Abstract
Malignant mixed germ cell tumors,though rare overall, are the most common type of malignant ovarian neoplasms in young and adolescent girls. These tumors are rapidly growing and can metastasize. We report a case of 13-yr-old girl who presented at SHKM GMC, Nalhar, Mewat, Haryana, India in December 2013 with huge abdominal lump of a malignant mixed germ cell tumor comprising both immature teratoma and embryonal carcinoma. This report illustrates the aggressiveness of this tumor and emphasises the need of early diagnosis and treatment.
Key Words: Malignant Mixed Germ Cell Tumors, Ovary, Immature Teratoma, Embryonal Carcinoma
Introduction
Germ cell tumors of ovary constitute 15-20% of all ovarian neoplasms. “Malignant germ cell tumors comprise less than 5% of all ovarian neoplasms” (1). “Malignant mixed germ cell tumors are still rare. Median age at presentation is second decade” (2). Malignant mixed germ cell tumors often show combination of two or more malignant components, occur in 8% of all cases of malignant ovarian germ cell tumors (MOGCT). The most common combination is that of Dysgerminoma with yolk sac tumor. The quality and quantity of various components may alter the therapeutic approach and prognosis, so it is essential that the tumor is sampled carefully and thoroughly analysed (3). Malignant mixed germ cell tumor of the ovary is a highly aggressive neoplasm occurring in young and adolescent girls that can present as disseminated disease at initial diagnosis. These tumors expand locally and metastasize through vascular and lymphatic invasion (4).
Here we report a rare case of malignant mixed germ cell tumor with a combination of immature teratoma and embryonal carcinoma in a 13-yr-old girl. We are yet to come across an individual case report with this combination in recent years.
Case report
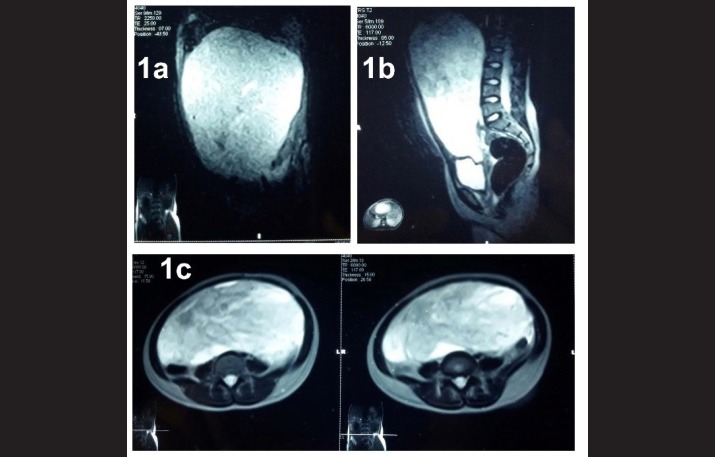
A 13 yr old girl presented to the Surgery Outpatient department (OPD) of SHKM GMC, Nalhar, Mewat, Haryana in December 2013 with pain in abdomen and huge abdominal lump since 2 months. Patient had attained menarche one year back and had normal menstrual history. She had fever, poor appetite, weight loss and backache. Physical examination showed a large abdominal mass. Hemoglobin was 8.5 g%. Complete Blood Count (CBC), Liver Function Tests (LFT), Kidney Function Tests (KFT) were with in normal limits. Serum α-fetoprotein (AFP) level was 612μg/L, serum human chorionic gonadotropin (βHCG)was 833.33mIU/mL, CA-125 was 339.4U/mL, lactate dehydrogenase (LDH) was 260U/L. Chest radiograph was normal. MRI revealed a 25x24x15 cm heterogenous, solid as well as cystic mass (Fig., 1a, 1b and 1c). Right ovary was unremarkable. Left sided salpingo-oophorectomy was done.
Fig. 1 (a, b, c).
MRI image revealing an intraabdominal_heterogenous, solid as well as cystic mass arising from left ovary
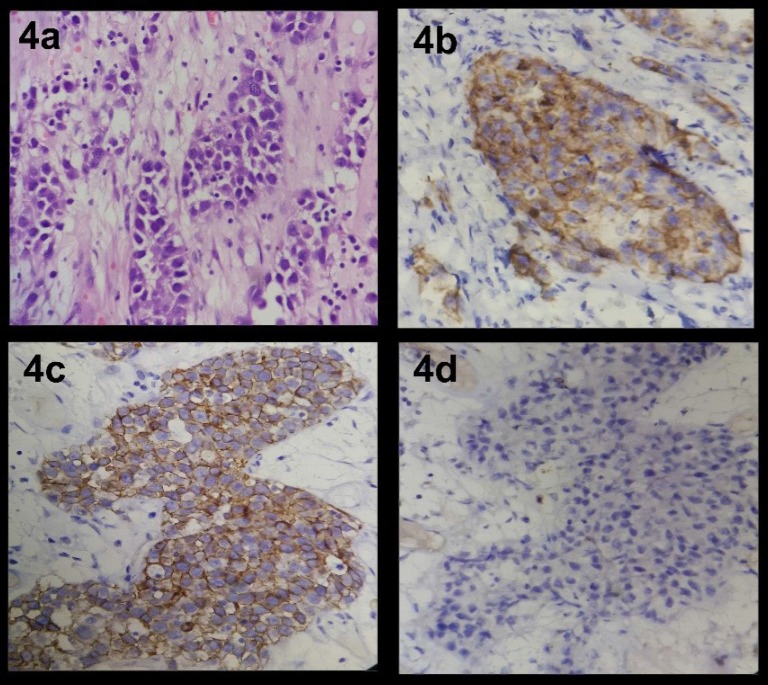
Gross examination revealed a large mass of 24x22x17 cm and weighing 3.8 kg, soft to firm to hard in consistency having bosselated external surface (Fig. 2a). Cut surface showed variegated appearance with solid greyish white, soft cystic areas with haemorrhage and necrosis. At places grey yellow mucoid areas were seen. No hair; bones; tooth were present (Fig. 2b). Microscopic examination revealed immature mesenchymal elements, epithelial elements represented by mucus secreting glands and islands of squamous cells. Few epithelial cells were seen forming nests and glands. They had pleomorphic nuclei with prominent nucleoli and showed multilayering. Mesenchymal areas showed prominent myxoid change and attempted cartilage formation. Large areas of necrosis and haemorrhage were present (Fig. 3a, 3b, 3c, 3d and Fig. 4a). Immunohistochemical studies revealed positive staining for CD30 and PLAP but negative staining for CD117 (Fig. 4b, 4c and 4d). The diagnosis was immature teratoma; grade 3 with coexistent embryonal carcinoma, left ovary. Immature teratoma component comprised 80% of the tumor. Random shave cut margins were positive for tumor. The fallopian tube showed normal histology. Omental biopsy was negative for tumor. Peritoneal fluid was negative for malignant cells on cytological examination. Patient had stage 1c disease. Imaging studies were not done postoperatively.
Fig. 2 (a).
Bosselated external surface of ovarian tumor. (b): Cut surface shows solid grey white, variegated and cystic appearance at places
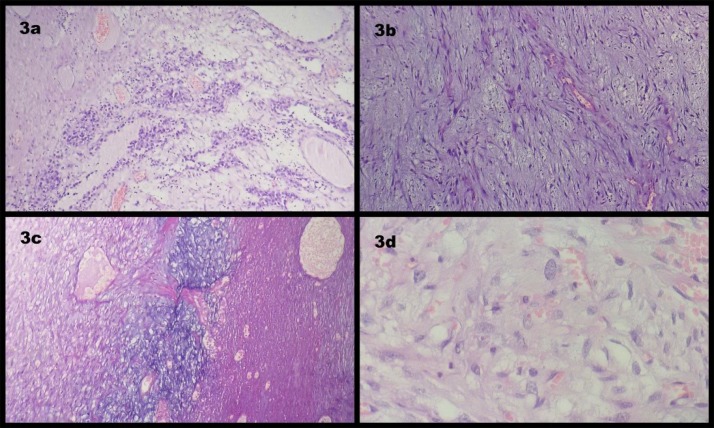
Fig. 3a.
Photomicrograph showing immature epithelial elements arranged in nests and glands. (H&E; x100). (3b): Photomicrograph showing immature mesenchymal elements. (H&E; x100). (3c): Photomicrograph showing immature mesenchymal elements with attempted cartilage formation and areas of necrosis. (H&E; x100). (3d): Photomicrograph showing sheets of tumor cells with large polygonal nuclei and prominent nucleoli; (Embryonal component) (H&E; x400
Fig. 4.
4 a: Photomicrograph revealing cells forming sheets and poorly formed glands. Nuclei are pleomorphic and show prominent nucleoli; (Embryonal component) (H&E; x400). (4b): Photomicrograph of embryonal component revealing positive immunostaining for CD30 (x400), (Dako, 1:100). (4c): Photomicrograph of embryonal component revealing positive immunostaining for PLAP (x400), (Dako, 1:100). (4d): Photomicrograph of embryonal component revealing negative immunostaining for CD117 (x400), (Dako, 1:100
After treatment the serum level of AFP was 7.10 ng/ml, βHCG was < 1.20mIU/mL, CA125 was 22.50 U/mL and LDH was 230 U/L.
Discussion
Germ cell tumors constitute 15-20% of all ovarian neoplasms (5). They are derived from the primitive germ cells of the embryonic gonad. These tumors include dysgerminoma, yolk sac tumor, choriocarcinoma, embryonal carcinoma, mature teratoma, immature teratoma and malignant ovarian mixed germ cell tumors. All ovarian germ cell tumors are malignant except for mature teratoma (4). Malignant ovarian germ cell tumors (MOGCT) comprise less than 5% of all ovarian neoplasms (5). “In children and adolescents, more than 60% of ovarian neoplasms are of germ cell origin, of which approximately 1/3rd are malignant” (1). In adults, the vast majority of germ cell tumors are benign (mature cystic teratomas) (4). Bhattacharyya et al. (6) found that out of a total 34 malignant ovarian tumors in girls below 20 years of age, 22 cases (66%) were malignant. Dysgerminoma was the commonest malignant germ cell tumor (41%). Malignant mixed germ cell tumor constituted 23.5% of all malignant germ cell tumors and 5.29% of all ovarian tumors. Mixed germ cell tumors of the ovary contain two or more malignant germ cell components. Mixed germ cell tumors constituted 14.3% of all MOGCT (7) while other study reported that MOGCT comprised 4.2% of all malignant ovarian tumors and malignant mixed germ cell tumors comprised 27% of all MOGCT (8).
The most common component of a MOGCT is dysgerminoma (80%), followed by yolk sac tumor (70%), immature teratoma (53%), choriocarcinoma (20%) and embryonal carcinoma (16%) (3). A mixture of dysgerminoma and yolk sac tumor is the most common combination (8, 9). In our case the combination was that of immature teratoma and embryonal carcinoma which is rare. Clinically, majority of patients with germ cell tumors present with abdominal pain, abdominal distension or a pelvic mass. Approximately 10% of patients will present with acute abdominal pain, usually caused by rupture, haemorrhage or torsion of the ovarian mass. A few patients can exhibit isosexual precocity, presumably due to HCG production by the tumor (10). In our case, the patient presented with rapidly increasing abdominal lump without any evidence of precocious puberty. Germ cell tumors secrete biologic markers that are quantifiable in serum and serve as a tool for monitoring treatment results and for detection of subclinical recurrences. Yolk sac tumor secretes AFP, while choriocarcinoma produces β-HCG. MOGCTs can secrete both β-HCG and AFP or none of the markers, depending on their components. Dysgerminoma does not secrete β-HCG or AFP and detection of either marker should prompt pathological re-examination to exclude the presence of non dysgerminomatous elements (11).
Treatment consists of salpingo-oophorectomy with adjunctive chemotherapy. In view of malignant germ cell tumors occurring almost exclusively in young females, preservation of their ovarian function and fertility is becoming an important although controversial issue. Normal gonadal function and fertility are possible after conservation surgery for ovarian germ cell malignancies, even with adjuvant chemotherapy (12).
Survival rates have dramatically improved with the use of combination regimes including vincristine/ dactinomycin/ cyclophosphamide (VAC) and platinum based combinations.
Currently, cure rates for patients with early stage MOGCT approach 100% (12).In our study, the patient had stage Ic disease despite the huge tumor size. This may be due to rapid progression of tumor because of which early diagnosis was done.
The prognosis of patients with stage I tumors can be predicted by the size and proportion of the various germ cell elements. Patients with tumors having a diameter greater than 10cm and containing more than 33% of endodermal sinus elements, choriocarcinoma or grade 3 immature teratoma have poor prognosis (3). Bilateral ovarian germ cell tumors are more often associated with advanced stage disease, high-risk histology and poor survival as compared to unilateral tumors (11).
Our patient has not yet attended post operatively for chemotherapy despite repeated contacts made by the hospital.
Conclusion
Malignant mixed germ cell tumor of the ovary is highly aggressive and grows rapidly in size. The importance of proper diagnostic workup and timely diagnosis in cases of pelvic masses in adolescent girls must be emphasised in order to provide early and adequate treatment; thus preventing recurrence and preserving prospects of reproductive future.
Conflict of Interest
The authors declare that there is no conflict of interests.
References
- 1.M Koshy, A Vijayanathan, V Vadiveloo. Malignant ovarian germ cell tumor: a rare combination. Biomed Imaging Interv J. 2005;1(2):e10. doi: 10.2349/biij.1.2.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munemane A, Munemane M. Malignant mixed germ cell tumours of ovary. A Report of two cases. Int J Med Sci Public Health. 2014;3(1):105–107. [cited October11, 2015] [Google Scholar]
- 3.Kurman RJ, Noris HJ. Malignant germ cell tumors of ovary. Human Pathol. 1977;8:551–64. doi: 10.1016/s0046-8177(77)80115-9. [DOI] [PubMed] [Google Scholar]
- 4.Kwok KKM, Loke TKL, Hui JPK, Lai MHY, Chan JCH. Malignant mixed germ cell tumor of the ovary in a 10 year old girl. JHK CollRadiol. 2008;11:92–5. [Google Scholar]
- 5.Gershenson DM, Del Junco G, Copeland LJ. Mixed germ cell tumors of the ovary. ObstetGynecol. 1984;64(2):200–6. [PubMed] [Google Scholar]
- 6.Bhattacharyya NK, De A, Bera P, Mongal S, Chakraborty S, Bandopadhyay R. Ovarian tumors in pediatric age group- A clinicopathologic study of 10 years’ cases in West Bengal, India. Ind J Med PaedOncol. 2010;31(2):54–7. doi: 10.4103/0971-5851.71656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abu-Zaid A, Nazer A, AlOmar O, Azzam A, Al-Eid HS, Elhassan TA et al. Incidence of malignant ovarian germ cell tumors (MOGCTs) in Saudi Arabia. HematolOncol Stem Cell Ther. 2014;7(1):41–3. doi: 10.1016/j.hemonc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Cicin I, Eralp Y, Saip P, Ayan I, Kebudi R, Iyibozkurt C. Malignant Ovarian germ cell tumors. Am J ClinOncol. 2009;32:191–6. doi: 10.1097/COC.0b013e3181841f2e. [DOI] [PubMed] [Google Scholar]
- 9.Kurman RJ, Norris HJ. Embryonal Carcinoma of the ovary. A clinicopathologic entity distinct from endodermal sinus tumor resembling embryonal carcinoma of adult testis. Cancer. 1976;38(6):2420–33. doi: 10.1002/1097-0142(197612)38:6<2420::aid-cncr2820380630>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Williams SD. Ovarian germ cell tumors: an update. SeminOncol. 1998;25(3):407–13. [PubMed] [Google Scholar]
- 11.Sekiya S, Seki K, Nagai Y. Rise of serum CA 125 in patients with pure ovarian yolk sac tumors. Int J GynaecolObstet. 1997;58:323–4. doi: 10.1016/s0020-7292(97)00094-5. [DOI] [PubMed] [Google Scholar]
- 12.Zanetta G, Bonazzi C, Cantu M. Survival and reproductive function after treatment of malignant germ cell ovarian tumors. J Clin Oncol. 2001;19(4):1015–20. doi: 10.1200/JCO.2001.19.4.1015. [DOI] [PubMed] [Google Scholar]
- 13.Haider M, Sanjeev K, Shelly S, Assaad S, Ramesh B, David L et al. Prognostic impact of Laterality in Malignant Ovarian Germ Cell Tumor. Int J Gynecol Can. 2011;21(2):257–62. doi: 10.1097/IGC.0b013e31820581e5. [DOI] [PubMed] [Google Scholar]