Abstract
Background:
Thyroid carcinoma is the most common malignancy of the endocrine system. Papillary thyroid carcinoma (PTC) is the most common type of thyroid cancer, accounting for 70-90% of well-differentiated thyroid malignancies. Thyroid papillary microcarcinoma is a subtype of papillary carcinoma that included tumors with less than 10mm diameter. As a result of diagnostic methods improvement, prevalence of this tumor is increasing. In this study we reviewed different characteristics of tumor.
Methods:
We searched various factors about this tumor in different databases (PubMed, Ovid, Google scholar, Iran medex and SID databases, from July 2012 until August 2013), after that, the articles were classified. Data of each article were extracted and sorted in tables. Data of each factor in different articles were summarized.
Results:
Etiology, clinical presentation, prognosis, histopathology, follow-up, diagnosis and also age, gender, tumor size and treatment were factors about this tumor described in details here.
Conclusion:
Awareness and better understanding of the characteristics of this tumor and manage it as an individual and valuable tumor can take an effective step in promoting public health practice.
Key Words: Thyroid cancer, papillary thyroid carcinoma, Thyroid Microcarcinoma
Introduction
Thyroid carcinoma is the most common malignancy of the endocrine system with incidence of approximately 9/100,000 per year. These cancers have a spectrum of behavior that ranges from incidentally detected and clinically inconsequential microcarcinomas to aggressive and virtually untreatable anaplastic malignant neoplasms (1, 2).
Most thyroid cancers present as thyroid nodules that are either asymptomatic or associated with local cervical symptoms or adenopathy. Less often, thyroid cancers first present with manifestations of metastatic disease, such as a pulmonary mass or bone pain (1).
Papillary and follicular thyroid cancers arise from follicular epithelium and often retain responsiveness to TSH, produce thyroglobulin, and concentrate iodide. They are distinguished by their histopathologic appearances and characteristic patterns of progression (1).
Papillary thyroid carcinoma is the most common form of thyroid cancer, accounting for 70–90% of well-differentiated thyroid malignancies. Whereas the mean age at diagnosis is 45 yr, papillary thyroid carcinoma does occur in children and increases in incidence with age (1, 2).
Papillary thyroid microcarcinoma (PTMC) is a specific subgroup of papillary thyroid carcinoma (PTC) and defined by WHO on the largest dimension of 1.0 cm or less. Most of PTMC are not detectable at clinical examination and are diagnosed incidentally during pathologic examination of thyroid specimens after surgery for benign thyroid diseases, or in autopsies. Characteristic cytologic features of PTC help make the diagnosis by FNA or after surgical resection; these include psammoma bodies, cleaved nuclei with an "orphan-Annie" appearance caused by large nucleoli, and the formation of papillary structures (2, 3).
Moreover, the increased accuracy of the pathologic thyroid examination, in particular with the thinness and the number of the anatomical slices obtained for thyroid specimens, led to a more frequent pathologic; diagnosis of incidental PTMC. On the other hand, the widespread use and the technical improvement of thyroid ultrasonography and fine-needle aspiration biopsy (FNAB) contributed to an increase in the rate of preoperative diagnosis of PTMC over the last few decades (3).
The advent of sensitive imaging devices has led to an increase in the detection rate of small thyroid cancers (4). Consequent greater access to diagnostic procedures resulted in increased detection of PTMC (5).With the widespread use of ultrasound, the detection rate for small thyroid nodules continues to increase (6). The improvement of fine-needle aspiration biopsy is useful for cancers larger than 10mm, and microcarcinomas may be detected by this method (7).Expanded thyroid ultrasound screening and fine needle aspiration biopsies (FNA), and greater specimen sampling by pathologists, account for some of the rise in PTMC incidence (8).
Thyroid cancer incidence rates have been steadily increasing all over the world including Iran (8-10). This trend has also included higher detection of papillary thyroid microcarcinoma due to the improvement of diagnostic methods. PTMC has a benign behavior with excellent prognosis in most cases (11, 12).
In this article, we reviewed the previous studies about PTMC to promote physicians awareness of this tumor characteristics and methods of treatment in order to diagnose and treat the tumor earlier, which may help to improve the prognosis of PTMC patients and decrease cancer mortality.
Materials and Methods
Search Strategy:
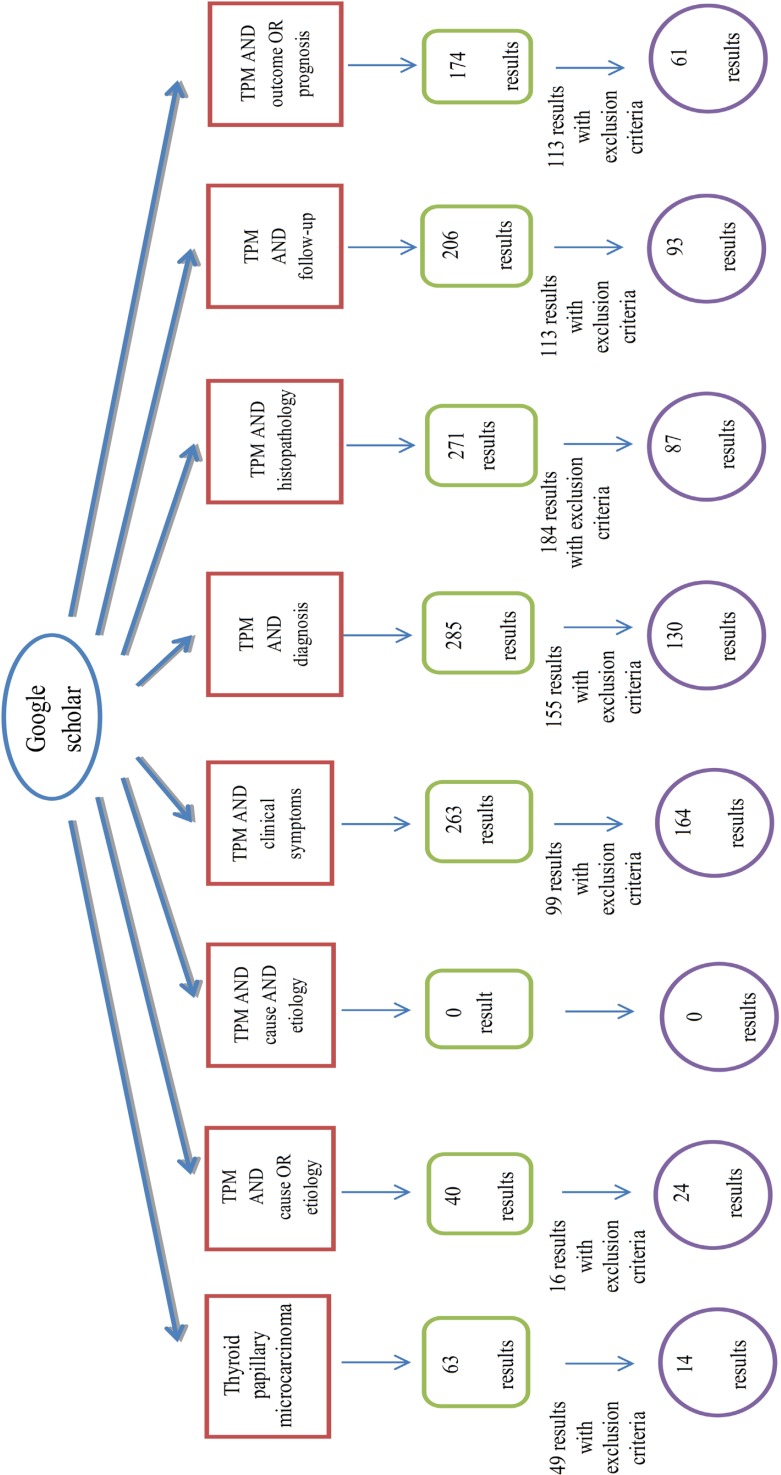
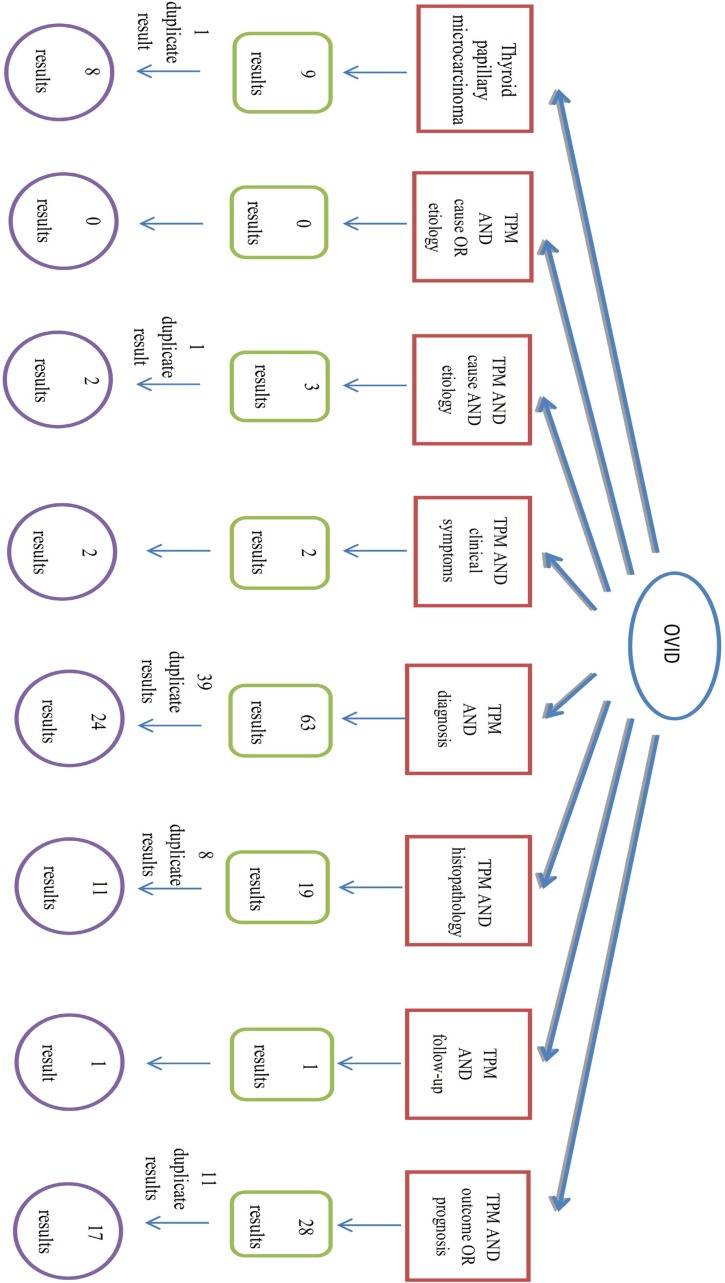
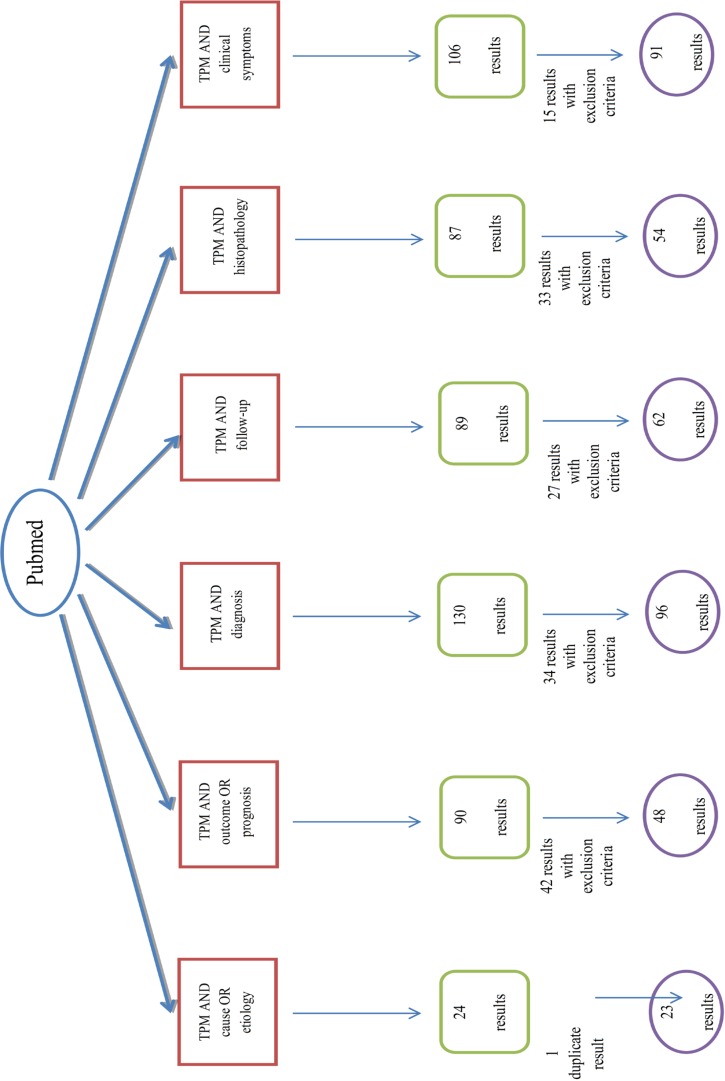
In this systematic review, at first step we used the relevant key words to our title, Thyroid Papillary Microcarcinoma (TPM); TPM AND etiology OR cause; TPM AND histopathology; TPM AND diagnosis; TPM AND clinical presentation OR clinical symptoms; TPM AND follow-up; TPM AND prognosis OR outcome for searching. Articles were searched in PubMed, Ovid, Google scholar, Iran medex and SID databases, July 2012 till August 2013 with no language limitations.
The search strategy is showed completely in Figures 1-5.
Fig. 1.
Algorithm of search in Google scholar database
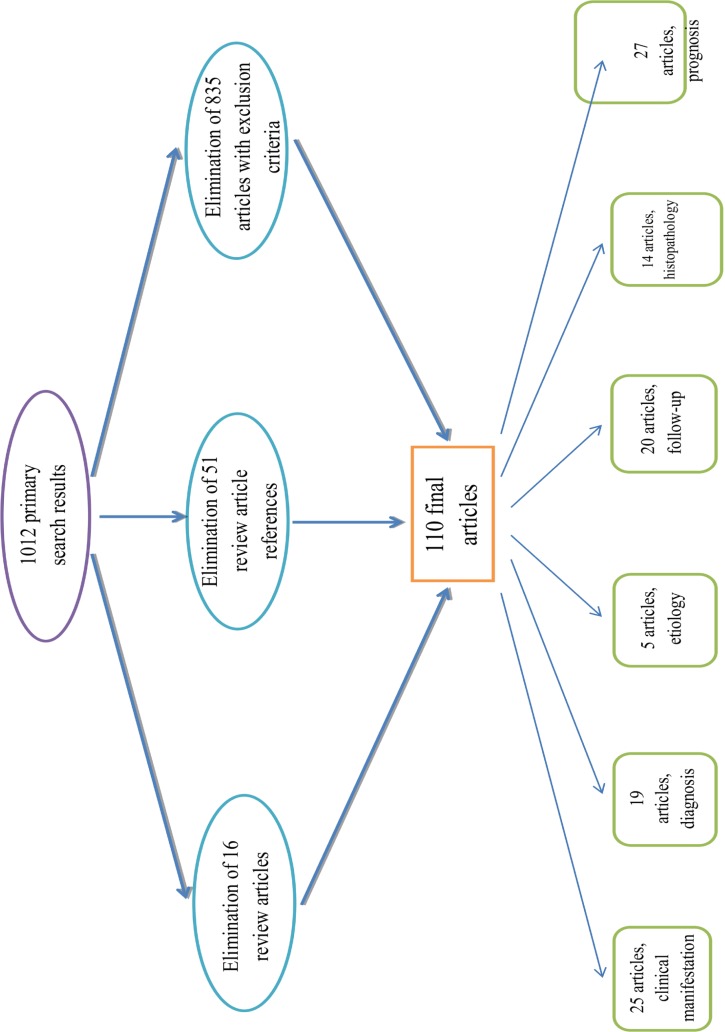
Fig.5.
Algorithm of classification of articles of search
Exclusion Criteria:
We excluded the articles with following criteria: a) Review articles about thyroid papillary microcarcinoma; b) Articles used as reference in review articles of TPM; c) Results which were books not articles; d) The same articles resulted from different databases; e) Articles about other thyroid tumors except TPM; f) and Articles about other TPM characteristics rather than those in this article title.Initially, we found 1012 articles but after implementation of exclusion criteria finally 110 articles were accepted for analysis.
At the next step, we divided articles in accordance with title and the main subject of each article into 6 groups: Etiology with 5 articles, clinical presentation with 25 articles, diagnosis with 19 articles, follow-up with 20 articles, histopathology with 14 articles and prognosis with 27 articles.
Data Extraction:
In this step we inserted each article data about main factors in the title as a table in Excell 2010 software. Each row was about one article and variables were in columns. Variables wereonthese factors as follows:Article title, year, number of TPM patients, age, sex, etiology, size, clinical presentation, prognosis, treatment, diagnosis, follow-upand histopathology. Data of case-report articles were not appropriate, so finally 87 out of 110 articles were sorted in the table.
Fig.2.
Algorithm of search in Ovid database
Fig.3.
Algorithm of search in PubMed database
Fig.4.
Algorithm of search in Iran medex and SID databases
Results:
We assessed 87 articles in our search .Our findings in articles are described below:
Sex: Among 25953 patients of papillary thyroid microcarcinoma (PTMC) in 75 articles(13-17,19,21-30,32,33,36,38,40-43,45-61,63-66,68,70-80,82-88,90-99) which evaluated the gender of patients, 4485 (18 percent) of them were male and 21468 (82 percent) were female. In 4 articles, females were less than males; in only one article male and female number were the same but 70 articles showed the preference of females.
Age: As result of 66 articles(13,15-17,19-21,23-30,32-33,36,38,40,42-43,45-46,48-49,51,53-61,65-68,71-80,82-85,87-92,94-98) which explained the age of their patients, mean age of PTMC patients concluded 47.2 years. The range of their age was between 8 to 88 years. In 29 articles(13-14,17,22,24-25,38,47-48,50,52,54,56,58,60,63-64,66,77-79, 82, 84, 86, 88, 91, 93-97, 99) the patient’s age was divided into 2 groups, one group included patients of 45 years-old or older, other group was patients younger than 45 years. Finally, we found that 7854 (59%) of patients were patients of the first group and 5395 (41%) of them were patients of the other group.
Tumor size: For this factor, one group contained patients with tumors of 5mm or smaller and the other group patients had tumors larger than 5mm, but as the patients had PTMC so all tumors were less than 10 mm in diameter. Overall, 4263 (34%) of patients formed the first group and 8224 (66%) were of the second group. Tumor size ranged between 0.1-10 mm with mean of 5.9 mm as described in 52 articles(13-15,17,19-21,23-26,28,32,35-38,42-43,46-49,51,54-56,58-59,61,64-65,67,71-77,79-80,82,84-85,87,90-92,95-97).
Etiology: For evaluation of this part 2 factors were assessed, “family history of thyroid cancer” and “patient’s history of radiation”. In 6 articles (13,17,24,63,68,99) mean of 19.3% of patients were positive for family history. On the other hand, only one article(32) with mean of 3% represented the history of radiation in patients.
Clinical presentation: Factors for evaluation of this part are described below; a) Presentation of autoimmune thyroid diseases: 3 articles(24,25,88) had this presentation in mean of 35.6% of their patients. b) Presentation of tumor in low stages of 1&2: The average of 77.5% of PTMC patients were in these stages as 17 articles(17,24,25,31,47-48,52,58,63,66,72-73,75,79-80,90,92) explained. c) Presentation of tumor in high stages of 3&4: Mean of 23.33% of patients in 15 articles(17,24-25,47-48,52,58,63,73,75,79-80,90,92) were in stage 3&4. d) Presentation of lymphocytic thyroiditis: 22 articles(22,37,39,42-43,48,55,59,62,64-65,67,70-71,73-74,79-80,82,88,96-97) presented this disease in average 19.85% of PTMC patients. e) Vascular emboli were the other factor that presented in 4% of patients only in one article (16). f) Multinodular goiter was presented in mean of 44% of patients in 22 articles(16,27,31,33,37,39-40,43-44,49-50,54-55,62,65,70,82,86,89,91,97,99). And finally 13 articles(16,24,37,44,50,54-55,68,74,82,88,91,99) described presentation of Graves’ disease in average of 12% of patients.
Prognosis: The first factor was lymph node metastasis, evaluated in 60 articles(13,15-17,19,21-22,24-26,28,31-33,36,40,44,47,50-54,56-61,63-69,71-73,75-79,82-84,86,88-92,94-99) by mean of 26 percent. Distant metastasis with mean of 3% was the other factor that 15 articles(17,25-26,28,32,42,46-47,54,61,63,82,86,91,93) expressed. Finally, 3 articles(15,47,58) described patients with low risk for tumor recurrence as a factor of prognosis in average of 29%.
Treatment: These factors in this part are explained, total or near-total thyroidectomy in 57 articles(14-20,22,24-26,28,31,33,36,40,44-47,49-51,53-57,59-61,65,67,70,74-77,79-80,82-84,86-96,98-99) with mean of 77%, lobectomy in 36 articles(15,25-26,28,36,40,44-45,47,50-51,53-57,60-61,67,70,74,76,79,82,84,86-87,89-92,94-96,99) by mean of 22% and radioactive iodine therapy with mean of 59% in 33 articles(16-17,20,24,28,32-33,40,47,49-51,53-59,61,67,73-75,79,82-83,86,88-89,91,93-94). Lymph node dissection with 2 types of central and lateral was also described. Central form was seen in 28 articles(14-15,18-20,22,25,38,40,44-45,47-48,54-55,59-60,74-77,84,86,88,91,95,99) with mean of 53% but lateral form in 15 articles(19-20,22,25,38,44-45,47,60,77,79,86,90,95,99) with mean of 17%.
Diagnosis: Tumor diagnosis was incidental or non-incidental. Twenty fivearticles(15,19,24,32,38,44-47,49-50,53-54,57,61,67,74-75,82-83,86-88,91,94) with mean of 57% diagnosed tumor incidentally versus 28 articles(15-16,19,24,28,32-33,38,45-47,49-50,53-54,57,61,63,67-68,73-74,82-83,86,88,91,94) in mean of 45% of patients diagnosed it non-incidentally.
Follow-up: Factors in this part were as follows:Temporary postoperative hypoparathyroidism in 8 articles(14,19,47,59,75,86,88,94) with mean of 24% of patients, permanent postoperative hypoparathyroidism in 10 articles(14,19,24,47,59-60,75,86,88,94) with mean of 3%, temporary postoperative recurrent laryngeal nerve paralysis in 6 articles(14,19,55,59,75,88) with mean of 3%, permanent postoperative recurrent laryngeal nerve paralysis in 5 articles(24,47,59-60,94) with mean of 1%, duration of patient’s follow-up in 37 articles(16-17,20,25,28,32,34,38,40,45-47,49-52,54-61,73-75,79,82,86-89,91,99) with mean of 63 months, number of disease-related deaths in 11 articles(17,28,45-46,50-52,54,61,86-87) with mean of 1.7%, number of tumor recurrence or distant metastasis during follow-up in 28 articles(17,20,32,34,38,45-47,50-52,54,56-61,72-73,79,82,87-88,91,93-94,99) with mean of 6% and number of disease-free patients at the end of follow-up in 9 articles(16-17,40,52,54,57,82,86,93) with average of 91%.
Histopathology: In this part, different clinical and paraclinical factors were assessed. Details of each factor were number of dissected central lymph nodes in 5 articles(14,19,75,80,94) with mean of 7.86 lymph node, number of dissected lateral lymph nodes in 1 article(19) with average of 15.75 lymph node, MACIS score was reported in 2 articles(14,92) that in both of them more than 90% of patients (93% & 97%) had score of less than 6 and less than 10% (3% & 7%) had score of more than 6. Location of thyroid tumor in 5 articles (14,20,72,77,96) with mean of 48.22% was in upper lobe and with mean of 33.76% was in lower lobe. 3 articles (19,27,67) with mean of 56.42% reported it in the right lobe and with mean of 34% in the left lobe. Solitary tumor was seen in 14 articles(14-15,17,20,25,27,41,55,57,73,86-88,91) with mean of 61.6%, as multiple tumors were reported in 7 articles(14,19,25-27,41,79) with mean of 24%. Intrathyroidal invasion was reported in 2 articles(17,24) with mean of 22% but extrathyoidal invasion was seen in 33 articles(13-14,17,21-22,24-25,31-32,45-46,48,50,54,58-61,63-65,72-73,76-80,82,84,86,91,97) and the mean expression of it was in 24.2% of patients. The other presentation of invasiveness was lymphovascular invasion with mean expression of 19.4% in 11 articles(14,21,24,32,47,51,56,64,75,80,88). Capsular invasion was another presentation of aggressiveness with average of 31.1% in 19 articles(14,16,20-21,24,51,54-55,67-68,75,88,90,92,94-98). Ipsilateral central pathologic lymph node positivity was reported in 31.3% of patients in only one article(14).
PTMC has different pathologic variants, classic variant which is most common was reported in 36 articles(15,17,20-21,24,26,30-31,33,35-37,40-44,47,50-51,54,59-60,63,65,69-71,76,84,88,91-92,97-98) with mean of 71% although 30 articles(15,17,20,26,30,33,36-37,40-44,47,50-51,54,65,67,70,76,78,83-84,88,91-92,97-98) with mean of 19% reported follicular variant. Other variants of PTMC in 19 articles(17,26,30,36-37,40,42,44,47,51,54,69-70,76,88,91-92,98) had average of 11%. Multifocality in 57 articles(15-17,20-22,24-26,28,31-33,37-38,43,45-48,50-59,61,63-65,67,69-73,75,77-82,86-88,90-92,94-97) had mean expression of 28%, in 8 articles(16,48,50,73-74,86-87,97) with mean of 25% the tumor was unilateral while bilaterality was reported in 26 articles(16-17,22,24,48,50,54-55,64,73-76,79-81,86-88,91-92,94-98) with mean of 23%.
Various paraclinical factors are detected in PTMC patients, Antimicrosomal antibody in only one article(22) with mean positivity of 5%, thyroglobulin antibody in 6 articles(17,22,24,40,49,99) with mean of 16%. One article (23) reported TSH level with mean level of 1.4 mIU/L in range of 1.07-1.72 mIU/L.
Expression of molecular characteristics in PTMC patients were reported in some articles. Fas ligand positivity in one article(24) in 62.7% of patients, BRAFV600E mutation in 7 articles(25,31,44,72,78,90,98) with mean of 51.28% of patients, P53 gene expression in 2 articles(66,67) with mean of 29.2% and cyclin D1 positivity in 2 articles(78,92) with mean of 60.3%. Some factors were only reported in one article, these factors are listed below: CK-19, HBME-1, galectin-3(34) expression in 98.7%, TSHR mRNA(40) in 59.4%, RET gene rearrangement(42) in 52.3%, Bcl-2 expression(66) in 94.8%, Bax expression(66) in 74.3%, S100A4(78) in 68.6%, P27 gene(78) expression in 36.8%, MUC1 gene expression(78) in 48.4%, FDG positivity(84) in 55% and finally surviving positivity(92) in 66.6% of patients were positive (Table 1).
Table 1.
Summary of articles’ results
| Main Factor | Subsidiary Factor | Number of Articles | Mean Value (%) |
|---|---|---|---|
| Gender | Male | 75 | 4485(18) |
| Female | 21468(82) | ||
| Age | Mean | 66 | 47.2 |
| Range | 8-88 | ||
| >= 45 yr | 29 | 7854(59) | |
| <45 yr | 5395(41) | ||
| Tumor Size | Mean | 52 | 5.9 |
| Range | 0.1-10 | ||
| >5 mm | 8224(66) | ||
| <=5 mm | 4263(34) | ||
| Etiology | Family history | 6 | (19.3) |
| Radiation history | 1 | (3) | |
| Clinical presentation | Autoimmune thyroid disease | 3 | (35.6) |
| Tumor stage 1&2 | 17 | (77.5) | |
| Tumor stage 3&4 | 15 | (23.33) | |
| Lymphocytic thyroiditis | 22 | (19.85) | |
| Vascular emboli | 1 | (4) | |
| Multinodular Goiter | 22 | (44) | |
| Graves’ disease | 13 | (12) | |
| Prognosis | Lymph node metastasis | 60 | (26) |
| Distant metastasis | 15 | (3) | |
| Low risk for recurrence | 3 | (29) | |
| Treatment | Total or near-total thyroidectomy | 57 | (77) |
| Lobectomy | 36 | (22) | |
| Radioactive iodine therapy | 33 | (59) | |
| Central lymph node dissection | 28 | (53) | |
| Lateral lymph node dissection | 15 | (17) | |
| Diagnosis | Incidental | 25 | (57) |
| Non-incidental | 28 | (45) | |
| Follow-up | Temporary postop. hypoparathyroidism | 8 | (24) |
| Permanent postop. hypoparathyroidism | 10 | (3) | |
| Temporary postop. recurrent laryngeal nerve paralysis | 6 | (3) | |
| Permanent postop. recurrent laryngeal nerve paralysis | 5 | (1) | |
| Duration of follow-up | 37 | 63 months | |
| Disease-related death | 11 | (1.7) | |
| recurrence or distant metastasis during follow-up | 28 | (6) | |
| Disease-free patients at end of follow-up | 9 | (91) | |
| Histopathology | No. of dissected central lymph nodes | 5 | 7.86 |
| No. of dissected lateral lymph nodes | 1 | 15.75 | |
| MACIS score <=6 | 2 | (95) | |
| MACIS score >6 | 2 | (5) | |
| Upper-lobe tumor location | 5 | (48.22) | |
| Lower-lobe tumor location | 5 | (33.76) | |
| Right-lobe tumor location | 3 | (56.42) | |
| Left-lobe tumor location | 3 | (34) | |
| Solitary tumor | 14 | (61.6) | |
| Multiple tumors | 7 | (24) | |
| Intrathyroidal invasion | 2 | (22) | |
| Extrathyroidal invasion | 33 | (24.2) | |
| Lymphovascular invasion | 11 | (19.4) | |
| Capsular invasion | 19 | (31.1) | |
| Ipsilateral central pathologic lymph node positivity | 1 | (31.3) | |
| Classic variant | 36 | (71) | |
| Follicular variant | 30 | (19) | |
| Other variants | 19 | (11) | |
| Multifocality | 57 | (28) | |
| Unilaterality | 8 | (25) | |
| Bilaterality | 26 | (23) | |
| Antimicrosomal antibody | 1 | (5) | |
| Thyroglobulin antibody | 6 | (16) | |
| Mean TSH level | 1 | 1.4 mIU/L | |
| Range of TSH level | 1 | 1.07-1.72 mIU/L | |
| Fas ligand positivity | 1 | (62.7) | |
| BRAFV600E mutation | 7 | (51.28) | |
| P53 expression | 2 | (29.2) | |
| Cyclin D1 positivity | 2 | (60.3) | |
| CK-19, HBME-1, galectin-3 expression | 1 | (98.7) | |
| TSHR mRNA | 1 | (59.4) | |
| RET gene rearrangement | 1 | (52.3) | |
| Bcl-2 expression | 1 | (94.8) | |
| Bax expression | 1 | (74.3) | |
| S100A4 positivity | 1 | (68.6) | |
| P27 expression | 1 | (36.8) | |
| MUC1 gene expression | 1 | (48.4) | |
| FDG positivity | 1 | (55) | |
| Survivin positivity | 1 | (66.6) |
Discussions:
Differentiated thyroid cancer is the most common endocrine malignancy, with rapidly increasing incidence worldwide. Up to 43% of all thyroid cancers are papillary thyroid microcarcinomas (PTMCs) and nearly 50% of new cases of papillary thyroid carcinoma (PTC) are PTMCs (13,72). PTMC is a type of PTC with a maximum diameter of 1.0 cm or less (72,78).PTMCs have been largely perceived as tumors that are characterized by benign behavior, of little clinical significance, and do not affect patients’ survival. However, PTMCs show very diverse disease extent with widely varying reported frequencies of the aggressive features (13, 15, 26).
These tumors are usually encountered during the histopathologic examination of the thyroid glands removed during necropsy or surgery for nonthyroid or nonmalignant thyroid disease (26). A papillary microcarcinoma may occasionally be the primary lesion of a lymph node metastasis presenting clinically as a neck mass (78).
High-resolution ultrasound-guided fine needle aspiration biopsy has led to a rapid rise in the incidence of papillary thyroid microcarcinoma. The wide availability of ultrasound (US) and fine needle aspiration biopsy (FNAB) and the improved accuracy of histopathologic examination of surgical specimens have been suggested to be reasons for the increased rate of detection (14,15,17,21,54,72,78,89).
PTMC is a specific subgroup of PTC that requires attention because of its increasing frequency among patients with PTC in clinical practice and the implications for patient management (77). According to these points, it is important to know more about this tumor and its characteristics. As systematic reviews evaluate a large number of patients therefore, reliable study of this subject can be obtained.
The clinical significance of PTMC is still unclear. Most PTMCs have an indolent course and excellent prognosis,although some are thought to be associated with recurrence, distant metastasis, or mortality (25,76). Papillary microcarcinomas have an excellent prognosis and may even behave like benign lesions (with even possible partial spontaneous regression) which can justify a minimalist therapeutic approach. However, microcarcinomas are “true” cancers, which may require aggressive treatment in some cases (16). The clinical importance of papillary thyroid microcarcinoma is debatable. Some authors (12-15,22-33,55) have reported a benign behavior with no progression, whereas others (9,66,73)have found a surprisingly aggressive cancer. Only rarely is lymph node or distant metastasis the first presentation of thyroid microcarcinoma (17).In this study, the value of lymph node metastasis was 26% whereas distant metastasis had been reported in 3% of PTMC patients (17).
Although the long-term outcome of PTMC is excellent, with a less than 1% cause-specific mortality rate at 20 years, it frequently spreads to the cervical lymph nodesand may occasionally metastasize to distant sites (54). Patients with PTMC have very low mortality. However, 4-16% of patients with PTMC develop recurrent disease with many of these patients developing distant metastasis (51). In our study mean rate of tumor related mortality was 1.7% and rate of recurrence or distant metastasis after follow-up was 6%.
Furthermore, PTMC is often multifocal (15.5–40% in surgical series and over 80% in systematic autopsy studies) and extrathyroidal tumor extension, a factor correlated with a higher risk of locoregional recurrence, is also observed (10–20% in surgical and pathologic studies) (54). The mean rate of tumor multifocality in our study was 28% and it was 24.2% for extrathyroidal extension.
Despite the absence of palpable neck nodes, PTMC has a considerable rate of lymph node metastasis to the central compartment (80).Extracapsular spread is thought to have predictive value for central compartment LN metastasis. Central compartment LN metastasis in PTMC is associated with a risk of disease recurrence and is a reliable prognostic factor (77). As in previous studies, central LNM was significantly related to bilaterality in our study (97). In this study, extracapsular invasion and bilaterality rates were 31.1% and 23%.
Although PTMC is relatively common, standard treatment for these tumors remains controversial. Some consider PTMC to be a less aggressive subset of papillary thyroid cancers that require only minimal treatment. However, other groups have reported a high incidence of metastasis from microcarcinomas and thus favor an aggressive surgical approach followed by ablation therapy (21, 77, 76). In general, total or near-total thyroidectomy is performed when tumor foci is preoperatively detected in a bilateral lobe. However, in PTMC confined to the unilateral lobe, two options regarding resection extent seem to be applicable: total thyroidectomyvs. Unilateral lobectomy (21). It is debatable whether total thyroidectomy or lobectomy is the appropriate treatment for patients with PTMC (54). In the American Thyroid Association guidelines, the recommended treatment in patients with isolated PTMC at low risk consists of thyroid lobectomy, whereas near-total or total thyroidectomy is considered the treatment of choice in patients with a history of radiation therapy to the head and neck or a first-degree member with differentiated thyroid cancer or age greater than 45 years (82). In our study total or near-total thyroidectomy was performed in 77% of patients versus 22% lobectomy.
The identification of patients with aggressive PTMC is of great importance because they need a radical therapeutic approach, as classical PTC, based on total thyroidectomy, lymphadenectomy, central compartment, and radioiodine therapy (82). Although there is a body of literature that advocates the use of I-131 therapy for patients with PTMC (particularly those patients with poor histological features), few studies have been able to demonstrate a clinical benefit of I-131 therapy for patients with PTMC (51). In our study, I-131 therapy was used in approximately half of patients in 59% of them.
Aggressive PTMC treatments can cause adverse outcomes, such as surgical damage to the recurrent laryngeal nerve, hypoparathyroidism, and the development of new primary cancers after radioiodine treatments (72). In the present study complications of thyroid surgery were divided into groups of temporary and permanent. Temporary hypoparathyroidism in 24%, permanent hypoparathyroidism in 3%, temporary recurrent laryngeal nerve paralysis in 3% and finally permanent form of paralysis was seen in 1% of patients.
Conclusion:
The numbers of detected PTMCs are increasing due to the development of diagnostic methods. This tumor can manifest as a marker of advanced papillary carcinomas. Although the tumor naturally is benign and develops slowly, sometimes it can present unfavorable with recurrence and distant metastasis. Overall, identifying this tumor and paying attention to its markers has double benefits, one of them is prevention of advanced tumors that need heavier and longer treatments and also the other benefit is managing the tumor as an distinct disease.
Since in systematic reviews a larger number of patients are analyzed, so there is confidence that recognition of each subject by this type of study is more reliable. At last we hope that by introducing characteristics of this tumor as a review article could have a little but useful role in cancer managements.
Acknowledgements
The authors declare that there is no conflict of interest.
References
- 1.Goldman L, Ausiello DA. Cecil Medicine. 23rd ed. Saunders Elsevier Publishers; 2007. [Google Scholar]
- 2.Longo DL, Kasper DL, Jameson JL, Fauci AS, Hauser SL, Loscalzo J. Harrison’s Principles of Internal Medicine. 16th ed. McGraw-Hill Publishers; 2012. [Google Scholar]
- 3.Lombardi CP, Bellantone R, De Crea C, Paladino NC, Fadda G, Salvatori M, et al. Papillary Thyroid Microcarcinoma: Extrathyroidal Extension, Lymph Node Metastases, and Risk Factors for Recurrence in a High Prevalence of Goiter Area. World J Surg. 2010(34):1214–21. doi: 10.1007/s00268-009-0375-x. [DOI] [PubMed] [Google Scholar]
- 4.Yang GCH, LiVolsi VA, Baloch ZW. Thyroid microcarcinoma: Fine-needle aspiration diagnosis and histologic follow-up. IJSP. 2002;10:133–9. doi: 10.1177/106689690201000206. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Li L, Wang YX, Feng XL, Zhao F, Zou SM, et al. Ultrasound findings of papillary thyroid microcarcinoma: A review of 113 consecutive cases with histopathologic correlation. Ultrasound Med Biol. 2012 Oct;38(10):1681–8. doi: 10.1016/j.ultrasmedbio.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Moon HJ, Son E, Kim EK, Yoon JH, Kwak JY. The diagnostic values of ultrasound and ultrasound-guided fine needle aspiration in subcentimeter-sized thyroid nodules. Ann Surg Oncol. 2012:52–9. doi: 10.1245/s10434-011-1813-1. [DOI] [PubMed] [Google Scholar]
- 7.Page C, Biet A, Boute P, Cuvelier Ph, Strunski V. Aggressive papillary’ thyroid microcarcinoma. Eur Arch Otorhinolaryngol. 2009 Dec;266(12):1959–63. doi: 10.1007/s00405-009-0952-5. [DOI] [PubMed] [Google Scholar]
- 8.Novosel T, Ritter HE, Gupta M, Harvey A, Mitchell J, Berber E, et al. Detection of circulating thyroid cancer cells in patients with thyroid microcarcinomas. Surgery. 2009:1081–9. doi: 10.1016/j.surg.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Goldust M, Samankan S, Sokouti M, Fakhrjoo A. A clinical epidemiologic study of thyroid carcinoma in patients under 25 years old in Tabriz, Iran (1995-2010) JPMA. 2012;62:1265. [PubMed] [Google Scholar]
- 10.Larijani B, Aghakhani S, Haghpanah V, Mosavi-Jarrahi A, Bastanhagh MH. Review in thyroid cancer in Iran. Austral-Asian J Cancer. 2005;4(4):199–203. [Google Scholar]
- 11.Arora N, Turbendian HK, Kato MA, Moo TA, Zarnegar R, Fahey TJ. Papillary Thyroid Carcinoma and Microcarcinoma: Is There a Need to Distinguish the Two? Thyroid. 2009;19(5):473–77. doi: 10.1089/thy.2008.0185. [DOI] [PubMed] [Google Scholar]
- 12.Törüner FB, Altinova AE, Taneri F, Aktürk M, Atasever T, Poyraz A, et al. Management of Papillary Thyroid Microcarcinomas: Our Clinical Experience. Turk Jem. 2006;3:53–6. [Google Scholar]
- 13.Yun M, Noh TW, Cho A, Choi YJ, Hong SW, Park CS, et al. Visually discernible (18F)fluorodeoxyglucose uptake in papillary thyroid microcarcinoma: A potential new risk factor. J Clin Endocrinol Metab. 2010;95:3182–8. doi: 10.1210/jc.2009-2091. [DOI] [PubMed] [Google Scholar]
- 14.Lim YC, Choi EC, Yoon YH, Kim EH, Koo BS. Central lymph node metastases in unilateral papillary thyroid microcarcinoma. Br J Surg. 2009 Mar;96(3):253–7. doi: 10.1002/bjs.6484. [DOI] [PubMed] [Google Scholar]
- 15.Zafon C, Baena JA, Castellví J, Obiols G, Monroy G, Mesa J. Differences in the Form of Presentation between Papillary Microcarcinomas and Papillary Carcinomas of Larger Size. J Thyroid Res. 2010 Dec 14;2011:639156. doi: 10.4061/2011/639156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page C, Biet A, Boute P, Cuvelier Ph, Strunski V. Aggressive papillary’ thyroid microcarcinoma. Eur Arch Otorhinolaryngol. 2009:1959–63. doi: 10.1007/s00405-009-0952-5. [DOI] [PubMed] [Google Scholar]
- 17.Tzvetov G, Hirsch D, Slutzky IS, Weinstein R, Manistersky Y, Kalmanovich R, et al. Well-differentiated thyroid carcinoma: comparison of microscopic and macroscopic disease. Thyroid. 2009(5):487–94. doi: 10.1089/thy.2008.0228. [DOI] [PubMed] [Google Scholar]
- 18.Kim YW, Wang SG, Lee JC, Lee BJ, Lee JW, Kim YK, et al. Clinically Related Factors and Features of Central Compartment Neck Lymph Nodes in Thyroid Micropapillary Carcinoma. Korean J Otorhinolaryngol-Head Neck Surg. 2009;52:232–6. [Google Scholar]
- 19.Chung YS, Kim JY, Bae JS, Song BJ, Kim JS, Jeon HM, et al. Lateral Lymph Node Metastasis in Papillary Thyroid Carcinoma: Results of Therapeutic Lymph Node Dissection. Thyroid. 2009;19:241–6. doi: 10.1089/thy.2008.0244. [DOI] [PubMed] [Google Scholar]
- 20.Garrel R, Tripodi C, Cartier C, Makeieff M, Crampette L, Guerrier B. Cervical lymphadenopathies signaling thyroid microcarcinoma. Case study and review of the literature. Eur Ann Otorhinolaryngol Head Neck Dis. 2011 Jun;128(3):115–9. doi: 10.1016/j.anorl.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Koo BS, Lim HS, Lim YC, Yoon YH, Kim YM, Park YH, et al. Occult Contralateral Carcinoma in Patients with Unilateral Papillary Thyroid Microcarcinoma. Ann Surg Oncol. 2010;17:1101–5. doi: 10.1245/s10434-009-0906-6. [DOI] [PubMed] [Google Scholar]
- 22.Kim HS, Choi YJ, Yun JS. Features of Papillary Thyroid Microcarcinoma in the Presence and Absence of Lymphocytic Thyroiditis. Endocr Pathol. 2010 Sep;21(3):149–53. doi: 10.1007/s12022-010-9124-9. [DOI] [PubMed] [Google Scholar]
- 23.Gerschpacher M, Göbl C, Anderwald C, Gessl A, Krebs M. Thyrotropin Serum Concentrations in Patients with Papillary Thyroid Microcancers. Thyroid. 2010;20:389–92. doi: 10.1089/thy.2009.0139. [DOI] [PubMed] [Google Scholar]
- 24.Bayraktaroglu T, Boztepe H, Kapran Y, Tanakol R, Alagöl F. Fas Ligand (FasL, Apo-1L / CD95L) Expression and Clinical Outcome in Papillary Microcarcinoma and Papillary Thyroid Carcinomas with Diameter Smaller than 1.5 Centimeters. Exp Clin Endocrinol Diabetes. 2010 Aug;118(8):537–43. doi: 10.1055/s-0029-1241205. [DOI] [PubMed] [Google Scholar]
- 25.Zheng X, Wei S, Han Y, Li Y, Yu Y, Yun X, et al. Papillary Microcarcinoma of the Thyroid: Clinical Characteristics and BRAFV600E Mutational Status of 977 Cases. Ann Surg Oncol. 2013;20:2266–73. doi: 10.1245/s10434-012-2851-z. [DOI] [PubMed] [Google Scholar]
- 26.Yang GCH, LiVolsi VA, Baloch ZW. Thyroid microcarcinoma: Fine-needle aspiration diagnosis and histologic follow-up. Int J Surg Pathol. 2002 Apr;10(2):133–9. doi: 10.1177/106689690201000206. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Li L, Wang YXJ, Feng XL, Zhao F, Zou SM, et al. Ultrasound findings of papillary thyroid microcarcinoma: A review of 113 consecutive cases with histopathologic correlation. Ultrasound Med Biol. 2012 Oct;38(10):1681–8. doi: 10.1016/j.ultrasmedbio.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 28.Lin JD, Huang BY, Chang HY. Clinical experience in the diagnosis of 127 papillary thyroid microcarcinomas. Endocr Relat Cancer. 1998:239–45. [Google Scholar]
- 29.Tanriover O, Comunoglu N, Eren B, Comunoglu C, Turkmen N, Dogan M, et al. Occult papillary thyroid carcinoma: prevalence at autopsy in Turkish people. Eur J Cancer Prev. 2011 Jul;20(4):308–12. doi: 10.1097/CEJ.0b013e32834473dc. [DOI] [PubMed] [Google Scholar]
- 30.Moon HJ, Son E, Kim EK, Yoon JH, Kwak JY. The diagnostic values of ultrasound and ultrasound-guided fine needle aspiration in subcentimeter-sized thyroid nodules. Ann Surg Oncol. 2012:52–9. doi: 10.1245/s10434-011-1813-1. [DOI] [PubMed] [Google Scholar]
- 31.Marchetti I, Iervasi G, Mazzanti CM, Lessi F, Tomei S, Naccarato AG, et al. Detection of the BRAFV600E Mutation in Fine Needle Aspiration Cytology of Thyroid Papillary Microcarcinoma Cells Selected by Manual Macrodissection: An Easy Tool to Improve the Preoperative Diagnosis. Thyroid. 2012;22(3):292–8. doi: 10.1089/thy.2011.0107. [DOI] [PubMed] [Google Scholar]
- 32.Zafon C, Baena JA, Castellvi J, Obiols G, Monroy G, Mesa J. Differences in the Form of Presentation between Papillary Microcarcinomas and Papillary Carcinomas of Larger Size. J Thyroid Res. 2010;2011:639156. doi: 10.4061/2011/639156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Törüner FB, Altinova AE, Taneri F, Aktürk M, Atasever T, Poyraz A, et al. Management of Papillary Thyroid Microcarcinomas: Our Clinical Experience. Turk Jem. 2006:53–6. [Google Scholar]
- 34.Tong J1, Wang Y, Da JP. Usefulness of CK19, HBME-1 and galectin-3 expressions in differential diagnosis of thyroid papillary microcarcinoma from benign lesions. Zhonghua Zhong Liu Za Zhi. 2011 Aug;33(8):599–604. [PubMed] [Google Scholar]
- 35.Batistatou A, Charalabopoulos K, Nakanishi Y, Vagianos C, Hirohashi S, Agnantis NJ, et al. Differential Expression of Dysadherin in Papillary Thyroid Carcinoma and Microcarcinoma: Correlation with E-cadherin. Endocr Pathol. 2008;19:197–202. doi: 10.1007/s12022-008-9035-1. [DOI] [PubMed] [Google Scholar]
- 36.Lee NS, Bae JS, Jeong SR, Jung CK, Lim DJ, Park WC, et al. Risk Factors of Lymph Node Metastasis in Papillary Thyroid Microcarcinoma. J Korean Surg Soc. 2010;78:82–6. [Google Scholar]
- 37.Pisello F, Geraci G, Sciumè C, Li Volsi F, Modica G. Total thyroidectomy of choice: papillary microcarcinoma. G Chir. 2007 Jan-Feb;28(1-2):13–9. [PubMed] [Google Scholar]
- 38.Xu YN, Wang JD. Surgical treatment of incidental and non-incidental papillary thyroid microcarcinoma. G Chir. 2010;31:205–9. [PubMed] [Google Scholar]
- 39.Sorrentino F, Atzeni J, Romano G, Buscemi G, Romano M. Differentiated microcarcinoma of the thyroid gland. G Chir. 2010;31:277–8. [PubMed] [Google Scholar]
- 40.Novosel T, Ritter HE, Gupta M, Harvey A, Mitchell J, Berber E, et al. Detection of circulating thyroid cancer cells in patients with thyroid microcarcinomas. Surgery. 2009;146:1081–9. doi: 10.1016/j.surg.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi Y, Lagarde F, Tsuda N, Funamoto S, Preston DL, Koyama K, et al. Papillary Microcarcinoma of the Thyroid Among Atomic Bomb Survivors. Cancer. 2010;116:1646–55. doi: 10.1002/cncr.24872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corvi R, Alfaro MM, Harach HR, Zini M, Papotti M, Romeo G. Frequent RET Rearrangements in Thyroid Papillary Microcarcinoma Detected by Interphase Fluorescence In Situ Hybridization. Lab Invest. 2001;81:1639–45. doi: 10.1038/labinvest.3780377. [DOI] [PubMed] [Google Scholar]
- 43.Kovács GL, Gonda G, Vadász G, Ludmány É, Uhrin K, Görömbey Z, et al. Epidemiology of Thyroid Microcarcinoma Found in Autopsy Series Conducted in Areas of Different Iodine Intake. Thyroid. 2005;15:152–7. doi: 10.1089/thy.2005.15.152. [DOI] [PubMed] [Google Scholar]
- 44.Virk RK, Van Dyke AL, Finkelstein A, Prasad A, Gibson J, Hui P, et al. BRAFV600E mutation in papillary thyroid microcarcinoma: a genotype–phenotype correlation. Mod Pathol. 2013 Jan;26(1):62–70. doi: 10.1038/modpathol.2012.152. [DOI] [PubMed] [Google Scholar]
- 45.Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y. Three Distinctly Different Kinds of Papillary Thyroid Microcarcinoma should be Recognized: Our Treatment Strategies and Outcomes. World J Surg. 2010;34:1222–31. doi: 10.1007/s00268-009-0359-x. [DOI] [PubMed] [Google Scholar]
- 46.Lin JD, Kuo SF, Chao TC, Hsueh C. Incidental and Nonincidental Papillary Thyroid Microcarcinoma. Ann Surg Oncol. 2008 Aug;15(8):2287–92. doi: 10.1245/s10434-008-9958-2. [DOI] [PubMed] [Google Scholar]
- 47.Besic N, Zgajnar J, Hocevar M, Petric R. Extent of Thyroidectomy and Lymphadenectomy in 254 Patients With Papillary Thyroid Microcarcinoma: A Single-Institution Experience. Ann Surg Oncol. 2009;16:920–8. doi: 10.1245/s10434-009-0332-9. [DOI] [PubMed] [Google Scholar]
- 48.Zhou YL, Gao EL, Zhang W, Yang H, Guo GL, Zhang XH, et al. Factors predictive of papillary thyroid micro-carcinoma with bilateral involvement and central lymph node metastasis: a retrospective study. World J Surg Oncol. 2012 May 19;10:67. doi: 10.1186/1477-7819-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Durante C, Attard M, Torlontano M, Ronga G, Monzani F, Costante G, et al. Identification and Optimal Postsurgical Follow-Up of Patients with Very Low-Risk Papillary Thyroid Microcarcinomas. J Clin Endocrinol Metab. 2010;95:4882–88. doi: 10.1210/jc.2010-0762. [DOI] [PubMed] [Google Scholar]
- 50.Mercante G, Frasoldati A, Pedroni C, Formisano D, Renna L, Piana S, et al. Patients with papillary microcarcinoma that extends into soft tissues or is metastatic to locoregional lymph nodes are at high risk for persistent or recurrent disease. Thyroid. 2009;19:1–10. [Google Scholar]
- 51.Creach KM, Siegel BA, Nussenbaum B, Grigsby PW. Radioactive iodine therapy decreases recurrence in thyroid papillary microcarcinoma. ISRN Endocrinol. 2012;2012:816386. doi: 10.5402/2012/816386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ross DS, Litofsky D, Ain KB, Bigos T, Brierley JD, Cooper DS, et al. Recurrence after treatment of micropapillary thyroid cancer. Thyroid. 2009;19:1043–8. doi: 10.1089/thy.2008.0407. [DOI] [PubMed] [Google Scholar]
- 53.Haymart MR, Cayo M, Chen H. Papillary thyroid microcarcinomas: Big decisions for a small tumor. Ann Surg Oncol. 2009;16:3132–9. doi: 10.1245/s10434-009-0647-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mercante G, Frasoldati A, Pedroni C, Formisano D, Renna L, Piana S, et al. Prognostic Factors Affecting Neck Lymph Node Recurrence and Distant Metastasis in Papillary Microcarcinoma of the Thyroid: Results of a Study in 445 Patients. Thyroid. 2009;19:707–6. doi: 10.1089/thy.2008.0270. [DOI] [PubMed] [Google Scholar]
- 55.Dănilă R, Grigorovici A, Ionescu L, Popovici R, Huţanu I, Ungureanu MC, et al. Thyroid Papillary Microcarcinoma Incidentally Found Following Thyroidectomies for Benignant Diseases. Chirurgia. 2008;4:273–80. [Google Scholar]
- 56.Buffet C, Golmard JL, Hoang C, Trésallet C, Du Pasquier Fédiaevsky L, Fierrard H, et al. Scoring system for predicting recurrences in patients with papillary thyroid microcarcinoma. Eur J Endocrinol. 2012 Aug;167(2):267–75. doi: 10.1530/EJE-12-0105. [DOI] [PubMed] [Google Scholar]
- 57.Vlassopoulou B, Vryonidou A, Ioannidis D, Koletti A, Klonaris N, Katsoulis C, et al. Long term follow-up and outcome in thyroid papillary microcarcinoma. Endocrine Abstracts. 2011;26:477. [Google Scholar]
- 58.Kim HJ, Kim NK, Choi JH, Kim SW, Jin SM, Suh S, et al. Radioactive iodine ablation does not prevent recurrences in patients with papillary thyroid microcarcinoma. Clin Endocrinol. 2013;78:614–20. doi: 10.1111/cen.12034. [DOI] [PubMed] [Google Scholar]
- 59.So YK, Seo MY, Son YI. Prophylactic central lymph node dissection for clinically node-negative papillary thyroid microcarcinoma: Influence on serum thyroglobulin level, recurrence rate, and postoperative complications. Surgery. 2012;151:192–8. doi: 10.1016/j.surg.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 60.Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Clinical Significance of Metastasis to the Central Compartment from Papillary Microcarcinoma of the Thyroid. World J Surg. 2006;30:91–9. doi: 10.1007/s00268-005-0113-y. [DOI] [PubMed] [Google Scholar]
- 61.Londero SC, Krogdahl A, Bastholt L, Overgaard J, Trolle W, Pedersen HB, et al. Papillary Thyroid Microcarcinoma in Denmark 1996–2008: A National Study of Epidemiology and Clinical Significance. Thyroid. 2013;23:1159–64. doi: 10.1089/thy.2012.0595. [DOI] [PubMed] [Google Scholar]
- 62.Pennelli N, Pennelli G, Merante Boschin I, Pelizzo MR. Thyroid intrafollicular neoplasia (TIN) as a precursor of papillary microcarcinoma. Ann Ital Chir. 2005 May-Jun;76(3):219–24. [PubMed] [Google Scholar]
- 63.Friguglietti CU, Dutenhefner SE, Brandão LG, Kulcsar MA. Classification of papillary thyroid microcarcinoma according to size and fine-needle aspiration cytology: behavior and therapeutic implications. Head Neck. 2011 May;33(5):696–701. doi: 10.1002/hed.21517. [DOI] [PubMed] [Google Scholar]
- 64.Pakdaman MN, Rochon L, Gologan O, Tamilia M, Garfield N, Hier MP, et al. Incidence and histopathological behavior of papillary microcarcinomas: Study of 429 cases. Otolaryngol Head Neck Surg. 2008 Nov;139(5):718–22. doi: 10.1016/j.otohns.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 65.Koperek O, Asari R, Niederle B, Kaserer K. Desmoplastic stromal reaction in papillary thyroid microcarcinoma. Histopathology. 2011 May;58(6):919–24. doi: 10.1111/j.1365-2559.2011.03791.x. [DOI] [PubMed] [Google Scholar]
- 66.Cvejic D, Selemetjev S, Savin S, Paunovic I, Petrovic I, Tatic S. Apoptosis and proliferation related molecules (Bcl-2, Bax, p53, PCNA) in papillary microcarcinoma versus papillary carcinoma of the thyroid. Pathology. 2008;40:475–80. doi: 10.1080/00313020802026989. [DOI] [PubMed] [Google Scholar]
- 67.Corapcioglu D, Sak SD, Delibasi T, Tonyukuk V, Kamel N, Uysal AR, Kocak S, et al. Papillary microcarcinomas of the thyroide gland and immunohistochemical analysis of expression of p53 protein in papillary microcarcinomas. J Transl Med. 2006 Jul 5;4:28. doi: 10.1186/1479-5876-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Akin S, Akin S, Aksoy DY, Bayraktar M. clinicopathological characteristics of patients with thyroid papillary microcarcinoma: preliminary results. Endocrine Abstracts. 2012;29:1766. [Google Scholar]
- 69.Yang GC, LiVolsi VA, Baloch ZW. Thyroid microcarcinoma: fine-needle aspiration diagnosis and histologic follow-up. Int J Surg Pathol. 2002;10:133–9. doi: 10.1177/106689690201000206. [DOI] [PubMed] [Google Scholar]
- 70.Siassakos D, Gourgiotis S, Moustafellos P, Dimopoulos N, Hadjiyannakis E. Thyroid microcarcinoma during thyroidectomy. Singapore Med J. 2008 Jan;49(1):23–5. [PubMed] [Google Scholar]
- 71.Fardella C, Jiménez M, González H, León A, Goñi I, Cruz F, et al. Pathological characteristics of thyroid microcarcinoma. A review of 402 biopsies. Rev Méd Chile. 2005;133:1305–10. doi: 10.4067/s0034-98872005001100005. [DOI] [PubMed] [Google Scholar]
- 72.Lin KL, Wang OC, Zhang XH, Dai XX, Hu XQ, Qu JM. The BRAF mutation is predictive of aggressive clinicopathological characteristics in papillary thyroid microcarcinoma. Ann Surg Oncol. 2010;17:3294–3300. doi: 10.1245/s10434-010-1129-6. [DOI] [PubMed] [Google Scholar]
- 73.Neuhold N, Schultheis A, Hermann M, Krotla G, Koperek O, Birner P. Incidental papillary microcarcinoma of the thyroid-further evidence of a very low malignant potential: A retrospective clinicopathological study with up to 30 years of follow-up. Ann Surg Oncol. 2011;18:3430–6. doi: 10.1245/s10434-011-1663-x. [DOI] [PubMed] [Google Scholar]
- 74.Lombardi CP, Bellantone R, De Crea C, Paladino NC, Fadda G, Salvatori M, et al. Papillary Thyroid Microcarcinoma: Extrathyroidal Extension, Lymph Node Metastases, and Risk Factors for Recurrence in a High Prevalence of Goiter Area. World J Surg. 2010;34:1214–21. doi: 10.1007/s00268-009-0375-x. [DOI] [PubMed] [Google Scholar]
- 75.Roh JL, Kim JM, Park CI. Central Cervical Nodal Metastasis from Papillary Thyroid Microcarcinoma: Pattern and Factors Predictive of Nodal Metastasis. Ann Surg Oncol. 2008 Sep;15(9):2482–6. doi: 10.1245/s10434-008-0044-6. [DOI] [PubMed] [Google Scholar]
- 76.Moon HJ, Kim EK, Yoon JH, Kwak JY. Clinical Implication of Elastography as a Prognostic Factor of Papillary Thyroid Microcarcinoma. Ann Surg Oncol. 2012;19:2279–87. doi: 10.1245/s10434-011-2212-3. [DOI] [PubMed] [Google Scholar]
- 77.Lee SH, Lee SS, Jin SM, Kim JH, Rho YS. Predictive Factors for Central Compartment Lymph Node Metastasis in Thyroid Papillary Microcarcinoma. Laryngoscope. 2008;118:659–62. doi: 10.1097/MLG.0b013e318161f9d1. [DOI] [PubMed] [Google Scholar]
- 78.Min HS, Choe G, Kim SW, Park YJ, Park do J, Youn YK, et al. S100A4 expression is associated with lymph node metastasis in papillary microcarcinoma of the thyroid. Mod Pathol. 2008 Jun;21(6):748–55. doi: 10.1038/modpathol.2008.51. [DOI] [PubMed] [Google Scholar]
- 79.Moon HJ, Kim EK, Chung WY, Yoon JH, Kwak JY. Minimal extrathyroidal extension in patients with papillary thyroid microcarcinomas: Is it a real prognostic factor? Ann Surg Oncol. 2011;18:1916–23. doi: 10.1245/s10434-011-1556-z. [DOI] [PubMed] [Google Scholar]
- 80.Kim BY, Jung CH, Kim JW, Lee SW, Kim CH, Kang SK, et al. Impact of Clinicopathologic Factors on Subclinical Central Lymph Node Metastasis in Papillary Thyroid Microcarcinoma. Yonsei Med J. 2012;53(5):924–30. doi: 10.3349/ymj.2012.53.5.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Connor MP, Wells D, Schmalbach CE. Variables Predictive of Bilateral Occult Papillary Microcarcinoma Following Total Thyroidectomy. Otolaryngol Head Neck Surg. 2011 Feb;144(2):210–5. doi: 10.1177/0194599810391616. [DOI] [PubMed] [Google Scholar]
- 82.Ardito G, Revelli L, Giustozzi E, Salvatori M, Fadda G, Ardito F, et al. Aggressive papillary thyroid microcarcinoma: prognostic factors and therapeutic strategy. Clin Nucl Med. 2013;38:25–8. doi: 10.1097/RLU.0b013e318279bc65. [DOI] [PubMed] [Google Scholar]
- 83.Czarniecka A, Wloch J, Lange D. Clinical picture of differentiated thyroid carcinoma in the T1 stage. Wiad Lek. 2001;54:225–33. [PubMed] [Google Scholar]
- 84.Byun BH, Jeong UG, Hong SP, Min JJ, Chong A, Song HC, et al. Prediction of central lymph node metastasis from papillary thyroid microcarcinoma by 18F-fluorodeoxyglucose PET/CT and ultrasonography. Ann Nucl Med. 2012;26:471–7. doi: 10.1007/s12149-012-0594-3. [DOI] [PubMed] [Google Scholar]
- 85.Bo YH, Ahn HY, Lee YH, Lee YJ, Kim JH, Ohn JH, et al. Malignancy Rate in Sonographically Suspicious Thyroid Nodules of Less than a Centimeter in Size Does Not Decrease with Decreasing Size. J Korean Med Sci. 2011;26:237–42. doi: 10.3346/jkms.2011.26.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pelizzo MR, Boschin IM, Toniato A, Piotto A, Bernante P, Pagetta C, et al. Papillary thyroid microcarcinoma (PTMC): Prognostic factors, management and outcome in 403 patient. EJSO. 2006;32:1144–8. doi: 10.1016/j.ejso.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 87.Kuo SF, Chao TC, Chang HY, Hsueh C, Yang CH, Lin JD. Prognostic Evaluation of Patients With Multicentric Papillary Thyroid Microcarcinoma. J Formos Med Assoc. 2011;110(8):511–7. doi: 10.1016/S0929-6646(11)60077-6. [DOI] [PubMed] [Google Scholar]
- 88.Giordano D, Gradoni P, Oretti G, Molina E, Ferri T. Treatment and prognostic factors of papillary thyroid microcarcinoma. Clin Otolaryngol. 2010;35:118–24. doi: 10.1111/j.1749-4486.2010.02085.x. [DOI] [PubMed] [Google Scholar]
- 89.Sethom A, Riahi I, Riahi K, Akkari K, Benzarti S, Miled I, et al. Care of thyroid microcarcinoma. About 13 cases. La Tunisie Medicale. 2011;89:23–5. [PubMed] [Google Scholar]
- 90.Kwak JY, Kim EK, Chung WY, Moon HJ, Kim MJ, Choi JR. Association of BRAFV600E Mutation with Poor Clinical Prognostic Factors and US Features in Korean Patients with Papillary Thyroid Microcarcinoma. Radiology. 2009;253(3):854–60. doi: 10.1148/radiol.2533090471. [DOI] [PubMed] [Google Scholar]
- 91.Kim TY, Hong SJ, Kim JM, Kim WG, Gong G, Ryu JS, et al. Prognostic parameters for recurrence of papillary thyroid microcarcinoma. BMC Cancer. 2008;8:296. doi: 10.1186/1471-2407-8-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Antonaci A, Consorti F, Mardente S, Natalizi S, Giovannone G, Della Rocca C. Survivin and cyclin D1 are jointly expressed in thyroid papillary carcinoma and microcarcinoma. Oncol Rep. 2008 Jul;20(1):63–7. [PubMed] [Google Scholar]
- 93.Friguglietti CUM, Kulcsar MAV. Thyroid Microcarcinoma: Experience and Management in Private Clinic. Arq Bras Endocrinol Metab. 2007;51(5):774–82. doi: 10.1590/s0004-27302007000500015. [DOI] [PubMed] [Google Scholar]
- 94.Caliskan M, Park JH, Jeong JS, Lee CR, Park SK, Kang SW, et al. Role of prophylactic ipsilateral central compartment lymph node dissection in papillary thyroid microcarcinoma. Endocr J. 2012;59(4):305–11. doi: 10.1507/endocrj.ej11-0366. [DOI] [PubMed] [Google Scholar]
- 95.Kwak JY, Kim EK, Kim MJ, Son EJ, Chung WY, Park CS, et al. Papillary Microcarcinoma of the Thyroid: Predicting Factors of Lateral Neck Node Metastasis. Ann Surg Oncol. 2009;16:1348–55. doi: 10.1245/s10434-009-0384-x. [DOI] [PubMed] [Google Scholar]
- 96.Kim KE, Kim EK, Yoon JH, Han KH, Moon HJ, Kwak JY. Preoperative Prediction of Central Lymph Node Metastasis in Thyroid Papillary Microcarcinoma Using Clinicopathologic and Sonographic Features. World J Surg. 2013;37:385–91. doi: 10.1007/s00268-012-1826-3. [DOI] [PubMed] [Google Scholar]
- 97.Zhao Q, Ming J, Liu C, Shi L, Xu X, Nie X, et al. Multifocality and Total Tumor Diameter Predict Central Neck Lymph Node Metastases in Papillary Thyroid Microcarcinoma. Ann Surg Oncol. 2013;20:746–52. doi: 10.1245/s10434-012-2654-2. [DOI] [PubMed] [Google Scholar]
- 98.Rossi ED, Martini M, Capodimonti S, Lombardi CP, Pontecorvi A, Vellone VG, et al. BRAF (V600E) Mutation Analysis on Liquid-Based Cytology-Processed Aspiration Biopsies Predicts Bilaterality and Lymph Node Involvement in Papillary Thyroid Microcarcinoma. Cancer Cytopathol. 2013 Jun;121(Jun):291–7. doi: 10.1002/cncy.21258. [DOI] [PubMed] [Google Scholar]
- 99.Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient Age Is Significantly Related to the Progression of Papillary Microcarcinoma of the Thyroid Under Observation. Thyroid. 2013;24(1):27–34. doi: 10.1089/thy.2013.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]