Abstract
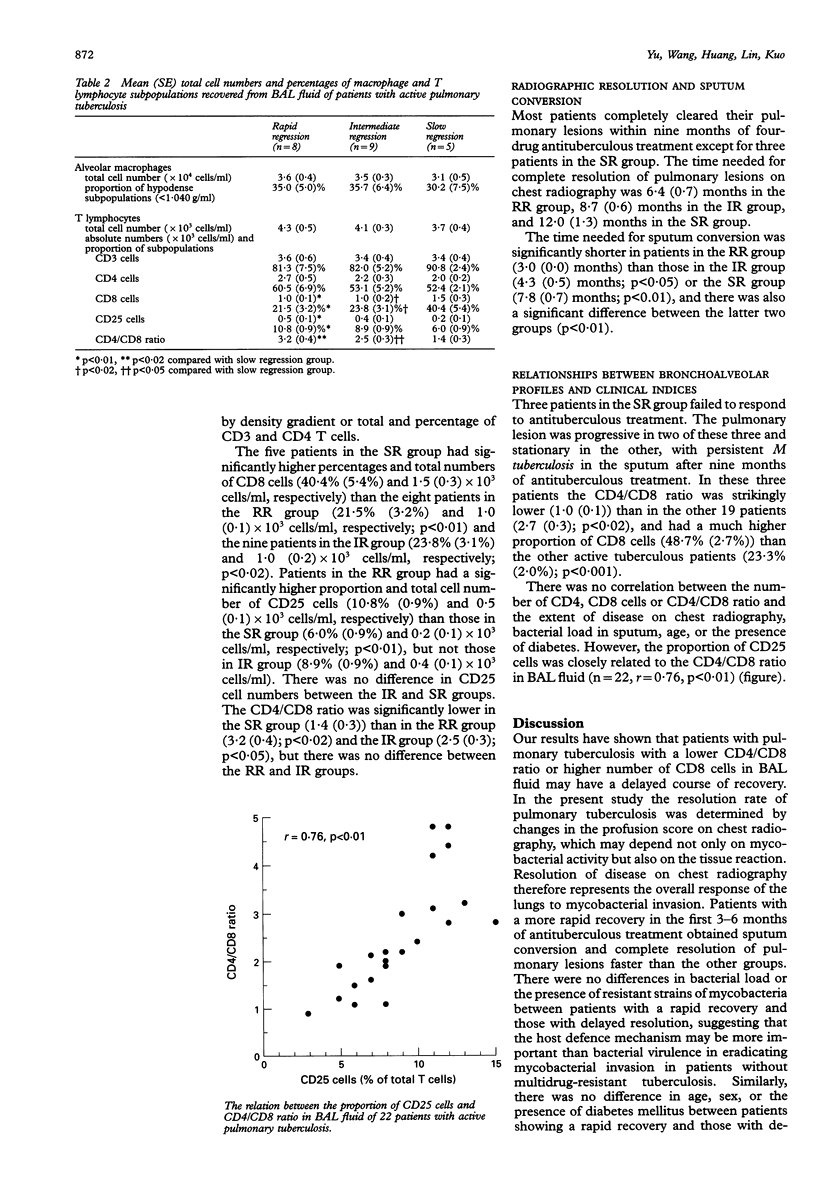
BACKGROUND--Effective host defence against mycobacterial infection chiefly depends on the interactions between macrophages and T lymphocytes. This study investigated the relation of cellular components and their activity of cells obtained by bronchoalveolar lavage (BAL) from the lower respiratory tract to disease regression in patients with active pulmonary tuberculosis without HIV infection. METHODS--Clinical indices including age, sex, the presence of diabetes, fever, the presence of resistant strains of mycobacteria, the bacterial load in sputum, and disease extent on chest radiography at presentation were assessed before commencing four-drug antituberculous therapy. Twenty two patients with active pulmonary tuberculosis were divided into rapid, intermediate, and slow regression groups. Subpopulations of alveolar macrophages separated using discontinuous Percoll density gradient centrifugation and T lymphocytes (with CD3, CD4, CD8, and CD25 monoclonal antibodies) were quantified. RESULTS--There were no differences among rapid, intermediate, and slow regression groups in terms of age, sex, the presence of diabetes, the presence of resistant strains of mycobacteria, or the bacterial load in sputum. No differences were found between the groups in terms of subpopulations of alveolar macrophages or numbers of CD3 and CD4 lymphocytes. By contrast, an increase in CD8 cells was shown in the slow regression group compared with the rapid and intermediate regression groups. CD25 cell numbers were increased in the rapid regression group compared with the slow regression group. The CD4/CD8 ratio was decreased in the slow regression group compared with the rapid and intermediate regression groups and the relation between the proportion of CD25 cells and the CD4/CD8 ratio in BAL fluid was significant. CONCLUSIONS--A decreased CD4/CD8 ratio with an increase in CD8 cells in the alveolar spaces was associated with slow disease regression in patients with active pulmonary tuberculosis without HIV infection, suggesting that the balance of T lymphocyte subsets may play a central part in the modulation of host defence against mycobacterial infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ainslie G. M., Solomon J. A., Bateman E. D. Lymphocyte and lymphocyte subset numbers in blood and in bronchoalveolar lavage and pleural fluid in various forms of human pulmonary tuberculosis at presentation and during recovery. Thorax. 1992 Jul;47(7):513–518. doi: 10.1136/thx.47.7.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J. S., Potts R. C., Kardjito T., Grange J. M. T4 lymphopenia in patients with active pulmonary tuberculosis. Clin Exp Immunol. 1985 Apr;60(1):49–54. [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar R., Malaviya A. N., Narayanan S., Rajgopalan P., Kumar R., Bharadwaj O. P. Spectrum of immune response abnormalities in different clinical forms of tuberculosis. Am Rev Respir Dis. 1977 Feb;115(2):207–212. doi: 10.1164/arrd.1977.115.2.207. [DOI] [PubMed] [Google Scholar]
- Chanez P., Bousquet J., Couret I., Cornillac L., Barneon G., Vic P., Michel F. B., Godard P. Increased numbers of hypodense alveolar macrophages in patients with bronchial asthma. Am Rev Respir Dis. 1991 Oct;144(4):923–930. doi: 10.1164/ajrccm/144.4.923. [DOI] [PubMed] [Google Scholar]
- Edwards D., Kirkpatrick C. H. The immunology of mycobacterial diseases. Am Rev Respir Dis. 1986 Nov;134(5):1062–1071. doi: 10.1164/arrd.1986.134.5.1062. [DOI] [PubMed] [Google Scholar]
- Ellner J. J. Pleural fluid and peripheral blood lymphocyte function in tuberculosis. Ann Intern Med. 1978 Dec;89(6):932–933. doi: 10.7326/0003-4819-89-6-932. [DOI] [PubMed] [Google Scholar]
- Godard P., Chaintreuil J., Damon M., Coupe M., Flandre O., Crastes de Paulet A., Michel F. B. Functional assessment of alveolar macrophages: comparison of cells from asthmatics and normal subjects. J Allergy Clin Immunol. 1982 Aug;70(2):88–93. doi: 10.1016/0091-6749(82)90234-2. [DOI] [PubMed] [Google Scholar]
- Iseman M. D. Treatment of multidrug-resistant tuberculosis. N Engl J Med. 1993 Sep 9;329(11):784–791. doi: 10.1056/NEJM199309093291108. [DOI] [PubMed] [Google Scholar]
- Katz P., Goldstein R. A., Fauci A. S. Immunoregulation in infection caused by Mycobacterium tuberculosis: the presence of suppressor monocytes and the alteration of subpopulations of T lymphocytes. J Infect Dis. 1979 Jul;140(1):12–21. doi: 10.1093/infdis/140.1.12. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. In vitro analysis of the cellular mechanisms involved in immunity to tuberculosis. Rev Infect Dis. 1989 Mar-Apr;11 (Suppl 2):S448–S454. doi: 10.1093/clinids/11.supplement_2.s448. [DOI] [PubMed] [Google Scholar]
- Kuo H. P., Yu C. T. Alveolar macrophage subpopulations in patients with active pulmonary tuberculosis. Chest. 1993 Dec;104(6):1773–1778. doi: 10.1378/chest.104.6.1773. [DOI] [PubMed] [Google Scholar]
- Rook G. A., Champion B. R., Steele J., Varey A. M., Stanford J. L. I-A restricted activation by T cell lines of anti-tuberculosis activity in murine macrophages. Clin Exp Immunol. 1985 Feb;59(2):414–420. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Taverne J., Leveton C., Steele J. The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology. 1987 Oct;62(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- Shiratsuchi H., Tsuyuguchi I. Analysis of T cell subsets by monoclonal antibodies in patients with tuberculosis after in vitro stimulation with purified protein derivative of tuberculin. Clin Exp Immunol. 1984 Aug;57(2):271–278. [PMC free article] [PubMed] [Google Scholar]
- Simon G. Radiology in epidemiological studies and some therapeutic trials. Br Med J. 1966 Aug 27;2(5512):491–494. doi: 10.1136/bmj.2.5512.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. R., Desai S. G., Jennings J., Engel D. T-cell differentiation antigens and antigenic lymphocyte reactivity in pleural effusions. Asian Pac J Allergy Immunol. 1986 Jun;4(1):19–27. [PubMed] [Google Scholar]
- Singhal M., Banavalikar J. N., Sharma S., Saha K. Peripheral blood T lymphocyte subpopulations in patients with tuberculosis and the effect of chemotherapy. Tubercle. 1989 Sep;70(3):171–178. doi: 10.1016/0041-3879(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Tsuyuguchi I., Shiratsuchi H., Teraoka O., Hirano T. Increase in T cells bearing IgG Fc receptors in peripheral blood of patients with tuberculosis by in vitro stimulation with purified protein derivative. Am Rev Respir Dis. 1980 Jun;121(6):951–957. doi: 10.1164/arrd.1980.121.6.951. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Allen P. M. The basis for the immunoregulatory role of macrophages and other accessory cells. Science. 1987 May 1;236(4801):551–557. doi: 10.1126/science.2437650. [DOI] [PubMed] [Google Scholar]



