Beijing 100084, China.
Yonggang Wu, Email: wyg931543@163.com, Beijing Neurosurgical Institute, Capital Medical University, Beijing 100050, China, Department of Neurosurgery, People’s Hospital of Xinjiang Uygur Autonomous Region, Urumqi 830001, China.
Chuzhong Li, Email: lichuzhong@163.com, Beijing Neurosurgical Institute, Capital Medical University, Beijing 100050, China.
Hong Wan, Email: wanhong96@163.com, Beijing Neurosurgical Institute, Capital Medical University, Beijing 100050, China.
Guilin Li, Email: liguilin40@hotmail.com, Beijing Neurosurgical Institute, Capital Medical University, Beijing 100050, China.
Yilin Sun, Email: sunyilin@hotmail.com, Beijing Neurosurgical Institute, Capital Medical University, Beijing 100050, China.
Shenyuan Yu, Email: daodaoysy@foxmail.com, Beijing Neurosurgical Institute, Capital Medical University, Beijing 100050, China.
Prashant Chittiboina, Email: prashant.chittiboina@nih.hhs.gov, Surgical Neurology Branch, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD 20892-1414, USA.
Blake Montgomery, Email: blake.montgomery@nih.hhs.gov, Surgical Neurology Branch, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD 20892-1414, USA.
Zhengping Zhuang, Email: Zhengping.Zhuang@nih.hhs.gov, Surgical Neurology Branch, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD 20892-1414, USA.
Yazhuo Zhang, Email: zyz2004520@yeah.net, Beijing Neurosurgical Institute, Capital Medical University, Beijing 100050, China.
References
- 1.DeLellis RA. Pathology and genetics of tumours of endocrine organs. IARC Press; Lyon: 2004. [Google Scholar]
- 2.Mete O, Ezzat S, Asa SL. Biomarkers of aggressive pituitary adenomas. J Mol Endocrinol. 2012;49:R69–78. doi: 10.1530/JME-12-0113. [DOI] [PubMed] [Google Scholar]
- 3.Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, McCutcheon IE. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101:613–619. doi: 10.1002/cncr.20412. [DOI] [PubMed] [Google Scholar]
- 4.Morita K, Takano K, Yasufuku-Takano J, Yamada S, Teramoto A, Takei M, Osamura RY, Sano T, Fujita T. Expression of pituitary tumour-derived, N-terminally truncated isoform of fibroblast growth factor receptor 4 (ptd-FGFR4) correlates with tumour invasiveness but not with G-protein alpha subunit (gsp) mutation in human GH-secreting pituitary adenomas. Clin Endocrinol (Oxf) 2008;68:435–441. doi: 10.1111/j.1365-2265.2007.03062.x. [DOI] [PubMed] [Google Scholar]
- 5.Gong J, Zhao Y, Abdel-Fattah R, Amos S, Xiao A, Lopes MB, Hussaini IM, Laws ER. Matrix metalloproteinase-9, a potential biological marker in invasive pituitary adenomas. Pituitary. 2008;11:37–48. doi: 10.1007/s11102-007-0066-2. [DOI] [PubMed] [Google Scholar]
- 6.Filippella M, Galland F, Kujas M, Young J, Faggiano A, Lombardi G, Colao A, Meduri G, Chanson P. Pituitary tumour transforming gene (PTTG) expression correlates with the proliferative activity and recurrence status of pituitary adenomas: a clinical and immunohistochemical study. Clin Endocrinol (Oxf) 2006;65:536–543. doi: 10.1111/j.1365-2265.2006.02630.x. [DOI] [PubMed] [Google Scholar]
- 7.Bottoni A, Piccin D, Tagliati F, Luchin A, Zatelli MC, Degli Uberti EC. MiR-15a and miR-16-1 down-regulation in pituitary adenomas. J Cell Physiol. 2005;204:280–285. doi: 10.1002/jcp.20282. [DOI] [PubMed] [Google Scholar]
- 8.Greenman Y, Melmed S. Diagnosis and management of nonfunctioning pituitary tumors. Annu Rev Med. 1996;47:95–106. doi: 10.1146/annurev.med.47.1.95. [DOI] [PubMed] [Google Scholar]
- 9.Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery. 1993;33:610–617. doi: 10.1227/00006123-199310000-00008. discussion 617–618. [DOI] [PubMed] [Google Scholar]
- 10.Salehi F, Agur A, Scheithauer BW, Kovacs K, Lloyd RV, Cusimano M. Biomarkers of pituitary neoplasms: a review (Part II) Neurosurgery. 2010;67:1790–1798. doi: 10.1227/NEU.0b013e3181faa680. discussion 1798. [DOI] [PubMed] [Google Scholar]
- 11.Morris DG, Musat M, Czirjak S, Hanzely Z, Lillington DM, Korbonits M, Grossman AB. Differential gene expression in pituitary adenomas by oligonucleotide array analysis. Eur J Endocrinol. 2005;153:143–151. doi: 10.1530/eje.1.01937. [DOI] [PubMed] [Google Scholar]
- 12.Kim K, Zakharkin SO, Allison DB. Expectations validity and reality in gene expression profiling. J Clin Epidemiol. 2010;63:950–959. doi: 10.1016/j.jclinepi.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zakharkin SO, Kim K, Mehta T, Chen L, Barnes S, Scheirer KE, Parrish RS, Allison DB, Page GP. Sources of variation in Affymetrix microarray experiments. BMC Bioinformatics. 2005;6:214. doi: 10.1186/1471-2105-6-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez MC, Andres-Barquin PJ, Martinez S, Bulfone A, Rubenstein JL, Israel MA. ENC-1: a novel mammalian kelch-related gene specifically expressed in the nervous system encodes an actin-binding protein. J Neurosci. 1997;17:3038–3051. doi: 10.1523/JNEUROSCI.17-09-03038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernandez MC, Andres-Barquin PJ, Holt I, Israel MA. Cloning of human ENC-1 and evaluation of its expression and regulation in nervous system tumors. Exp Cell Res. 1998;242:470–477. doi: 10.1006/excr.1998.4109. [DOI] [PubMed] [Google Scholar]
- 16.Kim TA, Lim J, Ota S, Raja S, Rogers R, Rivnay B, Avraham H, Avraham S. NRP/B, a novel nuclear matrix protein, associates with p110(RB) and is involved in neuronal differentiation. J Cell Biol. 1998;141:553–566. doi: 10.1083/jcb.141.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim TA, Ota S, Jiang S, Pasztor LM, White RA, Avraham S. Genomic organization, chromosomal localization and regulation of expression of the neuronal nuclear matrix protein NRP/B in human brain tumors. Gene. 2000;255:105–116. doi: 10.1016/s0378-1119(00)00297-3. [DOI] [PubMed] [Google Scholar]
- 18.Kim TA, Jiang S, Seng S, Cha K, Avraham HK, Avraham S. The BTB domain of the nuclear matrix protein NRP/B is required for neurite outgrowth. J Cell Sci. 2005;118:5537–5548. doi: 10.1242/jcs.02643. [DOI] [PubMed] [Google Scholar]
- 19.Liang XQ, Avraham HK, Jiang S, Avraham S. Genetic alterations of the NRP/B gene are associated with human brain tumors. Oncogene. 2004;23:5890–5900. doi: 10.1038/sj.onc.1207776. [DOI] [PubMed] [Google Scholar]
- 20.Hammarsund M, Lerner M, Zhu C, Merup M, Jansson M, Gahrton G, Kluin-Nelemans H, Einhorn S, Grander D, Sangfelt O, Corcoran M. Disruption of a novel ectodermal neural cortex 1 antisense gene, ENC-1AS and identification of ENC-1 overexpression in hairy cell leukemia. Hum Mol Genet. 2004;13:2925–2936. doi: 10.1093/hmg/ddh315. [DOI] [PubMed] [Google Scholar]
- 21.Seng S, Avraham HK, Jiang S, Yang S, Sekine M, Kimelman N, Li H, Avraham S. The nuclear matrix protein, NRP/B, enhances Nrf2-mediated oxidative stress responses in breast cancer cells. Cancer Res. 2007;67:8596–8604. doi: 10.1158/0008-5472.CAN-06-3785. [DOI] [PubMed] [Google Scholar]
- 22.Fujita M, Furukawa Y, Tsunoda T, Tanaka T, Ogawa M, Nakamura Y. Up-regulation of the ectodermal-neural cortex 1 (ENC1) gene, a downstream target of the beta-catenin/T-cell factor complex, in colorectal carcinomas. Cancer Res. 2001;61:7722–7726. [PubMed] [Google Scholar]
- 23.Holm R. Null cell adenomas and oncocytomas of the pituitary gland. Pathol Res Pract. 1995;191:348–352. doi: 10.1016/S0344-0338(11)80888-5. [DOI] [PubMed] [Google Scholar]
- 24.Janmey PA, Chaponnier C. Medical aspects of the actin cytoskeleton. Curr Opin Cell Biol. 1995;7:111–117. doi: 10.1016/0955-0674(95)80052-2. [DOI] [PubMed] [Google Scholar]
- 25.Widhalm G, Wolfsberger S, Preusser M, Fischer I, Woehrer A, Wunderer J, Hainfellner JA, Knosp E. Residual nonfunctioning pituitary adenomas: prognostic value of MIB-1 labeling index for tumor progression. J Neurosurg. 2009;111:563–571. doi: 10.3171/2008.4.17517. [DOI] [PubMed] [Google Scholar]
- 26.Gejman R, Swearingen B, Hedley-Whyte ET. Role of Ki- 67 proliferation index and p53 expression in predicting progression of pituitary adenomas. Hum Pathol. 2008;39:758–766. doi: 10.1016/j.humpath.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Noh TW, Jeong HJ, Lee MK, Kim TS, Kim SH, Lee EJ. Predicting recurrence of nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 2009;94:4406–4413. doi: 10.1210/jc.2009-0471. [DOI] [PubMed] [Google Scholar]
- 28.Dubois S, Guyetant S, Menei P, Rodien P, Illouz F, Vielle B, Rohmer V. Relevance of Ki-67 and prognostic factors for recurrence/progression of gonadotropic adenomas after first surgery. Eur J Endocrinol. 2007;157:141–147. doi: 10.1530/EJE-07-0099. [DOI] [PubMed] [Google Scholar]
- 29.Chacko G, Chacko AG, Kovacs K, Scheithauer BW, Mani S, Muliyil JP, Seshadri MS. The clinical significance of MIB- 1 labeling index in pituitary adenomas. Pituitary. 2010;13:337–344. doi: 10.1007/s11102-010-0242-7. [DOI] [PubMed] [Google Scholar]