Abstract
Background
Previous studies found genetic counseling increased participants' knowledge about hereditary breast and ovarian cancer (HBOC). However, most explored knowledge gain in unaffected women, and the scale most commonly used does not include items that may be more pertinent to BC survivors.
Aims
To explore whether genetic counseling impacts BC survivors' knowledge about HBOC and BC survivor specific information.
Methods
The National Center for Human Genome Research Knowledge Scale and five additional items specific to BC survivors were tested among BC survivors; before genetic counseling (Time 1), 2-3 weeks after genetic counseling (Time 2), and 6 months following genetic counseling (Time 3).
Results
A statistically significant change in knowledge over time was found x2 (2) = 56.78, p < .0005. Post hoc analyses revealed statistically significant increases in knowledge between Time 1 (median = 7.00) and Time 2 (median = 10.00; p < .005), and between Time 1 and Time 3 (median = 9.00; p < .005).
Conclusion
Knowledge increased following genetic counseling, but the highest total average score at any time was less than 70% out of 100%. Additional analyses revealed items with low rates of correct response at all three time points raising several concerns and the consideration of alternative approaches to measuring knowledge.
Keywords: Hereditary breast and ovarian cancer (HBOC), knowledge, genetic counseling, genetic testing, BRCA, breast cancer survivor
Introduction
Approximately 10 – 15% of breast cancer (BC) cases are attributed to inherited genetic mutations; the majority resulting from mutations in the BRCA1 and BRCA2 genes [1]. BC survivors without a mutation have a 17.2% cumulative risk to develop contralateral BC 25 years post diagnosis, whereas those with a BRCA1 or BRCA2 mutation have a cumulative risk of 44.1% and 33.5% [2] as well as a 12.7% and 6.8% risk to develop ovarian cancer, respectively [3-5].
This increased risk can be managed through prophylactic mastectomy [6], prophylactic oophorectomy [7], chemoprevention [8], or enhanced surveillance [9]. Therefore, the American Society of Clinical Oncology recommends genetic counseling for BC survivors who may be at risk for hereditary breast and ovarian cancer (HBOC), as part of survivorship care guidelines [10]. Genetic counseling includes pretest genetic counseling, and for those who decide to proceed with testing, posttest genetic counseling.
Pretest genetic counseling involves evaluation of personal and family medical history, consideration of the benefits and limitations of genetic testing, a discussion of the potential results and the implications of those results on the individual and their family members [11]. According to professional organizations such as the National Society of Genetic Counselors, an essential part of pretest genetic counseling is improving knowledge and understanding to facilitate informed decision making about testing and risk reducing procedures as well as identify other family members who may be at risk for hereditary disease [12,13].
Increasing knowledge about hereditary cancer risk and medical management options for those who undergo testing is vital to ensuring informed decisions about testing, understanding of risk and the measures to reduce their risk [14-16]. Previous studies found pretest genetic counseling increased patients' knowledge about HBOC [17], and that knowledge increase was maintained over time [17-20]. However, the majority of studies explored HBOC knowledge among unaffected individuals (i.e., who have not had cancer) [19,21], or a combination of those affected and unaffected [18,22,23], and used a knowledge scale, National Center for Human Genome Research Knowledge scale [NCHGRK; 24,25], that does not include items specifically relevant to BC survivors. Given their experience with cancer, BC survivors likely manage more health information than those who are unaffected, and may pay greater attention to information more relevant to their circumstance as a BC survivor. As part of a longitudinal study exploring the impact of genetic counseling and testing on cognitive and psychological outcomes, an earlier paper reported results from the first two time points, before and after the pre-test genetic counseling session. The paper reported differences in outcomes related to knowledge, cancer-related distress, and decisional conflict between patients who attended pretest genetic counseling prior to, versus those who attended after definitive surgical treatment [26]. A second paper reported on communication of test results to family members and providers among a subset of BC survivors who pursued genetic testing [27].
This paper longitudinally explores changes in general HBOC knowledge using the NCHGRK scale and five new items specific to BC survivors (Table 1) among BC survivors, before and after pre-test genetic counseling, and at a third time point, either six months after completion of their second assessment for those who did not pursue testing and six months after completion of their post-test counseling session for those who pursued testing. Specifically, we asked the following research questions:
Table 1.
Percentage of individuals who received correct scores by question over time.
| Items | Time 1 | Time 2 | Time 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No GT | GT | Total | No GT | GT | Total | No GT | GT | Total | |
| 1. All women who have an altered breast cancer gene get cancer. | 65.0% | 58.11% | 60.4%* | 85.0% | 71.62% | 75.0% | 75.0% | 79.45% | 78.1%*** |
| 2. Early-onset breast cancer is more likely due to an altered breast cancer gene than late-onset breast cancer. | 30% | 24.32% | 27.1%* | 60.0% | 59.72% | 57.3%** | 25.0% | 39.79% | 36.5% |
| 3. A father can pass down an altered breast cancer gene to his children. | 75% | 59.46% | 62.5%* | 75.0% | 90.54% | 87.5%** | 68.42% | 79.73% | 76.0%*** |
| 4. Having ovaries removed will definitely prevent ovarian cancer. | 30% | 31.51% | 31.3%* | 40.0% | 45.95% | 44.8% | 40.0% | 41.89% | 41.7% |
| 5. One half of all breast cancer cases occur in women who have inherited an altered breast cancer gene. | 35.0% | 28.38% | 30.2%* | 55.0% | 50.0% | 51.0% | 42.11% | 48.65% | 46.9%*** |
| 6. One in 10 women have inherited an altered breast cancer gene. | 15.0% | 13.51% | 14.6%* | 15.0% | 31.08% | 27.1% | 20.0% | 22.54% | 21.9%*** |
| 7. Ovarian cancer screening tests often do not detect cancer until after it spreads. | 30% | 39.19% | 37.5%* | 55.0% | 71.23% | 66.7% | 45.0% | 64.86% | 60.4% |
| 8. The sister of a woman with an altered breast cancer gene has a 50% risk of having the altered gene. | 45.0% | 58.11% | 55.2%* | 75.0% | 81.08% | 79.2% | 78.95% | 75.68% | 75.0%*** |
| 9. A woman who does not have an altered breast cancer gene can still get breast cancer. | 95.0% | 85.14% | 87.5% | 95.0% | 95.95% | 95.8% | 100% | 94.52% | 93.8%*** |
| 10. A woman who does not have an altered breast cancer gene can still get ovarian cancer. | 95.0% | 77.03% | 80.2%* | 95.0% | 91.89% | 92.7% | 94.74% | 89.19% | 89.6%*** |
| a11. A woman who has had her breasts removed can still get breast cancer. | 55% | 54.05% | 55.2% | 60.0% | 63.51% | 63.5% | 45.0% | 71.62% | 64.6% |
| a12. The best candidate for BRCA1/2 testing is someone who has had breast or ovarian cancer. | 35% | 36.49% | 37.5%* | 45.0% | 67.57% | 62.5% | 30.0% | 62.16% | 55.2%*** |
| a13. Women who have BRCA1/2 testing will always get positive or negative results. | 15.0% | 18.92% | 17.7%* | 50.0% | 63.51% | 59.4% | 47.37% | 66.22% | 60.4%*** |
| a14. Removing the ovaries (oophorectomy) reduces the risk of breast cancer. | 15.0% | 20.55% | 18.8% | 10.0% | 25.68% | 21.9% | 10.0% | 31.08% | 27.1% |
| a15. Breast cancer patients with an altered BRCA1 or BRCA2 gene are more likely to develop a second breast cancer. | 45.0% | 47.30% | 47.9%* | 60.0% | 75.68% | 72.9% | 52.63% | 63.51% | 60.4% |
Notes:
Items added by the investigators.
Significant difference at p ≤ .05 between time 1 and 2.
Significant difference at p ≤ .05 between time 2 and 3.
Significant difference at p ≤ .05 between time 1 and 3.
How does BC survivors' overall knowledge about HBOC change over time?
How does BC survivor specific knowledge about BC survivor specific HBOC information change over time?
After the initial genetic counseling session, some BC survivors are likely to decide to undergo genetic testing, and as such will attend a second genetic counseling session where they likely will receive similar information to the information they learned at the first genetic counseling session. This re-exposure to HBOC information may increase their overall knowledge and increase their recall at the third time point. Therefore the second two research questions are:
-
3
Is there a difference in HBOC knowledge over time based on the decision to undergo genetic testing?
-
4
How does BC survivor specific knowledge about BC survivor specific HBOC information change over time based on the decision to undergo genetic testing?
Methodology
The methods used for the study are reported in greater detail in previous reports [26,27] and are briefly summarized below.
Participants
Eligibility criteria included: 1) females with a genetic counseling appointment scheduled, 2) meeting National Comprehensive Cancer Network guidelines for referral to cancer genetic counseling, 3) ≥ 18 years of age, 4) a personal BC diagnosis, confirmed by medical record review, 5) no previous genetic counseling, 6) the ability to speak and write in English, and 7) having a mailing address and working telephone number. For the current study, analyses were limited to participants who completed the survey at all three study data collection time points described in the section below.
Procedure
Following Institutional Review Board approval, recruitment took place between April 2009 and July 2010 at a large cancer center in the South. Data were collected at three time points: Time 1 (T1) after the pretest genetic counseling appointment was scheduled, but before the pretest genetic counseling session, Time 2 (T2) two-three weeks after the pretest genetic counseling session, and Time 3 (T3) six months after the T2 assessment for those who did not have genetic testing, and six months after the post-test genetic counseling session for those who had genetic testing.
Measures
Participants completed a self-administered survey at each data collection point. Demographic and clinical characteristics were collected at T1. The majority of items on the knowledge scale were from the NCHGRK scale [24,25], originally consisting of eleven items, one of which was inadvertently excluded from the current survey (see Table 1). The NCHGRKS includes questions about HBOC regarding the prevalence of BRCA 1/2, hereditary patterns, associated future cancer risks, and risk reduction options. Our team developed five additional BC-specific pilot items regarding: 1) residual cancer risk post-mastectomy, 2) the importance for the affected woman to be the first in her family to be tested, 3) the possibility that genetic testing may not clarify personal BC etiology, 4) oophorectomy as a BC risk reduction option, and 5) the increased contralateral BC risk for BRCA mutation carriers (see Table 1). These pilot items were reviewed by a multidisciplinary team for face and content validity. The entire survey was pilot tested with a sample of five patients from the target audience. Response options to all scale items included “true,” “false,” and “don't know.” The correct response to each question was either “true” or “false,” where a correct answer received a score of one, an incorrect or “don't know” response received a score of zero. Total scores were calculated by summing the total number of correct responses for the NCHGRK scale (possible range: 0-10), the total number of correct responses for the BC survivor specific items (possible range: 0-5), and the total composite scale (possible range: 0-15). All items were equally weighted in calculating the total composite scale; therefore, the total composite scale score is more likely to reflect general knowledge from the NCHGRK scale, rather than BC survivor specific knowledge.
Data Analysis
Frequencies and percentages for participant demographic characteristics, practices, and confidence were obtained using SPSS 21. To assess and compare changes in knowledge over time using the original NCHGRK [24,25], five items specific to BC survivors constructed for this study, and a total composite scale using 10 items from the NCHGRK and the five items constructed for this study, Friedman's analyses of variance were conducted. To explore whether the second genetic counseling session impacted knowledge at T2 or T3, an independent samples Mann-Whitney U test was conducted to assess differences between those who received a second counseling session and those who did not.
Results
Of the 114 participants who consented and participated at T1, 100 completed the assessments at T2 and 96 at T3. Analyses for this study were conducted using data from 96 participants who completed the knowledge scales at all three time points. There were no significant demographic or clinical differences between those who participated at all three time points, and those who did not.
Participant demographic and clinical characteristics are presented in Table 2. On average, participants were 52 years old (SD = 10.48) at the time of study participation and were diagnosed with BC at age 49 (SD = 10.59). The majority was married or living with a partner (71.8%), white (84.4%), held at least an undergraduate degree (50.0%), had private insurance (52.1%), and an annual income of more than $50,000 per year (38.6%). Clinically, the majority was diagnosed in the three years prior to data collection (71.4%), reported diagnosis at Stage 1 or 2 (45.9%), and received surgical treatment for their BC (94.8%). After the first genetic counseling session, most (77.1%) underwent genetic testing for a mutation in the BRCA genes. Of those who underwent genetic testing, and chose to report their results to the study team, the majority (70.3%) received a negative result.
Table 2.
Sociodemographic and Clinical Characteristics of Participants (n = 96*).
| Characteristics, N = 96 | Mean (SD, range) |
|---|---|
| Current Age | 52.08 (10.48, 25 – 73) |
| Age at diagnosis | 49.01 (10.59, 24 – 69) |
|
| |
| N (%) | |
| Ethnicity | |
| Hispanic | 11 (11.5%) |
| Non-Hispanic | 80 (83.3%) |
| Race | |
| Asian | 3 (3.1%) |
| Black or African American | 6 (6.3%) |
| White | 81 (84.4%) |
| More than one race | 4 (4.2%) |
| Other | 2 (2.1%) |
| Ashkenazi Jewish | 9 (9.4%) |
| Marital Status | |
| Single or never married | 7 (7.3%) |
| Married | 68 (70.8%) |
| Divorced or separated | 17 (17.7%) |
| Widowed | 3 (3.1%) |
| Living with domestic partner | 1 (1.0%) |
| Education | |
| High school or less | 16 (16.6%) |
| Vocational school/some college | 32 (33.4%) |
| College graduate or beyond | 48 (50.0%) |
| Income | |
| ≤ 35K | 26 (27.1%) |
| > 35K to ≤ 50,000 | 15 (15.6%) |
| > 50,000 | 37 (38.6%) |
| Prefer not to answer | 17 (17.7%) |
| Health Insurance Coverage | |
| Private Insurance | 50 (52.1%) |
| Public Insurance | 29 (30.2%) |
| Other | 11 (11.5%) |
| No Insurance | 3 (3.1%) |
| Clinical Demographics | |
| Current stage | |
| Stage 0 | 17 (17.7%) |
| Stage 1 | 23 (24%) |
| Stage 2 | 21 (21.9%) |
| Stage 3 | 14 (14.6%) |
| Stage 4 | 8 (8.3%) |
| Surgical Treatment | |
| Lumpectomy | 34 (35.4%) |
| Unilateral mastectomy | 23 (24.0%) |
| Contralateral mastectomy | 2 (2.1%) |
| Bilateral mastectomy | 32 (33.3%) |
| Have not had surgery | 10 (10.4%) |
| Don't know | 2 (2.1%) |
| Had Genetic Testing for BRCA1/2 | |
| Yes | 74 (77.1%) |
| No | 20 (20.8%) |
| Results from those who had Genetic Testing | |
| Positive | 3 (4.1%) |
| Negative | 52 (70.3%) |
| Variant of Uncertain Significance | 1 (1.4%) |
Some percentages do not sum to 100% due to missing data.
Knowledge
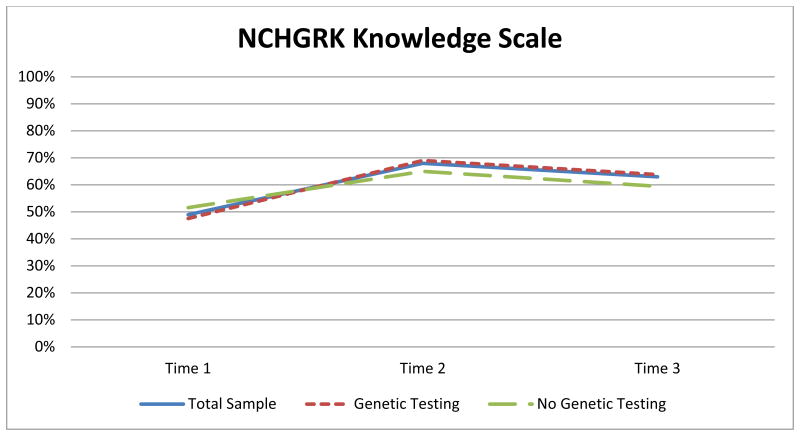
Results indicated a statistically significant difference over time using the NCHGRK comprised of ten items, x2(2) = 101.07, p < .005. Post hoc analysis revealed statistically significant differences in knowledge: an increase between a) T1 (median = 4.00) and T2 (median = 7.00) (p < .005), a decrease between b) T2 and T3 (median = 6.00) (p =.003), and an increase between c) T1 and T3 (p < .005) (Figure 1).
Figure 1.
Average scores at each time point for the 10 items from the NCHGRK knowledge scale.
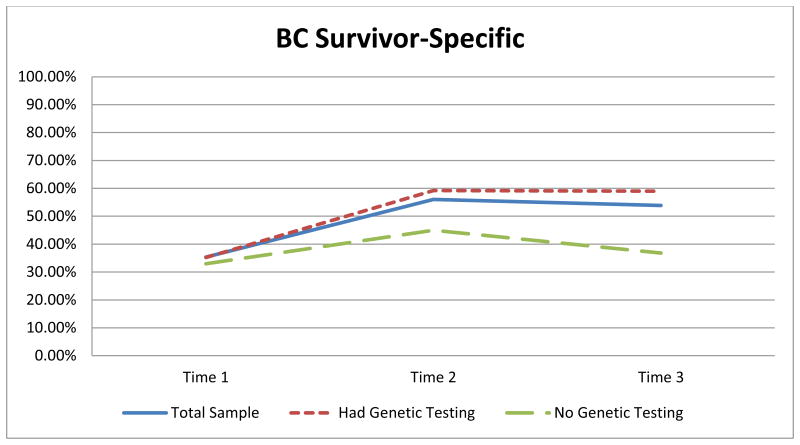
Results indicated a statistically significant difference overtime using the BC survivor-specific items constructed for this study comprised of five items, x2 (2) = 43.43, p < .005. Post hoc analyses revealed a statistically significant difference in knowledge: an increase between T1 (median = 2.00) and T2 (median = 3.00; p < .005), and an increase between T1 and T3 (median = 3.00) (p < .005), but no statistically significant change between T2 and T3 (p = .46) (Figure 2).
Figure 2.
Average scores at each time point for the additional five items.
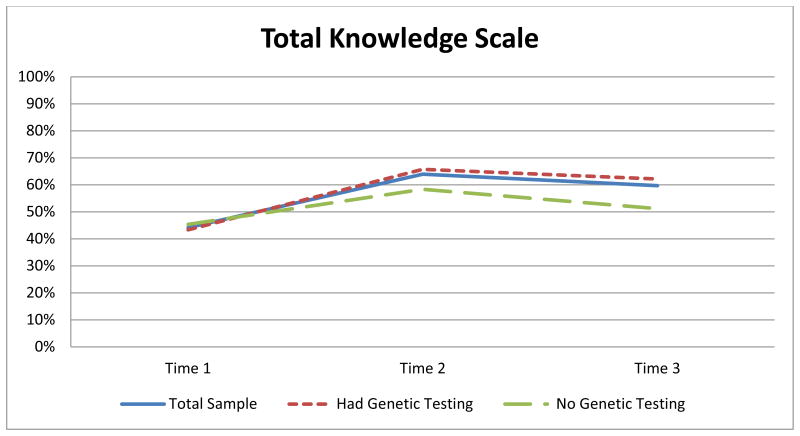
Results indicated a statistically significant change over time using the total composite scale (i.e., 10 items from the NCHGRK and five newly constructed items) x2 (2) = 56.78, p < .0005. Post hoc analyses revealed statistically significant changes in knowledge: an increase between T1 (median = 7.00) and T2 (median = 10.00; p < .005), a decrease between T2 and T3 (median = 9.00; p = .006), and an increase between T1 and T3 (p < .005; Figure 3).
Figure 3.
Average scores at each time point for the total knowledge scale in this study.
Of the 96 participants included in this analysis, 74 (77.1%) underwent genetic testing, 20 (20.83%) did not, and 2 (2.08%) did not provide this information. Those who proceeded with testing attended a second genetic counseling session between T2 and T3. Results (Table 3) indicated no statistically significant difference in scores on the NCHGRK at any of the three time points between those who underwent genetic testing and those who did not. There was no statistically significant difference in scores on the BC survivor-specific scores between those who underwent genetic testing and those who did not at T1. There was a statistically significant difference at T2 in BC survivor-specific knowledge with those who underwent genetic testing scoring higher than those who did not, U = 477.5, z = -2.51, p = .012, and at T3 with those who underwent genetic testing scoring higher than those who did not U = 365.5, z = -3.30, p = .001. There was no statistically significant difference in scores on the composite knowledge scale between those who underwent genetic testing and those who did not at T1 or T2. However, there was a statistically significant difference at T3 in the composite knowledge scores with those who underwent genetic testing scoring higher on the composite knowledge scale, U = 410.5, z = -2.23, p = .026. To further examine participants' knowledge on BC-specific items, average scores for each knowledge question by time were calculated (Table 1).
Table 3.
Results from independent samples Mann-Whitney U test assessing difference in knowledge between those who had genetic testing and those who did not at each time point.
| Knowledge Scales by Time | Genetic Testing | n | Median | U | z | p |
|---|---|---|---|---|---|---|
| NCHGRK | ||||||
|
| ||||||
| Time 1 | Yes | 73 | 50% | 786.00 | .529 | .597 |
| No | 20 | 50% | ||||
|
| ||||||
| Time 2 | Yes | 71 | 70% | 691.50 | -.880 | .379 |
| No | 20 | 70% | ||||
|
| ||||||
| Time 3 | Yes | 69 | 60% | 556.50 | -.686 | .493 |
| No | 18 | 60% | ||||
|
| ||||||
| BC Survivor Scale | ||||||
|
| ||||||
| Time 1 | Yes | 73 | 20% | 703.50 | -.254 | .799 |
| No | 20 | 30% | ||||
|
| ||||||
| Time 2 | Yes | 74 | 60% | 477.50 | -2.510 | .012* |
| No | 20 | 40% | ||||
|
| ||||||
| Time 3 | Yes | 74 | 60% | 365.50 | -3.304 | .001* |
| No | 19 | 40% | ||||
|
| ||||||
| Total Scale | ||||||
|
| ||||||
| Time 1 | Yes | 72 | 46.67% | 754.00 | .323 | .746 |
| No | 20 | 43.33% | ||||
|
| ||||||
| Time 2 | Yes | 71 | 66.67% | 533.50 | -1.705 | .088 |
| No | 20 | 56.67% | ||||
|
| ||||||
| Time 3 | Yes | 69 | 60% | 410.50 | -2.225 | .026* |
| No | 18 | 46.67% | ||||
Note:
significant at p ≤ .05
Discussion
Historically, outcomes of genetic counseling emphasized the use of knowledge measures, as education was determined an import aspect of genetic counseling [28]. Goals of genetic counseling were linked to those of other health education programs by promoting understanding of personal risk and promoting the uptake of prevention behaviors [29]. Previous studies examined the impact of genetic counseling on knowledge among unaffected women [19] or a combination of unaffected and affected women [18,22,23] and used a scale that does not include items more directly relevant to BC survivors. This study examined changes in knowledge of HBOC among BC survivors over time using the NCHGRK knowledge scale and five additional items constructed by the study team to measure knowledge specific to BC survivors. Similar to findings from previous studies, participants' knowledge about HBOC increased following pretest genetic counseling T1, and although it decreased between T2 and T3, knowledge was still significantly higher at T3 than at T1, prior to pre-test genetic counseling [17,18,20].
BC survivors in our study who underwent testing had a second genetic test results-disclosure genetic counseling session, therefore we explored whether there were differences in knowledge gains or maintenance resulting from re-exposure to the information. There was no difference between those who received the second genetic counseling session and those who did not on the NCHGRK knowledge scale at any of the three time points, however, BC survivors who underwent genetic testing scored higher at T2 and T3 on the BC survivor specific items.
There are several possible explanations for these findings. First, it may be that receiving information twice reinforced the information as predicted, but it is interesting that knowledge did not increase on the NCHGRK knowledge scale as well. Perhaps the information most relevant to their situation (i.e., BC survivor specific items) was most salient to women who believed they were at high risk for HBOC, and thus explains their decision to undergo testing. Specifically, women who believed genetic testing was a reasonable option following the pretest genetic counseling session may have given more attention to the issues particular to their situation as BC survivors, compared to those who opted not to pursue testing possibly due to a lower perceived risk for HBOC. It also is possible upon the counselor placed less emphasis on information specific to BC survivors in the pre-test genetic counseling session for those women who they believed were not appropriate genetic testing candidates, based on the genetic counselor's risk assessment. Second, it may be that the genetic counselor placed greater emphasis on survivor-specific information in the second genetic counseling session, thus increasing survivor specific knowledge following the post-test genetic counseling session. Finally, it may be that women who underwent genetic testing may have perceived a greater risk for HBOC and therefore attended to issues particular to their situation as BC survivors more than those who opted not to pursue testing due to a lower perceived risk for HBOC. Future research is needed to validate these findings and explore other explanations, if the findings hold.
Although increases in knowledge were significant, on average, across all three time points participants in this study scored lower than 70% on the NCHGRK knowledge scale, just above 50% on the five BC survivor-specific items, and just above 60% on the composite knowledge scale (NCHGRK knowledge scale with the five additional BC specific items). The low scores at T1 can be explained by the fact that participants had not undergone genetic counseling and thus have limited knowledge about HBOC. However, it is salient to note that total knowledge scores were barely above 60% at time point two and around 60% at time point three. Similar to the results of this study, previous studies found statistically significant differences in knowledge from pre-counseling to post-counseling, however an examination of the reported means reveals knowledge scores after genetic counseling in those studies remained at 70% or below following genetic counseling [30], and just over 70% when interventions to improve outcomes were tested [23,31], confirming consistently low overall average scores.
At T2, most participants (90.7%) made the decision about whether or not to have genetic testing (data not shown). An informed decision in health care can be characterized as a decision made “based on relevant, good quality information, and the resulting choice reflects the decision-maker's values” [32]. Results from this and previous studies raise questions about the quality of information that served as the basis for a patient's testing decision. If they do not have accurate knowledge about HBOC, is it still possible to make an informed decision regarding whether or not to undergo genetic testing? Although we did not examine decision making in this study, future research should consider how knowledge impacts decision making related to uptake of genetic testing.
At T3, it may seem a lower HBOC knowledge score is less concerning, especially since of the 74 (77%) who reported undergoing genetic testing, only three (3.1%) received a positive test result (data not shown). However, regardless of the test result, women undergoing genetic testing are encouraged to share results with her family members [33]. Yet, studies identified communication with family members to be a complex process that often happens when the woman believes “the time is right” which may be several months or years after genetic test results are known [34]. Therefore, ensuring accurate acquisition of, as well as recall and retrieval of HBOC knowledge over time is important for accurate and complete disclosure and dissemination of knowledge to other family members.
In addition to the overall low totals, scores on the five additional BC survivor specific items remained particularly low at all three time points. Examining the items individually, none of the BC survivor specific items scored above 65%, with the exception of the question about those with a BRCA mutation being more likely to develop a second BC (item #15), and scores on items eleven, twelve, and fourteen were notably low at all three time points, failing to change significantly over time. Correct responses on item six significantly increased from T1 to T2, but less than 30% of participants answered correctly at any of the time points.
Although the NCHGRK knowledge scale was developed in 1996, the core survey construct dimensions of HBOC (e.g., risks for cancer, patterns of inheritance, prevalence of BRCA mutations, and risk management options) remain important to understanding HBOC. However, the low average scores found in this study are consistent with findings in early applications of the scale [25]. In addition to considering whether certain items are not being covered in the genetic counseling session and whether the information is not being prioritized by the patients, it also may be valid to consider alternate methods of measuring knowledge.
It is unclear whether the educational-based or counseling-based (i.e., psychologically-based) model is more effective [35]. The educational-based model emphasizes supporting the patient through education, facilitating informed decision, providing impartial information, and correcting inaccurate information [36]. The counseling-based model emphasizes the provision of emotional support by facilitating the processing and integration of new information regarding their personal risk for cancer [36]. The current knowledge scale is based on information that providers and experts deemed is important for patients to understand, but what is important to patients may differ. A great deal of complex information is provided during a genetic counseling session, and genetic counselors are trained to tailor the session to meet the patient's needs [28]. It is likely genetic counselors focus content of the session on meeting the patients' needs in a manner that facilitates personal decision making, rather than ensuring “standard” educational content is discussed. Indeed, the goal of genetic counseling as a psychoeducational process is to, “facilitate clients' ability to use genetic information in a personally meaningful way that minimizes psychological distress and increases personal control [37].” As such, a standard knowledge scale may not accurately capture unique knowledge or other outcomes gained from genetic counseling sessions and minimizes other important outcomes that may influence patients' testing and treatment decisions.
For example, interviews with genetic counselors and their patients both identified the interpersonal interaction in the genetic counseling session as the most important determinate of a successful session [28]. However, results from a review of process studies examining the counselor-patient interaction found most genetic counseling sessions followed an educational approach whereby the content was primarily biomedical information and the interaction was dominated by the genetic counselor [35]. Rather than exclusively using process studies or objective quantitative measures like those used in this study, it may be more prudent to combine these methods such that the process and the outcome can be more implicitly examined.
Reviews of studies focused on other genetic counseling outcomes found subjective risk perception is more in-line with objective risk assessment following genetic counseling [38], most individuals demonstrated positive psychosocial wellbeing following a genetic counseling session [39], and genetic counseling did not cause negative affective outcomes [17]. Furthermore, results from this sample reported in an earlier manuscript found no changes in cancer distress or decisional conflict about genetic testing following the pre-test genetic counseling session [26]. However, it is unclear what aspects of genetic counseling caused these positive changes. Rather than examine these outcomes as independent from knowledge, future studies should examine the effects of knowledge on the aforementioned outcomes as a means to identify how these outcomes may be interacting.
This study examined knowledge gains during genetic counseling for BRCA mutations. Although this study examined knowledge related to HBOC, findings from this study may have implications for exploring the impact of genetic counseling on knowledge for other hereditary cancers. Understanding knowledge gains during traditional and newer approaches to delivering genetic counseling will become increasingly important as testing for multiple cancer predisposition genes through panel testing or whole exome/whole genome sequencing is progressively implemented in practice. As more genes are tested, the amount of information provided to the patient will inherently increase. Finding novel methods to assess whether or not patients understand the information provided to them in a manner that facilitates meaningful decision making will become all the more important.
Limitations
Although the demographics of our participants match the demographics commonly seen at the institution, participants in this study were primarily white, highly educated, and high SES limiting the generalizability of the study results. The portion of the knowledge scale developed for our study had lower reliability at T2 and T3 which could not be improved through item reduction, but there are questions regarding the utility of conducting reliability analysis on knowledge scales, particularly diverse knowledge scales [40]. Scales developed to measure several different aspects of a particular illness or disease, for example: cause, risk, treatment, often tap into what has been called “spotty knowledge” that is held by individuals as a result of receiving disjointed or sporadic information over time [40]. This may be particularly true when considering the variability that may occur in genetic counseling sessions to meet unique needs of patients. While the focus of this paper was the examination of changes in knowledge related to BC survivors, it is important to keep in mind that the changes in the composite knowledge scale are more likely to reflect the changes in knowledge relevant for both affected and unaffected individuals as there were 10 items reflecting this more “general” knowledge and only 5 items we considered to be BC specific. Additional studies are needed to assess and confirm validity of the knowledge items specific to BC survivors developed for this study. Data for this study were collected at a single institution, and genetic counseling was conducted by master's trained genetic counselors specializing in oncology, limiting the generalizability of this study to patients who may obtain genetic counseling in other contexts and/or from non-genetics providers.
Conclusion
BC survivors in this study increased in knowledge regarding HBOC following the receipt of cancer genetic counseling, and maintained that knowledge over time. However, on average, participants scored under 70% at all three time points. Additional analyses of the items on the scale identified specific areas in which knowledge does not seem to improve, raising questions about the educational content of the cancer genetic counseling session and/or the utility of the available measures used to assess HBOC knowledge.
References
- 1.Ellsworth RE, Decewicz DJ, Shriver CD, Ellsworth DL. Breast cancer in the personal genomics era. Current Genomics. 2010;11:146–161. doi: 10.2174/138920210791110951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhiem K, Engel C, Graeser M, Zachariae S, Kast K, Kiechle M, Ditsch N, Janni W, Mundhenke C, Golatta M, Varga D, Preisler-Adams S, Heinrich T, Bick U, Gadzicki D, Briest S, Meindl A, Schmutzler RK. The risk of contralateral breast cancer in patients from BRCA1/2 negative high risk families as compared to patients from BRCA1 or BRCA2 positive families: A retrospective cohort study. Breast Cancer Research. 2012;14:R156. doi: 10.1186/bcr3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao X, Fisher SG, Emami B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: A population-based study. International Journal of Radiation Oncology, Biology and Physics. 2003;56:1038–1045. doi: 10.1016/s0360-3016(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 4.Kauff ND, Mitra N, Robson ME, Hurley KE, Chuai S, Goldfrank D, Wadsworth E, Lee J, Cigler T, Borgen PI, Norton L, Barakat RR, Offit K. Risk of ovarian cancer in BRCA1 and BRCA2 mutation-negative hereditary breast cancer families. J Natl Cancer Inst. 2005;97:1382–1384. doi: 10.1093/jnci/dji281. [DOI] [PubMed] [Google Scholar]
- 5.Metcalfe KA, Lynch HT, Ghadirian P, Tung N, Olivotto IA, Foulkes WD, Warner E, Olopade O, Eisen A, Weber B, McLennan J, Sun P, Narod SA. The risk of ovarian cancer after breast cancer in BRCA1 and BRCA2 carriers. Gynecol Oncol. 2005;96:222–226. doi: 10.1016/j.ygyno.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 6.Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, Garber JE, Neuhausen SL, Matloff E, Eeles R. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finch A, Beiner M, Lubinski J, Lynch HT, Moller P, Rosen B, Murphy J, Ghadirian P, Friedman E, Foulkes WD. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 mutation. JAMA. 2006;296:185–192. doi: 10.1001/jama.296.2.185. [DOI] [PubMed] [Google Scholar]
- 8.Gronwald J, Tung N, Foulkes WD, Offit K, Gershoni R, Daly M, Kim-Sing C, Olsson H, Ainsworth P, Eisen A. Tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 carriers: An update. Int J Cancer. 2006;118:2281–2284. doi: 10.1002/ijc.21536. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. [9/15/2014];Hereditary breast and/or ovarian cancer syndrome management. 2014 available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 10.Khatcheressian JL, Hurley P, Bantug E, Esserman LJ, Grunfeld E, Halberg F, Hantel A, Henry NL, Muss HB, Smith TJ, Vogel VG, Wolff AC, Somerfield MR, Davidson NE. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:961–965. doi: 10.1200/JCO.2012.45.9859. [DOI] [PubMed] [Google Scholar]
- 11.Robson ME, Storm CD, Weitzel J, Wollins DS, Offit K. American Society of Clinical Oncology policy statement update: Genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010;28:893–901. doi: 10.1200/JCO.2009.27.0660. [DOI] [PubMed] [Google Scholar]
- 12.Riley B, Culver J, Skrzynia C, Senter L, Peters J, Costalas J, Callif-Daley F, Grumet S, Hunt K, Nagy R, McKinnon W, Petrucelli N, Bennett R, Trepanier A. Essential elements of genetic cancer risk assessment, counseling, and testing: Updated recommendations of the National Society of Genetic Counselors. J Genet Couns. 2012;21:151–161. doi: 10.1007/s10897-011-9462-x. [DOI] [PubMed] [Google Scholar]
- 13.Berliner J, Fay A, Cummings S, Burnett B, Tillmanns T. NSGC practice guideline: Risk assessment and genetic counseling for hereditary breast and ovarian cancer. J Genet Counsel. 2013;22:155–163. doi: 10.1007/s10897-012-9547-1. [DOI] [PubMed] [Google Scholar]
- 14.Domchek SM, Nathanson KL. Panel testing for inherited susceptibility to breast, ovarian, and colorectal cancer. Genetics in Medicine. 2014;16:827–829. doi: 10.1038/gim.2014.56. [DOI] [PubMed] [Google Scholar]
- 15.Gage M, Wattendorf D, Henry L. Translational advances regarding hereditary breast cancer syndromes. J Surg Oncol. 2012;105:444–451. doi: 10.1002/jso.21856. [DOI] [PubMed] [Google Scholar]
- 16.Grann V, Patel P, Jacobson J, Warner E, Heitjan D, Ashby-Thompson M, Hershman D, Neugut A. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat. 2011;125:837–847. doi: 10.1007/s10549-010-1043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braithwaite D, Emery J, Walter F, Prevost AT, Sutton S. Psychological impact of genetic counseling for familial cancer: A systematic review and meta-analysis. J Natl Cancer Inst. 2004;96:122–133. doi: 10.1093/jnci/djh017. [DOI] [PubMed] [Google Scholar]
- 18.Shedlosky-Shoemaker R, Ngo TL, Ferketich AK, Porter K, Leventhal H, Kelly KM. Exploring perceptions of genetic testing: An examination of perceived accuracy over time. Patient Educ Couns. 2010;78:34–39. doi: 10.1016/j.pec.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Meiser B, Butow P, Barratt A, Gattas M, Gaff C, Hann E, Gleeson M, Dudding T, Tucker K. Risk perceptions and knowledge of breast cancer genetics in women at increased risk of developing hereditary breast cancer. Psychol Health. 2001;16:297. [Google Scholar]
- 20.Kelly K, Leventhal H, Marvin M, Toppmeyer D, Baran J, Schwalb M. Cancer genetics knowledge and beliefs and receipt of results in Ashkenazi Jewish individuals receiving counseling for BRCA1/2 mutations. Cancer Control. 2004;11:236–244. doi: 10.1177/107327480401100405. [DOI] [PubMed] [Google Scholar]
- 21.Braithwaite D, Emery J, Walter F, Prevost AT, Sutton S. Psychological impact of genetic counseling for familial cancer: A systematic review and meta-analysis. J Natl Cancer Inst. 2004;96:122–133. doi: 10.1093/jnci/djh017. [DOI] [PubMed] [Google Scholar]
- 22.Kelly K, Ellington L, Schoenberg N, Agarwal P, Jackson T, Dickinson S, Abraham J, Paskett ED, Leventhal H, Andrykowski M. Linking genetic counseling content to short-term outcomes in individuals at elevated breast cancer risk. J Genet Couns. 2014 doi: 10.1007/s10897-014-9705-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Randall J, Butow P, Kirk J, Tucker K. Psychological impact of genetic counselling and testing in women previously diagnosed with breast cancer. Intern Med J. 2001;31:397–405. doi: 10.1046/j.1445-5994.2001.00091.x. [DOI] [PubMed] [Google Scholar]
- 24.Lerman C, Biesecker B, Benkendorf JL, Kerner J, Gomez-Caminero A, Hughes C, Reed MM. Controlled trial of pretest education approaches to enhance informed decision-making for BRCA1 gene testing. J Natl Cancer Inst. 1997;89:148–157. doi: 10.1093/jnci/89.2.148. [DOI] [PubMed] [Google Scholar]
- 25.Lerman C, Narod S, Schulman K, Hughes C, Gomez-Caminero A, Bonney G, Gold K, Trock B, Main D, Lynch J, Fulmore C, Snyder C, Lemon SJ, Conway T, Tonin P, Lenoir G, Lynch H. BRCA1 testing in families with hereditary breast-ovarian cancer: A prospective study of patient decision making and outcomes. JAMA. 1996;1885 [PubMed] [Google Scholar]
- 26.Christie J, Quinn GP, Malo T, Lee JH, Zhao XH, McIntyre J, Brzosowicz J, Jacobsen PB, Vadaparampil ST. Cognitive and psychological impact of BRCA genetic counseling in before and after definitive surgery breast cancer patients. Ann Sug Oncol. 2012;19:4003–4011. doi: 10.1245/s10434-012-2460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vadaparampil ST, Malo T, de la Cruz C, Christie J. Do breast cancer patients tested in the oncology care setting share BRCA mutation results with family members and health care providers? Journal of Cancer Epidemiology. 2012;2012:498062. doi: 10.1155/2012/498062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernhardt BA, Biesecker BB, Mastromarino CL. Goals, benefits, and outcomes of genetic counseling: Client and genetic counselor assessment. Am J Med Genet. 2000;94:189–197. doi: 10.1002/1096-8628(20000918)94:3<189::aid-ajmg3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 29.Biesecker BB. Goals of genetic counseling. Clin Genet. 2001;60:323–330. doi: 10.1034/j.1399-0004.2001.600501.x. [DOI] [PubMed] [Google Scholar]
- 30.Meiser B, Butow PN, Barratt AL, Schnieden V, Gattas M, Kirk J, Gaff C, Suthers G, Tucker K. Long-term outcomes of genetic counseling in women at increased risk of developing hereditary breast cancer. Patient Educ Couns. 2001;44:215–225. doi: 10.1016/s0738-3991(00)00191-9. [DOI] [PubMed] [Google Scholar]
- 31.Brain K, Gray J, Norman P, France E, Anglim C, Barton G, Parsons E, Clarke A, Sweetland H, Tischkowitz M. Randomized trial of a specialist genetic assessment service for familial breast cancer. J Natl Cancer Inst. 2000;92:1345–1351. doi: 10.1093/jnci/92.16.1345. [DOI] [PubMed] [Google Scholar]
- 32.Marteau TM, Dormandy E, Michie S. A measure of informed choice. Health Expectations. 2001;4:99–108. doi: 10.1046/j.1369-6513.2001.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godard B, Hurlimann T, Letendre M, Egalite N. Guidelines for disclosing genetic information to family members: From development to use. Familial Cancer. 2006;5:103–116. doi: 10.1007/s10689-005-2581-5. [DOI] [PubMed] [Google Scholar]
- 34.Chivers Seymour K, Addington-Hall J, Lucassen A, Foster C. What facilitates or impedes family communication following genetic testing for cancer risk? A systematic review and meta-synthesis of primary qualitative research J Genet Counsel. 2010;19:330–342. doi: 10.1007/s10897-010-9296-y. [DOI] [PubMed] [Google Scholar]
- 35.Meiser B, Irle J, Lobb E, Barlow-Stewart K. Assessment of the content and process of genetic counseling: A critical review of empirical studies. J Genet Counsel. 2008;17:434–451. doi: 10.1007/s10897-008-9173-0. [DOI] [PubMed] [Google Scholar]
- 36.Kessler S. Psychological aspects of genetic counseling. Ix Teaching and counseling J Genet Counsel. 1997;6:287–295. doi: 10.1023/A:1025676205440. [DOI] [PubMed] [Google Scholar]
- 37.Biesecker BB, Peters KF. Process studies in genetic counseling: Peering into the black box. Am J Med Genet. 2001;106:191–198. doi: 10.1002/ajmg.10004. [DOI] [PubMed] [Google Scholar]
- 38.Smerecnik CM, Mesters I, Verweij E, de Vries NK, de Vries H. A systematic review of the impact of genetic counseling on risk perception accuracy. J Genet Counsel. 2009;18:217–228. doi: 10.1007/s10897-008-9210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamilton JG, Lobel M, Moyer A. Emotional distress following genetic testing for hereditary breast and ovarian cancer: A meta-analytic review. Health Psychol. 2009;28:510. doi: 10.1037/a0014778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ondrusek N, Warner E, Goel V. Development of a knowledge scale about breast cancer and heredity (bchk) Breast Cancer Res Treat. 1999;53:69–75. doi: 10.1023/a:1006114710328. [DOI] [PubMed] [Google Scholar]