Abstract
Background
Self-reported unprotected vaginal sex appears to increase risk of bacterial vaginosis (BV). However, the validity of self-reports is questionable, given their inconsistency with more objective measures of recent semen exposure such as detection of prostate specific antigen (PSA). We examined whether recent unprotected sex, as measured both by PSA detection on vaginal swabs and by self-report, was associated with increased BV recurrence.
Methods
We analyzed randomized trial data from non-pregnant, BV-positive adult women recruited from a sexually transmitted diseases clinic. Participants received BV therapy at enrollment and were scheduled to return after 4, 12, and 24 weeks. BV (by Nugent score) and PSA were measured at each visit. We used Cox proportional hazards models to examine the association between PSA positivity and recurrent BV. We also evaluated associations between self-reported unprotected sex (ever/never since the last visit and in the last 48 hours, analyzed separately) and recurrent BV.
Results
PSA and BV results were available for 96 women who contributed 226 follow-up visits. PSA positivity was associated with increased BV recurrence (adjusted hazard ratio (aHR): 2.32, 95% confidence interval (CI): 1.28–4.21). In contrast, we observed no significant increase in BV recurrence among women self-reporting unprotected sex since the last visit (aHR: 1.63, 95% CI: 0.77–3.43) or in the last 48 hours (aHR: 1.28, 95% CI: 0.70–2.36).
Conclusions
Estimates from earlier studies linking self-reported unprotected sex and BV may be biased by misclassification. Biomarkers can improve measurement of unprotected sex, a critical exposure variable in sexual health research.
Keywords: prostate specific antigen, bacterial vaginosis, biomarker, unprotected sex, condom
INTRODUCTION
Bacterial vaginosis (BV) is a highly-prevalent vaginal condition among reproductive-age women. According to nationally-representative findings from women aged 15–49 in the United States, 23% of white women and 52% of black women have prevalent BV (1). Symptomatic women complain of discharge and a foul vaginal odor (2). Women with recurrent BV have reduced quality of life, including significantly reduced self-esteem and other adverse social and emotional effects (3). Prevalent BV is associated with myriad negative obstetric and reproductive outcomes, including spontaneous abortion, preterm delivery, post-partum infection, and increased risk of HIV acquisition and transmission (2).
The causes of BV, and the mechanisms by which it develops, are largely unknown. However several risk factors have been associated with increased BV risk, including non-white race, new or multiple sex partners, intravaginal hygiene practices including douching, high sexual frequency, and unprotected vaginal sex (4–7). In some studies, consistent condom users had significantly lower BV risk than women who did not use condoms (8–10). However, other studies have failed to find a significant association between condom use and BV, suggesting that if present, the protective benefit of condoms might be quite modest (11). The strength of the protective effect of condoms might be underestimated because self-reported condom use is a flawed measure. Studies testing for biological markers of semen exposure – primarily prostate-specific antigen (PSA) or Y-chromosome DNA – have consistently demonstrated that female research participants often under-report when asked about unprotected sex or condom use (12–14).
Given the documented bias in self-reported condom use as a measure of women’s recent engagement in unprotected vaginal sex, we hypothesized that published associations between self-reported condom use and BV might also be biased. The current analysis examines whether biomarker confirmation of recent semen exposure (via detection of PSA on vaginal swabs) or self-reported recent unprotected sex is associated with increased BV recurrence.
MATERIALS AND METHODS
Parent study
We describe a secondary analysis of data generated through a 24-week, randomized, double-blinded, placebo-controlled trial of vitamin D supplementation (clinicaltrials.gov NCT01450462). The primary outcome of the trial was BV recurrence. Detailed methods have been published previously (15). Briefly, 118 women were recruited from a public, urban sexually transmitted disease (STD) clinic and were randomized to receive either high-dose vitamin D supplements or placebo. To be eligible, women were BV-positive according to modified Amsel criteria (16), English speaking, between 18 and 50 years of age, pre-menopausal, and had at least one ovary. Exclusion criteria included current pregnancy or planned pregnancy in the next 6 months, current breastfeeding, current menstruation, or history of conditions incompatible with vitamin D supplementation. All participants received standard BV therapy at enrollment: 500 mg metronidazole orally twice daily for 7 days. Over 24 weeks, women in the vitamin D arm also received 50,000 international units of vitamin D3 in 9 doses; women in the control arm took placebo capsules on the same schedule. Participants returned for three follow-up visits, occurring 4 weeks, 12 weeks, and 24 weeks after enrollment. At the enrollment and 24-week visits, women received STD testing and treatment according to normal clinic procedures (17). At the 4- and 12-week visits, women were not examined by STD clinicians unless they had a clinical complaint. If a woman complaining of vaginal symptoms was found to have BV by Amsel criteria at one of these visits, she was treated with 500 mg metronidazole orally twice daily for 7 days. As reported previously, women in the vitamin D arm did not have reduced BV recurrence compared to women in the control arm, a finding confirmed through intention-to-treat and per-protocol analyses (15).
Biological measures
At enrollment and the 24-week visit, all participants underwent testing for infection with C. trachomatis, N. gonorrhoeae, T. vaginalis, syphilis, HIV, BV and candida. A separate swab was also collected and stored at each visit for later PSA evaluation. At the 4- and 12-week visits, because participants were not routinely examined by clinicians, they self-collected vaginal swabs in the clinic for later assessment of BV and PSA. Self-collected swabs have been validated as reliable for measurement of both BV (18) and PSA (19).
BV diagnosis by Nugent scoring (20) of stored, Gram-stained vaginal smears from each of the four visits was performed at the end of the trial by blinded assessors. BV was the primary outcome for this analysis. A Nugent score of 0–3 was coded as normal, 4–6 as intermediate, and 7–10 as BV.
Detection of PSA on vaginal swabs was the primary exposure variable for this analysis. Immediately after collection, vaginal swabs were transferred to −80°C until they were shipped to the CDC for quantification of PSA using the Abbott ARCHITECT total PSA assay (Abbott Diagnostics, Abbott Park, IL, USA) using previously described methods (21). Using the established threshold of 1.0 ng per mL of eluent for defining positivity, PSA can be detected with high (99–100%) sensitivity immediately following exposure to semen (22–23). The probability of positivity drops to 29% by 24 hours post-exposure and PSA levels return to background levels by 48 hours (23–24).
Behavioral measures
At all four study visits, women were interviewed by trained study staff about their sexual behavior. Responses were recorded using REDCap (25). To capture any self-reported unprotected sex since the last visit, we asked how many times each participant had had vaginal sex since the last visit, and during how many of those encounters a condom was used. Any act without a condom was considered evidence of unprotected sex. To measure more recent self-reported unprotected sex, we asked participants to enumerate all sex acts with men occurring in the last 24 hours, and separately, in the last 24–48 hours. For each time period we also captured the number of acts where condoms were used, whether a condom was in place for part or all of the act, and whether use was known to be compromised during any act (e.g. condom broke or slipped off during sex).
Ethical review
All women provided written informed consent prior to participating. The study was approved by the Ohio State University Institutional Review Board (OSU IRB). The STD clinic where the trial was conducted is an approved research site of the OSU IRB. All procedures were in accordance with the Helsinki Declaration of 1975, as revised in 2000.
Data analysis
All analyses were performed in SAS (Version 9.4, Cary, NC). We evaluated the agreement between semen exposure as detected by PSA positivity vs. self-report by comparing women’s self-reported unprotected vaginal sex in the previous 48 hours with PSA positivity. ‘Unprotected sex’ included any sex act where no condom was used, or where a condom was used for part but not all of the sex act, or when a condom broke or slipped off during sex.
The goal of this analysis was to examine the association between time-varying PSA and recurrent BV. This secondary analysis was specified a priori, at the start of the parent trial. All women were BV-positive at enrollment, and all received standard BV therapy at that visit. Thus, we assumed that all participants were at risk of recurrent BV during follow-up. As the outcome of interest was recurrent BV, women were removed from the analysis population after the first follow-up visit when they were found to be BV-positive. The analysis dataset was comprised of up to 3 records per participant (from the 4-, 12- and 24-week visits), with each record containing measures for both PSA and BV from that visit. We fit unadjusted and adjusted extended Cox proportional hazards models, to compare the risk of recurrent BV among women with a positive PSA result to the risk of recurrent BV among women who were PSA-negative. Because both PSA and BV were measured at each visit, exposure and outcome status for each woman were permitted to vary over time. No analytic provision was made to account for mid-study treatment, as such treatment occurred after the primary outcome (recurrent BV) and did not change women’s risk of that outcome. BV recurrence results are presented as hazard ratios (HRs) and 95% confidence intervals (CIs).
We adjusted for known BV risk factors, including sexual frequency since the last visit (0, 1–10, or more than 10 sex acts), age (dichotomized as 18–24 years vs. 25–40 years), race/ethnicity (black vs. all other race/ethnicities), and current use of hormonal contraception (including combined oral contraceptive pills, patch, or ring; injectable medroxyprogesterone acetate; and progesterone implant or IUD) (26). Treatment (vitamin D vs. placebo) was randomly assigned in the parent study and so unlikely to confound the association of interest (15). We assessed for modification of the PSA-BV association by treatment assignment by testing the significance of a product-interaction term between treatment assignment and PSA in the multivariable model.
Because distinguishing recurrent BV from prevalent BV is difficult, we also pursued a secondary strategy to examine the association between PSA positivity and prevalent BV. For this analysis women were not censored when they were diagnosed with BV; every positive BV case was included in the analysis. We fit unadjusted and adjusted log-binomial models using generalized estimating equations (GEE) with an unstructured working correlation matrix to produce a prevalence ratio (PR) and 95% CI. The PR compares the prevalence of BV among women with a positive PSA result to the prevalence of BV among women who were PSA-negative. As in our primary analysis, we adjusted for sexual frequency, age, race/ethnicity, and current use of hormonal contraception.
RESULTS
Screening, enrollment and follow-up
The trial started in September 2011 and concluded follow-up in January 2013. We screened 285 women and 118 enrolled (41% of the screened sample). Of the 118 enrolled women, 97 (82%) returned for follow-up. PSA results were available for 96 participants (99% of those who returned for follow-up) and are included in the present analysis.
Participant characteristics
The median age of included participants was 26.5 years (interquartile range (IQR): 22–34), and most women self-identified as Black (73%) (Table 1). Most women (n=58, 60%) reported currently using no method of contraception. Only 7% reported using condoms with every sex act in the last three months. Participants’ median number of male sex partners across the lifetime was 10 (IQR: 6–20 partners). Nearly a third of women (31%) reported sex with women at some point in their lives. At the enrollment visit, by design, all women had BV, and 9%, 8% and 17% were infected with C. trachomatis, N. gonorrhoeae, and T. vaginalis, respectively. No woman had syphilis at enrollment and 2% were HIV-positive. Nearly one in five women (19%) were PSA-positive (PSA level >1.0 ng/mL) at enrollment (Table 1).
Table 1.
Baseline demographic, behavioral and clinical characteristics of participants who returned for follow-up (n=96).
| N=96 | (%) | |
| Race/ethnicitya | ||
| Black | 70 | (73) |
| White | 26 | (27) |
| American-Indian/Alaskan Native | 4 | (4) |
| Asian/Pacific-Islander | 0 | (0) |
| Hispanic | 5 | (5) |
| Education | ||
| Finished high school | 83 | (86) |
| Finished college | 13 | (14) |
| Main partner is … | ||
| Man | 57 | (59) |
| Woman | 4 | (4) |
| No main partner | 35 | (36) |
| Employed full- or part-time | 56 | (58) |
| Ever pregnant | 65 | (68) |
| Currently using any method of contraceptionb | ||
| Hormonal methods (oral contraceptive pills, implants, injectable, patch, ring, hormonal IUD) | 9 | (24) |
| Male condoms | 22 | (58) |
| Female condoms | 1 | (3) |
| IUD (copper or unknown type) | 2 | (5) |
| Sterilization | 9 | (24) |
| Condom use, last 3 months | ||
| 0% of vaginal acts with men | 37 | (39) |
| >0% and <100% of vaginal acts with men | 45 | (47) |
| 100% of vaginal acts with men | 7 | (7) |
| Missing | 7 | (7) |
| Ever anal sex | 39 | (41) |
| Ever sex with women | 30 | (31) |
| Concurrent sexual partnerships in the last 3 months | 28 | (29) |
| Feminine hygiene products before, during or after sex in last 3 months | 37 | (39) |
| Ever douched | 79 | (82) |
| Ever past BV diagnosis (self-reported, prior to enrollment) | 75 | (78) |
| Infections diagnosed at enrollment | ||
| Chlamydia | 9 | (9) |
| Gonorrhea | 8 | (8) |
| Trichomoniasis | 16 | (17) |
| Syphilis | 0 | (0) |
| Yeast | 7 | (7) |
| Prevalent HIV (not new diagnoses) | 2 | (2) |
| Randomization arm | ||
| Vitamin D | 51 | (53) |
| Placebo | 45 | (47) |
| PSA level >1.0 ng/mL | 19 | (20) |
| Median | (IQR) | |
| Age, years) | 26.5 | (22–34) |
| Lifetime number male partners | 10 | (6–20) |
| Lifetime number female partnersc | 2 | (2–3) |
| Number vaginal sex acts with men, last 3 months | 11 | (4–33) |
Some women reported more than one race/ethnicity, so totals sum to greater than 100%.
Fifty-eight women (60%) reported using no method of contraception. The denominator for the proportions of women using each contraceptive method type is the total number of women reporting use of any method. Because women could report multiple methods, proportions sum to greater than 100%.
Among women who report any lifetime sex with women.
Agreement between semen exposure as detected by PSA positivity vs. self-report
Of the 187 follow-up visits included in the analysis of BV recurrence, PSA was detected from vaginal swabs at 22% (42 visits). At 12 of these visits (29% of PSA-positive visits), women reported no unprotected sex in the previous 48 hours; at the remaining 30 visits (71% of PSA-positive visits), women self-reported at least one unprotected act in the previous 48 hours. The proportion of visits where PSA was detected was similar at each visit: 24% at 4 weeks, 26% at 12 weeks, and 16% at 24 weeks.
Recent unprotected sex and recurrent BV
As measured by PSA positivity, recent unprotected sex was significantly associated with increased BV recurrence (Table 2). The unadjusted HR for the effect of PSA on recurrent BV was 2.15 (95% CI: 1.23–3.77). We saw no evidence of effect modification by treatment assignment (interaction term p=0.90), so the product-interaction term was dropped from the model. After multivariable adjustment the measure of effect increased slightly (adjusted HR: 2.32, 95% CI: 1.28–4.21).
Table 2.
Associations between recent unprotected sex and BV.
| Recurrent BV | Prevalent BV | |||||
|---|---|---|---|---|---|---|
| N (visits) |
HR | (95% CI) | N (visits) |
PR | (95% CI) | |
| Unprotected sex, measured by PSA | ||||||
| Unadjusted | 187 | 2.15 | (1.23 to 3.77) | 226 | 1.39 | (1.00 to 1.93) |
| Adjusted* | 186 | 2.32 | (1.28 to 4.21) | 225 | 1.55 | (1.16 to 2.07) |
| Any unprotected sex since last visit, measured by self-report | ||||||
| Unadjusted | 187 | 1.27 | (0.71 to 2.27) | 226 | 1.85 | (1.20 to 2.85) |
| Adjusted* | 186 | 1.63 | (0.77 to 3.43) | 225 | 1.92 | (1.24 to 2.99) |
| Any unprotected sex in last 48 hours, measured by self-report | ||||||
| Unadjusted | 186 | 1.25 | (0.71 to 2.20) | 225 | 1.27 | (0.90 to 1.81) |
| Adjusted* | 185 | 1.28 | (0.70 to 2.36) | 224 | 1.37 | (0.99 to 1.91) |
BV: bacterial vaginosis; PSA: prostate specific antigen; HR: hazard ratio; CI: confidence interval; PR: prevalence ratio
Adjusted models control for age, black race, recent douching, recent sexual frequency, and current use of hormonal contraception
In contrast, we observed no significant association between recent unprotected sex, as measured by self-report, and recurrent BV. Considering any self-reported unprotected sex since the last visit, the unadjusted HR for BV was 1.27 (95% CI: 0.71–2.27) and the adjusted HR was 1.63 (95% CI: 0.77–3.43). Considering any self-reported unprotected sex in the last 48 hours, the unadjusted HR for BV was 1.25 (95% CI: 0.71–2.20) and the adjusted HR was 1.28 (95% CI: 0.70–2.36) (Table 2).
Recent unprotected sex and prevalent BV
We next examined associations with prevalent BV. Women in whom PSA was detected had higher BV prevalence than PSA-negative women at every visit (Figure 1). PSA was significantly associated with prevalent BV (adjusted PR (aPR): 1.55, 95% CI: 1.16 to 2.07) (Table 2). Any self-reported unprotected sex since the last visit was also linked to increased BV prevalence (aPR: 1.92, 95% CI: 1.24 to 2.99), whereas we observed no significant association between any self-reported unprotected sex in the last 48 hours and prevalent BV (aPR: 1.37, 95% CI: 0.99 to 1.91) (Table 2).
Figure 1.
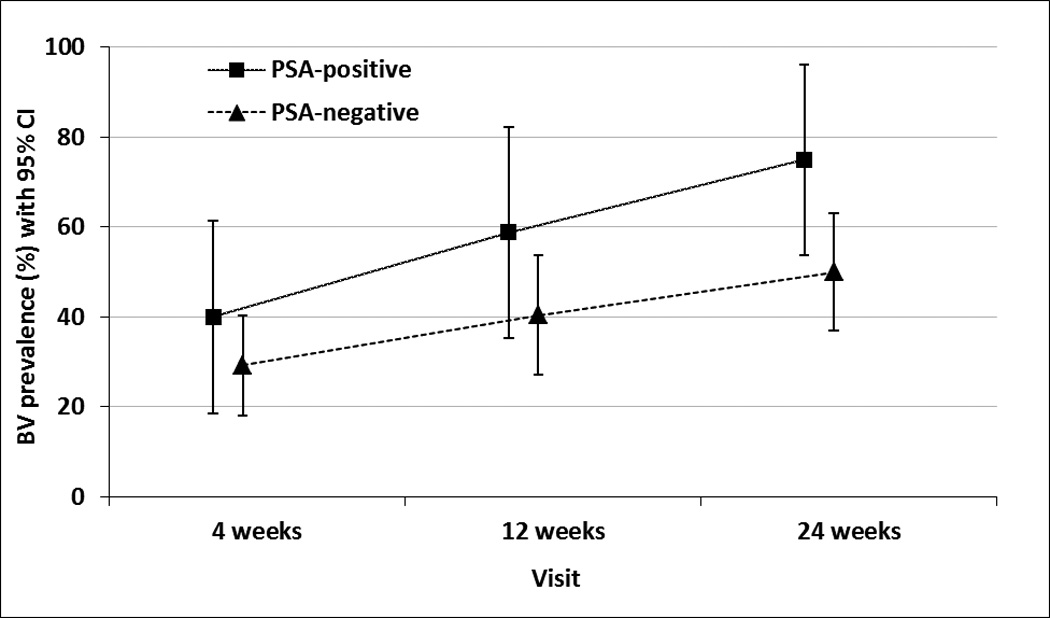
BV prevalence with 95% confidence intervals, by PSA status.
DISCUSSION
Among women participating in a longitudinal trial of BV recurrence, we found that recent, biomarker-confirmed semen exposure was significantly associated with recurrent BV in both unadjusted analysis and after controlling for documented BV risk factors. This finding sheds light on the prior inconsistent literature linking unprotected sex and BV risk, and suggests that the variation in earlier studies may relate to misclassification from self-reported condom use.
To our knowledge, only two previous studies have examined BV risk using biomarker confirmation of recent unprotected sex. Our findings are highly consistent with this prior work. In a study of 430 African women, Jespers et al. found that PSA detection was significantly correlated with incident BV (adjusted odds ratio (aOR): 2.35, 95% CI: 1.52–3.63). (27). Similar to our study, self-reported condom use at the last sex act was not linked to incident BV in that analysis (OR: 1.25, 95% CI: 0.79–1.96). The other published study was a secondary analysis of 871 HIV-infected and 439 HIV-uninfected participants in the HIV Epidemiology Research Study (HERS) study (28). Recent sex was classified not by PSA but by detection of spermatozoa on Gram stain, and HIV-positive participants with spermatozoa on Gram stain had significantly increased risk of incident BV (aOR: 1.5, 95% CI: 1.1–2.1). Among HIV-negative women, participants with spermatozoa on Gram stain also had significantly increased risk of incident BV (aOR, 1.9; 95% CI: 1.3–2.9).
As a secondary analysis of a clinical trial conducted within a relatively small sample (n=96 women), our analysis has important limitations. PSA is a validated, objective biomarker of recent semen exposure, but given its rapid degradation after sex, it can only reliably capture semen exposure within the last two days. However this limitation – potential misclassification of recent semen exposure – would bias the association between PSA and BV only if the likelihood of misclassification of PSA was also associated with BV (e.g., differential misclassification of the exposure with respect to the outcome). While we hypothesize that semen exposure, as characterized by PSA detection, is associated with BV, we do not have reason to suspect that misclassification of PSA is more likely among either BV-positive or BV-negative women. In contrast, several studies suggest that differential misclassification can occur when using self-reported condom data (29).
In addition, although our findings indicate that women with recent semen exposure as detected by PSA had increased BV recurrence, it was not possible given the trial design to determine whether BV had already recurred before the sex act captured by the PSA measurement. All women were BV-positive at enrollment, and our endpoint of interest was recurrent BV. However because we did not perform a test-of-cure for BV following metronidazole treatment, women classified as having recurrent BV during follow-up are likely a combination of truly recurrent BV and BV treatment failures. (The treatment success rate one month after BV treatment is approximately 80–90% (2, 30)). To address this limitation, we also examined the association between PSA-positivity and BV prevalence, including all BV-positive cases identified after enrollment. Similar to the BV recurrence analyses, women in whom PSA was detected had significantly increased BV prevalence, although the strength of this association was attenuated compared to the analysis of BV recurrence. Again similar to the BV recurrence analysis, self-reported unprotected sex in the last 48 hours was not significantly associated with prevalent BV. However, self-reported unprotected sex since the last visit was significantly associated with prevalent BV, whereas this variable was not significantly associated with recurrent BV.
Distinction between prevalent, incident, persistent, and recurrent BV presents a methodologic challenge for nearly all BV research, although we suggest that recurrent BV may be the most relevant endpoint to focus on from a public health perspective. Earlier studies have documented rapid and continuous changes in vaginal flora composition, including cycling in and out of a BV-positive state (e.g., 31). For most women, these BV episodes are transient and self-limiting. The more profoundly affected women are those who undergo multiple rounds of treatment with short-term relief of symptoms, only to present again within weeks with recurrent BV. Characterizing factors relevant to this population could reduce the significant BV-associated morbidities experienced by the subset of women with recurrent BV.
Our analysis also has significant compensating strengths. The incorporation of PSA as the primary exposure overcomes the well-documented biases introduced by reliance on self-reported condom use. The longitudinal design allows us to analyze and adjust for other time-varying factors identified in previous research as associated with BV, including douching (expected to increase BV recurrence) and use of hormonal contraception (expected to decrease BV recurrence). Ours is also the first study of PSA and BV among US women, as the Jespers study (27) enrolled women from Kenya, Rwanda and South Africa.
Although the etiology of BV remains murky, our findings provide strong support for the hypothesis that BV risk is correlated with sexual exposure. Counseling for STD clinic patients focuses on consistent use of condoms to reduce the spread of sexually transmitted infections, but given past data showing no value to treating male partners of BV-positive women (32), preventive counseling for BV often focuses on vaginal hygiene more than condom use. We suggest that women at risk of recurrent BV should be strongly counseled to use condoms with every sexual act.
Acknowledgements
The authors thank Mary Ellen Wewers, Raina Fichorova, Mysheika Williams Roberts, Rebecca Jackson, and the staff at the Sexual Health Clinic at Columbus Public Health for their support of this project. We thank James Partin and Dr. Carolyn Black at the Division of Scientific Resources, CDC for their technical expertise and support, and for providing PSA analyses. We also thank Charles Rivers and Jane Schwebke at the University of Alabama at Birmingham.
This manuscript describes a secondary analysis of a randomized trial of vitamin D supplementation. For that trial, Bio-Tech Pharmacal (Fayetteville, AR) donated both the vitamin D and placebo products. Bio-Tech Pharmacal had no involvement in any other aspect of the research, including protocol development, data collection, analysis, writing of results or any other component of trial implementation or interpretation.
Funding
This work was supported by KL2RR025754 through the Ohio State University Center for Clinical and Translational Science (OSU CCTS). The OSU CCTS is supported by the National Center for Advancing Translational Sciences (NCATS), 8UL1TR000090-05. ANT was also supported by R21AI095987 from the National Institute for Allergy and Infectious Diseases (NIAID). The US Centers for Disease Control and Prevention (CDC), Division of Reproductive Health, and the Division of Scientific Resources quantified prostate specific antigen for this project. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institutes of Health, NCATS, NIAID or CDC. Use of trade names is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services.
Footnotes
Conflicts of interest
No authors otherwise report any commercial or other association that might pose a conflict of interest.
REFERENCES
- 1.Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Obstet Gynecol. 2007 Jan;109(1):114–120. doi: 10.1097/01.AOG.0000247627.84791.91. [DOI] [PubMed] [Google Scholar]
- 2.Hillier S, Marrazzo JM, Holmes KK. Bacterial vaginosis. In: Holmes KK, Sparling PF, Mårdh P, et al., editors. Sexually transmitted diseases. 4th. New York: McGraw-Hill; 2008. [Google Scholar]
- 3.Bilardi JE, Walker S, Temple-Smith M, et al. The burden of bacterial vaginosis: women's experience of the physical, emotional, sexual and social impact of living with recurrent bacterial vaginosis. PLoS One. 2013 Sep 11;8(9):e74378. doi: 10.1371/journal.pone.0074378. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradshaw CS, Morton AN, Garland SM, et al. Higher-risk behavioral practices associated with bacterial vaginosis compared with vaginal candidiasis. Obstet Gynecol. 2005 Jul;106(1):105–114. doi: 10.1097/01.AOG.0000163247.78533.7b. [DOI] [PubMed] [Google Scholar]
- 5.McClelland RS, Richardson BA, Graham SM, et al. A prospective study of risk factors for bacterial vaginosis in HIV-1-seronegative African women. Sex Transm Dis. 2008 Jun;35(6):617–623. doi: 10.1097/OLQ.0b013e31816907fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradshaw CS, Vodstrcil LA, Hocking JS, et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis. 2013 Mar;56(6):777–786. doi: 10.1093/cid/cis1030. Epub 2012 Dec 12. [DOI] [PubMed] [Google Scholar]
- 7.Smart S, Singal A, Mindel A. Social and sexual risk factors for bacterial vaginosis. Sex Transm Infect. 2004 Feb;80(1):58–62. doi: 10.1136/sti.2003.004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guédou FA, Van Damme L, Deese J, et al. Behavioural and medical predictors of bacterial vaginosis recurrence among female sex workers: longitudinal analysis from a randomized controlled trial. BMC Infect Dis. 2013 May 8;13:208. doi: 10.1186/1471-2334-13-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwebke JR, Desmond R. Risk factors for bacterial vaginosis in women at high risk for sexually transmitted diseases. Sex Transm Dis. 2005 Nov;32(11):654–658. doi: 10.1097/01.olq.0000175396.10304.62. [DOI] [PubMed] [Google Scholar]
- 10.Hutchinson KB, Kip KE, Ness RB. Condom use and its association with bacterial vaginosis and bacterial vaginosis-associated vaginal microflora. Epidemiology. 2007 Nov;18(6):702–708. doi: 10.1097/EDE.0b013e3181567eaa. [DOI] [PubMed] [Google Scholar]
- 11.Verstraelen H, Verhelst R, Vaneechoutte M, et al. The epidemiology of bacterial vaginosis in relation to sexual behaviour. BMC Infect Dis. 2010 Mar 30;10:81. doi: 10.1186/1471-2334-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallo MF, Steiner MJ, Hobbs MM, et al. Biological markers of sexual activity: tools for improving measurement in HIV/sexually transmitted infection prevention research. Sex Transm Dis. 2013;40(6):447–452. doi: 10.1097/OLQ.0b013e31828b2f77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenbaum JE, Zenilman J, Melendez J, et al. Telling truth from Ys: an evaluation of whether the accuracy of self-reported semen exposure assessed by a semen Y-chromosome biomarker predicts pregnancy in a longitudinal cohort study of pregnancy. Sex Transm Infect. 2014 Sep;90(6):479–484. doi: 10.1136/sextrans-2013-051315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rose E, Diclemente RJ, Wingood GM, et al. The validity of teens' and young adults' self-reported condom use. Arch Pediatr Adolesc Med. 2009 Jan;163(1):61–64. doi: 10.1001/archpediatrics.2008.509. [DOI] [PubMed] [Google Scholar]
- 15.Turner AN, Carr Reese P, Fields KS, et al. A blinded, randomized controlled trial of high-dose vitamin D supplementation to reduce recurrence of bacterial vaginosis. Am J Obstet Gynecol. 2014 Nov;211(5):479.e1–479.e13. doi: 10.1016/j.ajog.2014.06.023. Epub 2014 Jun 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983 Jan;74(1):14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) Sexually Transmitted Diseases Treatment Guidelines, 2010. MMWR. 2010;59(RR-12) [PubMed] [Google Scholar]
- 18.Nelson DB, Bellamy S, Gray TS, et al. Self-collected versus provider-collected vaginal swabs for the diagnosis of bacterial vaginosis: an assessment of validity and reliability. J Clin Epidemiol. 2003 Sep;56(9):862–866. doi: 10.1016/s0895-4356(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 19.Bahamondes L, Diaz J, Marchi NM, et al. Prostate-specific antigen in vaginal fluid after exposure to known amounts of semen and after condom use: Comparison of self-collected and nurse collected samples. Hum Reprod. 2008;23:2444–2451. doi: 10.1093/humrep/den283. [DOI] [PubMed] [Google Scholar]
- 20.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991 Feb;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallo MF, Snead MC, Black CM, et al. Optimal methods for collecting and storing vaginal specimens for prostate-specific antigen testing in research studies. Contraception. 2013;87(6):830–835. doi: 10.1016/j.contraception.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawson ML, Maculuso M, Bloom A, et al. Objective markers of condom failure. Sex Transm Dis. 1998;25:427–432. doi: 10.1097/00007435-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Macaluso M, Lawson L, Akers R, et al. Prostate-specific antigen in vaginal fluid as a biologic marker of condom failure. Contraception. 1999;59:195–201. doi: 10.1016/s0010-7824(99)00013-x. [DOI] [PubMed] [Google Scholar]
- 24.Graves HC, Sensabaugh GF, Blake ET. Postcoital detection of a male-specific semen protein. Application to the investigation of rape. N Engl J Med. 1985;312:338–343. doi: 10.1056/NEJM198502073120603. [DOI] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. Epub 2008 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van de Wijgert JH, Verwijs MC, Turner AN, et al. Hormonal contraception decreases bacterial vaginosis but oral contraception may increase candidiasis: implications for HIV transmission. AIDS. 2013 Aug 24;27(13):2141–2153. doi: 10.1097/QAD.0b013e32836290b6. [DOI] [PubMed] [Google Scholar]
- 27.Jespers V, Crucitti T, Menten J, et al. Vaginal Biomarkers Study Group. Prevalence and correlates of bacterial vaginosis in different sub-populations of women in sub-Saharan Africa: a cross-sectional study. PLoS One. 2014 Oct 7;9(10):e109670. doi: 10.1371/journal.pone.0109670. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallo MF, Warner L, King CC, et al. Association between semen exposure and incident bacterial vaginosis. Infect Dis Obstet Gynecol. 2011;2011:842652. doi: 10.1155/2011/842652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallo MF, Steiner MJ, Hobbs MM, et al. Biological markers of sexual activity: tools for improving measurement in HIV/STI prevention research. Sex Transm Dis. 2013;40:447–452. doi: 10.1097/OLQ.0b013e31828b2f77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradshaw CS, Tabrizi SN, Fairley CK, et al. The association of Atopobium vaginae and Gardnerella vaginalis with bacterial vaginosis and recurrence after oral metronidazole therapy. J Infect Dis. 2006;194(6):828–836. doi: 10.1086/506621. [DOI] [PubMed] [Google Scholar]
- 31.Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86(4):297–302. doi: 10.1136/sti.2009.040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta SD. Systematic review of randomized trials of treatment of male sexual partners for improved bacteria vaginosis outcomes in women. Sex Transm Dis. 2012 Oct;39(10):822–830. doi: 10.1097/OLQ.0b013e3182631d89. [DOI] [PubMed] [Google Scholar]


