Abstract
Background and Purpose
Alberta Stroke Program Early Computed Tomographic Score (ASPECTS) has been used to estimate diffusion-weighted imaging (DWI) lesion volume in acute stroke. We aimed to assess correlations of DWI-ASPECTS with lesion volume in different middle cerebral artery (MCA) subregions and reproduce existing ASPECTS thresholds of a malignant profile defined by lesion volume ≥100 mL.
Methods
We analyzed data of patients with MCA stroke from a prospective observational study of DWI and fluid-attenuated inversion recovery in acute stroke. DWI-ASPECTS and lesion volume were calculated. The population was divided into subgroups based on lesion localization (superficial MCA territory, deep MCA territory, or both). Correlation of ASPECTS and infarct volume was calculated, and receiver-operating characteristics curve analysis was performed to identify the optimal ASPECTS threshold for ≥100-mL lesion volume.
Results
A total of 496 patients were included. There was a significant negative correlation between ASPECTS and DWI lesion volume (r=−0.78; P<0.0001). With regards to lesion localization, correlation was weaker in deep MCA region (r=−0.19; P=0.038) when compared with superficial (r=−0.72; P<0.001) or combined superficial and deep MCA lesions (r=−0.72; P<0.001). Receiver-operating characteristics analysis revealed ASPECTS≤6 as best cutoff to identify ≥100-mL DWI lesion volume; however, positive predictive value was low (0.35).
Conclusions
ASPECTS has limitations when lesion location is not considered. Identification of patients with malignant profile by DWI-ASPECTS may be unreliable. ASPECTS may be a useful tool for the evaluation of noncontrast computed tomography. However, if MRI is used, ASPECTS seems dispensable because lesion volume can easily be quantified on DWI maps.
Keywords: brain ischemia, diffusion magnetic resonance imaging, magnetic resonance imaging, middle cerebral artery, neuroimaging, severity of illness index, stroke
The Alberta Stroke Program Early Computed Tomographic Score (ASPECTS) has been introduced as a simple and reliable tool to assess the extent of early ischemic signs on CT in the anterior circulation. The score is a topographical 10-point scoring system to quantify abnormalities indicating brain ischemia on noncontrast CT scans, in which a score of 10 indicates a normal CT, whereas a score of 0 reflects involvement of all 10 brain regions included. ASPECTS is now widely used to identify patients with stroke at risk for an unfavourable clinical outcome1 both in clinical practice as in the context of clinical trials. Although originally designed for evaluation of CT ASPECTS may also be applied for standardized scoring of magnetic resonance diffusion-weighted imaging (DWI),2 DWI-ASPECTS was found to be superior in detecting ischemic changes when compared with noncontrast CT alone,3 reflecting the higher sensitivity of DWI for acute cerebral ischemia.
It has been reported that patients with large lesions on DWI-MRI have a high risk of developing symptomatic intracerebral hemorrhage (SICH) and a worse clinical outcome after intravenous thrombolysis. The Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) study identified a threshold of ≥100-mL lesion volume,4 characterizing a malignant course (ie, high risk of SICH and poor outcome). It has been suggested to use DWI-ASPECTS to identify patients with acute stroke and large DWI lesions, thus avoiding the need for measuring lesion volumes in the acute setting. DWI-ASPECTS was already found to predict SICH after thrombolysis.5 In addition, several studies have tried to define ASPECTS cutoff values identifying large DWI lesion volumes6,7
However, there are limitations to ASPECTS. First, the score is limited to the anterior circulation. Moreover, the anatomic regions covered by ASPECTS are not equally distributed across the middle cerebral artery (MCA) territory. There are 3 score items allotted to subcortical regions, which are located in close proximity, whereas 7-score items represent cortical brain areas distributed across the entire MCA territory. It has already been argued that ASPECTS regions are weighed unequally and in favor of the striatocapsular region.8
The aim of the present study was to assess the performance of DWI-ASPECTS as surrogate for ischemic lesion volume with regard to the affected region of the MCA territory and to reproduce existing threshold values to identify patients with a malignant profile defined by extensive DWI lesion volume.
Methods
We analyzed patient data from the Predictive Value of Fluid-Attenuated Inversion Recovery (PRE-FLAIR) study database. The details of this study have been published previously.9 PRE-FLAIR was a multicenter observational study of patients with acute ischemic stroke who underwent multiparametric MRI within 12 hours of symptom onset.
Demographic data, time from symptom onset to MRI, severity of neurological deficit on admission as assessed with the National Institutes of Health Stroke Scale (NIHSS), stroke cause according to Trial of Org 10 172 in Acute Stroke Treatment (TOAST)10 definitions on admission were recorded. DWI-ASPECTS was rated by a neurologist experienced in stroke imaging. DWI lesion volumes were calculated by a semiautomatic thresholding approach using an in-house developed software tool (AnToNIa), as described previously.9 For the present analysis, we excluded patients with anterior or posterior cerebral artery infarction and bihemispherial ischemic lesions. Thus, we included only patients with MCA infarction. We further divided the population into 3 subgroups based on the localization of DWI lesions: involvement of superficial MCA territory alone, involvement of the deep MCA territory alone, and finally involvement of both superficial and deep MCA territories.
Correlations among DWI-ASPECTS, DWI lesion volume, and NIHSS were calculated using Pearson correlation coefficient. Group comparison was performed using the Kruskal–Wallis or Mann–Whitney U test. Receiver operating characteristic curves were generated to determine the optimal DWI-ASPECTS cutoff point to characterize a DWI lesion volume ≥100 mL.4 All statistical analysis was performed using SPSS 21.
Results
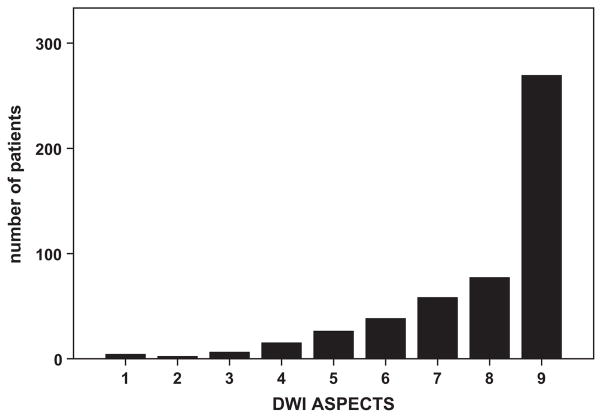
Of 543 patients included in the final analysis in the PRE-FLAIR study, we excluded 47 patients with DWI lesion outside the MCA territory or no visible DWI lesion. Thus, 496 patients with MCA territory stroke were included in the present analysis (47% women; mean age, 66 years; SD±15 years). Mean onset-to-MRI delay was 244±170 minutes (see the Table for patient characteristics). Figure 1 shows the distribution of DWI-ASPECTS values.
Table.
Baseline Clinical and Imaging Parameters in Different Subgroups and Overall Study Population
| All Patients MCA | Superficial MCA (n=279) | Deep MCA (n=128) | Both (n=88) | Group Comparison | |
|---|---|---|---|---|---|
| Age (mean, SD) | 66±15 | 67.1±14.7 | 64.3±15.8 | 65±14.8 | P=0.203 |
| Sex | |||||
| Men | 263 (53%) | 163 (58.4%) | 55 (43%) | 45 (51.1%) | |
| Women | 233 (47%) | 116 (41.6%) | 73 (57%) | 43 (48.9%) | P=0,014 |
| NIHSS (mean, SD) | 10.3±7.1 | 8.7±6.5 | 9.4±6.4 | 16.8±6.6 | P<0.0001 |
| Cause (TOAST) | P=0.679 | ||||
| Large artery atherosclerosis | 139 (28%) | 71 (25.4%) | 41 (32%) | 26 (29.5%) | |
| Cardioembolism | 150 (30.2%) | 95 (34.1%) | 28 (21.9%) | 27 (30.7%) | |
| Small-vessel occlusion | 21 (4.2%) | 4 (1.4%) | 17 (13.3%) | 0 | |
| Stroke of other determined cause | 36 (7.3%) | 14 (5%) | 11 (8.6%) | 11 (12.5%) | |
| Stroke of undetermined cause | 64 (12.9%) | 43 (15.4%) | 14 (10.9%) | 7 (8%) | |
| ASPECTS (median, IQR) | 9, 7–9 | 9, 7–9 | 9, 9–9 | 6, 5–7 | P<0.0001 |
| DWI lesion volume (mean, SD), Ml | 25±38.6 | 21.2±30.8 | 7.2±17.7 | 67.8±54.6 | P<0.0001 |
Group comparison was conducted using the Kruskal–Wallis Test. ASPECTS indicates Alberta Stroke Program Early Computed Tomographic Score; DWI, diffusion-weighted imaging; IQR, interquartile range; MCA, middle cerebral artery; NIHSS, National Institutes of Health Stroke Scale; and TOAST, Trial of Org 10 172 in Acute Stroke Treatment.
Figure 1.
Distribution of diffusion-weighted imaging (DWI) Alberta Stroke Program Early Computed Tomographic Score (ASPECTS) values across 496 patients.
The subgroups defined by involvement of the different MCA regions showed significant differences in mean DWI lesion volume, NIHSS, and median ASPECTS (Table). Patients with DWI lesions in both superficial and deep MCA territories had larger DWI lesion volumes, higher NIHSS values, and lower ASPECTS scores than both other groups. In addition, patients with superficial MCA stroke lesions had higher DWI lesion volumes than patients with deep MCA stroke lesions (21.2 versus 7.2 mL), whereas median ASPECTS seemed comparable between groups (9 versus 9).
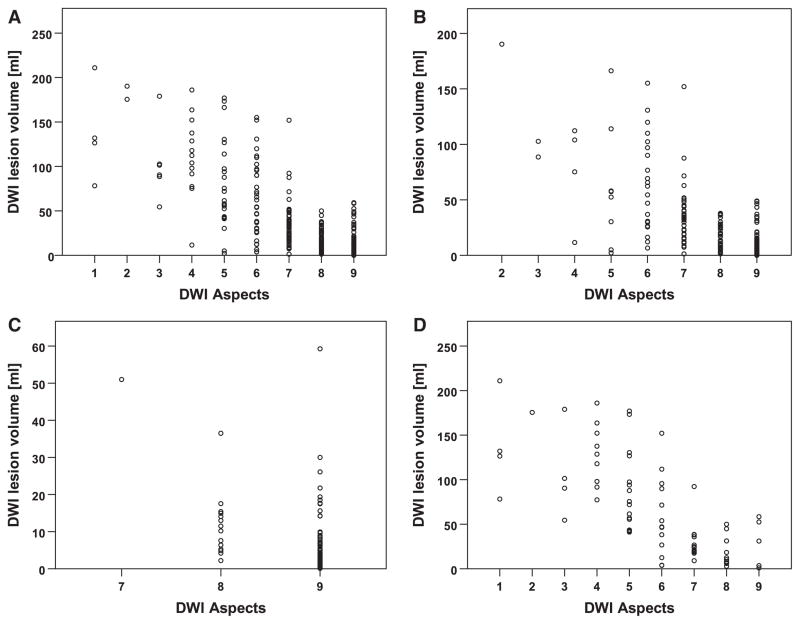
There was a significant negative correlation between DWI-ASPECTS and DWI lesion volume for all patients (r=−0.78; P<0.0001). Looking at the different subgroups, strong negative correlations between DWI-ASPECTS and DWI lesion volume were detected for superficial stroke lesions (r=−0.72; P<0.0001) and in the combined superficial and deep MCA region group (r=−0.72; P<0.0001). In the deep MCA region group, correlation of DWI-ASPECTS with lesion volume was considerably weaker (r=−0.19; P=0.0383). This is also reflected in the plots of DWI lesion volume by ASPECTS value (Figure 2).
Figure 2.
Scatterplot of diffusion-weighted imaging (DWI) lesion volume per Alberta Stroke Program Early Computed Tomographic Score (ASPECTS) value in all middle cerebral artery (MCA) strokes and different subgroups. A, For all analyzed MCA strokes; (B), superficial MCA territory involved; (C), deep MCA territory involved; and (D), superficial and deep MCA territory involved.
Correlations of DWI-ASPECTS and initial NIHSS revealed a similar pattern. A significant negative correlation was found for the entire sample (r=−0.49; P<0.0001) and for the groups with involvement of superficial MCA regions alone (r=−0.41; P<0.0001) or superficial and deep MCA regions (r=−0.39; P<0.0001), wheeras correlation was much weaker in patients with involvement of deep MCA regions alone (r=−0.18; P=0.0455).
Figure 3 shows the receiver operating characteristic curve for ASPECTS to identify a DWI lesion ≥100 mL. Area under the curve was 0.938 with a 95% confidence interval of 0.891 to 0.985. ASPECTS ≤6 was the best predictor of DWI lesion >100 mL with high sensitivity 0.935 (95% confidence interval, 0.772–0.989), specificity 0.877 (0.841–0.905), and negative predictive value 0.995 (0.979–0.999), but low positive predictive value 0.349 (0.250–0.463).
Figure 3.
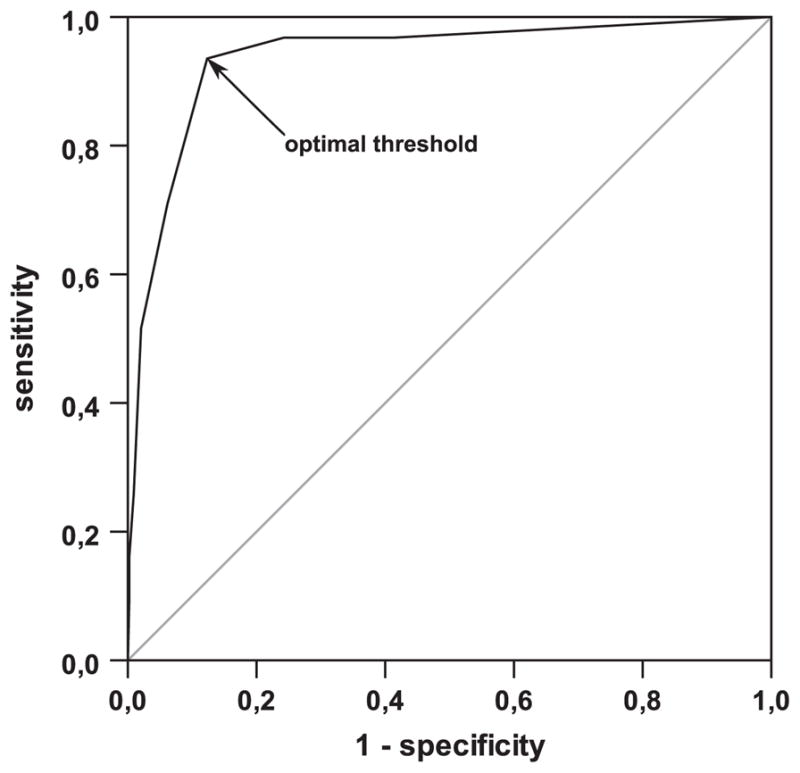
Receiver-operating characteristics curve to determine an Alberta Stroke Program Early Computed Tomographic Score (ASPECTS) value for optimal detection of a diffusion-weighted imaging lesion volume >100 mL; ASPECTS≤6 is identified as optimal threshold (arrow); area under the curve is 0.938 with a 95% confidence interval of 0.891 to 0.985.
The previously described ASPECTS threshold of ≤36,7 to identify lesion volume ≥100 mL applied to our sample resulted in the following predictive values: sensitivity 0.258 (0.125–0.449), specificity 0.990 (0.975–0.997), negative predictive value 0.950 (0.924–0.967), and positive predictive value 0.666 (0.354–0.887).
Discussion
In our analysis of DWI-ASPECTS and DWI lesion volume in a large sample of patients with acute ischemic stroke, we found that the performance of ASPECTS as substitute for stroke lesion volume depends on lesion location. Although there was a strong correlation of ASPECTS with DWI lesion volume in the stroke lesions involving the superficial MCA region, a much weaker correlation was found for lesions confined to the deep MCA territory. Thus, our study demonstrates for the first time in a large sample of patients an unequal weighing of ASPECTS, treating lesions in the basal ganglia differently from those in the superficial MCA territory. This is in line with a previous theoretical demonstration of an unequal distribution of tissue volume per ASPECTS region of interest.8
ASPECTS was originally designed to overcome limitations of noncontrast CT imaging in acute stroke, including high inter-rater variability and to standardize assessment of early ischemic changes. It is increasingly used to identify patients likely to benefit from reperfusion treatment both in clinical routine as in clinical stroke trials. ASPECTS predicts functional outcome and risk of SICH after thrombolysis, a threshold value of ≤7 on CT and ≤6 on DWI-MRI was proposed to isolate patients at high risk.1,11 Although originally designed to be used with CT imaging, it has also been used for standardized assessment and quantification of acute stroke lesions on DWI.5 In contrast to CT, DWI allows accurate delineation and volume measurement of acute ischemic lesions and thus enables comparison of ASPECTS with effective lesion volumes. In addition, DWI-ASPECTS was found to be superior to noncontrast CT ASPECTS in detecting ischemic changes,3 which again highlights the superiority of MRI in detecting acute ischemic brain lesions.
In the present data, we found a wide range of lesion volumes per ASPECTS value. Nevertheless, there was a strong correlation of lesion volume and ASPECTS when all patients with MCA infarction were included in the analysis. This is in line with previous studies, which also report a strong negative correlation between ASPECTS and DWI lesion volume.6,7
However, already in 2006 Phan et al8 discussed an unequal weighing of different MCA regions in ASPECTS, favoring the basal ganglia. They speculated that this could lead to unjustified exclusion of patients from recombinant tissue-type plasminogen activator treatment based on ASPECTS thresholds that do not account for different lesion locations. Our study supports this concern by the finding of a striking disparity of ASPECTS-DWI lesion volume correlations between the superficial and the deep MCA regions. For stroke lesions in the deep MCA territory, ASPECTS showed only a poor correlation to DWI lesion volume. This means that estimation of lesion volume in striatocapsular stroke by ASPECTS is unreliable. Whether ASPECTS favors lesions in the basal ganglia, as suggested before or whether it should be considered as a disadvantage depends on the point of view. In any case, ASPECTS performs rather poorly as a surrogate of lesion volume in striatocapsular stroke, which raises concerns about the use of ASPECTS to exclude patients from treatment or stroke trials without consideration of lesion location. However, it has to be considered that virtually all patients with stroke lesions restricted to the deep MCA territory score ≥7 on DWI-ASPECTS, a value that usually allows inclusion in stroke trials using ASPECTS thresholds. Nevertheless, as soon as ASPECTS is used to compare lesion volumes between patients groups, differences in stroke location between those groups may introduce a significant bias.
Of note, a similar phenomenon was found for correlation of ASPECTS with NIHSS with strong correlations for lesions involving the superficial MCA territory but rather weak correlation for strokes confined to the deep MCA region. Thus, evaluation of striatocapsular stroke by ASPECTS seems to be inaccurate in multiple regards.
The volume of the ischemic lesion is a strong predictor of clinical outcome and is also correlated with the risk of SICH. In DEFUSE, DWI lesions ≥100 mL4 were associated with a poor outcome and a high risk of SICH. A tissue volume of 100 mL is also a widely accepted estimate for one third of the MCA territory,12 a cutoff that is frequently used to define large infarctions, which may not benefit from reperfusion treatment, but which is subject to notorious discussions and poor interobserver agreement.13 Previous studies have already aimed at determining an ASPECTS cutoff value for ≥100-mL lesion volume.6,7 In our study, a threshold value of ASPECTS≤6 was the best predictor for ≥100-mL DWI lesion volume. This is close to the originally proposed cutoff value of ASPECTS≤71 for identifying patients at high risk for ICH and poor clinical outcome irrespective of an explicit tissue volume. However, a low positive predictive value of only 0.35 limits the value of ASPECTS≤6 as predictor of large DWI lesion volume in our study. Using this cutoff to exclude patients might thus lead to unjustified exclusion of patients from treatment or clinical trials if exclusion of patients with large (≥100 mL) DWI lesion is intended. However, negative predictive value is high (ie, one can assume that patients with ASPECTS>6 have a lesion volume <100 mL). Previous publications considering ASPECTS as a possible substitute for lesion volume measurement suggested a threshold value of ≤3.6,7 Using this value in our data set resulted in only low sensitivity (0.26).
There are limitations to our study. We studied patients with acute stroke and no lower threshold for symptom severity or lesion volumes including patients where thrombolysis was not considered. This probably leads to a higher number of patients with relatively low lesion volume and the different distribution could explain at least part of the disparity in predictive values when compared with previous studies. Additional studies should assess the validity of DWI-ASPECTS in a balanced population of infarct volumes.
Conclusions
If MRI or multimodal CT are not available, ASPECTS may be considered a simple and useful tool to help in the assessment of acute ischemic lesions on noncontrast CT in a semi-quantitative way and in the identification of patients with large acute stroke who may not benefit from reperfusion treatment. Nevertheless, comparison of ASPECTS with lesion volume measurements reveals relevant limitations, including a wide variation of lesion volumes for each score value and rather poor correlation with stroke lesion volume in the deep MCA territory. These limitations may impair decisions aiming at the exclusion of patients with large stroke lesions. Finally, the use of ASPECTS for evaluation of DWI seems somewhat anachronistic.14 Acute stroke lesions are visible with high contrast on DWI and tools for fast and easy volume measurement on MRI images are now widely available. Thus, if MRI is used for stroke imaging, there should be no need to use a substitute for stroke lesion volume measurement because lesion volume can easily be quantified on DWI maps.
Acknowledgments
We thank the Echoplanar Imaging Thrombolysis Evaluation Trial investigators, the University of California, Los Angeles Stroke investigators, and all our colleagues from the Departments of Neurology and Neuroradiology, and medical and technical staff in the participating centres for their support.
Sources of Funding
Predictive Value of Fluid-Attenuated Inversion Recovery received funding from the Else Kröner-Fresenius-Stiftung (2009_A36). Funding was provided for central data collection and analysis; there was no funding for inclusion of patients. In Berlin, data were collected within the 1000+ study, which has received funding from the Federal Ministry of Education and Research via the grant Center for Stroke Research Berlin (01 EO 0801). This work was further supported by the European Union Seventh Framework Programme grant agreement No 202213 and No 223153 (European Stroke Network), the Volkswagen Foundation, and the Deutsche Forschungsgemeinschaft. Echoplanar Imaging Thrombolysis Evaluation Trial was funded by the National Health and Medical Research Council (Australia), the National Stroke Foundation (Australia), and the Heart Foundation of Australia. The Valeur Precitive des Parametres Imagerie par Résonance Magnétique à la Phase Aigue de l’Accident Vasculaire Cerebral: Application à la Gestion des Essais Thérapeutics (VIRAGE) study is supported by French national grant Programme Hospitalier de Recherche Clinique (PHRC). This work was supported by Seton/UT Southwestern Clinical Research Institute of Austin, Department of Neurology and Neurotherapeutics, UT Southwestern Medical Center, Austin, TX, and the National Institute of Neurological Disorders and Stroke, Bethesda, MD.
Appendix
STIR and VISTA Imaging Steering Committee Members: Steven Warach (chair), Gregory Albers, Stephen Davis, Geoffrey Donnan, Marc Fisher, Anthony Furlan, James Grotta, Werner Hacke, Dong-Wha Kang, Chelsea Kidwell, Walter Koroshetz, Kennedy R. Lees, Michael Lev, David S. Liebeskind, A. Gregory Sorensen, Vincent Thijs, Götz Thomalla, Joanna Wardlaw, and Max Wintermark.
PRE-FLAIR Investigators: Götz Thomalla, Bastian Cheng, Martin Ebinger, Qing Hao, Thomas Tourdias, Ona Wu, Jong S. Kim, Lorenz Breuer, Oliver C. Singer, Marie Luby, Steven Warach, Soren Christensen, Andras Treszl, Nils D. Forkert, Ivana Galinovic, Michael Rosenkranz, Tobias Engelhorn, Martin Köhrmann, Matthias Endres, Dong-Wha Kang, Vincent Dousset, A. Gregory Sorensen, David S. Liebeskind, Jochen B. Fiebach, Jens Fiehler, and Christian Gerloff.
Footnotes
Disclosures
Dr Fiebach has received fees as a board member, consultant, or lecturer from Boehringer Ingelheim, Lundbeck, Siemens, Sygnis, and Synarc. Dr Gerloff has received fees as a consultant or lecture fees from Bayer Vital, Boehringer Ingelheim, EBS technologies, Glaxo Smith Kline, Lundbeck, Pfizer, Sanofi Aventis, Silk Road Medical and UCB. Dr Liebeskind has received fees as a consultant for Stryker and Covidien. Dr Thomalla has received fees as a consultant or lecturer from Bayer, Boehringer Ingelheim and Covidien and a research grant from the Else Kröner-Fresenius-Stiftung. Dr Tourdias has received a national grant from the French Government (PHRC). Dr Wu was supported, in part, by grants from the National Institutes of Health (R01NS059775, P50NS051343, and R01NS063925), received consulting fees from Penumbra Inc and received royalties from General Electric, Olea and Imaging Biometrics. The other authors report no conflicts.
References
- 1.Barber P, Demchuk A, Zhang J, Buchan A. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 2.Barber P, Hill MD, Eliasziw M, Demchuk M, Pexman JHW, Hudon ME, et al. Imaging of the brain in acute ischaemic stroke: comparison of computed tomography and magnetic resonance diffusion-weighted imaging. J Neurol Neurosurg Psychiatry. 2005;76:1528–1533. doi: 10.1136/jnnp.2004.059261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitomi M, Kimura K, Aoki J, Iguchi Y. Comparison of CT and DWI findings in ischemic stroke patients within 3 hours of onset. J Stroke Cerebrovasc Dis. 2014;23:37–42. doi: 10.1016/j.jstrokecerebrovasdis.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 5.Singer OC, Kurre W, Humpich MC, Lorenz MW, Kastrup A, Liebeskind DS, et al. Risk assessment of symptomatic intracerebral hemorrhage after thrombolysis using DWI-ASPECTS. Stroke. 2009;40:2743–2748. doi: 10.1161/STROKEAHA.109.550111. [DOI] [PubMed] [Google Scholar]
- 6.de Margerie-Mellon C, Turc G, Tisserand M, Naggara O, Calvet D, Legrand L, et al. Can DWI-ASPECTS substitute for lesion volume in acute stroke? Stroke. 2013;44:3565–3567. doi: 10.1161/STROKEAHA.113.003047. [DOI] [PubMed] [Google Scholar]
- 7.Lin K, Lee SA, Zink WE. What ASPECTS value best predicts the 100-mL threshold on diffusion weighted imaging? Study of 150 patients with middle cerebral artery stroke. J Neuroimaging. 2011;21:229–231. doi: 10.1111/j.1552-6569.2010.00487.x. [DOI] [PubMed] [Google Scholar]
- 8.Phan TG, Donnan GA, Koga M, Mitchell LA, Molan M, Fitt G, et al. The ASPECTS template is weighted in favor of the striatocapsular region. Neuroimage. 2006;31:477–481. doi: 10.1016/j.neuroimage.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 9.Thomalla G, Cheng B, Ebinger M, Hao Q, Tourdias T, Wu O, et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4.5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet Neurol. 2011;10:978–986. doi: 10.1016/S1474-4422(11)70192-2. [DOI] [PubMed] [Google Scholar]
- 10.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 11.Nezu T, Koga M, Kimura K, Shiokawa Y, Nakagawara J, Furui E, et al. Pretreatment ASPECTS on DWI predicts 3-month outcome following rt-PA: SAMURAI rt-PA Registry. Neurology. 2010;75:555–561. doi: 10.1212/WNL.0b013e3181eccf78. [DOI] [PubMed] [Google Scholar]
- 12.van der Zwan A, Hillen B, Tulleken CA, Dujovny M. A quantitative investigation of the variability of the major cerebral arterial territories. Stroke. 1993;24:1951–1959. doi: 10.1161/01.str.24.12.1951. [DOI] [PubMed] [Google Scholar]
- 13.Von Kummer R. Effect of training in reading CT scans on patient selection for ECASS II. Neurology. 1998;51:S50–S52. doi: 10.1212/wnl.51.3_suppl_3.s50. [DOI] [PubMed] [Google Scholar]
- 14.Bivard A, Parsons M. ASPECTaSaurus (a dinosaur)? Int J Stroke. 2012;7:564. doi: 10.1111/j.1747-4949.2012.00854.x. [DOI] [PubMed] [Google Scholar]