Summary
Immunoadhesins are recombinant proteins that combine the ligand‐binding region of a receptor or adhesion molecule with immunoglobulin constant domains. All FDA‐approved immunoadhesins are designed to modulate the interaction of a human receptor with its normal ligand, such as Etanercept (Enbrel®), which interferes with the binding of tumour necrosis factor (TNF) to the TNF‐alpha receptor and is used to treat inflammatory diseases such as rheumatoid arthritis. Like antibodies, immunoadhesins have long circulating half‐lives, are readily purified by affinity‐based methods and have the avidity advantages conferred by bivalency. Immunoadhesins that incorporate normal cellular receptors for viruses or bacterial toxins hold great, but as yet unrealized, potential for treating infectious disease. As decoy receptors, immunoadhesins have potential advantages over pathogen‐targeted monoclonal antibodies. Planet Biotechnology has specialized in developing anti‐infective immunoadhesins using plant expression systems. An immunoadhesin incorporating the cellular receptor for anthrax toxin, CMG2, potently blocks toxin activity in vitro and protects animals against inhalational anthrax. An immunoadhesin based on the receptor for human rhinovirus, ICAM‐1, potently blocks infection of human cells by one of the major causes of the common cold. An immunoadhesin targeting the MERS coronavirus is in an early stage of development. We describe here the unique challenges involved in designing and developing immunoadhesins targeting infectious diseases in the hope of inspiring further research into this promising class of drugs.
Keywords: Fc fusion, anthrax, Middle East respiratory syndrome, rhinovirus, Nicotiana benthamiana
Introduction
Plants offer a highly attractive system for the large‐scale, economical production of vaccines, antibodies and other therapeutic proteins. Much of our work at Planet Biotechnology has focused on a class of therapeutic recombinant proteins known as immunoadhesins. An immunoadhesin is a chimeric antibody‐like protein that combines the ligand‐binding region of a receptor or adhesion molecule with immunoglobulin constant domains. Not every immunoglobulin Fc fusion protein can be considered an immunoadhesin. For example, Eftrenonacog alfa is a recombinant protein in which factor IX, the blood clotting factor, has been fused with Fc to achieve an extended half‐life (Miguelino and Powell, 2014). Immunoadhesins are generally intended to function in much the same manner as many monoclonal antibodies (mAbs): by binding with exquisite specificity to a particular ligand, blocking the interaction of that ligand with its native receptor. The term was coined in 1989 to describe recombinant fusions of CD4, the receptor for HIV‐1 and portions of immunoglobulin G (Capon et al., 1989). An immunoadhesin combining the extracellular portion of the human tumour necrosis factor‐alpha receptor (TNFR) and the human IgG1 hinge and Fc regions was first developed in 1991 as a TNF inhibitor (Ashkenazi et al., 1991; Peppel et al., 1991) and eventually given the name Etanercept. FDA approved for rheumatoid arthritis and other inflammatory indications under the trade name Enbrel®, it has been the highest selling biopharmaceutical for at least the last 10 years. FDA has approved five other immunoadhesins, all of which are designed to interfere with human receptor/ligand interactions: Alefacept (CD2‐directed LFA‐3) (Strober and Menon, 2007), Abatacept (CTLA‐4) (Korhonen and Moilanen, 2009), Belatacept (CTLA‐4) (Larsen et al., 2005), Rilonacept (IL‐1 and IL‐1 receptor accessory protein) (Hoffman et al., 2012) and Aflibercept (VEGF receptor 1 and 2) (Holash et al., 2002). Immunoadhesins and other immunoglobulin Fc fusion proteins approved and in development have recently been comprehensively reviewed (Linderholm and Chamow, 2014a,b).
Immunoadhesins have three critical advantages over soluble receptor fragments that are related to half‐life, affinity and ease of purification. While free receptor fragments are typically very short lived in circulation, the ability of Fc to bind to the neonatal Fc receptor (FcRn) imparts an extended circulating half‐life (Capon et al., 1989; Kurschner et al., 1992; Rath et al., 2015; Weinblatt et al., 1999). Most immunoadhesins are bivalent homodimers, and bivalency can result in increased affinity or avidity for the ligand (Ashkenazi and Chamow, 1997). Finally, binding of Fc to Protein A facilitates purification.
In addition to their superiority to soluble receptors, immunoadhesins have an underappreciated advantage over similarly targeted mAbs: low immunogenicity. The development of antidrug antibodies (ADAs) is common following repeated dosing with human mAbs, and patients who develop substantial ADA levels may experience loss of efficacy. Typically, this immune response is an anti‐idiotype immune response (Bartelds et al., 2010; van Schouwenburg et al., 2013b). Immunoadhesins elicit lower ADA titers than mAbs, and these ADAs are generally non‐neutralizing (van Schouwenburg et al., 2013a). This may be because the linker region connecting receptor and Fc is often the only foreign part of these molecules.
None of the FDA‐approved immunoadhesins target infectious disease, although immunoadhesins are well suited for that purpose because many pathogenic viruses and bacterial toxins gain access to host cells by binding to cell surface receptors. Immunoadhesins derived from host receptors have the potential to be effective therapeutics, with possible advantages over anti‐infective mAbs. Immunoadhesins that bind to viruses or toxins can not only interfere with binding to host receptors, but via Fc effector functions can promote clearance and target the virus or toxin to antigen‐presenting cells, jump‐starting an active immune response. In addition, as decoy molecules for toxins and viral receptor‐binding proteins, immunoadhesins may be less vulnerable to the development of escape mutants than monoclonal antibodies. The reasons for this will be elaborated in section ‘CMG2‐Fc’.
We have specialized in producing anti‐infective immunoadhesins in plants, and the following sections describe five such proteins that were or are being developed. Each molecule presented unique challenges related to protein design, development of assays for quantification and biological activity, stability in planta, purification and in some cases preclinical animal testing. Some of these proteins remain promising commercial candidates while others encountered obstacles that have precluded further development.
ICAM‐1‐Fc
Human rhinoviruses: a major cause of the common cold
The common cold is generally thought of as a mild disease, but significant complications resulting from colds, such as otitis media, sinusitis and asthma exacerbations, are common. Human rhinoviruses (HRV) cause up to 50% of all adult colds and 25% of colds in children (Bella and Rossmann, 1999; Sperber and Hayden, 1988), with the cost to society running to billions of dollars per year. Of the 102 characterized HRV serotypes, 91 (the major group) share as their receptor the cell surface glycoprotein intercellular adhesion molecule‐1 (ICAM‐1) (Greve et al., 1989; Staunton et al., 1989). ICAM‐1 has five extracellular immunoglobulin‐like domains, a hydrophobic transmembrane domain, and a short cytoplasmic domain. The binding site for HRV is located within N‐terminal domain 1 (Greve et al., 1991; Staunton et al., 1990). ICAM‐1 is expressed on many cells important in immune and inflammatory responses, as well as on respiratory epithelial cells (Casasnovas et al., 1998). Recombinant, soluble forms of ICAM‐1 (sICAM‐1) consisting of the extracellular domains can block infection of human cells in vitro by all major group but not minor group HRV serotypes (which use a different receptor) (Crump et al., 1993; Greve et al., 1991; Marlin et al., 1990; Ohlin et al., 1994).
sICAM‐1 attenuates HRV infection in vivo
The HRV host range is restricted to higher primates. In chimpanzees, sICAM‐1 was effective in preventing HRV infection when co‐administered with HRV or when the virus was administered 10 min later (Huguenel et al., 1997). A human clinical trial funded by Boehringer Ingelheim Pharmaceuticals showed that sICAM‐1 as a nasal spray reduced the severity of experimental HRV colds and did not result in any adverse effects or evidence of absorption through the nasal mucosa (Turner et al., 1999). Also, there was no inhibition of the development of serotype‐specific anti‐HRV antibodies. It appears that Boehringer Ingelheim abandoned development of sICAM‐1 as a common cold therapeutic because of the impracticality of the dosing regimen—a 50% reduction of symptom score required 6 daily doses (4.4 mg/day) over 7 days—and because of the cost of production in Chinese hamster ovary (CHO) cells.
In the early 1990s, researchers at Harvard produced a series of ICAM‐1 immunoadhesins in CHO cells and demonstrated that they were at least an order of magnitude more potent than monovalent sICAM‐1 in neutralizing HRV in vitro (Martin et al., 1993). Against a panel of nine major HRV serotypes, an ICAM‐1‐IgA1 immunoadhesin was between 50 and 143 times more potent on a weight basis than sICAM‐1 (Crump et al., 1994). These results suggested that an ICAM‐1 immunoadhesin had more potential than sICAM‐1 to become an effective therapy for colds caused by HRV. However, the levels of these immunoadhesins in cell culture supernatants were very low—less than 1 mg/L (Staunton et al., 1992).
Expression of ICAM‐1‐IgA2 in plants
We generated a construct for plant expression of an ICAM‐1 immunoadhesin comprising the five extracellular domains of human ICAM‐1 and the human IgA2(n) constant region (Cα1‐Cα3) (Chintalacharuvu et al., 1994), with the amino acids KDEL appended to the carboxy‐terminus to retain the protein in the endoplasmic reticulum and enhance accumulation (Schouten et al., 1996; Triguero et al., 2005). We chose the IgA2(n) allotype because it was thought to be less susceptible to proteolytic cleavage in the hinge region than IgA1 (Senior and Woof, 2005). The gene sequence was synthesized using codons frequently used by highly expressed tobacco genes and incorporating published strategies for codon optimization (Angenon et al., 1990; Chiapello et al., 1988; De Amicis and Marchetti, 2000; Sawant et al., 1999, 2001). Transcription was placed under the control of the 35S promoter in a derivative of the binary plasmid vector pBINPLUS (van Engelen et al., 1995). Tobacco (Nicotiana tabacum) was transformed using Agrobacterium tumefaciens strain LBA4404 (Hoekema et al., 1983). An elite line containing a single transgene insertion was selected from a large number of T0 plants and self‐crossed to produce a homozygous T2 line. T3 plants from this line were used to evaluate expression, develop a purification process and characterize in vitro potency and in vivo safety of ICAM‐1‐IgA2.
The concentration of ICAM‐1‐IgA2 in leaves of T3 plants increased in a nearly linear fashion with respect to time after seeding in the greenhouse. At flowering (~day 200), expression in leaves reached a maximum of approximately 600 mg/kg fresh weight of leaves (Figure S1).
ICAM‐1‐IgA2 purification
The purification of ICAM‐1‐IgA2 from transgenic tobacco was accomplished by tissue extraction in an aqueous buffer followed by clarification and ultrafiltration/diafiltration to generate a stable concentrate. This concentrate was then subjected to a three‐column purification: anion exchange chromatography to remove impurities, capture on a Lens culinaris agglutinin (LCA) affinity column followed by elution with methyl α‐D‐glucopyranoside, and a final polishing by anion exchange chromatography. Chromatography was followed by final concentration, buffer exchange, filtration and frozen storage. The purification of ICAM‐1‐IgA2 yielded monomeric, dimeric and multimeric glycosylated species (Figure 1) with a typical final yield of 36%, based on ELISA estimate of ICAM‐1‐IgA2 in crude juice.
Figure 1.
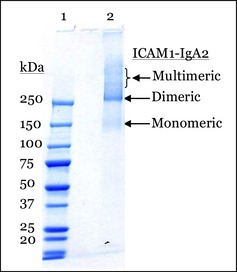
Coomassie‐stained polyacrylamide gel of ICAM‐1‐IgA2. Lane 1, Bio‐Rad all‐blue molecular weight markers; Lane 2, purified ICAM‐1‐IgA2.
In vitro potency of ICAM‐1‐IgA2
The ability of any potential therapeutic to inhibit infection by HRV in vitro can be measured by a cytopathic effect (CPE) assay (Andries et al., 1988; Arruda et al., 1992). In this assay, HeLa cells (which express ICAM‐1) are exposed to HRV that has been pre‐incubated with a range of inhibitor concentrations. After 3 days, the extent of cell killing is quantified using a dye that stains living cells. ICAM‐1‐IgA2 (plant‐made) was compared to ICAM‐1‐IgG and sICAM‐1 (R&D Systems, Minneapolis, MN) against a standard serotype (HRV‐39) in this assay, and the EC50 values (concentration at which cytopathic effect is reduced 50%) were computed (Figure 2). These results indicated that ICAM‐1‐IgA2 was six times more potent, on a weight basis, than sICAM‐1 against HRV‐39. This was not as high as the potency reported for ICAM‐1‐IgA1, perhaps reflecting differences in the hinge length or overall molecular geometry between IgA1 and IgA2 (Furtado et al., 2004).
Figure 2.
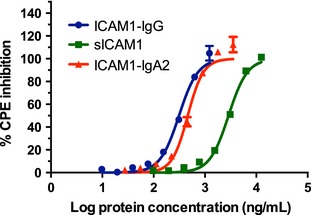
Inhibition of cytopathic effect (CPE). Dilutions of sICAM‐1, ICAM‐1‐IgG and ICAM‐1‐IgA2 were co‐incubated with HRV‐39 (MOI of 0.2) for 30 min before adding to adherent Hela‐Ohio1 cells (20 000 cells/well) in 96‐well plates. Cells were incubated for 48 h at 32 °C, then stained for viability with 0.1% crystal violet. The percentage of CPE inhibition was calculated relative to cells exposed to virus alone. Means ± standard deviations of three wells per dilution are shown.
Dr Fred Hayden and co‐workers (University of Virginia) conducted cell‐based toxicity tests with plant‐made ICAM‐1‐IgA2 and more extensive CPE assays against a range of HRV serotypes. ICAM‐1‐IgA2 showed no evidence of toxicity on any of three human cell lines when tested at up to 50 μg/mL. Depending on cell type used in the CPE assay, ICAM‐1‐IgA2 was 1.4–102 times more potent than sICAM‐1 against 16 standard HRV serotypes and 1.4–21.3 times more active against 30 clinical HRV isolates. As expected, there was no inhibitory effect against two minor group HRV serotypes.
ICAM‐1‐IgA2 protein stability variants
To investigate the effect of N‐terminal amino acid choice on protein stability and in planta accumulation, we made seven new N‐terminal amino acid variants of ICAM‐1‐IgA2. In addition to the native Q (Gln) N‐terminus, our variants included substitution of Q with P, or addition of L, M, V, G, LAP or LAPG to the N‐terminus. These variants were expressed transiently in N. tabacum, and the ex vivo protein half‐life of each ICAM‐1‐IgA2 variant was measured in the presence of cycloheximide (Geyer et al., 2010). The variants with V and LAPG at the N‐terminus had the longest half‐lives, at approximately 2‐fold that of the wild‐type; while the M variant had a half‐life approximately ½ that of the wild‐type protein. The higher in planta stability of the LAPG variant led to higher accumulation in stable transgenic N. tabacum plants, where the highest expressing LAPG T0 lines had ICAM‐1‐IgA2 levels 10–20‐fold higher than the highest expressing native ICAM‐1‐IgA2 T0 lines generated at the same time. However, the addition of LAPG at the N‐terminus resulted in an approximately 3‐fold reduction in potency as determined by CPE assay (Figure S2).
Preclinical testing of ICAM‐1‐IgA2
A nasal dosing study in rats sought to identify acute toxicity of ICAM‐1‐IgA2 and to determine whether any toxicity was reversible. The dosage volumes and ICAM‐1‐IgA2 concentrations were chosen based on the successful study of sICAM‐1 in humans for safety and efficacy against HRV infection (Turner et al., 1999). Both studies delivered the same volume per nasal passage surface area (1.55 μL/cm2 in 6 applications over 6 h) but up to 4.5‐fold more moles of ICAM‐1 in rats than in humans.
Rats were observed for 10 min after each dosing for signs of distress; none was noted. Macroscopic and histopathologic examination of all respiratory tissues identified only minimal‐to‐mild acute inflammation of the anterior turbinates of the nares in 9 of 15 animals sacrificed 1 h after the last dose, but this was not dose dependent and delivery by pipette was the suspected cause: rats’ nostrils close when under anaesthesia and were opened by the pipette during dosing. This irritation was not present in animals sacrificed on day 14. No ICAM‐1‐IgA2 was detected in serum at either sacrifice time. As the limit of detection by ELISA was 16 ng/mL, less than 0.0015% of ICAM‐1‐IgA2 delivered to the nose was present in serum at either sampling time. A similar multidose study with rabbits confirmed that delivery by pipette could cause irritation while drip administration did not. No test article‐related observations were noted upon macroscopic and histopathologic examinations of rabbit respiratory tissues.
Thus, ICAM‐1‐IgA2 appears to cause little or no acute toxicity in rats or rabbits. The absence of toxicity in these preliminary studies, along with the in vitro virus neutralization data, indicates that ICAM‐1‐IgA2 warrants further development.
CMG2‐Fc
Anthrax: still a threat to public health
Bacillus anthracis is a soilborne anaerobic spore forming bacterium that causes multiple pathologies in man (Hicks et al., 2012; Joshi et al., 2004; Lowe and Glomski, 2012; Sweeney et al., 2011). Inhalation of B. anthracis endospores can initiate an acute and severe systemic bacterial infection (inhalational anthrax), which, unless treated immediately with aggressive antibiotic therapy, is essentially 100% fatal. The limited effectiveness of antibiotics in symptomatic patients is likely because the causal agent, B. anthracis, elaborates two toxins, lethal toxin (LeTx) and edema toxin (EdTx) that continue to damage tissue even after the bacterium is dead.
Protective antigen (PA), the common component of both LeTx and EdTx, binds to a host cell surface receptor and facilitates the uptake of the toxic proteins Lethal Factor and edema Factor (Liu et al., 2009; Rainey et al., 2005; Scobie and Young, 2005; Scobie et al., 2003). Following the 2001 anthrax attack (Borio et al., 2001; Jernigan et al., 2001, 2002; Williams et al., 2002), several companies initiated programs to develop anti‐PA monoclonal antibodies (mAbs) for treatment of inhalational anthrax. These mAbs either prevent PA from binding to its cellular receptor or interfere with PAs ability to facilitate toxin entry and cell death (Leysath et al., 2009; Liu et al., 2009; Migone et al., 2009; Mohamed et al., 2005; Peterson et al., 2006, 2007; Subramanian et al., 2005; Vitale et al., 2006).
PA binds to two related cell surface proteins, tumor endothelial marker 8 (TEM8) and capillary morphogenesis protein 2 (CMG2), with CMG2 being the major receptor mediating lethality in vivo (Banks et al., 2005; Bradley et al., 2001; Liu et al., 2009; Scobie et al., 2003). The affinity of CMG2 for PA is quite high, between 200 and 900 pm, depending on the measurement method used (Liu et al., 2009; Wigelsworth et al., 2004). A soluble version of the CMG2 extracellular domain inhibited intoxication of human and animal cells when it was premixed with PA (Scobie et al., 2003), suggesting that a CMG2 immunoadhesin could be a viable alternative to anti‐PA antibodies.
Designing a CMG2 immunoadhesin
Some of the challenges of immunoadhesin design can be illustrated by our early attempts to produce a stable and functional CMG2 immunoadhesin. Our first design used amino acids 34–232 of human CMG2 fused to all three constant domains of human IgG1 (all of our constructs begin with a 19 amino acid signal peptide from a murine immunoglobulin light chain). The choice of this fragment of CMG2 was based on the sequence of the soluble CMG2 that had previously been shown to inhibit LeTx in vitro (Scobie et al., 2003) and using all three heavy chain constant domains had worked well for our ICAM‐1‐IgA2 immunoadhesin. The open reading frame was cloned into a binary vector, transformed into A. tumefaciens and used for transient expression in Nicotiana benthamiana using a vacuum‐assisted whole‐plant infiltration method (Kapila et al., 1997; Vaquero et al., 1999). Expressed CMG2‐IgG immunoadhesin was purified by Protein A chromatography and visualized on Coomassie‐stained SDS‐PAGE.
The predicted monomeric molecular weight of this original CMG2‐IgG construct was 58 kDa. In reduced samples, a minor fraction of the purified protein was in a 53 kDa band, while the majority of protein was in three smaller bands between 29 and 34 kDa (Figure 3, lane 2). All of the bands visualized by Coomassie blue also reacted with anti‐human IgG in Western blot analysis (not shown). N‐terminal sequencing of these smaller fragments revealed that they began with amino acid sequences in the middle of the first IgG1 constant domain (Cγ1), suggesting the presence there of a proteolytically sensitive site. Examination of the CMG2 extracellular domain crystal structure (Lacy et al., 2004; Santelli et al., 2004) suggested that amino acids 207–218 of our original CMG2‐IgG (aa 221–232 of CMG2) were not necessary for the structure of the domain or for PA binding function. We then made three new CMG2‐IgG variants: V1, without aa 221–232 of CMG2; V2a, without the Cγ1 domain; and V2b, without either. Only V2b lacked a significant fraction of degraded protein fragments (Figure 3, lane 4), so it was this variant that we used for further development.
Figure 3.
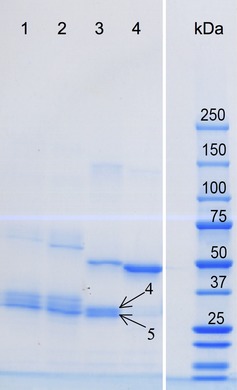
Coomassie‐stained SDS‐PAGE gel of CMG2‐Fc variants. Each lane was loaded with 5 μg protein eluted from Protein A chromatography. Samples were reduced with 100 mm DTT. Lane 1, original CMG2‐Fc; lane 2, V1; lane 3, V2a; lane 4, V2b. Bands labelled 4 and 5 (with arrows) had N‐terminal ends between aa 221 and 232 of CMG2.
CMG2‐Fc was further modified by the introduction of an Asn297Gln mutation in the Fc region, making a nonglycosylated variant. Neither in vitro potency, as measured by a standard cell‐based lethal toxin neutralization assay, nor in vivo potency, as measured in a B. anthracis spore challenge study in rabbit, were significantly affected by the presence or absence of N‐glycosylation (Wycoff et al., 2011). Following these early studies, only the aglycosyl form was further developed. Aglycosyl CMG2‐Fc in the pTRAkc plant expression vector (Schinkel et al., 2005) in A tumefaciens GV3101 (English et al., 1997) was used to transform N. tabacum. Transgenic CMG2‐Fc N. tabacum plants were bred through the T3 generation, which, at maturity, accumulated CMG2‐Fc to approximately 30 mg/kg fresh weight. This is about 10 times higher than the level reported by Andrianov et al. (2010) for a CMG2‐Fc construct that incorporated a slightly smaller portion of CMG2 lacking two cysteines that participate in a disulphide bond in the native protein (Lacy et al., 2004). That CMG2‐Fc also was about 10‐fold less potent in vitro than the one we produced, suggesting, again, that the specific design of an immunoadhesin can be crucial in determining its expression and biological activity. In a cell‐based toxin neutralization assay (TNA), the potency of an anthrax antitoxin is expressed as the EC50, or the concentration of antitoxin that reduces cytotoxicity 50% (Li et al., 2008). The EC50 for our CMG2‐Fc (53 ng/mL) is similar to the reported EC50 for the anti‐PA antibodies raxibacumab (Zhang et al., 2003), Anthim™ (Mohamed et al., 2005) and AVP‐21D9 (Sawada‐Hirai et al., 2004).
In vivo efficacy
We have demonstrated efficacy of CMG2‐Fc against inhalational anthrax in both rabbit and cynomolgus macaque models. In a ‘postexposure prophylaxis’ study, rabbits were challenged by nasal instillation of 100 LD50 of B. anthracis Ames spores. CMG2‐Fc was administered intravenously within an hour after spore challenge. Doses of 2 and 4 mg CMG2‐Fc/kg body weight were 100% effective in preventing death (6/6 survivors). Doses of 1 and 0.5 mg CMG2‐Fc/kg resulted in 33% survival (2/6 survivors at each dose). Survivors were re‐challenged with B. anthracis Ames spores 1 month after the first challenge (when CMG2‐Fc was expected to be completely absent from circulation), and all four animals survived. This study provided a proof of concept for a postinfection therapeutic indication for CMG2‐Fc (Wycoff et al., 2011).
In a ‘trigger‐to‐treat’ study, twenty‐one rabbits given an aerosol challenge with 200 LD50 were treated with CMG2‐Fc following a significant increase in body temperature (SIBT) and these animals were confirmed bacteremic (17/21) and toxemic (20/21) before treatment. Fifty‐seven per cent of animals treated with 30 mg/kg CMG2‐Fc survived, while 86% of animals treated with 15 mg/kg CMG2‐Fc and 43% of animals treated with 7.5 mg/kg CMG2‐Fc survived (Figure 4a). All of the untreated animals succumbed to disease. A one‐sided Fisher's exact test revealed significantly higher survival rates in the groups that received 30 mg/kg (P = 0.0350) and 15 mg/kg (P = 0.0023) antitoxin compared to the untreated control group.
Figure 4.
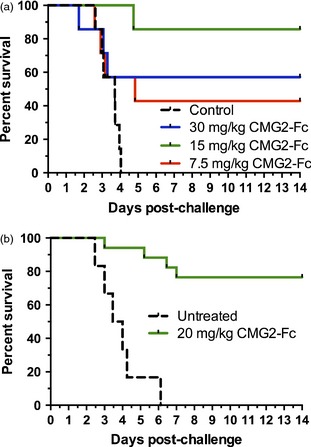
Kaplan–Meier curves representing time‐to‐death and survival for B. anthracis‐challenged animals. Animals were challenged with 200 LD 50 B. anthracis (Ames) spores then treated as indicated after evidence of infection. A, Rabbits received CMG2‐Fc or PBS intravenously after sustained increase in body temperature. B, Cynomolgus macaques received CMG2‐Fc or PBS intravenously after detection of PA in serum.
Two studies in cynomolgus macaques have demonstrated CMG2‐Fc efficacy against inhalational anthrax. In a postexposure prophylaxis study, cynomolgus macaques were challenged with ~200 LD50 of B. anthracis (Ames) spores via small particle aerosol and then administered CMG2‐Fc intravenously 20–40 min after challenge. A dose of 15 mg/kg was protective, with three of four animals receiving the drug surviving (75%), while all eight untreated animals died (P = 0.02). At a dose of 2 mg/kg only 1 of 4 animals survived, a result not significantly different from untreated controls. In a trigger to treat study, twenty‐four (24) cynomolgus macaques were challenged with ~200 LD50 of B. anthracis (Ames) spores via small particle aerosol. Seventeen of these were then administered CMG2‐Fc at 20 mg/kg intravenously after PA was detected in serum (24–42 h after challenge), while six animals were not treated. All six untreated control animals succumbed to disease. Only 13 of the animals that received treatment were confirmed positive for blood culture prior to receiving treatment. Of these confirmed positive animals, ten survived (77%) (Figure 4b). A one‐sided Fisher's exact test revealed a significantly higher survival rate in the treated, confirmed blood culture‐positive group compared to the untreated control group (P = 0.003).
Inherent superiority of immunoadhesins over monoclonal antibodies
Published reports indicate that anti‐PA mAbs and CMG2‐Fc are about equally effective in vivo against inhalation anthrax, with reported survival following doses of 15–40 mg/kg in trigger‐to‐treat non‐human primate models of 60–75% (Malkevich et al., 2014; Migone et al., 2009; Riddle et al., 2011). So far the evidence suggests that CMG2‐Fc and anti‐PA mAbs inhibit the toxicity of anthrax lethal toxin by similar mechanisms. By binding with high affinity to PA, both types of molecules prevent PA from carrying out its normal function.
One way in which an immunoadhesin‐like CMG2‐Fc may be superior to a mAb that binds the same toxin is in the differing susceptibility of the two molecules to deliberate modification of their target. It is possible to engineer PA to eliminate the epitope for a particular mAb without affecting PA's ability to bind to CMG2 on the cell surface. By contrast, any changes to PA that reduce binding to CMG2‐Fc also reduce PA's binding to the cell surface and thus its toxicity. For example, amino acid substitution at any of four residues in PA eliminated binding (and thus neutralization) by the anti‐PA mAb 14B7 (the precursor of Anthim™) without greatly reducing the in vitro toxicity of LeTx containing the mutant PA (Rosovitz et al., 2003). By contrast, CMG2‐Fc was fully effective at neutralizing LeTx containing any of these mutations (Wycoff et al., 2011).
Animal pharmacokinetics studies
A pharmacokinetics study in rabbits showed that elimination of CMG2‐Fc fit best with a two‐compartment model, with a 5‐day terminal half‐life. A study in cynomolgus macaques evaluated the pharmacokinetics of CMG2‐Fc delivered both intravenously (IV) and intramuscularly (IM). The calculated terminal IV and IM half‐lives were 241 and 222 h, respectively, values similar to human mAbs in this species (Deng et al., 2010, 2011).
Animal safety/immunogenicity studies
Pilot 2‐week repeat‐dose toxicology studies in rats and cynomolgus macaques found that dosages of up to 62.5 mg/kg of CMG2‐Fc were well‐tolerated; no test article‐related mortality or changes in clinical observations, food consumption, body weight, urinalysis parameters, gross necropsy findings or organ weights occurred. Overall, no test article‐related adverse findings were identified in these studies. Although animals developed antibodies against the human Fc, no antidrug antibodies against human CMG2 were detected.
Pilot‐scale CMG2‐Fc purification from transgenic N. tabacum
We have developed a pilot‐scale manufacturing process for CMG2‐Fc that was used to supply high‐quality product for animal efficacy and preclinical safety testing. Our goal was to prepare a drug product suitable for testing in humans using good manufacturing practices (GMP). Manufacturing CMG2‐Fc using GMP means purifying CMG2‐Fc from plant biomass, preparing and releasing drug product for use, after testing and judging its attributes of identity, potency, purity, strength, stability, appearance and safety (e.g. endotoxin, aggregates, alkaloids, host antigens, etc.). These activities must be reliably reproducible and documented, to show that materials and procedures are specified, acceptable for their purpose and properly used or performed.
We have scaled CMG2‐Fc production from 0.3 to 25 g per lot while establishing procedures for obtaining the expected quality (attributes and behavior) of intermediates and product. The effort included the creation of a Quality System, an infrastructure comprising manufacturing (make drug product), facilities (maintain the manufacturing environment), quality control (test attributes of the raw materials, environment and purifications) and quality assurance (coordinate and review manufacturing‐associated actions).
The flow chart for pilot‐scale manufacturing (Figure 5) shows upstream processing, which is specific to obtaining raw protein from plant biomass, and downstream processing, which is similar to biopharmaceutical purifications from a variety of sources and involves chromatography, formulation and filling. All operations except freezing occur at room temperature. In a typical pilot‐scale manufacturing run, T3 N. tabacum plants transgenic for CMG2‐Fc were grown to maturity in a greenhouse and 500 kg of stems and leaves were harvested from approximately 1875 sq ft of raised beds approximately 110 days after seeding, when CMG2‐Fc has accumulated to 30–40 mg/kg plant biomass. Biomass was stored overnight in bins. The next day, the biomass was pulped using a grinder (Reitz‐Bepex stacked‐blade disintegrator), and then squeezed in a screw press (Vincent CP‐4) with a buffer to produce approximately 420 L of juice (Figure 5), which was green, opaque and largely free of cellulosic solids.
Figure 5.
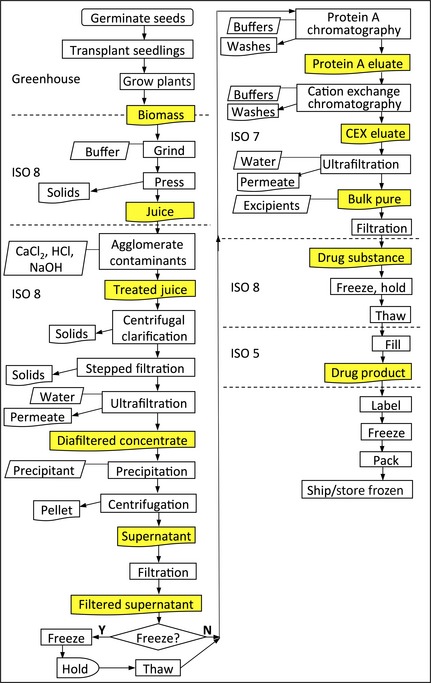
CMG2‐Fc pilot‐scale manufacturing flow with inputs, outputs, processes and environments. Discrete product outputs (yellow) are shown during upstream (left) and downstream (right) processing. CMG2‐Fc from batches of 500 kg biomass (left) are combined for chromatography and lots of 25 grams of drug product (right).
Juice was treated to agglomerate solids by briefly reducing the pH to 4.5 before returning it to pH 6.5–7. While this results in the loss of about 35% of the CMG2‐Fc found in juice, we found that brief acidification was necessary to produce, following centrifugation, a clarified juice that was amenable to chromatography. Continuous centrifugation using an Alfa Laval Clara 80, a stacked‐disc clarifier, removed about 60% of total protein and >99% of all agglomerated solids, initially present at 8–10% (v/v). Bioburden was not significantly reduced during clarification, but filtration through 1 μm and 0.2 μm reduced bioburden to less than 1 cfu/mL without loss of CMG2‐Fc. A diafiltered concentrate was prepared, using 4 m2 of 30 kDa MWCO cassettes, reducing volume up to 80‐fold, with a step yield of >90% for CMG2‐Fc and about 1% for phenolics. The diafiltered concentrate was subjected to a proprietary precipitation to remove significant amounts of protein, color and nucleic acids before a final filtration and frozen storage. At this point, CMG2‐Fc specific activity was increased over 20‐fold, volume was reduced up to 80‐fold and phenolics reduced >99% at an overall yield of 60%–65% of product, or 9–12 g CMG2‐Fc from 500 kg biomass.
In downstream processing, we combined filtered supernatants for protein A chromatography to prepare 25 g lots of drug product. Polishing by cation exchange chromatography reduced endotoxin and aggregates to acceptable levels (<0.1 EU/mg and <5% aggregates by SEC‐HPLC) (Figure S3). The step yield from Protein A chromatography was >95% while the step yield from cation exchange chromatography was 70%. The drug substance was concentrated and buffer‐exchanged into phosphate‐buffered saline for filling into a final container. The overall yield of product at this scale is 38% at >90% purity. The drug product was prepared at 30–50 mg/mL, depending upon the study, and stored frozen.
The largest task remaining for developing pilot‐scale manufacturing is to increase CMG2‐Fc purity from >90% to >95% and further reduce nucleic acid and host antigen content. Improving step yields will mitigate product losses that will accompany the introduction of additional polishing step(s), which will likely include anion exchange chromatography. We plan to replace acid/base titration, to agglomerate juice solids, with the addition of our proprietary precipitant because subsequent clarification is excellent and product recovery is nearly 100%.
Improved expression of CMG2‐Fc using RNA‐optimized Fc
It is widely believed that recombinant protein production in plants can be more economical than mammalian cell culture production, but publications describing rigorous cost analyses are limited (Buyel and Fischer, 2012; Kaufman and Kalaitzandonakes, 2011; Tuse et al., 2014). Kaufman and Kalaitzandonakes (2011) estimated the final per unit cost of a mAb from corn (expressed in seed at 323 mg mAb/kg of seed) to be 2/3 as much as the same mAb produced using a mammalian cell culture system (assuming 1.5 g/L). The cost model used in that paper indicated that increases in mAb expression should result in substantial reduction in both production and purification costs.
Protein expression in plants has been improved by optimizing codon usage to reflect frequently used codons of the expression host (Batard et al., 2000; Horvath et al., 2000; Huang et al., 2001; Joensuu et al., 2009; Kang et al., 2004a,b; Rouwendal et al., 1997; Suo et al., 2006). Generally, codon optimization strategies (synonymous codon mutations) are concerned with increasing the translation rate (Ikemura, 1985; Kane, 1995) and pay little attention to the possibility of affecting transcription or mRNA stability. However, a few publications have shown that codon usage can dramatically affect mRNA levels (Kudla et al., 2006, 2009; Nguyen et al., 2004; Tokuoka et al., 2008).
In an effort to improve antibody expression, we compared a number of synonymous codon variants of the human IgG1 and IgA2 heavy chains using transient agro‐infiltration in N. benthamiana and N. tabacum (combining all variants with the same kappa chain). These codon variants generated significant differences in protein accumulation (up to 20‐fold). Surprisingly, the mRNA levels produced by the gene variants were considerably different (up to 80‐fold), despite using the same promoters and the same upstream and downstream UTRs in all expression constructs. Interestingly, we found a generally inverse association between protein expression level and codon adaptation index (CAI), although all of the genes tested had relatively high CAIs (0.80–0.95), and hence, other gene features likely outweighed CAI for impact on expression. We found that the best correlate of mRNA and protein accumulation was computationally predicted mRNA thermostability (folding minimum Gibbs free energy), with high mRNA thermostability (low Gibbs free energy) leading to high mRNA and protein levels. This was a surprising finding because researchers often try to prevent strong mRNA secondary structures, assuming that these structures impede the progress of ribosomes. We postulate that thermodynamically stable mRNA secondary structures play a key role in facilitating high protein accumulation in plants and could be an effective approach for improving production of biotherapeutics in plants. We called the best expressing of these synonymous codon variants the ‘RNA‐optimized’ Fc sequence.
We compared expression of two CMG2‐Fc gene constructs, one containing a N. tabacum codon‐optimized Fc sequence generated by a commonly used online algorithm and the other incorporating the RNA‐optimized Fc sequence. The two genes had the same CAI (0.87) and GC contents of 43.8% and 43.9% for the codon‐optimized and RNA‐optimized sequences, respectively. By A. tumefaciens‐mediated transient expression in both N. tabacum and N. benthamiana leaves, we observed at least 7‐fold higher CMG2‐Fc protein accumulation when using the RNA‐optimized Fc sequence (Figure 6). We also generated stable transgenic tobacco plants (N. tabacum, cultivar Wisconsin 38) by A. tumefaciens‐mediated transformation of the two CMG2‐Fc gene variants described above. The RNA‐optimized gene variants expressed CMG2‐Fc at higher levels (up to 200 mg/kg) and produced a larger number of detectable transformants than their codon‐optimized counterparts (Figure 6).
Figure 6.
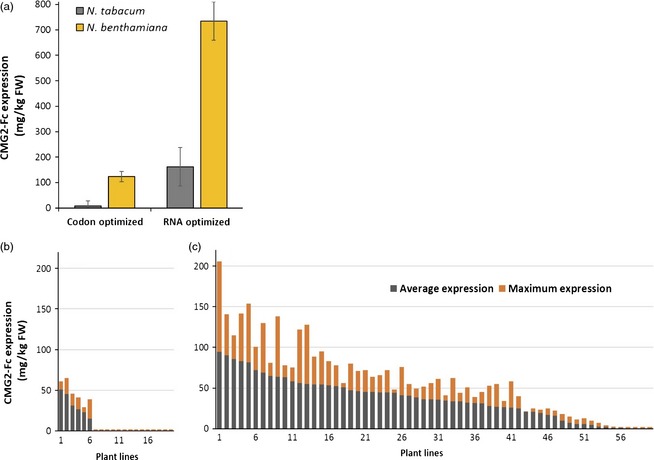
Stable and transient expression of CMG2‐Fc synonymous codon variants. (a) Transient expression of CMG2‐Fc gene variants in Nicotiana spp. by A. tumefaciens syringe infiltration (OD600 = 0.1). A. tumefaciens carrying the P19 post‐transcriptional gene silencing suppressor was co‐infiltrated into N. benthamiana leaves. Leaf samples were harvested 7 days postinfiltration, and CMG2‐Fc expression was measured by ELISA. (b and c) N. tabacum was transformed with either the codon‐optimized CMG2‐Fc gene (b) or the RNA‐optimized sequence (c). Approximately 120 individual T0 plants were screened for each transformation. Plants were screened by ELISA at 3‐week intervals until flowering stage. Each bar represents a plant (only expressing plants are shown). Grey bar, average expression. Orange bar, maximum expression detected in that plant.
When producing CMG2‐Fc and other immunoadhesins by vacuum‐assisted agro‐infiltration of N. benthamiana we routinely co‐infiltrate with an A. tumefaciens strain designed to introduce P19, a viral suppressor of post‐transcriptional gene silencing (PTGS) (Garabagi et al., 2012; Voinnet et al., 2003). Arzola et al. (2011) evaluated co‐expression of one of our CMG2‐Fc constructs with nine different PTGS suppressor proteins. P1 resulted in the greatest enhancement, with expression of CMG2‐Fc at 3.5 days postinfiltration ten‐fold higher when compared to the absence of PTGS suppression.
Conclusions regarding CMG2‐Fc development
There are currently three biologicals licensed for treatment of inhalational anthrax: the anti‐PA mAbs raxibacumab (GSK) and Anthim (Elusys Therapeutics, Pine Brook, NJ) and the human serum‐derived Anthrasil (Emergent Biosolutions, Gaithersburg, MD). Both of these drugs have been approved under FDA's so‐called ‘Animal Rule’, which provides a framework for demonstrating safety and efficacy of drugs such as CMG2‐Fc that cannot ethically be tested for efficacy in humans (Department of Health and Human Services, 2002). We have demonstrated the efficacy of plant‐produced CMG2‐Fc against inhalational anthrax in rabbits and cynomolgus macaques and demonstrated the safety of repeated doses as high as 62.5 mg/kg in pilot rat and cynomolgus macaque studies. We are currently seeking funding for IND‐enabling animal toxicology studies, which will be required before a Phase I clinical trial.
DPP4‐Fc
MERS: an emerging infectious disease
Middle East respiratory syndrome (MERS), caused by the MERS coronavirus (MERS‐CoV), is a newly emerging human health threat with an estimated 40% case fatality rate (Majumder et al., 2014). MERS‐CoV is closely related to bat coronaviruses, leading to early speculation that bats might be the source of human infections (Cui et al., 2013; Ithete et al., 2013; Lau et al., 2013). More recent studies have found that MERS‐CoV is widespread in dromedary camels (Abdel‐Moneim, 2014; Alexandersen et al., 2014; Ferguson and Van Kerkhove, 2014; Haagmans et al., 2014; Meyer et al., 2014) and that the virus has been circulating in camels since at least 1992 (Alagaili et al., 2014). It seems fairly clear now that camels are the origin of many of the human infections (Azhar et al., 2014; Kupferschmidt, 2014; Memish et al., 2014). There are currently no effective therapies against the virus.
Like other coronaviruses, the MERS‐CoV virion uses a large surface spike (S) glycoprotein for interaction with and entry into target cells. The S glycoprotein consists of an N‐terminal S1 subunit containing the receptor‐binding domain (RBD), followed by a membrane‐proximal S2 domain, a transmembrane domain and a C‐terminal intracellular domain (Qian et al., 2008).
Through co‐purification with the MERS‐CoV S1 domain, Raj and colleagues determined that dipeptidyl peptidase 4 (DPP4) functions as a MERS‐CoV cellular receptor (Raj et al., 2013). DPP4 is expressed on the surface of several cell types, including those found in human airways. In support of its role as a MERS‐CoV receptor, a polyclonal antiserum directed against DPP4 inhibited MERS‐CoV infection of human bronchial epithelial cells and human hepatoma‐7 cells, and soluble DPP4 inhibited Vero cell infection by MERS‐CoV (Raj et al., 2013). Nonsusceptible cells transformed to express cell surface human DPP4 became susceptible to infection (Raj et al., 2013).
DPP4 is a serine protease, but its enzymatic function does not appear to be essential for viral entry. It cleaves peptide bonds to release proline‐containing dipeptides from the N‐terminus of physiologically important polypeptides (Hopsu‐Havu and Glenner, 1966) and has been proposed as an important regulator of different physiological and pathophysiological conditions (Bengsch et al., 2012; Mentlein, 1999; Miyazaki et al., 2012; Moran et al., 2012; Shigeta et al., 2012). In addition to the cell surface form a soluble DPP4, identical to the 727 amino acid extracellular domain, is found in serum (at about 6 μg/mL). The extracellular domain consists of an N‐terminal eight‐bladed β‐propeller domain (S39 to D496) and a C‐terminal α/β hydrolase domain (N497 to P766). The MERS‐CoV S1 protein receptor‐binding domain (RBD) contacts the DPP4 β‐propeller domain and has no contact with the hydrolase domain.
Several potential MERS‐CoV therapies have been reported, including some small molecules (Dyall et al., 2014; Hart et al., 2014; Lu et al., 2014). MAbs against either DPP4 (Ohnuma et al., 2013) or the MERS‐CoV RBD can block infection in vitro (Du et al., 2014; Jiang et al., 2014; Ying et al., 2014). The mAb approaches, however, have potential drawbacks. Targeting DPP4, a widespread human cell surface antigen, with an antibody could have pleiotropic effects in patients. MAbs against the virus RBD may be effective, but the development of neutralization escape mutants is a very real possibility, as seen with anti‐SARS mAbs (Ng et al., 2014; Rockx et al., 2010). Alanine‐scanning mutations in the RBD of MERS‐CoV pseudoviruses, while having little effect on infectivity of the virus, significantly reduced the inhibitory effect of even very potent mAbs on pseudovirus entry (Du et al., 2014; Jiang et al., 2014). There is evidence that variability in the RBD sequence in natural MERS‐CoV isolates may have originated from selective pressure of neutralizing antibodies in vivo (Tang et al., 2014).
Designing and testing a DPP4 immunoadhesin
We are pursuing the immunoadhesin approach to develop a MERS‐CoV therapeutic. Sequences encoding the human DPP4 extracellular domain (aa 39–766) or just the DPP4 β‐propeller domain (either aa 39–496 or 39–504) were PCR‐amplified from a human DPP4 clone and cloned into the plant binary vector pTRAkc upstream of and in‐frame with RNA‐optimized Fc sequences from human IgG1, IgA1 or IgA2. The IgA constructs were truncated to remove the 18‐amino acid C‐terminal IgA tail piece, a sequence that enables dimeric IgA formation but significantly reduces IgA expression in plants (Hadlington et al., 2003) and is not required for binding Fc alpha receptors (Brunke et al., 2013). All constructs included a C‐terminal KDEL peptide.
The expression vectors were introduced into A. tumefaciens GV3101::pMP90RK (Maclean et al., 2007) and transient expression in whole N. benthamiana plants was initiated by vacuum‐infiltrating recombinant Agrobacterium. DPP4‐Fc fusions were purified from plant homogenates using Protein A chromatography for IgG Fc fusions or CaptureSelect IgA Affinity Matrix (Life Technologies, Carlsbad, CA) for IgA Fc fusions. Expression levels ranged from 140 to 440 mg DPP4‐Fc/kg fresh plant weight. These numbers are not high, but not unexpected because the DPP4 nucleotide sequence was human (not codon‐ or RNA‐optimized for plants).
The DPP4‐Fc proteins were analysed by SDS‐PAGE (Figure 7a/b) and size exclusion chromatography (Figure 7c). The majority of DPP4‐Fc was in the expected dimeric form under nonreducing conditions and monomeric form when reduced. Purity was estimated by scanning gels for band intensities using a BioRad Gel Doc EZ Imager. The IgG Fc fusions were 93%–96% pure, the IgA Fc fusions 76%–87% pure.
Figure 7.
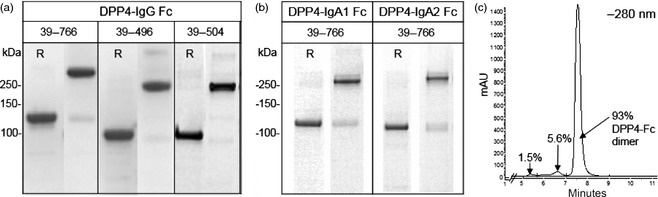
Characterization of plant‐made DPP4‐Fc. DPP4‐Fc variants, with either full‐length DPP4 extracellular domain (aa 39–766) or the β‐propeller domain (aa 39–496, or aa 39–504) fused to human Fc from IgG1 (a), or IgA (b), were purified from plants. Reduced (R) and nonreduced samples were separated by SDS‐PAGE and stained with Coomassie dye. (c) Size exclusion chromatography 280 nm trace of a representative sample, DPP4(39‐766)‐FcG1.
A fusion of the entire DPP4 extracellular domain (amino acids 39–766) to IgG1 Fc (designated DPP4(39‐766)‐FcG1) bound the S1 domain of recombinant Spike protein in an ELISA with an EC50 of 0.04 μg/mL, a 30‐fold enhancement compared to soluble DPP4 (EC50 of 1.2 μg/mL). A smaller Fc fusion with just the β‐propeller domain, DPP4(39‐496)‐FcG1, did not bind as well (EC50 of 3.2 μg/mL). Because structural analysis of DPP4 (Engel et al., 2003) suggested that the N‐terminal extension of blade I (Phe53–Tyr58) can tighten the propeller structure by interacting with the immediate C‐terminal extension to blade VIII (Glu499–Met503), we produced a third fusion, DPP4(39‐504)‐FcG1 and tested that as well, but found that it also bound poorly to S1 (Figure 8).
Figure 8.
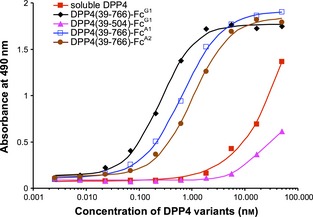
Dose‐dependent binding of DPP4‐Fc variants to MERS‐CoV spike protein. The ability of the DPP4‐Fc variants to bind to the S1 protein of MERS‐CoV was tested using a ligand‐binding ELISA. Dilutions of DPP4‐Fc variants or soluble DPP4 were added to S1 protein coated on microtiter plates. Bound DPP4‐Fc was detected using goat anti‐DPP4 IgG and reported with donkey anti‐goat IgG labelled with HRP.
The ELISA results suggest that a fusion of the DPP4 extracellular domain to IgG1 Fc binds somewhat better to MERS‐CoV S1 than similar IgA1 and IgA2 fusions. Work is underway to test the neutralizing potency of these immunoadhesins in vitro against both VSV‐based pseudovirus (Du et al., 2010) and live MERS‐CoV (Raj et al., 2013). We anticipate that, unlike a mAb, DPP4‐Fc should not subject the virus to selection for neutralization escape mutants, as any mutation that decreases binding to DPP4‐Fc will likely decrease binding to the native receptor, resulting in an attenuated virus.
Basigin‐Fc
Erythrocyte invasion by the merozoite form of Plasmodium falciparum is essential for the pathogenesis of malaria. Crosnier et al. (2011) showed that the erythrocyte antigen basigin (BSG) is a receptor for PfRh5, a parasite ligand that is essential for blood stage growth. Erythrocyte invasion could be inhibited by a soluble pentamerized basigin, by basigin knockdown, or by anti‐basigin antibodies.
We posited that a basigin immunoadhesin might be a useful malaria‐preventive agent for travellers to malaria‐endemic regions, and set out to produce and test a basigin‐Fc in plants. The DNA sequence for the human basigin extracellular domain (amino acids 140–317, Genbank accession P35613, isoform 1) was codon optimized for expression in N. tabacum and mutated to prevent glycosylation at three potential N‐linked glycosylation sites, as had been performed by Crosnier (2011). The Fc sequence of human IgG1 was cloned in frame downstream of the BSG sequence in the pTRAkc plant expression vector (Maclean et al., 2007). The expression vector was introduced into A. tumefaciens, and expression in N. benthamiana plants was accomplished as above. Basigin‐Fc was purified from plant extracts by Protein A chromatography, and its purity and assembly into dimers was characterized by SDS‐PAGE.
Approximately 238 μg Basigin‐Fc per gram fresh weight was eluted from Protein A, but as the column was saturated, this represents a minimum estimate for the level that accumulated in the plant. Basigin‐Fc bound to recombinant PfRh5 (gift of G. Wright, Wellcome Trust Sanger Institute) in ELISA (Figure S4), but the protein was not able to inhibit parasite growth in a standard in vitro assay (Hui et al., 1994), at concentrations up to 100 μg/mL, probably because the dimeric basigin‐Fc did not have the same avidity as the pentameric form tested by Crosnier (2011). As this is well above any practical dose for human administration, this project was discontinued.
DAF‐Fc
Hantaviruses as a health threat
Hantaviruses (family Bunyaviridae) are emerging zoonotic viruses, harbored by small mammals, which cause febrile diseases in humans. New World hantaviruses are responsible for hantavirus cardiopulmonary syndrome (HCPS) in the Americas, a syndrome that results in respiratory failure, cardiogenic shock and subsequent death in 30%–50% of cases. Sin Nombre virus (SNV) is a prototypical New World hantavirus that causes the vast majority of HCPS cases in the United States (Kanerva et al., 1998; Mertz et al., 2006; Zaki et al., 1995). There is no approved antiviral therapy for HCPS.
Decay‐accelerating factor (DAF) is one of the cell surface proteins postulated to mediate, at least in vitro, hantavirus entry into cultured cells, yet its direct role in virus entry is not well understood. Nevertheless, the pretreatment of cells with anti‐DAF antibodies or recombinant human DAF from mouse cells (rhDAF) resulted in inhibition of Hantavirus infection (Krautkramer and Zeier, 2008; Popugaeva et al., 2012). DAF is expressed at high levels on the luminal surface of endothelial cells and has been subverted by many bacterial and viral pathogens, including echoviruses (Bergelson et al., 1994; Ward et al., 1994) and human enteroviruses (Powell et al., 1999).
Designing and testing a DAF immunoadhesin
Based on the forgoing evidence, we rationalized that a decoy receptor based on DAF might act as a broad spectrum therapeutic against hantavirus infections. The multivalent binding avidity of hantavirus particles for cellular DAF receptors is very high with a KDa of ~26 pm (Buranda et al., 2010). A bivalent DAF‐Fc might be expected to bind virus particles with an affinity much greater than monomeric rhDAF.
One challenge in designing a DAF immunoadhesin is the presence of an N‐linked glycosylation site in the DAF protein. The role of this N‐glycan in binding to Hantavirus is not known, so the effect of differences in N‐glycan structure resulting from plant expression could not be predicted. Thus, we made three glycosylation variants based on a prototype DAF‐Fc construct with 252 aa of human DAF + hinge and Fc of human IgG1 (aglycosyl). The variants included the following: (i) complex DAF‐Fc, with complex‐type N‐glycan in DAF with plant‐specific (α1,3‐fucose and β1,2‐xylose) residues; (ii) high mannose DAF‐Fc with C‐terminal SEKDEL, resulting in high mannose‐type N‐glycan in DAF; and (iii) aglycosyl DAF‐Fc, in which the glycosylation site in DAF is abolished by changing the Asn residue to Gln. All three DAF‐Fc variants expressed at high levels in N. benthamiana (540 mg of protein/kg leaves) and bound in ELISA to recombinant human CD97 (Figure S5), the receptor for cellular DAF (Hamann et al., 1996), significantly better than rhDAF.
However, while monomeric rhDAF was able to inhibit the binding of fluorescently labelled SNV to cultured Tanoue B cells by 90%, an 18‐fold higher molar concentration of plant‐made DAF‐Fc reduced virus binding by only 50% (Figure S6) (Buranda et al., 2014). The reason for this is unclear. There were differences between all of the DAF‐Fc glycoforms and the glycoforms on the rhDAF. It is possible that the specific nature of the DAF N‐glycan is important for binding to virus, but not for binding to CD97. It is also possible that the DAF‐Fc fusion perhaps is sterically unfavourable to Hantavirus binding, unlike the soluble rhDAF. Additional engineering may be needed to generate a fully functional DAF immunoadhesin.
Conclusions
Anti‐infective immunoadhesins can, in principle, be used against any infectious disease with a known cellular receptor, but despite this, none have achieved FDA approval for this purpose; the potential of these proteins as anti‐infective agents has therefore not been fully exploited. Immunoadhesins may be particularly useful against pathogens with high mutation rates or multiple pathogens that share cellular receptors. Also, immunoadhesins can be developed for emerging diseases as soon as their receptors are elucidated. Important design considerations should be whether to use the full or partial receptor, whether to mutate the receptor to improve binding affinity, as well as linker choice and Fc isotype choice.
We reviewed here our and others' experience producing antiviral and antitoxin immunoadhesins in plants. Plants have certain advantages over other expression systems for the production of immunoadhesins, which cannot easily be made in bacterial expression systems because of their complexity and requirement for intra‐ and interchain disulphide bonds. Because they act primarily as receptor antagonists, high immunoadhesin concentrations may be needed to achieve a biological effect, depending on receptor affinity and biological ligand concentration. In cases where high immunoadhesin doses are required, the economics of plant production become more important.
The challenges involved in developing a successful anti‐infective immunoadhesin may be somewhat greater than for a similarly targeted monoclonal antibody. We found, with CMG2‐Fc, that our initial design needed to be modified to achieve a stable, functional protein. The very first immunoadhesin, CD4‐Fc, while exhibiting great promise as an HIV therapeutic 25 years ago based on in vitro data, has been disappointing in the clinic (Fletcher et al., 2007; Schooley et al., 1990). However, recently published work showed that judicious protein engineering improved the stability, solubility and potency of CD4. Six copies of the engineered single domain CD4 and two copies of an antibody domain (m36.4) that target the coreceptor‐binding site on gp120 were fused to IgG1 and kappa constant regions. The resulting hexavalent immunoadhesin was 200 times as potent as a clinically tested sCD4‐Fc in vitro and neutralized a much broader range of HIV‐1 isolates, including several that were not neutralized by the broadly neutralizing monoclonal antibody 2G12 (Chen et al., 2014). A concern raised by the paper's authors was that the relatively complex structure of the fusion protein might pose potential challenges for large‐scale production. Perhaps expression in plants should be considered.
Supporting information
Figure S1 Increase in accumulation of ICAM‐1‐IgA2 with time in greenhouse‐grown plants.
Figure S2 Inhibition of cytopathic effect (CPE).
Figure S3 Characterization of PBI‐220 (lot E00050) by size exclusion HPLC and stain free SDS‐PAGE.
Figure S4 Plant‐made Basigin‐Fc binds recombinant PfRh5 in ELISA.
Figure S5 RhCD97 binding activity of plant‐made DAF‐Fc variants and rhDAF in a functional ELISA.
Figure S6 RhDAF is better than plant‐made DAF‐Fc variants at blocking the binding of fluorescently labelled hantavirus particles [inactivated Sin Nombre virus (SNV)] to Tanoue B cells.
Acknowledgements
The authors would like to acknowledge the help of the following individuals: Kimberly M. Underwood and Carolyn E. Crump conducted CPE assays with ICAM‐1‐IgA2 at the University of Virginia Health Sciences Center. Gabriel Meister and Lisa Henning conducted rabbit challenge studies with CMG2‐Fc at Battelle Biomedical Research Center. Kasi Russell‐Lodrigue, Stephanie Killeen and Rachel Redmann conducted non‐human primate studies with CMG2‐Fc at the Tulane National Primate Research Center. George Hui performed parasite growth inhibition assays with Basigin‐Fc at the University of Hawaii School of Medicine. Tione Buranda conducted Hantavirus binding assays with DAF‐Fc at the University of New Mexico.
Keith Wycoff, Archana Belle, James Maclean and Y Tran are employed by Planet Biotechnology Inc., and thus have a potential conflict of interest.
References
- Abdel‐Moneim, A.S. (2014) Middle East respiratory syndrome coronavirus (MERS‐CoV): evidence and speculations. Arch. Virol. 159, 1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagaili, A.N. , Briese, T. , Mishra, N. , Kapoor, V. , Sameroff, S.C. , Burbelo, P.D. , de Wit, E. , Munster, V.J. , Hensley, L.E. , Zalmout, I.S. , Kapoor, A. , Epstein, J.H. , Karesh, W.B. , Daszak, P. , Mohammed, O.B. and Lipkin, W.I. (2014) Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. MBio, 5, e00884–00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen, S. , Kobinger, G.P. , Soule, G. and Wernery, U. (2014) Middle East respiratory syndrome coronavirus antibody reactors among camels in Dubai, United Arab Emirates, in 2005. Transbound. Emerg. Dis. 61, 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrianov, V. , Brodzik, R. , Spitsin, S. , Bandurska, K. , McManus, H. , Koprowski, H. and Golovkin, M. (2010) Production of recombinant anthrax toxin receptor (ATR/CMG2) fused with human Fc in planta. Protein Expr. Purif. 70, 158–162. [DOI] [PubMed] [Google Scholar]
- Andries, K. , Dewindt, B. , De Brabander, M. , Stokbroekx, R. and Janssen, P.A. (1988) In vitro activity of R 61837, a new antirhinovirus compound. Arch. Virol. 101, 155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angenon, G. , Van Monatagu, M.M. and Depicker, A. (1990) Analysis of the stop codon context in plant nuclear genes. FEBS Lett. 271, 144–146. [DOI] [PubMed] [Google Scholar]
- Arruda, E. , Crump, C.E. , Marlin, S.D. , Merluzzi, V.J. and Hayden, F.G. (1992) In vitro studies of the antirhinovirus activity of soluble intercellular adhesion molecule‐1. Antimicrob. Agents Chemother. 36, 1186–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzola, L. , Chen, J. , Rattanaporn, K. , Maclean, J.M. and McDonald, K.A. (2011) Transient co‐expression of post‐transcriptional gene silencing suppressors for increased in planta expression of a recombinant anthrax receptor fusion protein. Int. J. Mol. Sci. 12, 4975–4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi, A. and Chamow, S.M. (1997) Immunoadhesins as research tools and therapeutic agents. Curr. Opin. Immunol. 9, 195–200. [DOI] [PubMed] [Google Scholar]
- Ashkenazi, A. , Marsters, S.A. , Capon, D.J. , Chamow, S.M. , Figari, I.S. , Pennica, D. , Goeddel, D.V. , Palladino, M.A. and Smith, D.H. (1991) Protection against endotoxic shock by a tumor necrosis factor receptor immunoadhesin. Proc. Natl Acad. Sci. USA, 88, 10535–10539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar, E.I. , El‐Kafrawy, S.A. , Farraj, S.A. , Hassan, A.M. , Al‐Saeed, M.S. , Hashem, A.M. and Madani, T.A. (2014) Evidence for camel‐to‐human transmission of MERS coronavirus. N. Engl. J. Med. 370, 2499–2505. [DOI] [PubMed] [Google Scholar]
- Banks, D.J. , Barnajian, M. , Maldonado‐Arocho, F.J. , Sanchez, A.M. and Bradley, K.A. (2005) Anthrax toxin receptor 2 mediates Bacillus anthracis killing of macrophages following spore challenge. Cell. Microbiol. 7, 1173–1185. [DOI] [PubMed] [Google Scholar]
- Bartelds, G.M. , de Groot, E. , Nurmohamed, M.T. , Hart, M.H. , van Eede, P.H. , Wijbrandts, C.A. , Crusius, J.B. , Dijkmans, B.A. , Tak, P.P. , Aarden, L. and Wolbink, G.J. (2010) Surprising negative association between IgG1 allotype disparity and anti‐adalimumab formation: a cohort study. Arthritis. Res. Ther. 12, R221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batard, Y. , Hehn, A. , Nedelkina, S. , Schalk, M. , Pallett, K. , Schaller, H. and Werck‐Reichhart, D. (2000) Increasing expression of P450 and P450‐reductase proteins from monocots in heterologous systems. Arch. Biochem. Biophys. 379, 161–169. [DOI] [PubMed] [Google Scholar]
- Bella, J. and Rossmann, M.G. (1999) Review: rhinoviruses and their ICAM receptors. J. Struct. Biol. 128, 69–74. [DOI] [PubMed] [Google Scholar]
- Bengsch, B. , Seigel, B. , Flecken, T. , Wolanski, J. , Blum, H.E. and Thimme, R. (2012) Human Th17 cells express high levels of enzymatically active dipeptidylpeptidase IV (CD26). J. Immunol. 188, 5438–5447. [DOI] [PubMed] [Google Scholar]
- Bergelson, J.M. , Chan, M. , Solomon, K.R. , St John, N.F. , Lin, H. and Finberg, R.W. (1994) Decay‐accelerating factor (CD55), a glycosylphosphatidylinositol‐anchored complement regulatory protein, is a receptor for several echoviruses. Proc. Natl Acad. Sci. USA, 91, 6245–6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borio, L. , Frank, D. , Mani, V. , Chiriboga, C. , Pollanen, M. , Ripple, M. , Ali, S. , DiAngelo, C. , Lee, J. , Arden, J. , Titus, J. , Fowler, D. , O'Toole, T. , Masur, H. , Bartlett, J. and Inglesby, T. (2001) Death due to bioterrorism‐related inhalational anthrax: report of 2 patients. JAMA, 286, 2554–2559. [DOI] [PubMed] [Google Scholar]
- Bradley, K.A. , Mogridge, J. , Mourez, M. , Collier, R.J. and Young, J.A. (2001) Identification of the cellular receptor for anthrax toxin. Nature, 414, 225–229. [DOI] [PubMed] [Google Scholar]
- Brunke, C. , Lohse, S. , Derer, S. , Peipp, M. , Boross, P. , Kellner, C. , Beyer, T. , Dechant, M. , Royle, L. , Liew, L.P. , Leusen, J.H. and Valerius, T. (2013) Effect of a tail piece cysteine deletion on biochemical and functional properties of an epidermal growth factor receptor‐directed IgA2 m(1) antibody. MAbs, 5, 936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buranda, T. , Wu, Y. , Perez, D. , Jett, S.D. , BonduHawkins, V. , Ye, C. , Edwards, B. , Hall, P. , Larson, R.S. , Lopez, G.P. , Sklar, L.A. and Hjelle, B. (2010) Recognition of decay accelerating factor and alpha(v)beta(3) by inactivated hantaviruses: toward the development of high‐throughput screening flow cytometry assays. Anal. Biochem. 402, 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buranda, T. , Swanson, S. , Bondu, V. , Schaefer, L. , Maclean, J. , Mo, Z. , Wycoff, K. , Belle, A. and Hjelle, B. (2014) Equilibrium and kinetics of Sin Nombre hantavirus binding at DAF/CD55 functionalized bead surfaces. Viruses, 6, 1091–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyel, J.F. and Fischer, R. (2012) Predictive models for transient protein expression in tobacco (Nicotiana tabacum L.) can optimize process time, yield, and downstream costs. Biotechnol. Bioeng. 109, 2575–2588. [DOI] [PubMed] [Google Scholar]
- Capon, D.J. , Chamow, S.M. , Mordenti, J. , Marsters, S.A. , Gregory, T. , Mitsuya, H. , Byrn, R.A. , Lucas, C. , Wurm, F.M. , Groopman, J.E. , Broder, S. and Smith, D.H. (1989) Designing CD4 immunoadhesins for AIDS therapy. Nature, 337, 525–531. [DOI] [PubMed] [Google Scholar]
- Casasnovas, J.M. , Stehle, T. , Liu, J.H. , Wang, J.H. and Springer, T.A. (1998) A dimeric crystal structure for the N‐terminal two domains of intercellular adhesion molecule‐1. Proc. Natl Acad. Sci. USA, 95, 4134–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W. , Feng, Y. , Prabakaran, P. , Ying, T. , Wang, Y. , Sun, J. , Macedo, C.D. , Zhu, Z. , He, Y. , Polonis, V.R. and Dimitrov, D.S. (2014) Exceptionally potent and broadly cross‐reactive, bispecific multivalent HIV‐1 inhibitors based on single human CD4 and antibody domains. J. Virol. 88, 1125–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiapello, H. , Lisacek, F. , Caboche, M. and Hénaut, A. (1988) Codon usage and gene function are related in sequences of Arabidopsis thaliana . Gene, 209, GC1–GC38. [DOI] [PubMed] [Google Scholar]
- Chintalacharuvu, K.R. , Raines, M. and Morrison, S.L. (1994) Divergence of human alpha‐chain constant region gene sequences. A novel recombinant alpha 2 gene. J. Immunol. 152, 5299–5304. [PubMed] [Google Scholar]
- Crosnier, C. , Bustamante, L.Y. , Bartholdson, S.J. , Bei, A.K. , Theron, M. , Uchikawa, M. , Mboup, S. , Ndir, O. , Kwiatkowski, D.P. , Duraisingh, M.T. , Rayner, J.C. and Wright, G.J. (2011) Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum . Nature, 480, 534–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump, C.E. , Arruda, E. and Hayden, F.G. (1993) In vitro inhibitory activity of soluble ICAM‐1 for the numbered serotypes of human rhinovirus. Antivir. Chem. Chemother. 4, 323–327. [Google Scholar]
- Crump, C.E. , Arruda, E. and Hayden, F.G. (1994) Comparative antirhinoviral activities of soluble intercellular adhesion molecule‐1 (sICAM‐1) and chimeric ICAM‐1/immunoglobulin A molecule. Antimicrob. Agents Chemother. 38, 1425–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, J. , Eden, J.S. , Holmes, E.C. and Wang, L.F. (2013) Adaptive evolution of bat dipeptidyl peptidase 4 (dpp4): implications for the origin and emergence of Middle East respiratory syndrome coronavirus. Virol. J. 10, 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Amicis, F. and Marchetti, S. (2000) Intercodon dinucleotides affect codon choice in plant genes. Nucleic Acids Res. 28, 3339–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, R. , Loyet, K.M. , Lien, S. , Iyer, S. , DeForge, L.E. , Theil, F.P. , Lowman, H.B. , Fielder, P.J. and Prabhu, S. (2010) Pharmacokinetics of humanized monoclonal anti‐tumor necrosis factor‐α antibody and its neonatal Fc receptor variants in mice and cynomolgus monkeys. Drug Metab. Dispos. 38, 600–605. [DOI] [PubMed] [Google Scholar]
- Deng, R. , Iyer, S. , Theil, F.P. , Mortensen, D.L. , Fielder, P.J. and Prabhu, S. (2011) Projecting human pharmacokinetics of therapeutic antibodies from nonclinical data: what have we learned? MAbs, 3, 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health and Human Services (2002) 21 CFR Parts 314 and 601. New Drug and biological drug products; Evidence needed to demonstrate effectiveness of new drugs when human efficacy studies are not ethical or feasible In Federal Register Vol. 67, No. 105, pp. 37988–37998. [PubMed] [Google Scholar]
- Du, L. , Zhao, G. , Zhang, X. , Liu, Z. , Yu, H. , Zheng, B.J. , Zhou, Y. and Jiang, S. (2010) Development of a safe and convenient neutralization assay for rapid screening of influenza HA‐specific neutralizing monoclonal antibodies. Biochem. Biophys. Res. Commun. 397, 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, L. , Zhao, G. , Yang, Y. , Qiu, H. , Wang, L. , Kou, Z. , Tao, X. , Yu, H. , Sun, S. , Tseng, C.T. , Jiang, S. , Li, F. and Zhou, Y. (2014) A conformation‐dependent neutralizing monoclonal antibody specifically targeting receptor‐binding domain in MERS‐CoV spike protein. J. Virol. 88, 7045–7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall, J. , Coleman, C.M. , Hart, B.J. , Venkataraman, T. , Holbrook, M.R. , Kindrachuk, J. , Johnson, R.F. , Olinger, G.G. Jr , Jahrling, P.B. , Laidlaw, M. , Johansen, L.M. , Lear‐Rooney, C.M. , Glass, P.J. , Hensley, L.E. and Frieman, M.B. (2014) Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob. Agents Chemother. 58, 4885–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Engelen, F.A. , Molthoff, J.W. , Conner, A.J. , Nap, J.P. , Pereira, A. and Stiekema, W.J. (1995) pBINPLUS: an improved plant transformation vector based on pBIN19. Transgenic Res. 4, 288–290. [DOI] [PubMed] [Google Scholar]
- Engel, M. , Hoffmann, T. , Wagner, L. , Wermann, M. , Heiser, U. , Kiefersauer, R. , Huber, R. , Bode, W. , Demuth, H.U. and Brandstetter, H. (2003) The crystal structure of dipeptidyl peptidase IV (CD26) reveals its functional regulation and enzymatic mechanism. Proc Natl Acad Sci U S A, 100, 5063–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English, J.J. , Davenport, G.F. , Elmayan, T. , Vaucheret, H. and Baulcombe, D. (1997) Requirement of sense transcription for homology‐dependent virus resistance and trans‐inactivation. Plant J. 12, 597–603. [Google Scholar]
- Ferguson, N.M. and Van Kerkhove, M.D. (2014) Identification of MERS‐CoV in dromedary camels. Lancet Infect. Dis. 14, 93–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher, C.V. , DeVille, J.G. , Samson, P.M. , Moye Jr, J.H. , Church, J.A. , Spiegel, H.M. , Palumbo, P. , Fenton, T. , Smith, M.E. , Graham, B. , Kraimer, J.M. , Shearer, W.T. and Pediatric Aids Clinical Trials Group, P.S.G. (2007) Nonlinear pharmacokinetics of high‐dose recombinant fusion protein CD4‐IgG2 (PRO 542) observed in HIV‐1‐infected children. J. Allergy Clin. Immunol. 119, 747–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado, P.B. , Whitty, P.W. , Robertson, A. , Eaton, J.T. , Almogren, A. , Kerr, M.A. , Woof, J.M. and Perkins, S.J. (2004) Solution structure determination of monomeric human IgA2 by X‐ray and neutron scattering, analytical ultracentrifugation and constrained modelling: a comparison with monomeric human IgA1. J. Mol. Biol. 338, 921–941. [DOI] [PubMed] [Google Scholar]
- Garabagi, F. , Gilbert, E. , Loos, A. , McLean, M.D. and Hall, J.C. (2012) Utility of the P19 suppressor of gene‐silencing protein for production of therapeutic antibodies in Nicotiana expression hosts. Plant Biotechnol. J. 10, 1118–1128. [DOI] [PubMed] [Google Scholar]
- Geyer, B.C. , Kannan, L. , Cherni, I. , Woods, R.R. , Soreq, H. and Mor, T.S. (2010) Transgenic plants as a source for the bioscavenging enzyme, human butyrylcholinesterase. Plant Biotechnol. J. 8, 873–886. [DOI] [PubMed] [Google Scholar]
- Greve, J.M. , Davis, G. , Meyer, A.M. , Forte, C.P. , Yost, S.C. , Marlor, C.W. , Kamarck, M.E. and McClelland, A. (1989) The major human rhinovirus receptor is ICAM‐1. Cell, 56, 839–847. [DOI] [PubMed] [Google Scholar]
- Greve, J.M. , Forte, C.P. , Marlor, C.W. , Meyer, A.M. , Hoover‐Litty, H. , Wunderlich, D. and McClelland, A. (1991) Mechanisms of receptor‐mediated rhinovirus neutralization defined by two soluble forms of ICAM‐1. J. Virol. 65, 6015–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagmans, B.L. , Al Dhahiry, S.H. , Reusken, C.B. , Raj, V.S. , Galiano, M. , Myers, R. , Godeke, G.J. , Jonges, M. , Farag, E. , Diab, A. , Ghobashy, H. , Alhajri, F. , Al‐Thani, M. , Al‐Marri, S.A. , Al Romaihi, H.E. , Al Khal, A. , Bermingham, A. , Osterhaus, A.D. , AlHajri, M.M. and Koopmans, M.P. (2014) Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect. Dis. 14, 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlington, J.L. , Santoro, A. , Nuttall, J. , Denecke, J. , Ma, J.K. , Vitale, A. and Frigerio, L. (2003) The C‐terminal extension of a hybrid immunoglobulin A/G heavy chain is responsible for its Golgi‐mediated sorting to the vacuole. Mol. Biol. Cell, 14, 2592–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann, J. , Vogel, B. , van Schijndel, G.M. and van Lier, R.A. (1996) The seven‐span transmembrane receptor CD97 has a cellular ligand (CD55, DAF). J. Exp. Med. 184, 1185–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart, B.J. , Dyall, J. , Postnikova, E. , Zhou, H. , Kindrachuk, J. , Johnson, R.F. , Olinger, G.G. Jr , Frieman, M.B. , Holbrook, M.R. , Jahrling, P.B. and Hensley, L. (2014) Interferon‐beta and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell‐based assays. J. Gen. Virol. 95, 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks, C.W. , Sweeney, D.A. , Cui, X. , Li, Y. and Eichacker, P.Q. (2012) An overview of anthrax infection including the recently identified form of disease in injection drug users. Intensive Care Med. 38, 1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekema, A. , Hirsch, P.R. , Hooykaas, P.J.J. and Schilperoort, R.A. (1983) A binary plant vector strategy based on separation of the vir‐ and T‐region of A. tumefaciens Ti plasmid. Nature, 303, 179–180. [Google Scholar]
- Hoffman, H.M. , Throne, M.L. , Amar, N.J. , Cartwright, R.C. , Kivitz, A.J. , Soo, Y. and Weinstein, S.P. (2012) Long‐term efficacy and safety profile of rilonacept in the treatment of cryopryin‐associated periodic syndromes: results of a 72‐week open‐label extension study. Clin. Ther. 34, 2091–2103. [DOI] [PubMed] [Google Scholar]
- Holash, J. , Davis, S. , Papadopoulos, N. , Croll, S.D. , Ho, L. , Russell, M. , Boland, P. , Leidich, R. , Hylton, D. , Burova, E. , Ioffe, E. , Huang, T. , Radziejewski, C. , Bailey, K. , Fandl, J.P. , Daly, T. , Wiegand, S.J. , Yancopoulos, G.D. and Rudge, J.S. (2002) VEGF‐Trap: a VEGF blocker with potent antitumor effects. Proc. Natl Acad. Sci. USA, 99, 11393–11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopsu‐Havu, V.K. and Glenner, G.G. (1966) A new dipeptide naphthylamidase hydrolyzing glycyl‐prolyl‐beta‐naphthylamide. Histochemie, 7, 197–201. [DOI] [PubMed] [Google Scholar]
- Horvath, H. , Huang, J. , Wong, O. , Kohl, E. , Okita, T. , Kannangara, C.G. and von Wettstein, D. (2000) The production of recombinant proteins in transgenic barley grains. Proc. Natl Acad. Sci. USA, 97, 1914–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J. , Sutliff, T.D. , Wu, L. , Nandi, S. , Benge, K. , Terashima, M. , Ralston, A.H. , Drohan, W. , Huang, N. and Rodriguez, R.L. (2001) Expression and purification of functional human alpha‐1‐Antitrypsin from cultured plant cells. Biotechnol. Prog. 17, 126–133. [DOI] [PubMed] [Google Scholar]
- Huguenel, E.D. , Cohn, D. , Dockum, D.P. , Greve, J.M. , Fournel, M.A. , Hammond, L. , Irwin, R. , Mahoney, J. , McClelland, A. , Muchmore, E. , Ohlin, A.C. and Scuderi, P. (1997) Prevention of rhinovirus infection in chimpanzees by soluble intercellular adhesion molecule‐1. Am. J. Respir. Crit. Care Med. 155, 1206–1210. [DOI] [PubMed] [Google Scholar]
- Hui, G.S. , Gosnell, W.L. , Case, S.E. , Hashiro, C. , Nikaido, C. , Hashimoto, A. and Kaslow, D.C. (1994) Immunogenicity of the C‐terminal 19‐kDa fragment of the Plasmodium falciparum merozoite surface protein 1 (MSP1), YMSP1(19) expressed in S. cerevisiae . J. Immunol. 153, 2544–2553. [PubMed] [Google Scholar]
- Ikemura, T. (1985) Codon usage and tRNA content in unicellular and multicellular organisms. Mol. Biol. Evol. 2, 13–35. [DOI] [PubMed] [Google Scholar]
- Ithete, N.L. , Stoffberg, S. , Corman, V.M. , Cottontail, V.M. , Richards, L.R. , Schoeman, M.C. , Drosten, C. , Drexler, J.F. and Preiser, W. (2013) Close relative of human middle East respiratory syndrome coronavirus in bat, South Africa. Emerg. Infect. Dis. 19, 1697–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan, J.A. , Stephens, D.S. , Ashford, D.A. , Omenaca, C. , Topiel, M.S. , Galbraith, M. , Tapper, M. , Fisk, T.L. , Zaki, S. , Popovic, T. , Meyer, R.F. , Quinn, C.P. , Harper, S.A. , Fridkin, S.K. , Sejvar, J.J. , Shepard, C.W. , McConnell, M. , Guarner, J. , Shieh, W.J. , Malecki, J.M. , Gerberding, J.L. , Hughes, J.M. and Perkins, B.A. (2001) Bioterrorism‐related inhalational anthrax: the first 10 cases reported in the United States. Emerg. Infect. Dis. 7, 933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan, D.B. , Raghunathan, P.L. , Bell, B.P. , Brechner, R. , Bresnitz, E.A. , Butler, J.C. , Cetron, M. , Cohen, M. , Doyle, T. , Fischer, M. , Greene, C. , Griffith, K.S. , Guarner, J. , Hadler, J.L. , Hayslett, J.A. , Meyer, R. , Petersen, L.R. , Phillips, M. , Pinner, R. , Popovic, T. , Quinn, C.P. , Reefhuis, J. , Reissman, D. , Rosenstein, N. , Schuchat, A. , Shieh, W.J. , Siegal, L. , Swerdlow, D.L. , Tenover, F.C. , Traeger, M. , Ward, J.W. , Weisfuse, I. , Wiersma, S. , Yeskey, K. , Zaki, S. , Ashford, D.A. , Perkins, B.A. , Ostroff, S. , Hughes, J. , Fleming, D. , Koplan, J.P. and Gerberding, J.L. (2002) Investigation of bioterrorism‐related anthrax, United States, 2001: epidemiologic findings. Emerg. Infect. Dis. 8, 1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, L. , Wang, N. , Zuo, T. , Shi, X. , Poon, K.M. , Wu, Y. , Gao, F. , Li, D. , Wang, R. , Guo, J. , Fu, L. , Yuen, K.Y. , Zheng, B.J. , Wang, X. and Zhang, L. (2014) Potent neutralization of MERS‐CoV by human neutralizing monoclonal antibodies to the viral spike glycoprotein. Sci. Transl. Med. 6, 234ra259. [DOI] [PubMed] [Google Scholar]
- Joensuu, J.J. , Brown, K.D. , Conley, A.J. , Clavijo, A. , Menassa, R. and Brandle, J.E. (2009) Expression and purification of an anti‐Foot‐and‐mouth disease virus single chain variable antibody fragment in tobacco plants. Transgenic Res. 18, 685–696. [DOI] [PubMed] [Google Scholar]
- Joshi, S.G. , Cymet, H.B. , Kerkvliet, G. and Cymet, T. (2004) Anthrax in America 2001‐2003. J. Natl Med. Assoc. 96, 344–350. [PMC free article] [PubMed] [Google Scholar]
- Kane, J.F. (1995) Effects of rare codon clusters on high‐level expression of heterologous proteins in Escherichia coli . Curr. Opin. Biotechnol. 6, 494–500. [DOI] [PubMed] [Google Scholar]
- Kanerva, M. , Mustonen, J. and Vaheri, A. (1998) Pathogenesis of Puumala and other hantavirus infections. Rev. Med. Virol. 8, 67–86. [DOI] [PubMed] [Google Scholar]
- Kang, T.‐J. , Loc, N.‐H. , Jang, M.‐O. and Yang, M.‐S. (2004a) Modification of the cholera toxin B subunit coding sequence to enhance expression in plants. Mol. Breed. 13, 143–153. [Google Scholar]
- Kang, T.J. , Han, S.C. , Jang, M.O. , Kang, K.H. , Jang, Y.S. and Yang, M.S. (2004b) Enhanced expression of B‐subunit of Escherichia coli heat‐labile enterotoxin in tobacco by optimization of coding sequence. Appl. Biochem. Biotechnol. 117, 175–187. [DOI] [PubMed] [Google Scholar]
- Kapila, J. , De Rycke, R. , Van Monatagu, M.M. and Angenon, G. (1997) An Agrobacterium‐mediated transient gene expression system for intact leaves. Plant Sci. 122, 101–108. [Google Scholar]
- Kaufman, J. and Kalaitzandonakes, N. (2011) The economic potential of plant‐made pharmaceuticals in the manufacture of biologic pharmaceuticals. J. Commer. Biotechnol. 17, 173–182. [Google Scholar]
- Korhonen, R. and Moilanen, E. (2009) Abatacept, a novel CD80/86‐CD28 T cell co‐stimulation modulator, in the treatment of rheumatoid arthritis. Basic Clin. Pharmacol. Toxicol. 104, 276–284. [DOI] [PubMed] [Google Scholar]
- Krautkramer, E. and Zeier, M. (2008) Hantavirus causing hemorrhagic fever with renal syndrome enters from the apical surface and requires decay‐accelerating factor (DAF/CD55). J. Virol. 82, 4257–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla, G. , Lipinski, L. , Caffin, F. , Helwak, A. and Zylicz, M. (2006) High guanine and cytosine content increases mRNA levels in mammalian cells. PLoS Biol. 4, e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla, G. , Murray, A.W. , Tollervey, D. and Plotkin, J.B. (2009) Coding‐sequence determinants of gene expression in Escherichia coli . Science, 324, 255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmidt, K. (2014) The camel connection. Science, 343, 1422–1423, 1425. [DOI] [PubMed] [Google Scholar]
- Kurschner, C. , Garotta, G. and Dembic, Z. (1992) Construction, purification, and characterization of new interferon gamma (IFN gamma) inhibitor proteins. Three IFN gamma receptor‐immunoglobulin hybrid molecules. J. Biol. Chem. 267, 9354–9360. [PubMed] [Google Scholar]
- Lacy, D.B. , Wigelsworth, D.J. , Scobie, H.M. , Young, J.A. and Collier, R.J. (2004) Crystal structure of the von Willebrand factor A domain of human capillary morphogenesis protein 2: an anthrax toxin receptor. Proc. Natl Acad. Sci. USA, 101, 6367–6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen, C.P. , Pearson, T.C. , Adams, A.B. , Tso, P. , Shirasugi, N. , Strobert, E. , Anderson, D. , Cowan, S. , Price, K. , Naemura, J. , Emswiler, J. , Greene, J. , Turk, L.A. , Bajorath, J. , Townsend, R. , Hagerty, D. , Linsley, P.S. and Peach, R.J. (2005) Rational development of LEA29Y (belatacept), a high‐affinity variant of CTLA4‐Ig with potent immunosuppressive properties. Am. J. Transplant. 5, 443–453. [DOI] [PubMed] [Google Scholar]
- Lau, S.K. , Li, K.S. , Tsang, A.K. , Lam, C.S. , Ahmed, S. , Chen, H. , Chan, K.H. , Woo, P.C. and Yuen, K.Y. (2013) Genetic characterization of Betacoronavirus lineage C viruses in bats reveals marked sequence divergence in the spike protein of pipistrellus bat coronavirus HKU5 in Japanese pipistrelle: implications for the origin of the novel Middle East respiratory syndrome coronavirus. J. Virol. 87, 8638–8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leysath, C.E. , Monzingo, A.F. , Maynard, J.A. , Barnett, J. , Georgiou, G. , Iverson, B.L. and Robertus, J.D. (2009) Crystal structure of the engineered neutralizing antibody M18 complexed to domain 4 of the anthrax protective antigen. J. Mol. Biol. 387, 680–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Soroka, S.D. , Taylor, T.H. Jr , Stamey, K.L. , Stinson, K.W. , Freeman, A.E. , Abramson, D.R. , Desai, R. , Cronin, L.X. , Oxford, J.W. , Caba, J. , Pleatman, C. , Pathak, S. , Schmidt, D.S. , Semenova, V.A. , Martin, S.K. , Wilkins, P.P. and Quinn, C.P. (2008) Standardized, mathematical model‐based and validated in vitro analysis of anthrax lethal toxin neutralization. J. Immunol. Methods, 333, 89–106. [DOI] [PubMed] [Google Scholar]
- Linderholm, A.L. and Chamow, S.M. (2014a) Immunoglobulin Fc‐fusion proteins part 1: their design and manufacture. Bioprocess Int. 12, 30–35. [Google Scholar]
- Linderholm, A.L. and Chamow, S.M. (2014b) Immunoglobulin Fc‐fusion proteins part 2: therapeutic uses and clinical development. Bioprocess Int. 12, 2–7. [Google Scholar]
- Liu, S. , Crown, D. , Miller‐Randolph, S. , Moayeri, M. , Wang, H. , Hu, H. , Morley, T. and Leppla, S.H. (2009) Capillary morphogenesis protein‐2 is the major receptor mediating lethality of anthrax toxin in vivo. Proc. Natl Acad. Sci. USA, 106, 12424–12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, D.E. and Glomski, I.J. (2012) Cellular and physiological effects of anthrax exotoxin and its relevance to disease. Front Cell. Infect. Microbiol. 2, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, L. , Liu, Q. , Zhu, Y. , Chan, K.H. , Qin, L. , Li, Y. , Wang, Q. , Chan, J.F. , Du, L. , Yu, F. , Ma, C. , Ye, S. , Yuen, K.Y. , Zhang, R. and Jiang, S. (2014) Structure‐based discovery of Middle East respiratory syndrome coronavirus fusion inhibitor. Nat. Commun. 5, 3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean, J. , Koekemoer, M. , Olivier, A.J. , Stewart, D. , Hitzeroth, I.I. , Rademacher, T. , Fischer, R. , Williamson, A.L. and Rybicki, E.P. (2007) Optimization of human papillomavirus type 16 (HPV‐16) L1 expression in plants: comparison of the suitability of different HPV‐16 L1 gene variants and different cell‐compartment localization. J. Gen. Virol. 88, 1460–1469. [DOI] [PubMed] [Google Scholar]
- Majumder, M.S. , Rivers, C. , Lofgren, E. and Fisman, D. (2014) Estimation of MERS‐coronavirus reproductive number and case fatality rate for the spring 2014 Saudi Arabia outbreak: insights from publicly available data. PLoS Curr. doi: 10.1371/currents.outbreaks.98d2f8f3382d84f390736cd5f5fe133c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkevich, N.V. , Hopkins, R.J. , Bernton, E. , Meister, G.T. , Vela, E.M. , Atiee, G. , Johnson, V. , Nabors, G.S. , Aimes, R.T. , Ionin, B. and Skiadopoulos, M.H. (2014) Efficacy and safety of AVP‐21D9, an anthrax monoclonal antibody, in animal models and humans. Antimicrob. Agents Chemother. 58, 3618–3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin, S.D. , Staunton, D.E. , Springer, T.A. , Stratowa, C. , Sommergruber, W. and Merluzzi, V.J. (1990) A soluble form of intercellular adhesion molecule‐1 inhibits rhinovirus infection. Nature, 344, 70–72. [DOI] [PubMed] [Google Scholar]
- Martin, S. , Casasnovas, J.M. , Staunton, D.E. and Springer, T.A. (1993) Efficient neutralization and disruption of rhinovirus by chimeric ICAM‐ 1/immunoglobulin molecules. J. Virol. 67, 3561–3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish, Z.A. , Cotten, M. , Meyer, B. , Watson, S.J. , Alsahafi, A.J. , Al Rabeeah, A.A. , Corman, V.M. , Sieberg, A. , Makhdoom, H.Q. , Assiri, A. , Al Masri, M. , Aldabbagh, S. , Bosch, B.J. , Beer, M. , Muller, M.A. , Kellam, P. and Drosten, C. (2014) Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg. Infect. Dis. 20, 1012–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentlein, R. (1999) Dipeptidyl‐peptidase IV (CD26)–role in the inactivation of regulatory peptides. Regul. Pept. 85, 9–24. [DOI] [PubMed] [Google Scholar]
- Mertz, G.J. , Hjelle, B. , Crowley, M. , Iwamoto, G. , Tomicic, V. and Vial, P.A. (2006) Diagnosis and treatment of new world hantavirus infections. Curr. Opin. Infect Dis. 19, 437–442. [DOI] [PubMed] [Google Scholar]
- Meyer, B. , Muller, M.A. , Corman, V.M. , Reusken, C.B. , Ritz, D. , Godeke, G.J. , Lattwein, E. , Kallies, S. , Siemens, A. , van Beek, J. , Drexler, J.F. , Muth, D. , Bosch, B.J. , Wernery, U. , Koopmans, M.P. , Wernery, R. and Drosten, C. (2014) Antibodies against MERS coronavirus in dromedary camels, United Arab Emirates, 2003 and 2013. Emerg. Infect. Dis. 20, 552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migone, T.‐S. , Subramanian, G.M. , Zhong, J. , Healey, L.M. , Corey, A. , Devalaraja, M. , Lo, L. , Ullrich, S. , Zimmerman, J. , Chen, A. , Lewis, M. , Meister, G. , Gillum, K. , Sanford, D. , Mott, J. and Bolmer, S.D. (2009) Raxibacumab for the treatment of inhalational anthrax. N. Engl. J. Med. 361, 135–144. [DOI] [PubMed] [Google Scholar]
- Miguelino, M.G. and Powell, J.S. (2014) Clinical utility and patient perspectives on the use of extended half‐life rFIXFc in the management of hemophilia B. Patient Prefer. Adherence, 8, 1073–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki, M. , Kato, M. , Tanaka, K. , Tanaka, M. , Kohjima, M. , Nakamura, K. , Enjoji, M. , Nakamuta, M. , Kotoh, K. and Takayanagi, R. (2012) Increased hepatic expression of dipeptidyl peptidase‐4 in non‐alcoholic fatty liver disease and its association with insulin resistance and glucose metabolism. Mol. Med. Rep. 5, 729–733. [DOI] [PubMed] [Google Scholar]
- Mohamed, N. , Clagett, M. , Li, J. , Jones, S. , Pincus, S. , D'Alia, G. , Nardone, L. , Babin, M. , Spitalny, G. and Casey, L. (2005) A high‐affinity monoclonal antibody to anthrax protective antigen passively protects rabbits before and after aerosolized Bacillus anthracis spore challenge. Infect. Immun. 73, 795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, G.W. , O'Neill, C. , Padfield, P. and McLaughlin, J.T. (2012) Dipeptidyl peptidase‐4 expression is reduced in Crohn's disease. Regul. Pept. 177, 40–45. [DOI] [PubMed] [Google Scholar]
- Ng, O.W. , Keng, C.T. , Leung, C.S. , Peiris, J.S. , Poon, L.L. and Tan, Y.J. (2014) Substitution at aspartic acid 1128 in the SARS coronavirus spike glycoprotein mediates escape from a s2 domain‐targeting neutralizing monoclonal antibody. PLoS ONE, 9, e102415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, K.L. , llano, M. , Akari, H. , Miyagi, E. , Poeschla, E.M. , Strebel, K. and Bour, S. (2004) Codon optimization of the HIV‐1 vpu and vif genes stabilizes their mRNA and allows for highly efficient Rev‐independent expression. Virology, 319, 163–175. [DOI] [PubMed] [Google Scholar]
- Ohlin, A. , Hoover‐Litty, H. , Sanderson, G. , Paessens, A. , Johnston, S.L. , Holgate, S.T. , Huguenel, E. and Greve, J.M. (1994) Spectrum of activity of soluble intercellular adhesion molecule‐1 against rhinovirus reference strains and field isolates. Antimicrob. Agents Chemother. 38, 1413–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma, K. , Haagmans, B.L. , Hatano, R. , Raj, V.S. , Mou, H. , Iwata, S. , Dang, N.H. , Jan Bosch, B. and Morimoto, C. (2013) Inhibition of middle east respiratory syndrome coronavirus (MERS‐CoV) infection by anti‐CD26 monoclonal antibody. J. Virol. 87, 13892–13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppel, K. , Crawford, D. and Beutler, B. (1991) A tumor necrosis factor (TNF) receptor‐IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. J. Exp. Med. 174, 1483–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, J.W. , Comer, J.E. , Noffsinger, D.M. , Wenglikowski, A. , Walberg, K.G. , Chatuev, B.M. , Chopra, A.K. , Stanberry, L.R. , Kang, A.S. , Scholz, W.W. and Sircar, J. (2006) Human monoclonal anti‐protective antigen antibody completely protects rabbits and is synergistic with ciprofloxacin in protecting mice and guinea pigs against inhalation anthrax. Infect. Immun. 74, 1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, J.W. , Comer, J.E. , Baze, W.B. , Noffsinger, D.M. , Wenglikowski, A. , Walberg, K.G. , Hardcastle, J. , Pawlik, J. , Bush, K. , Taormina, J. , Moen, S. , Thomas, J. , Chatuev, B.M. , Sower, L. , Chopra, A.K. , Stanberry, L.R. , Sawada, R. , Scholz, W.W. and Sircar, J. (2007) Human monoclonal antibody AVP‐21D9 to protective antigen reduces dissemination of the Bacillus anthracis Ames strain from the lungs in a rabbit model. Infect. Immun. 75, 3414–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popugaeva, E. , Witkowski, P.T. , Schlegel, M. , Ulrich, R.G. , Auste, B. , Rang, A. , Kruger, D.H. and Klempa, B. (2012) Dobrava‐Belgrade hantavirus from Germany shows receptor usage and innate immunity induction consistent with the pathogenicity of the virus in humans. PLoS ONE, 7, e35587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell, R.M. , Ward, T. , Goodfellow, I. , Almond, J.W. and Evans, D.J. (1999) Mapping the binding domains on decay accelerating factor (DAF) for haemagglutinating enteroviruses: implications for the evolution of a DAF‐binding phenotype. J. Gen. Virol. 80(Pt 12), 3145–3152. [DOI] [PubMed] [Google Scholar]
- Qian, K. , Xie, F. , Gibson, A.W. , Edberg, J.C. , Kimberly, R.P. and Wu, J. (2008) Functional expression of IgA receptor FcalphaRI on human platelets. J. Leukoc. Biol. 84, 1492–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey, G.J. , Wigelsworth, D.J. , Ryan, P.L. , Scobie, H.M. , Collier, R.J. and Young, J.A. (2005) Receptor‐specific requirements for anthrax toxin delivery into cells. Proc. Natl Acad. Sci. USA, 102, 13278–13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj, V.S. , Mou, H. , Smits, S.L. , Dekkers, D.H. , Muller, M.A. , Dijkman, R. , Muth, D. , Demmers, J.A. , Zaki, A. , Fouchier, R.A. , Thiel, V. , Drosten, C. , Rottier, P.J. , Osterhaus, A.D. , Bosch, B.J. and Haagmans, B.L. (2013) Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus‐EMC. Nature, 495, 251–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath, T. , Baker, K. , Dumont, J.A. , Peters, R.T. , Jiang, H. , Qiao, S.W. , Lencer, W.I. , Pierce, G.F. and Blumberg, R.S. (2015) Fc‐fusion proteins and FcRn: structural insights for longer‐lasting and more effective therapeutics. Crit. Rev. Biotechnol. 35, 235–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle, V. , Leese, P. , Blanset, D. , Adamcio, M. , Meldorf, M. and Lowy, I. (2011) Phase I study evaluating the safety and pharmacokinetics of MDX‐1303, a fully human monoclonal antibody against Bacillus anthracis protective antigen, in healthy volunteers. Clin. Vaccine Immunol. 18, 2136–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockx, B. , Donaldson, E. , Frieman, M. , Sheahan, T. , Corti, D. , Lanzavecchia, A. and Baric, R.S. (2010) Escape from human monoclonal antibody neutralization affects in vitro and in vivo fitness of severe acute respiratory syndrome coronavirus. J. Infect. Dis. 201, 946–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosovitz, M.J. , Schuck, P. , Varughese, M. , Chopra, A.P. , Mehra, V. , Singh, Y. , McGinnis, L.M. and Leppla, S.H. (2003) Alanine‐scanning mutations in domain 4 of anthrax toxin protective antigen reveal residues important for binding to the cellular receptor and to a neutralizing monoclonal antibody. J. Biol. Chem. 278, 30936–30944. [DOI] [PubMed] [Google Scholar]
- Rouwendal, G.J. , Mendes, O. , Wolbert, E.J. and Douwe de Boer, A. (1997) Enhanced expression in tobacco of the gene encoding green fluorescent protein by modification of its codon usage. Plant Mol. Biol. 33, 989–999. [DOI] [PubMed] [Google Scholar]
- Santelli, E. , Bankston, L.A. , Leppla, S.H. and Liddington, R.C. (2004) Crystal structure of a complex between anthrax toxin and its host cell receptor. Nature, 430, 905–908. [DOI] [PubMed] [Google Scholar]
- Sawada‐Hirai, R. , Jiang, I. , Wang, F. , Sun, S. , Nedellec, R. , Ruther, P. , Alvarez, A. , Millis, D. , Morrow, P.R. and Kang, A.S. (2004) Human anti‐anthrax protective antigen neutralizing monoclonal antibodies derived from donors vaccinated with anthrax vaccine adsorbed. J. Immune Based Ther. Vaccines, 2, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant, S.V. , Singh, P.K. , Gupta, S.K. , Madnala, R. and Tuli, R. (1999) Conserved nucleotide sequences in highly expressed genes in plants. J. Genet. 78, 123–131. [Google Scholar]
- Sawant, S.V. , Kiran, K. , Singh, P.K. and Tuli, R. (2001) Sequence architecture downstream of the initiator codon enhances gene expression and protein stability in plants. Plant Physiol. 126, 1630–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel, H. , Schiermeyer, A. , Soeur, R. , Fischer, R. and Schillberg, S. (2005) Production of an active recombinant thrombomodulin derivative in transgenic tobacco plants and suspension cells. Transgenic Res. 14, 251–259. [DOI] [PubMed] [Google Scholar]
- Schooley, R.T. , Merigan, T.C. , Gaut, P. , Hirsch, M.S. , Holodniy, M. , Flynn, T. , Liu, S. , Byington, R.E. , Henochowicz, S. , Gubish, E. , Spriggs, D. , Kufe, D. , Schindler, J. , Dawson, A. , Thomas, D. , Hanson, D.G. , Letwin, B. , Liu, T. , Gulinello, J. , Kennedy, S. , Fisher, R.F. and Ho, D.D. (1990) Recombinant soluble CD4 therapy in patients with the acquired immunodeficiency syndrome (AIDS) and AIDS‐related complex. A phase I‐II escalating dosage trial. Ann. Intern. Med. 112, 247–253. [DOI] [PubMed] [Google Scholar]
- Schouten, A. , Roosien, J. , van Engelen, F.A. , de Jong, G.A.M. , Borst‐Vrenssen, A.W.M. , Zilverentant, J.F. , Bosch, D. , Steidema, W.J. , Gommers, F.J. , Schots, A. and Bakker, J. (1996) The C‐terminal KDEL sequence increases the expression level of a single‐chain antibody designed to be targeted to both the cytosol and the secretory pathway in transgenic tobacco. Plant Mol. Biol. 30, 781–793. [DOI] [PubMed] [Google Scholar]
- van Schouwenburg, P.A. , Rispens, T. and Wolbink, G.J. (2013a) Immunogenicity of anti‐TNF biologic therapies for rheumatoid arthritis. Nat. Rev. Rheumatol. 9, 164–172. [DOI] [PubMed] [Google Scholar]
- van Schouwenburg, P.A. , van de Stadt, L.A. , de Jong, R.N. , van Buren, E.E. , Kruithof, S. , de Groot, E. , Hart, M. , van Ham, S.M. , Rispens, T. , Aarden, L. , Wolbink, G.J. and Wouters, D. (2013b) Adalimumab elicits a restricted anti‐idiotypic antibody response in autoimmune patients resulting in functional neutralisation. Ann. Rheum. Dis. 72, 104–109. [DOI] [PubMed] [Google Scholar]
- Scobie, H.M. and Young, J.A. (2005) Interactions between anthrax toxin receptors and protective antigen. Curr. Opin. Microbiol. 8, 106–112. [DOI] [PubMed] [Google Scholar]
- Scobie, H.M. , Rainey, G.J. , Bradley, K.A. and Young, J.A. (2003) Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc. Natl Acad. Sci. USA, 100, 5170–5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior, B.W. and Woof, J.M. (2005) The influences of hinge length and composition on the susceptibility of human IgA to cleavage by diverse bacterial IgA1 proteases. J. Immunol. 174, 7792–7799. [DOI] [PubMed] [Google Scholar]
- Shigeta, T. , Aoyama, M. , Bando, Y.K. , Monji, A. , Mitsui, T. , Takatsu, M. , Cheng, X.W. , Okumura, T. , Hirashiki, A. , Nagata, K. and Murohara, T. (2012) Dipeptidyl peptidase‐4 modulates left ventricular dysfunction in chronic heart failure via angiogenesis‐dependent and ‐independent actions. Circulation, 126, 1838–1851. [DOI] [PubMed] [Google Scholar]
- Sperber, S.J. and Hayden, F.G. (1988) Chemotherapy of rhinovirus colds. Antimicrob. Agents Chemother. 32, 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staunton, D.E. , Merluzzi, V.J. , Rothlein, R. , Barton, R. , Marlin, S.D. and Springer, T.A. (1989) A cell adhesion molecule, ICAM‐1, is the major surface receptor for rhinoviruses. Cell, 56, 849–853. [DOI] [PubMed] [Google Scholar]
- Staunton, D.E. , Dustin, M.L. , Erickson, H.P. and Springer, T.A. (1990) The arrangement of the immunoglobulin‐like domains of ICAM‐1 and the binding sites for LFA‐1 and rhinovirus. Cell, 61, 243–254. [DOI] [PubMed] [Google Scholar]
- Staunton, D.E. , Ockenhouse, C.F. and Springer, T.A. (1992) Soluble intercellular adhesion molecule 1‐immunoglobulin G1 immunoadhesin mediates phagocytosis of malaria‐infected erythrocytes. J. Exp. Med. 176, 1471–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober, B.E. and Menon, K. (2007) Alefacept for the treatment of psoriasis and other dermatologic diseases. Dermatol. Ther. 20, 270–276. [DOI] [PubMed] [Google Scholar]
- Subramanian, G.M. , Cronin, P.W. , Poley, G. , Weinstein, A. , Stoughton, S.M. , Zhong, J. , Ou, Y. , Zmuda, J.F. , Osborn, B.L. and Freimuth, W.W. (2005) A phase 1 study of PAmAb, a fully human monoclonal antibody against Bacillus anthracis protective antigen, in healthy volunteers. Clin. Infect. Dis. 41, 12–20. [DOI] [PubMed] [Google Scholar]
- Suo, G. , Chen, B. , Zhang, J. , Duan, Z. , He, Z. , Yao, W. , Yue, C. and Dai, J. (2006) Effects of codon modification on human BMP2 gene expression in tobacco plants. Plant Cell Rep. 25, 689–697. [DOI] [PubMed] [Google Scholar]
- Sweeney, D.A. , Hicks, C.W. , Cui, X. , Li, Y. and Eichacker, P.Q. (2011) Anthrax infection. Am. J. Respir. Crit. Care Med. 184, 1333–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, X.C. , Agnihothram, S.S. , Jiao, Y. , Stanhope, J. , Graham, R.L. , Peterson, E.C. , Avnir, Y. , Tallarico, A.S. , Sheehan, J. , Zhu, Q. , Baric, R.S. and Marasco, W.A. (2014) Identification of human neutralizing antibodies against MERS‐CoV and their role in virus adaptive evolution. Proc. Natl Acad. Sci. USA, 111, E2018–E2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuoka, M. , Tanaka, M. , Ono, K. , Takagi, S. , Shintani, T. and Gomi, K. (2008) Codon optimization increases steady‐state mRNA levels in Aspergillus oryzae heterologous gene expression. Appl. Environ. Microbiol. 74, 6538–6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triguero, A. , Cabrera, G. , Cremata, J.A. , Yuen, C.T. , Wheeler, J. and Ramirez, N.I. (2005) Plant‐derived mouse IgG monoclonal antibody fused to KDEL endoplasmic reticulum‐retention signal is N‐glycosylated homogeneously throughout the plant with mostly high‐mannose‐type N‐glycans. Plant Biotechnol. J. 3, 449–457. [DOI] [PubMed] [Google Scholar]
- Turner, R.B. , Wecker, M.T. , Pohl, G. , Witek, T.J. , McNally, E. , St. George, R. , Winther, B. and Hayden, F.G. (1999) Efficacy of tremacamra, a soluble intercellular adhesion molecule 1, for experimental rhinovirus infection: a randomized clinical trial. JAMA, 281, 1797–1804. [DOI] [PubMed] [Google Scholar]
- Tuse, D. , Tu, T. and McDonald, K.A. (2014) Manufacturing economics of plant‐made biologics: case studies in therapeutic and industrial enzymes. BioMed Res. Int. 2014, 256135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero, C. , Sack, M. , Chandler, J. , Drossard, J. , Schuster, F. , Monecke, M. , Schillberg, S. and Fischer, R. (1999) Transient expression of a tumor‐specific single‐chain fragment and a chimeric antibody in tobacco leaves. Proc. Natl Acad. Sci. USA, 96, 11128–11133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale, L. , Blanset, D. , Lowy, I. , O'Neill, T. , Goldstein, J. , Little, S.F. , Andrews, G.P. , Dorough, G. , Taylor, R.K. and Keler, T. (2006) Prophylaxis and therapy of inhalational anthrax by a novel monoclonal antibody to protective antigen that mimics vaccine‐induced immunity. Infect. Immun. 74, 5840–5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet, O. , Rivas, S. , Mestre, P. and Baulcombe, D. (2003) An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J. 33, 949–956. [DOI] [PubMed] [Google Scholar]
- Ward, T. , Pipkin, P.A. , Clarkson, N.A. , Stone, D.M. , Minor, P.D. and Almond, J.W. (1994) Decay‐accelerating factor CD55 is identified as the receptor for echovirus 7 using CELICS, a rapid immuno‐focal cloning method. EMBO J. 13, 5070–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinblatt, M.E. , Kremer, J.M. , Bankhurst, A.D. , Bulpitt, K.J. , Fleischmann, R.M. , Fox, R.I. , Jackson, C.G. , Lange, M. and Burge, D.J. (1999) A trial of etanercept, a recombinant tumor necrosis factor receptor: Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N. Engl. J. Med. 340, 253–259. [DOI] [PubMed] [Google Scholar]
- Wigelsworth, D.J. , Krantz, B.A. , Christensen, K.A. , Lacy, D.B. , Juris, S.J. and Collier, R.J. (2004) Binding stoichiometry and kinetics of the interaction of a human anthrax toxin receptor, CMG2, with protective antigen. J. Biol. Chem. 279, 23349–23356. [DOI] [PubMed] [Google Scholar]
- Williams, J.L. , Noviello, S.S. , Griffith, K.S. , Wurtzel, H. , Hamborsky, J. , Perz, J.F. , Williams, I.T. , Hadler, J.L. , Swerdlow, D.L. and Ridzon, R. (2002) Anthrax postexposure prophylaxis in postal workers, Connecticut, 2001. Emerg. Infect. Dis. 8, 1133–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wycoff, K.L. , Belle, A. , Deppe, D. , Schaefer, L. , Maclean, J.M. , Neuss, S. , Trilling, A.K. , Liu, S. , Leppla, S.H. , Geren, I.N. , Pawlik, J. and Peterson, J.W. (2011) Recombinant anthrax toxin receptor‐Fc fusion proteins produced in plants protect rabbits against inhalational anthrax. Antimicrob. Agents Chemother. 55, 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying, T. , Du, L. , Ju, T.W. , Prabakaran, P. , Lau, C.C. , Lu, L. , Liu, Q. , Wang, L. , Feng, Y. , Wang, Y. , Zheng, B.J. , Yuen, K.Y. , Jiang, S. and Dimitrov, D.S. (2014) Exceptionally potent neutralization of MERS‐CoV by human monoclonal antibodies. J. Virol. 88, 7796–7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki, S.R. , Greer, P.W. , Coffield, L.M. , Goldsmith, C.S. , Nolte, K.B. , Foucar, K. , Feddersen, R.M. , Zumwalt, R.E. , Miller, G.L. and Khan, A.S. (1995) Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am. J. Pathol. 146, 552–579. [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Askins, J. , Fleming, R. , Sturm, B. , Poortman, C. , Viriassov, P. , Peterson, B. , Flynn, M. , Miao, Y. , Zukauskas, D. , Smith, R. , Laird, M. and Choi, G. (2003) Selection of potent neutralizing human monoclonal antibodies to protective antigen of Bacillus anthracis [abstract 3976]. In 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy p. 277 (Chicago). Washington, DC: American Society for Microbiology. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Increase in accumulation of ICAM‐1‐IgA2 with time in greenhouse‐grown plants.
Figure S2 Inhibition of cytopathic effect (CPE).
Figure S3 Characterization of PBI‐220 (lot E00050) by size exclusion HPLC and stain free SDS‐PAGE.
Figure S4 Plant‐made Basigin‐Fc binds recombinant PfRh5 in ELISA.
Figure S5 RhCD97 binding activity of plant‐made DAF‐Fc variants and rhDAF in a functional ELISA.
Figure S6 RhDAF is better than plant‐made DAF‐Fc variants at blocking the binding of fluorescently labelled hantavirus particles [inactivated Sin Nombre virus (SNV)] to Tanoue B cells.


