Abstract
Objective
We studied the pharmacokinetics and pharmacodynamics of boosted soft-gel lopinavir/ritonavir to assess if the area under the plasma concentration versus time curve (AUC) is altered in pregnancy and whether changes in AUC impacted HIV-1 control.
Methods
We enrolled pregnant women ≥13 years of age between 22 to 30 weeks gestation who expected to be on stable lopinavir/ritonavir for ≥8 weeks pre-delivery and ≥24 weeks post-delivery. Pharmacokinetic evaluations for lopinavir and ritonavir occurred at 36 weeks gestation and 6 and 24 weeks postpartum.
Results
Ten women underwent intensive pharmacokinetic evaluations for lopinavir and ritonavir at 36 weeks gestation and at 6 and 24 weeks postpartum. Estimated geometric mean (GM) AUC 0–6h (95% CI) for lopinavir were not significantly different at 26.5 (17.0, 41.4) and 41.9 (26.1, 67.5) mcg*hr/mL at 36 weeks gestation and 6 weeks postpartum, respectively (within-subject GM ratio 0.60 (0.25, 1.43); p=0.19). At 36 weeks gestation, 5 of 10 women had viral load <50 copies/mL and at 6 weeks postpartum 5 of 9 had viral load <50 copies/mL. Nine of ten infants for whom data were available were HIV negative.
Conclusion
Despite below target lopinavir levels (< 52 mcg*hr/mL except at 2 postpartum measurements), women maintained virologic control postpartum. Higher doses of lopinavir/ritonavir during pregnancy may not be necessary in all women.
Keywords: lopinavir, ritonavir, pregnancy, HIV viral load, pharmacokinetics
Introduction
Current Department of Health and Human Services United States guidelines recommend combination antiretroviral therapy for all pregnant women to prevent perinatal transmission of HIV [1]. Lopinavir/ritonavir (LPV/r) twice daily remains a preferred protease inhibitor (PI) for antiretroviral naïve pregnant women.
Drug absorption, distribution, metabolism and excretion constitute the basis of drug pharmacokinetics and may all undergo changes during pregnancy. Changes become increasingly pronounced as pregnancy advances and peak during the third trimester [2, 3].
Pregnancy induces gastrointestinal and liver metabolic enzymes including cytochrome P450 3A4 responsible for PI metabolism, resulting in reductions in PI drug exposure [4]. Increases of 35% in CYP3A4 activity have been reported, which is consistent with the 28% decrease in lopinavir exposure reported for pregnant women taking the soft-gel formulation during late pregnancy compared to the postpartum period [2, 4]. Stek et al found that only 3 of 17 (18%) pregnant women in their third trimester met the target lopinavir area under the curve (AUC) of ≥52 mcg*hr/mL, the 10th percentile for lopinavir AUC in non-pregnant adults [2]. Pregnancy decreases the absorption of drugs including PIs requiring low gastric pH for stability and increases drug distribution due to increases in plasma volume of up to 45%. In addition reductions in albumin and alpha-1 acid glycoprotein concentrations decrease protein binding of PIs thus increasing the free drug fraction. This may partially compensate for the reductions noted for total drug [5].
For women on LPV/r-based treatment, we sought to assess pharmacokinetic (PK) changes from third trimester to early postpartum, and to describe accompanying viral load profiles at 6 and 24 weeks postpartum.
Methods
Study Population and Design
AIDS Clinical Trials Group (ACTG) Protocol A5150 was a multi-center, prospective, observational study designed to characterize the incidence, magnitude, mechanisms and consequences of postpartum viral rebound during the initial 24 weeks postpartum in HIV-1 infected pregnant women [6]. ACTG A5153s was a substudy designed to evaluate the pharmacologic exposure and virologic response in women treated with LPV/r and nelfinavir. Only LPV/r data summaries are presented here.
Eligibility criteria for A5150 and A5153s have been previously described [6, 7]. The population comprised pregnant women ≥13 years between 22–30 weeks gestation on a nelfinavir or LPV/r based highly active antiretroviral regimen who planned to be on stable treatment for ≥8 weeks pre-delivery and ≥ 24 weeks post-delivery. Concurrent use of medications known to interfere with absorption or metabolism of lopinavir or ritonavir was not allowed. Standard treatment doses were administered for soft gel LPV/r (400/100 mg BID to all but one subject who, on a decision by her primary HIV provider, received 533/133 mg BID during her entire study participation).
Each site’s local institutional review board approved the substudy and informed consent was obtained from all women. Each participating woman’s primary care provider determined the choice of antiretroviral (ARV) medications used and remained responsible for her management throughout the study.
PK evaluations occurred during the third trimester antepartum (at 36 weeks gestation visit) and at visit week 6 and 24 postpartum. Women were asked to record the times and doses of their study drugs for 48 hours prior to each PK study visit using the study provided diary and calendar of their scheduled medications. On the day of PK sampling, a standardized meal was consumed 30 minutes prior to the observed study dose. Plasma samples were collected immediately pre-dose and at 1, 2, 4 and 6 hours post-dose. Women included in the PK analysis were instructed to take their prescribed regimens at the same dosages for at least 2 weeks prior to each PK visit to assure steady-state conditions. Self-reported adherence was measured by a standard validated ACTG questionnaire at each study visit.
Analysis of protease inhibitor concentrations and viral load determinations
For quantitation of lopinavir and ritonavir, liquid chromatography/tandem mass spectrometry methods were employed by the Drug Research Unit of the University of California, San Francisco. Methods were approved by the DAIDS funded Pharmacology Quality Assurance Program [8]. Inter-assay coefficient of variation for lopinavir and ritonavir were less than 11%. Lower limit of quantitation for lopinavir and ritonavir were 0.04 and 0.025 mcg/mL, respectively.
Plasma HIV-1 RNA levels were measured with the Roche Amplicor HIV-1 Monitor (Version 1.5; lower limit of quantification detection <50 copies/mL). HIV serostatus was collected retrospectively in the newborn infants.
Pharmacokinetic and statistical analysis
PK parameters were estimated for lopinavir and ritonavir using standard non-compartmental analysis. An abbreviated area under the plasma concentration versus time curve (AUC) from 0 to 6 hours post-dose was calculated using the linear trapezoidal rule. Dose normalized AUC was calculated using reference doses of LPV/r: 400/100 mg BID and as (observed AUC)*(reference)/(specific subject’s dose) for the one woman who was not on the reference dose. The percentage of women exceeding the target lopinavir AUC of ≥52 mcg*hr/mL was assessed in a post-hoc analysis [2]. Concentrations below the lower limit of quantitation were assigned a value of half that limit. The minimum concentration (Cmin) was calculated as the concentration obtained at the pre-dose sample time and the maximum concentration (Cmax) was calculated as the maximum concentration observed over the 0–6 hours of sampling. If the previous dose was taken >16 hours prior to the observed dose, data from that PK visit were excluded. Within-subject mean change in PK-parameter (AUC, Cmin, Cmax) from antepartum to postpartum was assessed on the natural log(ln)-scale with a paired t-test.
Results
Eleven women enrolled between October 2003 and December 2004. Data from one woman were excluded because her last previous dose was taken >16 hours before the observed dose at her only PK visit (third trimester). The remaining 10 women contributed data to at least one of the PK parameters (AUC, Cmin or Cmax). Women were enrolled at six AIDS Clinical Trials Units.
Demographics for the 10 women at entry to the parent study were median (minimum, maximum) age 29 (19, 39) years; 5 Black non-Hispanic and 3 Hispanic; median weight 93.8 kg (69.5, 147.6); median CD4 400 cells/mm3 (36, 658); and median HIV RNA log10 2.2 copies/mL (1.7, 4.6). Although women were scheduled to have three PK visits (36 weeks gestation and 6 and 24 weeks postpartum), only two of ten women had three evaluable PK visits. Four had an evaluable third trimester and 6-week postpartum visit, but no 24 week visit. Two had only the third trimester visit and two had only the 6 week postpartum visit (1 woman at each time point contributed only Cmin data). Visits were missed due to withdrawal of consent (3 visits), illness (1 visit), unable to come to clinic (3 visits), early delivery (1 visit), work schedule (1 visit), changing family situation (1 visit), move out of the area (1 visit), and discontinued ARVs (1 visit).
All women remained on the same nucleoside analogue component of their ARV regimen for their PK visits (zidovudine/lamivudine (4), abacavir/zidovudine/lamivudine (2), tenofovir-containing regimen (3), and zidovudine/didanosine (1)).
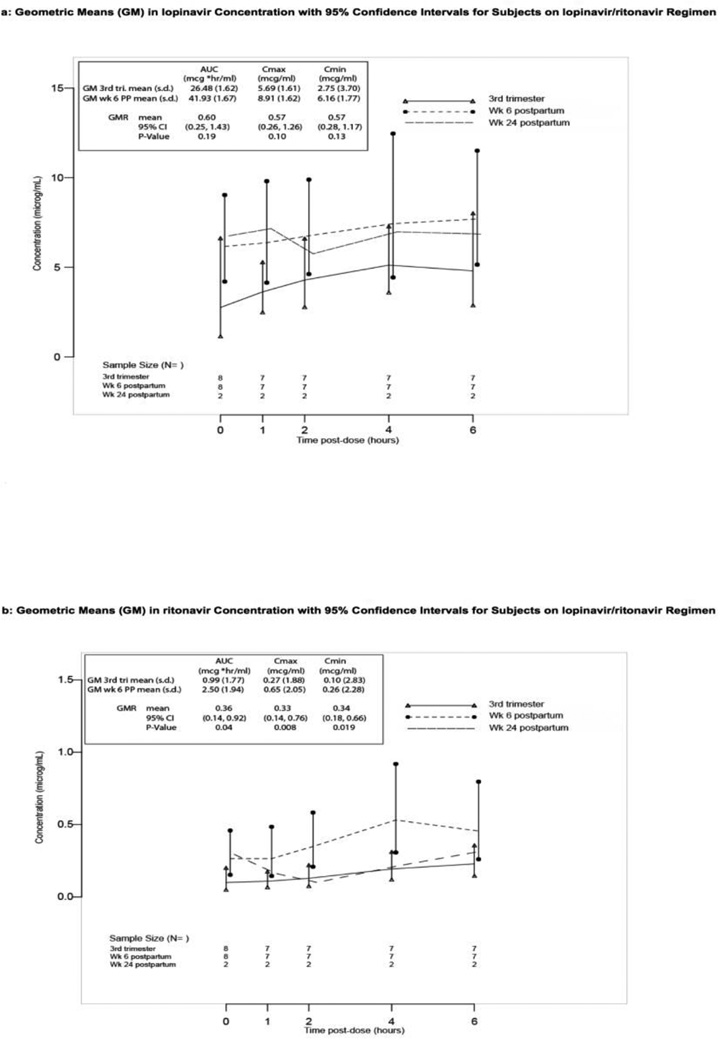
Figures 1a and 1b present mean lopinavir (1a) and ritonavir (1b) concentrations over the sampling time points and summarize the comparison of antepartum and week 6 postpartum PK parameters. Estimated lopinavir geometric mean (GM) AUC0–6h (95% confidence interval (CI)) was 26.5 (17.0, 41.4) mcg*hr/mL for 7 women at 36 weeks gestation and 41.9 (26.1, 67.5) mcg*hr/mL for 7 women at 6 weeks postpartum. None of the 7 women with week 36 gestation PK data had a lopinavir AUC of ≥52 mcg*hr/mL. In post-hoc analysis only two women had a lopinavir AUC of ≥52 mcg*hr/mL at a postpartum PK visit (53.15 and 98.40), both at week 6 postpartum). For the 6 women with lopinavir PK data at both time points, the within-subject estimated geometric mean ratio (GMR) week 6 postpartum/week 36 gestation was 0.60 (95% CI 0.25, 1.43), a 40% lower average AUC during pregnancy that was not statistically significantly different from 1 (p=0.19) (Figure 1a). Insufficient data were available to compare third trimester to week 24 postpartum lopinavir concentrations.
Figure 1.
Drug exposure at antepartum and postpartum visits; geometric mean (GM) with 95% confidence interval (CI); along with within-subject geometric mean ratio (GMR) of antepartum to week 6 post partum (reference) for PK parameters area under the curve (AUC0–6h), Cmax and Cmin. 3rd tri.=third trimester (36 weeks gestation visit), PP=postpartum. Panel a: lopinavir; Panel b: ritonavir
For ritonavir, data from the six women with both antepartum and week 6 postpartum PK data indicated 36 weeks gestation values were significantly lower on average, with an estimated GM of 0.99 (0.58,1.68) compared to 2.5 (1.4,4.6) mcg*hr/mL at 6 weeks postpartum, resulting in a 64% lower AUC during pregnancy (within-subject GMR (95% CI) of 0.36 (0.14, 0.92), p=0.04) (Figure 1b). Peak concentrations (Cmax) for ritonavir were reduced significantly at 36 weeks gestation compared to postpartum values (Figure 1b). Insufficient data were available to compare third trimester to week 24 postpartum ritonavir concentrations.
Table 1 details viral load results for each woman. Third trimester HIV RNA level was <50 copies/mL of plasma in 5 of 10 (50%) women and remained <50 copies/mL in 4 of these 5 women at 6 weeks postpartum (Subject #5 on the higher LPV/r dose, 59 copies/mL) and in 2 of the 3 women with data at 24 weeks postpartum. For the 2 women converting from <50 copies/mL to ≥50 copies/mL postpartum, only 1 had viral load measurements exceeding 200 copies/mL postpartum (at 24 weeks postpartum) and this woman had stopped her antiretroviral therapy. Four of 5 women with viral load >200 copies/mL at any point in the study reported adherence problems around the time of the elevated viral load measurement (Table 1). Of the 6 women with both antepartum and 6 week postpartum PK data (Subjects 2, 4, 5, 8, 9, and 10), 3 had viral loads <50 copies/mL at both time points, 1 went from <50 copies/mL to 59 copies/mL, 1 went from 316 copies/mL to <50 copies/mL, and 1 with reported adherence issues at multiple time points went from 933 copies/mL to 50,362 copies/mL. This last woman had the smallest increase from third trimester to week 6 postpartum in her AUC0–6h for both lopinavir and ritonavir. The woman with the decrease in her HIV-1 RNA level had very high week 6 postpartum AUC0–6h levels of lopinavir and ritonavir
Table 1.
HIV RNA levels (copies/mL) ante- and postpartum
| Subject # | Started LPV/r, # wks before delivery |
Antepartum | Delivery | 6 wk PP | 24 wk PP | Comment |
|---|---|---|---|---|---|---|
| 1 | 24.1+ | 63** | NA | *NA | *NA | Withdrew at Wk 39 gestation |
| 2 | 21.3 | <50 | <50 | <50 | 16,534* | Self discontinued meds @ 12 wks PP |
| 3 | 16.1 | <50 | NA | <50 | 199* | |
| 4 | 19.7 | 933 | 13,804 | 50,362 | 39,449* | Missed some meds week prior to delivery, off meds for 6 days at wk 4 PP, self discontinued meds at 21 wks PP |
| 5 | 15.1 | 316 | 65 | <50 | 25,168* | Off meds for 3 days prior to wk 24 PP PK observed dose |
| 6 | 14.3 | 363 | 155 | 96* | *NA | Last dose 34 hrs prior to wk 6 PP PK observed dose |
| 7 | 29.1 | 1995* | 7244 | 71* | *NA | |
| 8 | 20.3 | <50 | <50 | 59 | *NA | On LPV/r 533/133 mg bid at each PK visit# |
| 9 | 23.3 | <50 | <50 | <50 | <50 | |
| 10 | 136.3 | <50 | <50 | <50 | <50 |
On LPV/r 24.1 weeks at time of withdrawal,
No PK visit or this PK visit data excluded from analysis,
Cmin data only,
only subject on higher dose LPV/r during pregnancy.
LPV/r = lopinavir/ritonavir, PP=postpartum
One woman withdrew consent prior to delivery and her baby’s HIV status is unknown. The remaining nine infants were HIV negative.
Discussion
We compared the pharmacokinetics of lopinavir and ritonavir during pregnancy to postpartum values and investigated viral load response during and following pregnancy. Although the pharmacokinetics of these drugs during pregnancy has been investigated previously, viral load response data during and after pregnancy in the context of PK information are limited. Studies have demonstrated similar plasma concentrations of lopinavir with soft-gel lopinavir/ritonavir compared to the current hard-tablet formulation in both pregnant and non-pregnant adults, although the hard-tablet formulation has less inter-subject variability [9].
Our study estimated an average but not statistically significant 40% reduction in lopinavir exposure in the context of pregnancy. Based on the confidence intervals this was consistent with true values between as low as 75% reduction to as high as 43% increase (GMR (95% CI) 0.6 (0.25, 1.43)). This is consistent with previous reports of a 28% reduction in lopinavir exposure during pregnancy in a study of 17 women [2]. We also observed a significantly lower AUC in ritonavir exposure during pregnancy compared to week 6 postpartum with an estimated within-subject geometric mean ratio on the AUC of 0.36 (64% reduction), which was consistent with values ranging from 0.14 to 0.92.
The majority of women maintained postpartum plasma viral loads at low (<200 HIV-1 RNA copies/mL) to unquantifiable levels (<50 copies/mL). When evaluating the association between ARV exposure and virologic control, women with lower lopinavir AUC estimates in their third trimester were still able to maintain virologic suppression during and following pregnancy, albeit the sample size for this analysis was very small. For the 5 women with viral load measurements exceeding 200 copies/mL at any time point antepartum to 24 weeks postpartum, results were attributable in all but one case to problems with adherence and reports of self-discontinuation of ARV medications. Despite the variability in LPV/r exposure, viral load control was relatively stable with no indication that PK variability translated into problems with virologic control.
Current Department of Health and Human Services United States guidelines state that some experts recommend increasing the dose of lopinavir/ritonavir from 400/100 mg bid to 600/150 mg bid in the second and third trimester to compensate for PK changes in pregnancy [1]. This increased dose has been shown to result in AUC similar to non-pregnant women on 400/100 mg bid, but is less well tolerated [10, 11]. Whether this dose increase is necessary remains unclear. Outside the US, LPV/r PK studies in Thai and European pregnant women have generally found adequate lopinavir levels in the third trimester though lower than postpartum [2–15]. One Thai study found 5/26 (19%) Thai women below the lopinavir 52 mcg*h/mL threshold in the third trimester, but all attained delivery viral load < 400 copies/mL [2, 14]. All of our antepartum women (N=7) had lopinavir AUC < 52 mcg*hr/mL and 5 of 7 week 6 postpartum and 2 of 2 week 24 postpartum women were below this cutoff.
Drug protein binding decreases in pregnancy [5,16]. Investigators have found that the unbound fraction of lopinavir increases during pregnancy [5,12,16]. Of two US studies, one demonstrated an 18% increase in unbound lopinavir and another found that the unbound Cmin exposures of 12 pregnant women were > 70 times the target concentrations at standard doses [7,16]. These effects may compensate for the reduction in total lopinavir AUC previously reported and may partially explain the stable viral load control we observed despite variability in total drug exposure. On 1/28/15, the FDA using these data updated the Kaletra (lopinavir/ritonavir) label to include dosing recommendations in pregnant women. Lopinavir/ritonavir is now recommended to be dosed 400/100 mg po bid in pregnant women with no documented lopinavir-associated mutations. Once daily dosing is not recommended in pregnancy. There are insufficient data to recommend dosing in pregnant women with any documented lopinavir-associated mutations [17].
Women exhibited substantial variability in their PK disposition that did not appear to translate into changes in viral load control. While our sample size is small, our data add to the growing body of data that dose adjustment of LPV/r during pregnancy may not be necessary for many women, particularly those with controlled viremia and lopinavir-sensitive virus.
Acknowledgements
This work was supported in part by the AIDS Clinical Trials Group (ACTG) funded by the National Institute of Allergy and Infectious Diseases, ACTG Central Group grant AI38858 and AI68636; ACTG Statistical Data Analysis Center grant number AI38855 and AI68634; University of Washington Center for AIDS Research (AI27757) and ACTG Virology Specialty Laboratory (AI38858;AI068636); and in part by the General Clinical Research Center Units funded by the National Center for Research Resources.
Appendix
The study team gratefully acknowledges the participation of the study volunteers. In addition to the authors, other members of the study team include Patricia Lizak BS for performing the PK assays, Joseph Mrus, MD, MSc, Karin L. Klingman, MD (Division of AIDS, National Institute of Allergy and Infectious Disease), protocol medical officer; Heather D. Watts, MD (Eunice Kennedy Shriver National Institute of Child Health and Human Development), Jane Hitti, MD, MPH (University of Washington Medical Center), Pamela Juba, MD (University of Florida, Jacksonville), co-investigators; Joan Gormley, BSN (The Miriam Hospital), site representative; Linda Gedeon and Ann Walawander (Frontier Science and Technology Research Foundation), data managers; Estelle M. Piwowar-Manning, MT (Johns Hopkins University HIV Specialty Labs), laboratory technologist; Jennifer Nowak, Travis Behm, and Jim Tutko (Frontier Science and Technology Research Foundation), laboratory data coordinators; and Catherine Olufs, Rona Taylor, and Michael D. Stewart, community representatives.
Participating Sites and Site Personnel
C. Bradley Hare, MD and Deborah Zeitschel, RN, MSN, UCSF AIDS CRS (AI695020); Joan A. Swiatek, APN-NP, and Michael J. Hussey, MD, Rush University Medical Center (AI069471); Françoise Kramer, MD, and LaShonda Spencer, MD, Los Angeles County + University of Southern California Medical Center, PACTU/Maternal-Child-Adolescent HIV Center (N01-HD-3-3162, HHSN 2672080000IC); Debra Goldman, ARNP, and Shelia B. Dunaway, MD, University of Washington AIDS CRS (AI-069434); Janet Forcht, RN, and Margie Vasquez, RN, New York University/NYC HHC at Bellevue Hospital Center (AI069532); Warren A. Andiman, MD, and Leslie Hurst, BS, Yale University School of Medicine.
Footnotes
Presented in part at the 14th Conference on Retroviruses and Opportunistic Infections, February 25–28, 2007. Los Angeles, CA. Abstract #S-156.
ClinicalTrials.gov identifier: NCT00041964
Conflict of Interest:
No competing interest declared.
References
- 1.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. [Accessed (9/4/14)];2014 Mar 28;:1–225. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf.
- 2.Stek AM, Mirochnick M, Capparelli E, Best BM, Hu C, Burchett SK, et al. Reduced lopinavir exposure during pregnancy. AIDS. 2006;20:1931–1939. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- 3.Baroncelli S, Villani P, Floridia M, Pirillo MF, Galluzzo DM, Cusato M, et al. Trough concentrations of lopinavir, nelfinavir, and nevirapine with standard dosing in human immunodeficiency virus-infected pregnant women receiving 3-drug combination regimens. Ther Drug Monit. 2008;30:604–610. doi: 10.1097/FTD.0b013e3181867a6e. [DOI] [PubMed] [Google Scholar]
- 4.Tracy TS, Venkataramanan R, Glover DD, Caritis SN. Temporal changes in drug metabolism (CYP1A2, CYP2D6, and CYP3A Activity) during pregnancy. Am J Obstet Gynecol. 2005;192:633–639. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 5.Aweeka FT, Stek A, Best BM, Hu C, Holland D, Hermes A, et al. Lopinavir protein binding in HIV-1-infected pregnant women. HIV Medicine. 2010;11:232–238. doi: 10.1111/j.1468-1293.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sha BE, Tierney C, Cohn SE, Sun X, Coombs RW, Frenkel LM, et al. Postpartum viral load rebound in HIV-1-infected women treated with highly active antiretroviral therapy: AIDS Clinical Trials Group Protocol A5150. HIV Clin Trials. 2011;12(1):9–23. doi: 10.1310/hct1201-9. PMCID PMC3227722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aweeka FT, Tierney C, Stek A, Sun X, Cohn SE, Coombs R, et al. ACTG 5153s: Pharmacokinetic Exposure and Virological Response in HIV-1 Infected Pregnant Women Treated with Protease Inhibitors. 14th Conference on Retroviruses and Opportunistic Infections; February 25–28, 2007; Los Angeles, CA. Abstract #739. [Google Scholar]
- 8.DiFrancesco R, Rosenkranz SL, Taylor CR, Pande PG, Siminski SM, Jenny RW, et al. Clinical pharmacology quality assurance program: Models for longitudinal analysis of antiretroviral proficiency testing for international laboratories. Ther Drug Monit. 2013;35:631–642. doi: 10.1097/FTD.0b013e31828f5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khuong-Josses MA, Azerad D, Boussairi A, Ekoukou D. Comparison of lopinavir levels between the two formulations (soft-gel capsule and tablet) in HIV-infected pregnant women. HIV Clin Trials. 2007;8(4):254–255. doi: 10.1310/hct0804-254. [DOI] [PubMed] [Google Scholar]
- 10.Mirochnick M, Best BM, Stek AM, Capparelli E, Hu C, Burchett SK, et al. Lopinavir exposure with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2008;49:485–491. doi: 10.1097/QAI.0b013e318186edd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Best BM, Stek AM, Mirochnick M, Hu C, Holland D, Hermes A, et al. Lopinavir tablet pharmacokinetics with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2010;54:381–388. doi: 10.1097/qai.0b013e3181d6c9ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert JS, Else LJ, Jackson V, Breiden J, Gibbons S, Dickinson L, et al. Therapeutic drug monitoring of lopinavir/ritonavir in pregnancy. HIV Medicine. 2011;12:166–173. doi: 10.1111/j.1468-1293.2010.00865.x. [DOI] [PubMed] [Google Scholar]
- 13.Ramautarsing RA, van der Lugt J, Gorowara M, Kerr SJ, Burger D, Ruxrungtham K, Phanuphak P, et al. Thai HIV-1-infected women do not require a dose increase of lopinavir/ritonavir during the third trimester of pregnancy. AIDS. 2011;25:1299–1303. doi: 10.1097/QAD.0b013e328347f7e9. [DOI] [PubMed] [Google Scholar]
- 14.Cressey TR, Jourdain G, Rawangban B, Varadisai S, Kongpanichkul R, Sabsanong P, et al. Pharmacokinetics and virologic response of zidovudine/lopinavir/ritonavir initiated during the third trimester of pregnancy. AIDS. 2010;24:2193–2200. doi: 10.1097/QAD.0b013e32833ce57d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyons F, Lechelt M, De Ruiter A. Steady-state lopinavir trough levels in third trimester of pregnancy. AIDS. 2007;21:1053–1054. doi: 10.1097/QAD.0b013e3281053a1e. [DOI] [PubMed] [Google Scholar]
- 16.Patterson KB, Durmond JB, Prince HA, Jenkins AJ, Scarsi KK, Wang R, et al. Protein binding of lopinavir and ritonavir during four phases of pregnancy: Implications for treatment guidelines. J Acquir Immune Defic Syndr. 2013;63:51–58. doi: 10.1097/QAI.0b013e31827fd47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaletra (lopinavir/ritonavir) [package insert] Chicago, IL: AbbVie Inc.; 2015. [Google Scholar]