Abstract
Aggrecan, the major proteoglycan in cartilage, serves to protect cartilage tissue from damage and degradation during the progression of osteoarthritis (OA). In cartilage extracellular matrix (ECM) aggrecan exists in an aggregate composed of several aggrecan molecules that bind to a single filament of hyaluronan. Each molecule of aggrecan is composed of a protein core and glycosaminoglycan sides chains, the latter of which provides cartilage with the ability to retain water and resist compressive loads. During the progression of OA, loss of aggrecan is considered to occur first, after which other cartilage matrix components become extremely susceptible to degradation. Proteolytic cleavage of the protein core of aggrecan by enzymes such as aggrecanases, prevent its binding to HA and lower cartilage mechanical strength. Here we present the use of HA-binding or collagen type II-binding molecules that functionally mimic aggrecan but lack known cleavage sites, protecting the molecule from proteolytic degradation. These molecules synthesized with chondroitin sulfate backbones conjugated to hyaluronan- or collagen type II- binding peptides, are capable of diffusing through a cartilage explant and adhering to the ECM of this tissue. The objective of this study was to test the functional efficacy of these molecules in an ex vivo osteoarthritic model to discern the optimal molecule for further studies. Different variations of chondroitin sulfate conjugated to the binding peptides were diffused through aggrecan depleted explants and assessed for their ability to enhance compressive stiffness, prevent CS degradation, and modulate catabolic (MMP-13 and ADAMTS-5) and anabolic (aggrecan and collagen type II) gene expression. A pilot in vivo study assessed the ability to retain the molecule within the joint space of an osteoarthritic guinea pig model. The results indicate chondroitin sulfate conjugated to hyaluronan-binding peptides is able to significantly restore equilibrium modulus and prevent CS degradation. All molecules demonstrated the ability to lower catabolic gene expression in aggrecan depleted explants. In order to enhance biosynthesis and regeneration, the molecules need to be coupled with an external stimulant such as a growth factor. The chondroitin sulfate molecule synthesized with HA-binding peptides demonstrated adherence to cartilage tissue and retention up to 6 hours in an ambulatory joint. Further studies will monitor the in vivo residence time and ability of the molecules to act as a disease-modifying agent.
Keywords: Chondroitin sulfate, collagen type II, hyaluronan, intra-articular, resist proteases, cartilage matrix degradation
Graphical Abstract
Introduction
An effective intra-articular injection to treat osteoarthritis (OA) requires the therapy to have sufficient residence time in the joint space to diffuse into and be retained within a tightly woven extracellular matrix (ECM) composed of collagen type II fibers and aggregating proteoglycans1–3. The pathophysiology during the progression of OA makes this feat difficult. The ECM of cartilage is primarily composed of collagen fibrils (15–22% wet weight collagen type II) and proteoglycans (4–7% wet weight)4. The interaction between these two networks is known to affect fibrillogenesis and control the overall biology of the tissue 5–6. During OA, the cartilage ECM is host to a number of proteolytic enzymes, specifically matrix metalloproteases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS), shown to cleave collagen type II and aggrecan respectively7–8. Proteolytic cleavage of aggrecan occurs prior to the cleavage of collagen type II, and the presence of aggrecan has been suggested to protect cartilage by hindering diffusion of proteases into the matrix9–11. However, aggrecan is also susceptible to cleavage. ADAMTS cleaves aggrecan within specific amino acid sequences, releasing fragments from the cartilage matrix12–13. These fragments, unable to rebind to hyaluronan (HA), diffuse out of the matrix and expose collagen type II and HA to enzymatic degradation, which in turn releases new HA and collagen type II fragments that stimulate an increase in pro-inflammatory cytokines and MMP expression14–16. A therapeutic designed to treat OA has to not only enter the tight ECM mesh, but also shield the ECM from proteolytic cleavage.
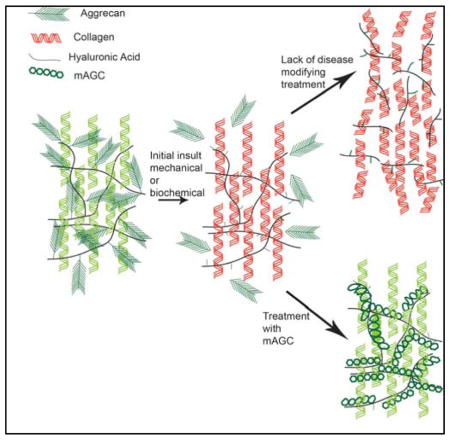
Towards this approach, our laboratory has designed HA-binding or collagen type II-binding molecules that have been shown to functionally mimic aggrecan, but lack the known cleavage sites, making the molecule less susceptible to proteolytic degradation. The molecules, designed to functionally mimic aggrecan, do not mimic the ‘bottle brush structure’ of aggrecan, where molecules composed of a protein core and glycosaminoglycan (GAG) side chains, primarily chondroitin sulfate, bind to a filament of HA. The protein core of aggrecan consists of three main globular domains and an inter-globular domain (IGD), the latter of which is highly susceptible to protease cleavage17. The GAG side chains in aggrecan provide cartilage tissue with the ability to resist compressive loads. The design of the molecules discussed in this paper focuses on the functional benefits of chondroitin sulfate (CS) and provides a design that allows binding to cartilage ECM components without the protease susceptible IGD region. The design consists of a CS backbone functionalized with either HA-binding peptide GAHWQFNALTVR or collagen type II-binding peptide WYRGRL18–19. The HA binding peptide has been previously used to enhance cartilage lubrication by tethering to poly (ethylene glycol) chains and the WYRGRL peptide has been tethered to poly(propylene sulfide) nanoparticles to enhance drug uptake19–20. In our study the peptides were coupled to CS by adding a glycine-cysteine spacer changing the sequences to CS-GAHWQFNALTVRGGGC and CS-WYRGRLGC respectively. Our lab has extensively used this chemistry to conjugate peptides to glycosaminoglycan backbones. Previously, we have demonstrated the ability of these molecules to protect HA and collagen type I from hyaluronidase and MMP based degradation, respectively21–22. We have also shown that when a biomaterial incorporates the mimic, less ECM is lost to proteolytic degradation23. Further, in a preliminary evaluation, we have noted the molecule’s ability to suppress MMP-13 activity and preserve subchondral bone volume in an aggressive rat model of osteoarthritis 24.
In this present study we investigated the capability of chondroitin sulfate augmented with HA- and collagen type II-binding peptides, the molecules that bind specifically to cartilage matrix components, to diffuse through an aggrecan depleted ex vivo cartilage matrix. As both designs incorporate a GAG backbone, their ability to restore compressive stiffness of an aggrecan-depleted matrix was tested. Further, the ability of these molecules to protect CS from proteolytic degradation was evaluated by monitoring CS fragmentation and the anabolic and catabolic mRNA expression of chondrocytes within the matrix. Finally we evaluated the ability of the molecules to adhere to cartilage tissue in vivo. We hypothesize that by protecting cartilage ECM molecules from proteolysis, the degradation cycle seen in OA will decline, thus supporting tissue preservation.
Materials & Methods
Reagents
Peptide GAHWQFNALTVRGGGC (GAH) and WYRGRLGC (WYRGRL) were purchased from Genscript (Piscataway, NJ). N-[β-maleimidopropionic acid] hydrazide, trifluoroacetic acid salt (BMPH) was purchased from Pierce (Rockford, IL). All other supplies were purchased from VWR (West Chester, PA) or Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
Synthesis of molecules
Synthesis of collagen type I and HA binding proteoglycans has been previously described by our laboratory21, 25. Utilizing the same methods, chondroitin sulfate augmented with collagen type II and HA binding peptides were synthesized. Briefly, 2μM chondroitin-6 sulfate was oxidized by reacting with 30 mM sodium periodate for 48 hours to form reactive aldehyde functional groups. A heterbifunctional crosslinker N-(b-Maleimidopropionic acid) hydrazide, trifluoroacetic acid salt (BMPH) and sodium cyanoborohydride were added to the oxidized CS and reacted for 4 hours. A glycine-cysteine spacer was added by the manufactuerer to the C terminus of the respective binding peptides to yield GAHWQFNALTVRGGGC (HA binding peptide, referred to as GAH) and WYRGRLGC (collagen type II binding peptide, referred to as WYRGRL. Ten binding peptides of either GAH or WYRGRL were conjugated to the CS-BMPH molecule by allowing the thiol group on the peptides to react with the maleimide group of BMPH for 2 hours. Peptides were added at a 1: 1 molar ratio of peptide:CS. The end product of the functionalized molecules yielded CS-BMPH-WYRGRL (type II-binding molecule) and CS-BMPH-GAH (HA-binding molecule). Molecules with a biotin label were synthesized by reacting one mole of WYRGRLbiotin or GAHbiotin per mole of CS-BMPH followed by addition of nine moles of unlabeled WYRGRL or GAH respectively. Molecules were separated using size exclusion chromatography with an ÄKTA Purifier FPLC (GE Healthcare) and a column packed with polyacrylamide beads (Bio-Rad Labs). After synthesis, molecules were lyophilized and stored at −80 °C until further testing and have been labeled as: mAGC (HA binding), mAGCb (HA binding, biotin labeled), mAG(II)C (collagen type II binding) and mAG(II)Cb (collagen type II binding, biotin labeled).
Tissue harvest
Cartilage explants were obtained from three month old bovine knee joints obtained from an abattoir within 24 hours of slaughter (Dutch Valley Veal). The explants were removed using a 3 mm diameter cork borer from the load bearing region of the femoral condoyle and washed three times in serum free DMEM medium (50 μg/mL ascorbic acid 2-phosphate, 100 μg/mL sodium pyruvate, 0.1 % bovine serum albumin, 100 units/mL penicillin, 100 μg/mL streptomycin and 25 mM HEPES). Explants used for diffusion and mechanical tests were stored in Hank’s Balanced Salt Solution (HBSS) containing a mix of protease inhibitors at −20 °C until further testing. Explants required for ex vivo inflammatory model were weighed in a sterile hood and cultured for three days in 5 % FBS supplemented media prior to testing.
Diffusion of molecules through cartilage explant
Native aggrecan was removed from harvested cartilage explants using a previously described protocol26. Briefly, plugs were treated with 0.5 % (w/v) trypsin in HBSS for 3 hours at 37 °C. After trypsin treatment, explants were washed three times in HBSS and incubated with 20 % FBS to inactivate residual trypsin activity. Molecules dissolved in distilled water at 10 μM concentration were diffused through the articular surface of cartilage explants by placing 10 μL of the solution on the surface every ten minutes for one hour at room temperature. The molecules diffused included:1) mAGCb, 2) mAG(II)Cb, 3) mAGCb+mAG(II)C and 4) mAGC+mAG(II)Cb. Normal cartilage and aggrecan depleted cartilage were treated with 1X PBS as positive and negative controls respectively. After diffusion, explants were washed three times with 1X PBS and stored at −20 °C until further testing.
Histological assessment of diffusion
A midsagittal cut was made through the explant to examine diffusion through the center of the tissue. The two halves were embedded in O.C.T compound (Tissue Tek), sectioned at 7 μm thickness, fixed in cold acetone, air dried and washed in distilled water. All experimental groups were incubated with streptavidin-horseradish peroxidase (HRP) stain for 30 minutes, washed, counter stained with 3,3′-diaminobenzidine for 10 minutes, washed and finally stained with hematoxylin for 5 minutes. All washes were done with distilled water. The effectiveness of aggrecan depletion was assessed using Safranin O stain and counter stained with Fast Green stain. Tissue sections were deparaffinized in xylene for five minutes followed by a gradual series of ethanol dehydration. The sections were rinsed in distilled water and stained with Fast Green for ten minutes. This was followed by a rinse in 1 % acetic acid and a stain in 0.1 % aqueous Safranin O stain for fifteen minutes. The tissue sections were dried with 100 % ethanol and mounted with synthetic resin. Cell viability after trypsin treatment was assessed using Live-Dead assay (Invitrogen). The slices were dehydrated on clear coverslip and scanned using Aperio ScanScope at 1X magnification using Aperio’s ImageScope Viewer.
Bulk compression testing
Displacement-controlled unconfined compression was performed on an AR G2 rheometer with force transducers capable of detecting normal forces in the range of 0.01–50 N (TA Instruments). The explants were glued to the bottom of a hydrophobic printed slide (Tekdon) and covered in a 1X PBS bath. A 20 mm diameter stainless steel parallel plate geometry head was lowered until initial contact was made. Explant height was measured using a digital micrometer (Duratool). Compressive loads from 0–30 % nominal strain (at 5 % intervals) were applied to the explants through a stepwise loading that involved a ramp duration of 5 sec (i.e. a strain rate of 1.0 %/sec) and hold time of 30 sec. Equilibrium modulus values were obtained by using the slope of equilibrium stress values, computed during each hold section, versus respective strain values, based on a linear fit model.
Ex vivo inflammatory model
An ex vivo model stimulating early OA like conditions was designed by removing aggrecan from cartilage explants. Freshly harvested explants were cultured for three days in DMEM/F-12 medium (50 μg/mL ascorbic acid 2-phosphate, 100 μg/mL sodium pyruvate, 0.1% bovine serum albumin, 100 units/mL penicillin, 100μg/mL streptomycin and 25 mM HEPES) media supplemented with 5 % FBS27. Native aggrecan was removed from cartilage explants using a trypsin treatment. A Live-Dead assay (Invitrogen) was used to assess cell viability after trypsin treatment. Following trypsin treatment, molecules were diffused through aggrecan depleted cartilage explants as described earlier. The explants were cultured for three weeks. Experimental conditions were: 1) Normal cartilage, 2) aggrecan depleted cartilage (AD), 3) AD+mAGC, 4) AD+mAG(II)C and 5) AD+mAGC+mAG(II)C. Culture medium was replaced every two days for 21 days. Media aliquots collected were stored at −80 °C for fragmentation assessment. After culture period explants were stored in RNA later solution (Ambion) at −80 °C for protein and gene assessment.
ECM fragmentation assessment
Glycosaminoglycan degradation was monitored by measuring CS released every two days in cell culture media using the dimethylmethylene blue (DMMB) dye assay and computed with a chondroitin-6-sulfate standard curve28. CS degradation is reported as day to day measurement as well as cumulative release over a seven day culture period.
Real-time PCR
Quantitative real-time polymerase chain reaction (rtPCR) was used to assess anabolic and catabolic mRNA expression. Due to known zonal variations in cartilage endogenous gene expression only the superficial zone of each explant was used for analysis 29. A slice was taken from the top 10–20 % of the tissue to represent the superficial zone of cartilage30. Total mRNA from this zone was extracted using Nucleospin RNA II (Clontech) according to manufacturer’s protocols. Extracted mRNA from all samples was quantified using Nanodrop 2000 spectrophotometer (Thermo Scientific) and reverse transcribed into cDNA using High Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems). Real-time PCR was performed using Taqman Gene Expression Assays (Applied Biosystems) with the primers for the following genes: GAPDH (Bt03210913_g1), aggrecan (Bt03212186_m1), collagen type II (Bt03251861_m1), MMP-13(Bt03214050_m1) and ADAMTS5(Bt04230783_m1). 40 ng of cDNA template was prepared per 20 μL reaction for the genes of interest and the endogenous gene. Real-time PCR analysis was carried out using a Taqman PCR Master Mix and 7500 Real-Time PCR System (Applied Biosystems). Data was analyzed using the comparative CT method, where average CT values were first normalized to GAPDH gene expression followed by relative quantification to the positive control (normal cartilage).
Joint residence pilot study
The study was approved by the institutional animal care committee of Purdue University.
The best performing molecule based on results obtained thus far was injected into the joint space of 5 month old male Dunkin Hartley guinea pigs (average weight = 1139 ± 46 g, n=3). The guinea pigs were anesthetized with 1–5 % isofluorane in oxygen (~3–5 mL/min). Treatment with either 100μL of 10μM mAGCb or 100μL 1X PBS was administered as an intra-articular injection to either the right or left knee joint through the patellar tendon using a 29GX1/2″ needle (Covidien). Animals were euthanized after a time point of 6 hours and 1 week using 1–5 % isofluorane followed by an overdose of Euthasol. The femoral condyle of each joint was isolated. A mid sagittal cut was made through the condyles to harvest the medial and lateral sections. The sections were placed into a plastic embedding tray containing O.C.T and frozen by placing the tray into a solution of isopentane and dry ice. 15 μm sections of the frozen tissue were taken using a cryotome. Prior to staining, the slices were fixed in 4 % paraformaldehyde for 5 minutes followed by a blocking step with a solution containing 10 % donkey serum, 0.2 % Triton X-100 and 0.02 % sodium azide for 5 minutes. The slices were then stained with DAPI (1:500) and Streptavidin Alexa Fluor 555 (1:200) for 30 minutes followed by 3X rinsing with 1X PBS for 5 minutes each. The tissue was imaged using a fluorescent microscope (Leica).
Statistical analysis
Statistical significance was analyzed with a one way ANOVA followed by a post-hoc pairwise comparison (Scheffe’s Test, α= 0.05). Each experiment has been repeated twice, with at least n=5 in each data set.
Results
Diffusion of molecules through cartilage explant
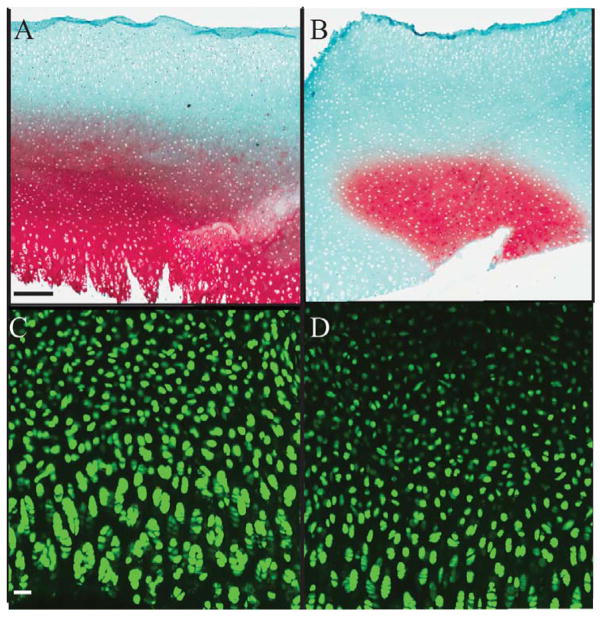
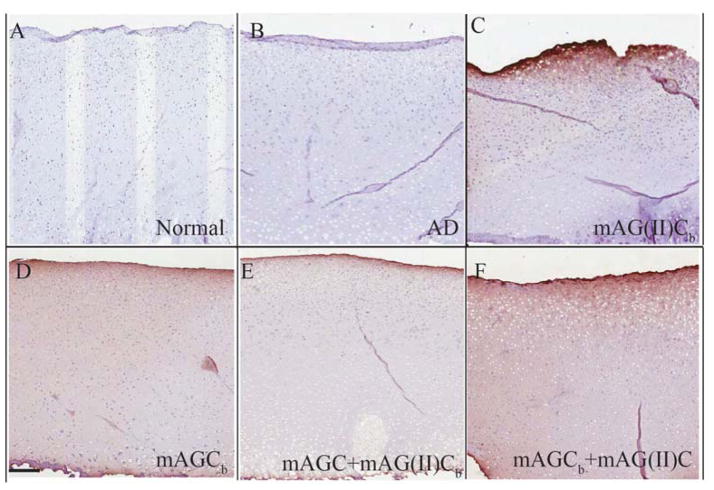
The trypsin treatment effectively removed native aggrecan without harming chondrocytes in the cartilage explants (Figure 1). Effectiveness of aggrecan removal using trypsin was determined by staining with Safranin O & Fast Green stains which bind to glycosaminoglycans in cartilage (Figure 1A&B). Aggrecan depleted matrices showed <50% staining for glycosaminoglycans (red stain) as compared to normal cartilage (Figure 1B). Survival of the explants post trypsin treatment was determined by staining with Live (green stain)-Dead (red stain) Assay (Figure 1C&D). The lack of red staining in the explants confirms survival of chondrocytes in the explants (Figure 1D). The extent of diffusion of the molecules through the cartilage explant depended on the type of binding ligand used (Figure 2). Collagen type II-binding mAG(II)C, diffused approximately 150 μm into the cartilage matrix and showed a strong affinity for the articular surface of the explant. Hyaluronan binding, mAGC, diffused throughout the entire tissue section. Addition of both molecules (mAGC+mAG(II)C) to the same explant resulted in a lower staining intensity compared to individual diffusion of the proteoglycans. Normal and aggrecan depleted controls show no specific binding to streptavidin-HRP.
Figure 1.
Trypsin treatment sufficiently removes native aggrecan from bovine ex vivo tissue. Safranin O & Fast Green staining (red) for GAG in bovine explants indicates <50% of residual GAG remains in aggrecan depleted matrices (Figure 1B) as compared to normal cartilage (Figure 1A). Live (green)-dead (red) stain of chondrocytes in the explant demonstrate living cells (green) in both explants indicating no death post trypsin treatment (Figure 1C, D). Scale bar for Figure A&B: 200μm, Figure C&D: 50 μm
Figure 2.
Diffusion of molecules through bovine cartilage explant. Streptavidin HRP counter stained with diaminobenzidine was used on all experimental groups to probe for biotin labeled mAG(II)Cb (collagen type II binding) (figure 2C), mAGCb (HA binding) (figure 2D), mAGC + mAG(II)Cb (figure 2E) and mAGCb + mAG(II)C (figure 2F) diffused through aggrecan depleted cartilage explants. Normal cartilage (figure 2A) and aggrecan depleted cartilage (figure 2B) were treated with 1X PBS. Images represent the mid sagittal section of the explant and represent diffusion from articular surface towards the deeper zone. Scale bar 200 μm
Bulk compression testing
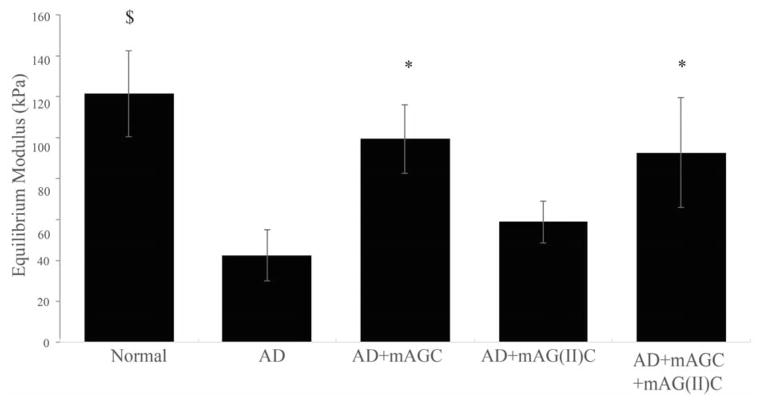
The molecule mAGC significantly restored the equilibrium modulus of aggrecan depleted cartilage explants (p<0.05) (Figure 3). The modulus of the mAGC augmented explants were statistically similar to that of normal cartilage explants (p>0.05). Similarly, addition of both molecules (mAGC+mAG(II)C) showed a significantly larger increase in modulus as compared to aggrecan depleted explants. The addition of mAG(II)C on its own did not restore modulus values. Aggrecan depleted cartilage contructs had significantly lower moduli as compared to normal cartilage (p<0.005). The average modulus values ranged from 42.41 ± 12.49 kPa, 99.36 ±16.85 kPa, 58.69±10.32 kPa and 92.62±26.74 kPa for aggrecan depleted, mAGC augmented, mAG(II)C augmented and mAGC+mAG(II)C augmented contructs respectively.
Figure 3.
The addition of chondroitin sulfate augmented molecules in aggrecan depleted (AD) explants increases equilibrium modulus. Addition of mAGC (HA binding) significantly restores stiffness of cartilage explants to a higher extent as compared to mAG(II)C (collagen type II binding). Significance, denoted as *, specifies an increase in equilibrium modulus for AD+mAGC and AD+mAGC+mAG(II)C when compared to AD explants (p<0.005). Significance, denoted as $, specifies a statistical difference between Normal and AD explants. Data is presented as mean± SEM (n=5).
ECM fragmentation assessment
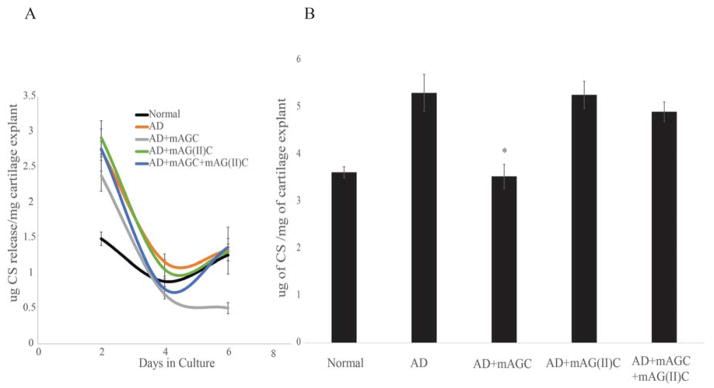
The amount of CS released into cell culture media decreased consdierably over the course of the 21-day culture period. The cumulative amount of CS seen in culture media over a period of 7 days was used for assessment. The amount of CS released from the ECM into the culture was significantly lower for explants that were treated with mAGC as compared to explants that were aggrecan depleted and left untreated (p<0.01). No significant difference was seen for explants treated with mAG(II)C or mAGC+mAG(II)C (Figure 4).
Figure 4.
CS released into cell culture media over a seven day culture period. CS release was measured every two days (Figure 4A) and measured as a culmination of seven day culture period (Figure 4B). CS loss was measured by a DMMB assay. The addition of mAGC (HA binding), has a significant effect on preventing loss of CS from the scaffolds (p<0.01). * denotes statistical significance between explants treated with mAGC after aggrecan depletion and explants left untreated. Bars represent average ± SEM (n=9).
Real-time PCR Analysis
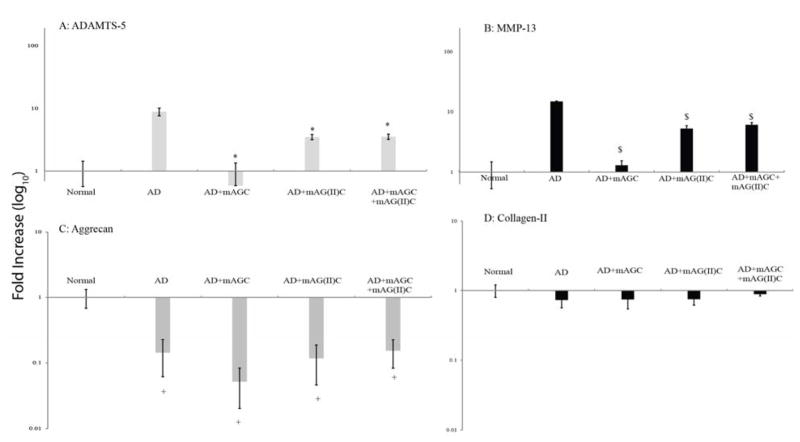
Expression of MMP-13 and ADAMTS-5 increased in aggrecan depleted explants (Figure 5). The addition of mAGC, mAG(II)C and mAGC+mAG(II)C to aggrecan depleted explants significantly lowered ADAMTS-5 and MMP-13 expression (p<0.0001). There is no statistical difference in MMP-13 expression between the three treatment groups. However, ADAMTS-5 expression in AD+mAGC is significantly different from the other two treatment groups (p<0.05). Expression of collagen type II mRNA showed no significant difference and remained the same for all explants, whereas aggrecan expression significantly decreased for all groups as compared to normal cartilage (p<0.05) (Figure 5).
Figure 5.
Real-time PCR analysis for catabolic (ADAMTS-5 and MMP-13) and anabolic (Aggrecan and Collagen) expressed by chondrocytes in superficial zone of cartilage explants. Values have been normalized to endogenous GAPDH expression. A fold increase was quantified by normalizing to positive control (normal cartilage). Addition of mAGC (HA binding), mAG(II)C (collagen type II binding) and combination of mAGC+mAG(II)C to aggrecan depleted explants significantly lowers the expression of both catabolic genes (p<0.0001). Significance denoted as * (Figure 5A) and $ (Figure 5B) were compared to expression seen in aggrecan depleted explants. Bars represent average ±SEM(n=7). Anabolic gene expression in aggrecan depleted explants and those treated with the various molecules showed a significant decrease in aggrecan expression (Figure 5C). There is no difference in collagen type II expression (Figure 5D). Significance denoted as + and is compared to expression seen in normal cartilage explants Bars represent average ±SEM (n=5).
Joint residence pilot study
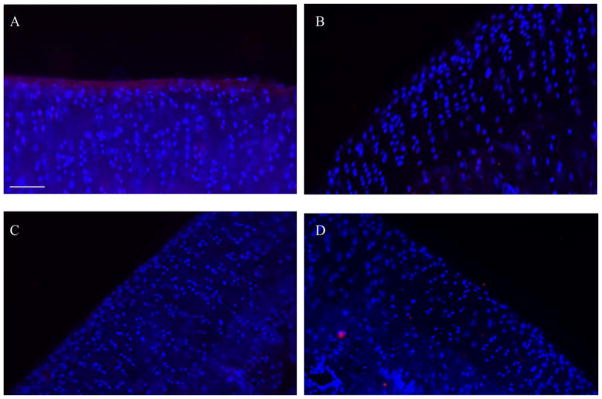
Since mAGCb had shown the most promising results, this mimic was injected into the Dunkin Hartley guinea pig model. After 6 hours, mAGCb is still clearly seen adhered to the surface of cartilage as indicated by the streptavidin counterstain (red, Figure 6). No streptavidin staining is seen in cartilage tissue from the knee that received 1X PBS. No mAGCb staining is noted in either joint of the animals sacrificed at 1 week time point.
Figure 6. In vivo localization of mAGCb on articular cartilage surface.
Animals were injected with mAGCb (HA binding) or 1X PBS and euthanized 6 hours or 1 week after injection. Tissue stained with streptavidin 555 (red) and DAPI (blue). Streptavidin staining on cartilage joints injected with mAGCb is evident on surface after 6 hours (Figure 7A) as compared to the control groups injected with 1X PBS and stained similarly (Figure 7B). After 1 week, joint surface does not show presence of mAGCb (Figure 7C) and is similar to the control group injected with 1X PBS (Figure 7D) Images shown are representative of n= 3 samples where each animal received a treatment and control. Scale bar =75μm
Discussion
While investigating therapeutics that will suppress OA symptoms, numerous studies on intra-articular injections of non-steroidal anti-inflammatory drugs have reported limited retention in synovial fluid and joint space1–2, 31. Discovery of peptides that bind to specific ECM components of cartilage have paved the path for developing therapies that can be retained within the matrix. Here, we utilize bio-molecular ligands that bind to HA and collagen type II to design and deliver molecules that adhere to an aggrecan depleted cartilage matrix. The design, synthesis and characterization of several combinations of these molecules and their ability to resist in vitro proteolytic degradation has been previously described by our laboratory21–23, 32. We have also demonstrated the use of non-degradable nanoparticles to selectively deliver cell penetrating anti-inflammatory peptides to ‘inflamed’ cartilage explants33. As a potential complementary therapeutic to the anti-inflammatory peptide system, in this present study we describe the use of these molecules in ex vivo and in vivo OA models, and extrapolate their potential as an intra articular therapy.
Trypsin treatment to remove aggrecan has been used by other researchers to study diffusion of molecules through cartilage tissue and as an ex vivo model for osteoarthritis34–35. In this study we utilized this platform to explore the extent of diffusion of molecules specifically designed to adhere to cartilage components, to create an ex vivo model of early stage OA, and to evaluate the efficacy of the molecules in such an environment. While a complete characterization of cellular activity after trypsin treatment was not performed, the viability of chondrocytes was examined. Histological staining of the explants indicated that greater than 50 % of the aggrecan had been removed, making this model ideal to test the diffusion of our molecules. Enzymatic degradation of cartilage tissue has been used before to study mechanical changes that occur in the tissue at early stages of OA, making this a good model to study the ability of the molecules to diffuse and restore compressive stiffness. Although the use of trypsin digested explants for cell culture studies does not adequately reflect real OA like conditions, the model allows one to monitor the protein and genetic consequences of lack of aggrecan in an explant culture. In a previous study where we treated cartilage explants with synovial fluid from OA patients, we noted a sharp decrease in amount of CS seen in culture media as well as a decrease in ADAMTS expression when explants were treated with mAGC24. These results are consistent with those seen in this present trypsin treated cartilage explant study. To adequately capture early stages of OA in an explant culture, use of pro-inflammatory cytokines, such as IL-1β can be used and will be explored in future studies.
Transport of molecules through cartilage, an avascular tissue, primarily occurs by passive diffusion36. Further, the diffusion of molecules is controlled by permeability of the tissue, which in turn depends on the fixed charge density (or total GAG content) of the tissue37. Removal of proteoglycans from a cartilage matrix decreases the fixed charge density and allows for a higher diffusion coefficient38. Here, we report diffusion of two separate molecules (mAGC and mAG(II)C), each approximately 40 kDa. The extent of diffusion of the molecules depended on the binding ligand and its proximity to the articular surface. For instance, the collagen type II-binding molecule was retained primarily at the surface of articular cartilage. The upper zone of cartilage is known to have the highest concentration of densely packed collagen type II fibers39. The tight collagen type II spacing and the molecule’s affinity to its binding ligand work to retained mAG(II)C near the articular surface. Conversely, the concentration of HA in articular cartilage increases from surface to the deep zone40 and the ligand mAGC diffused further into the tissue to bind to HA. This difference in diffusion, based on the heterogeneity of cartilage’s composition, has also been shown with other similarly sized molecules41. Simultaneous diffusion of both molecule’s through the same explant showed similar results where when mAG(II)C is biotin labeled, the edges appear darker, whereas when mAGC is biotin labeled the molecule appears to be diffused throughout. The diffusion of both molecule’s through the same explant appeared to have resulted in lower staining compared to individual. While both molecules are approximately 40 kDa in size, their ligand specificity and collective high negative charge may have hindered adequate diffusion. Diffusion of the molecules with aggrecan present, such as seen in healthy cartilage, has not been tested as this situation is not envisioned to merit the need for an intra articular treatment. Other studies of molecular diffusion through cartilage have shown effective diffusion of uncharged molecules up to 500 kDa41.
A second aspect of this study was to test the ability of the designed molecules to restore compressive stiffness of cartilage explants. The presence of aggrecan in cartilage ECM is known to provide a high compressive stiffness to the tissue4. Bovine cartilage explants tested under unconfined compression have shown stiffness values ranging from 0.1–0.5MPa42. Loss in proteoglycan content causes a decrease in compressive stiffness and an increase in tissue permeability, resulting in rapid degradation43. The incorporation of chondroitin sulfate augmented molecules into aggrecan depleted matrices increased the compressive stiffness of the explant depending on the extent of their diffusion. mAGC, diffused through the entire tissue section and significantly restored compressive strength, whereas mAG(II)C bound to the surface of the tissue and did not significantly change the equilibrium modulus. When both mAGC+mAG(II)C were diffused, the modulus increased significantly as compared to untreated trypsin treated tissue explants. These results are consistent with the diffusion images, where addition of mAGC and mAGC+mAG(II)C showed the darkest staining, indicating these molecules were able to diffuse and restore the mechanical properties to the original value. In this aggrecan depleted model we have shown the ability to restore modulus values using mAGC to statistically similar values seen in normal cartilage explants. The ability of these molecules to enhance compressive stiffness of cartilage at different levels of aggrecan depletion using this OA model has yet to be determined.
The ability of chondroitin sulfate augmented with HA-binding or collagen type II-binding peptides to resist degradation and promote repair of cartilage ECM components was tested by culturing cartilage explants for three weeks. Since media aliquots past seven days showed negligible amount of CS, only data from the first seven days were used. Aggrecan depleted explants showed a loss of CS on Day 2 of the culture. Of the three different molecules tested, the presence of mAGC after aggrecan depletion significantly reduced the fragmentation of CS into cell culture media by Day 4. The addition of mAGC+mAG(II)C did not reduce CS release into the media. Based on our diffusion and mechanical results the combination of mAGC+mAG(II)C had shown statistically similar results to mAGC alone. It is speculated this inefficiency could have risen due to the fact that the combination treatment consists of twice the concentration of CS added since both mAGC and mAG(II)C were added. In our previous work with mAGC we noted the mimic’s ability to prevent collagen and HA degradation in tissue analogs as well as the ability to lower CS degradation from collagen analogs21, 23. These observations support the hypothesis that a biomimetic less susceptible to degradation prevents loss of ECM components. Large fragments of the cartilage ECM, such as CS, have been collected in synovial fluid of OA patients and have shown to instigate inflammation in the joint by perpetuating pro inflammatory mediators12, 44. It is anticipated that the ability of mAGC to retard fragmentation of CS will provide a protective environment for regeneration and repair. In future studies we will directly examine the ability of this mimic to prevent loss of HA and type II collagen from explants.
The catabolic genetic expressions seen in our experimental setup resulted in two interesting findings. First, the removal of aggrecan resulted in a sharp increase in MMP-13 and ADAMTS-5, highlighting the transcriptional consequences of lack of aggrecan during early stages of OA. Secondly, incorporation of augmented chondroitin sulfate molecules into aggrecan depleted matrices drastically reduced catabolic gene expression, showing their ability to recoup from the deleterious effects of aggrecan loss and ECM degradation. The results from anabolic gene expressions in this experimental setup also highlighted some interesting findings. Gene expressions in aggrecan depleted explants, depicting an earlier stage of OA, showed a constant expression of collagen type II but a lowered aggrecan expression. These results seem to suggest that in very early stage OA collagen type II expression is not yet affected, while the propensity of chondrocytes to express aggrecan is lowered. Further this data suggests that as ECM degradation progresses the ability of cells to express aggrecan remains stunted despite treatment with augmented chondroitin sulfate molecules. While it is difficult to accurately discern whether this reduction in anabolic expression is the result of ECM-based degradation leading to lower compensation by the chondrocytes, the reduction is an artifact of trypsin treatment, or the presence of mAGC signals sufficient aggrecan presence, the data suggests that future studies should look into alternate strategies to promote anabolic activity including differing chondroitin sulfate concentration, or combination of mAGC with another therapeutic. Further, while we did not measure the expression of inflammatory mediators that could have risen due to trypsin treatment, it is possible that the lack of increase in anabolic genes in our experiments were a result of the ex vivo OA model utilized. Pro-inflammatory cytokines have been shown to block the pathway for synthesis of new ECM components and decrease cartilage biosynthesis by diminishing the effect of anabolic factors such as insulin-like-growth-factor-1 (IGF-1) or transforming growth factor β (TGF- β)45–46. Future studies will look into using treatment with IL-1 β as an in vitro model for OA. This will discern whether treatment with mAGC promotes or hinders synthesis of new aggrecan. To enhance growth and biosynthesis in our future studies an anabolic stimulant, such as TGF-β, might also be considered in the molecule’s design.
The delivery of mAGC was assessed in a well-established in vivo model for OA. Dunkin-Hartley guinea pigs are known to show OA related changes in the joint as early as 2 months of age. It is known that the severity of the OA lesions in this animal model increases steadily with age and by 12 months severe degeneration of the condyles and meniscus is noted47–48. In this light, at 5 months of age the guinea pigs tested for our study perhaps were in a mild-moderate stage of OA. However, the development of mAGC as an intra-articular injection is intended to be tested clinically at mild-moderate stages of OA. In this pilot study, the primary objective was to test whether mAGC had the ability to adhere to cartilage tissue and remain attached in an ambulatory joint. We noted that despite mild-moderate OA like environment, mAGC remained attached at 6 hours, but was not present at 1 week time point. A 1 week time point was employed to replicate injection frequencies performed clinically. A future study at 24–72 hour time point study would help determine the peptide’s kinetics at shorter time intervals. mAGC’s ability to adhere to severe OA tissue or normal cartilage in vivo has yet to be tested. An effective intra-articular therapy for OA is deemed to require some residence time in the joint. Various NSAIDs injected into the joint space have shown residence time to be as short as 1–5 hours2. Intra-articular injections of hyaluronic acid appear to decrease in intensity starting at 48 hours up to 28 days in goat OA models. These studies indicate joint clearance is affected by molecular weights with smaller molecules being removed more rapidly via mechanisms involving lymphatic transport whereas larger molecules appear to be removed via phagocytosis2, 49. However, as the possible biological mechanisms which may be involved after receipt of these treatments remain unknown, we also do not know whether enhancing residence time of these treatments will provide prolonged analgesic and disease modifying effects. For future time point studies we will monitor the effect of prolonged mAGC joint residence times and its ability to act as a disease modifying agent.
Conclusion
In this study we diffused two chondroitin sulfate molecules augmented with HA or collagen type II binding peptides alone, or in combination, through an aggrecan depleted cartilage explant and monitored their ability to restore strength, prevent degradation and promote biosynthesis. Our findings showed that of the three treatments, chondroitin sulfate augmented with HA-binding peptides was able to diffuse through the entire explant, significantly restore compressive stiffness bringing the modulus value similar to native tissue and reduce catabolic gene expression. Our results also highlight the importance of the presence of aggrecan in promoting aggrecan expression. Of all molecules tested, chondroitin sulfate augmented with HA-binding peptides showed the highest ability of inhibiting CS degradation and preventing the rise of catabolic gene expression. The HA-binding molecule also demonstrated adherence to cartilage tissue in an ambulatory joint in vivo. These results indicate chondroitin sulfate augmented with HA-binding peptides can serve well as an anti-inflammatory agent that adheres to and protects the ECM of cartilage. However, to promote matrix biosynthesis, this molecule may need to be coupled with an anabolic stimulant.
Acknowledgments
Funding source for all authors: Supported by the Purdue University Faculty Scholars Fund and the National Institutes of Health National Institute of Arthritis and Musculoskeltal and Skin Disorders (NIAMS) through R01AR065398. The funding source did not play any role in preparation of the manuscript.
Footnotes
Author contributions: All authors have contributed to acquisition and analysis of data and were involved in drafting, revising and final approval of the manuscript.
Conflict of interest: Dr. Panitch is a Founder of Symic Biomedical. Symic Biomedical has licensed the rights to develop the proteoglycan mimics described herein.
References
- 1.Gerwin N, Hops C, Lucke A. Intraarticular drug delivery in osteoarthritis. Adv Drug Deliv Rev. 2006;58(2):226–42. doi: 10.1016/j.addr.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 2.Owen SG, Francis HW, Roberts MS. Disappearance kinetics of solutes from synovial fluid after intra-articular injection. British journal of clinical pharmacology. 1994;38(4):349–55. doi: 10.1111/j.1365-2125.1994.tb04365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knudson CB, Knudson W. Cartilage proteoglycans. Seminars in cell & developmental biology. 2001;12(2):69–78. doi: 10.1006/scdb.2000.0243. [DOI] [PubMed] [Google Scholar]
- 4.Mow VC, Ratcliffe A, Poole AR. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13(2):67–97. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 5.Muir H. Proteoglycans as organizers of the intercellular matrix. Biochem Soc Trans. 1983;11(6):613–22. doi: 10.1042/bst0110613. [DOI] [PubMed] [Google Scholar]
- 6.Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–52. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 7.Fosang AJ, Rogerson FM, East CJ, Stanton H. ADAMTS-5: the story so far. European cells & materials. 2008;15:11–26. doi: 10.22203/ecm.v015a02. [DOI] [PubMed] [Google Scholar]
- 8.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Critical reviews in oral biology and medicine : an official publication of the American Association of Oral Biologists. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 9.Ellis AJ, Curry VA, Powell EK, Cawston TE. The prevention of collagen breakdown in bovine nasal cartilage by TIMP, TIMP-2 and a low molecular weight synthetic inhibitor. Biochem Biophys Res Commun. 1994;201(1):94–101. doi: 10.1006/bbrc.1994.1673. S0006-291X(84)71673-1 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Kozaci LD, Buttle DJ, Hollander AP. Degradation of type II collagen, but not proteoglycan, correlates with matrix metalloproteinase activity in cartilage explant cultures. Arthritis and rheumatism. 1997;40(1):164–74. doi: 10.1002/art.1780400121. [DOI] [PubMed] [Google Scholar]
- 11.Pratta MA, Yao W, Decicco C, Tortorella MD, Liu RQ, Copeland RA, Magolda R, Newton RC, Trzaskos JM, Arner EC. Aggrecan protects cartilage collagen from proteolytic cleavage. The Journal of biological chemistry. 2003;278(46):45539–45. doi: 10.1074/jbc.M303737200. [DOI] [PubMed] [Google Scholar]
- 12.Lohmander LS, Neame PJ, Sandy JD. The structure of aggrecan fragments in human synovial fluid. Evidence that aggrecanase mediates cartilage degradation in inflammatory joint disease, joint injury, and osteoarthritis. Arthritis and rheumatism. 1993;36(9):1214–22. doi: 10.1002/art.1780360906. [DOI] [PubMed] [Google Scholar]
- 13.Sandy JD, Flannery CR, Neame PJ, Lohmander LS. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular domain. The Journal of clinical investigation. 1992;89(5):1512–6. doi: 10.1172/JCI115742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fosang AJ, Hardingham TE. Isolation of the N-terminal globular protein domains from cartilage proteoglycans. Identification of G2 domain and its lack of interaction with hyaluronate and link protein. The Biochemical journal. 1989;261(3):801–9. doi: 10.1042/bj2610801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klatt AR, Paul-Klausch B, Klinger G, Kuhn G, Renno JH, Banerjee M, Malchau G, Wielckens K. A critical role for collagen II in cartilage matrix degradation: collagen II induces pro-inflammatory cytokines and MMPs in primary human chondrocytes. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2009;27(1):65–70. doi: 10.1002/jor.20716. [DOI] [PubMed] [Google Scholar]
- 16.Schmitz I, Ariyoshi W, Takahashi N, Knudson CB, Knudson W. Hyaluronan oligosaccharide treatment of chondrocytes stimulates expression of both HAS-2 and MMP-3, but by different signaling pathways. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 18(3):447–54. doi: 10.1016/j.joca.2009.10.007. S1063-4584(09)00279-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiani C, Chen L, Wu YJ, Yee AJ, Yang BB. Structure and function of aggrecan. Cell research. 2002;12(1):19–32. doi: 10.1038/sj.cr.7290106. [DOI] [PubMed] [Google Scholar]
- 18.Mummert ME, Mohamadzadeh M, Mummert DI, Mizumoto N, Takashima A. Development of a peptide inhibitor of hyaluronan-mediated leukocyte trafficking. The Journal of experimental medicine. 2000;192(6):769–79. doi: 10.1084/jem.192.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothenfluh DA, Bermudez H, O’Neil CP, Hubbell JA. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nature materials. 2008;7(3):248–54. doi: 10.1038/nmat2116. [DOI] [PubMed] [Google Scholar]
- 20.Singh A, Corvelli M, Unterman SA, Wepasnick KA, McDonnell P, Elisseeff JH. Enhanced lubrication on tissue and biomaterial surfaces through peptide-mediated binding of hyaluronic acid. Nature materials. 2014;13(10):988–95. doi: 10.1038/nmat4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernhard JC, Panitch A. Synthesis and characterization of an aggrecan mimic. Acta biomaterialia. 2012;8(4):1543–50. doi: 10.1016/j.actbio.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 22.Stuart K, Paderi J, Snyder PW, Freeman L, Panitch A. Collagen-binding peptidoglycans inhibit MMP mediated collagen degradation and reduce dermal scarring. PloS one. 2011;6(7):e22139. doi: 10.1371/journal.pone.0022139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma S, Panitch A, Neu CP. Incorporation of an aggrecan mimic prevents proteolytic degradation of anisotropic cartilage analogs. Acta biomaterialia. 2013;9(1):4618–25. doi: 10.1016/j.actbio.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 24.Sharma S, Lee A, Choi K, Kim K, Youn I, Trippel SB, Panitch A. Biomimetic aggrecan reduces cartilage extracellular matrix from degradation and lowers catabolic activity in ex vivo and in vivo models. Macromolecular bioscience. 2013;13(9):1228–37. doi: 10.1002/mabi.201300112. [DOI] [PubMed] [Google Scholar]
- 25.Paderi JE, Panitch A. Design of a synthetic collagen-binding peptidoglycan that modulates collagen fibrillogenesis. Biomacromolecules. 2008;9(9):2562–6. doi: 10.1021/bm8006852. [DOI] [PubMed] [Google Scholar]
- 26.Poole AR, Pidoux I, Reiner A, Tang LH, Choi H, Rosenberg L. Localization of proteoglycan monomer and link protein in the matrix of bovine articular cartilage: An immunohistochemical study. J Histochem Cytochem. 1980;28(7):621–35. doi: 10.1177/28.7.6156200. [DOI] [PubMed] [Google Scholar]
- 27.Niikura T, Reddi AH. Differential regulation of lubricin/superficial zone protein by transforming growth factor beta/bone morphogenetic protein superfamily members in articular chondrocytes and synoviocytes. Arthritis and rheumatism. 2007;56(7):2312–21. doi: 10.1002/art.22659. [DOI] [PubMed] [Google Scholar]
- 28.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochimica et biophysica acta. 1986;883(2):173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 29.Eleswarapu SV, Leipzig ND, Athanasiou KA. Gene expression of single articular chondrocytes. Cell and tissue research. 2007;327(1):43–54. doi: 10.1007/s00441-006-0258-5. [DOI] [PubMed] [Google Scholar]
- 30.Darling EM, Athanasiou KA. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2005;23(2):425–32. doi: 10.1016/j.orthres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 31.St Onge RA, Dick WC, Bell G, Boyle JA. Radioactive xenon (133Xe) disappearance rates from the synovial cavity of the human knee joint in normal and arthritic subjects. Ann Rheum Dis. 1968;27(2):163–6. doi: 10.1136/ard.27.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paderi JE, Stuart K, Sturek M, Park K, Panitch A. The inhibition of platelet adhesion and activation on collagen during balloon angioplasty by collagen-binding peptidoglycans. Biomaterials. 2011;32(10):2516–23. doi: 10.1016/j.biomaterials.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 33.Bartlett RL, 2nd, Sharma S, Panitch A. Cell-penetrating peptides released from thermosensitive nanoparticles suppress pro-inflammatory cytokine response by specifically targeting inflamed cartilage explants. Nanomedicine : nanotechnology, biology, and medicine. 2012 doi: 10.1016/j.nano.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JI, Sato M, Ushida K, Mochida J. Measurement of diffusion in articular cartilage using fluorescence correlation spectroscopy. BMC biotechnology. 2011;11:19. doi: 10.1186/1472-6750-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Millroy SJ, Poole AR. Pig articular cartilage in organ culture. Effect of enzymatic depletion of the matrix on response of chondrocytes to complement-sufficient antiserum against pig erythrocytes. Annals of the rheumatic diseases. 1974;33(6):500–8. doi: 10.1136/ard.33.6.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Hara BP, Urban JP, Maroudas A. Influence of cyclic loading on the nutrition of articular cartilage. Ann Rheum Dis. 1990;49(7):536–9. doi: 10.1136/ard.49.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maroudas A. Biophysical chemistry of cartilaginous tissues with special reference to solute and fluid transport. Biorheology. 1975;12(3–4):233–48. doi: 10.3233/bir-1975-123-416. [DOI] [PubMed] [Google Scholar]
- 38.Torzilli PA, Arduino JM, Gregory JD, Bansal M. Effect of proteoglycan removal on solute mobility in articular cartilage. Journal of biomechanics. 1997;30(9):895–902. doi: 10.1016/s0021-9290(97)00059-6. [DOI] [PubMed] [Google Scholar]
- 39.Comper WD. Physicochemical aspects of cartilage extracellular matrix. In: Hall B, Newman S, editors. Cartilage: Molecular Aspects. Boston: CRC Press; 1991. pp. 59–96. [Google Scholar]
- 40.Parkkinen JJ, Hakkinen TP, Savolainen S, Wang C, Tammi R, Agren UM, Lammi MJ, Arokoski J, Helminen HJ, Tammi MI. Distribution of hyaluronan in articular cartilage as probed by a biotinylated binding region of aggrecan. Histochemistry and cell biology. 1996;105(3):187–94. doi: 10.1007/BF01462291. [DOI] [PubMed] [Google Scholar]
- 41.Leddy HA, Guilak F. Site-specific molecular diffusion in articular cartilage measured using fluorescence recovery after photobleaching. Annals of biomedical engineering. 2003;31(7):753–60. doi: 10.1114/1.1581879. [DOI] [PubMed] [Google Scholar]
- 42.Korhonen RK, Laasanen MS, Toyras J, Rieppo J, Hirvonen J, Helminen HJ, Jurvelin JS. Comparison of the equilibrium response of articular cartilage in unconfined compression, confined compression and indentation. Journal of biomechanics. 2002;35(7):903–9. doi: 10.1016/s0021-9290(02)00052-0. [DOI] [PubMed] [Google Scholar]
- 43.Mansour JM, Mow VC. The permeability of articular cartilage under compressive strain and at high pressures. The Journal of bone and joint surgery. American volume. 1976;58(4):509–16. [PubMed] [Google Scholar]
- 44.Struglics A, Larsson S, Pratta MA, Kumar S, Lark MW, Lohmander LS. Human osteoarthritis synovial fluid and joint cartilage contain both aggrecanase- and matrix metalloproteinase-generated aggrecan fragments. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2006;14(2):101–13. doi: 10.1016/j.joca.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 45.Dingle JT. The role of catabolin in the control of cartilage matrix integrity. The Journal of rheumatology Supplement. 1983;11:38–44. [PubMed] [Google Scholar]
- 46.Trippel SB. Growth factor inhibition: potential role in the etiopathogenesis of osteoarthritis. Clinical orthopaedics and related research. 2004;(427 Suppl):S47–52. [PubMed] [Google Scholar]
- 47.Bendele AM, Hulman JF. Spontaneous cartilage degeneration in guinea pigs. Arthritis and rheumatism. 1988;31(4):561–5. doi: 10.1002/art.1780310416. [DOI] [PubMed] [Google Scholar]
- 48.Kraus VB, Huebner JL, DeGroot J, Bendele A. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the guinea pig. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18(Suppl 3):S35–52. doi: 10.1016/j.joca.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jackson DW, Simon TM. Intra-articular distribution and residence time of Hylan A and B: a study in the goat knee. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2006;14(12):1248–57. doi: 10.1016/j.joca.2006.05.015. [DOI] [PubMed] [Google Scholar]