Abstract
Objectives
Sepsis is one of the most common reasons of increased mortality and morbidity in the intensive care unit. The changes in CRP levels and hemogram parameters and their combinations may help to distinguish sepsis from non-sepsis SIRS. The aim of this study is to investigate the CRP and hemogram parameters as an indicator of sepsis.
Methods
A total of 2777 patients admitted to the ICU of two centers between 2006–2013 were evaluated retrospectively. The patients were diagnosed as SIRS (-), non-sepsis SIRS and sepsis. The patients who were under 18 years old, re-admitted, diagnosed with hematological disease, on corticosteroid and immunosuppressive therapy, SIRS (-), culture negative, undocumented laboratory values and outcomes were excluded. 1257 patients were divided into 2 groups as non-sepsis SIRS and sepsis. The patients’ demographic data, CRP levels, hemogram parameters, length of ICU stay and mortality were recorded.
Results
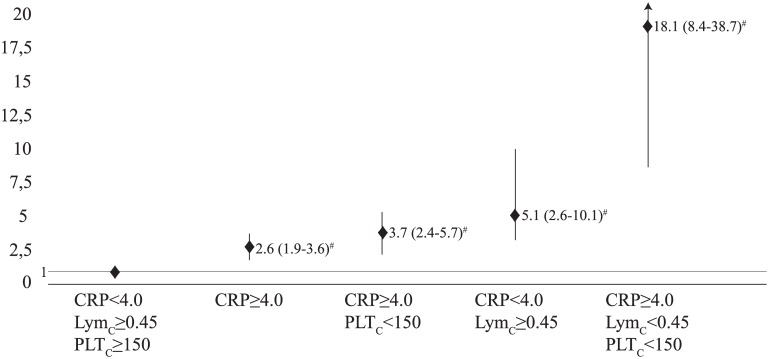
1257 patients were categorized as non-sepsis SIRS (816, 64.9%) and sepsis (441, 35.1%). In the multivariate analysis, the likelihood of sepsis was increased 3.2 (2.2–4.6), 1.7 (1.2–2.4), 1.6 (1.2–2.1), 2.3 (1.4–3.8), 1.5 (1.1–2.1) times by the APACHE II≥13, SOFA score≥4, CRP≥4.0, LymC<0.45 and PLTC<150 respectively (p<0.001 p = 0.007 p = 0.004 p<0.001 p = 0.027). The likelihood of sepsis was increased 18.1 (8.4–38.7) times by the combination of CRP≥4.0, lymC<0.45 and PLTC<150 (P<0.001).
Conclusions
While WBCC, NeuC, Neu%, NLCR and EoC are far from being the indicators to distinguish sepsis from non-sepsis SIRS, the combinations of CRP, LymC and PLTC can be used to determine the likelihood of sepsis.
Introduction
Systemic inflammatory response syndrome (SIRS) which occurs due to infection or non-infectious reasons is a clinical status. SIRS is the occurrence of at least two of the following criterias: fever>38°C or <36°C, heart rate>90 min-1, respiratory rate >20 min-1, white blood cell count (WBCC)>12000 or >4000 L-1 [1]. In the last guideline, SIRS criterias are diagnostic criteria for sepsis [2]. However, at the ICU admission, the patients often display SIRS criterion but sepsis is not diagnosed in a considerable number of these patients. It is known that sepsis is one of the most common reasons of increased mortality and morbidity in the intensive care unit (ICU) [3]. Therefore, it is crucial to distinguish sepsis from non-sepsis SIRS at the ICU admission. C-reactive protein (CRP) which is produced in liver is an acute phase reactant and it is known that CRP is comprised of five subunits and deposited at sites of inflammation [4]. In the last guideline, increase in CRP levels by 2 standard deviation (SD) is defined as a diagnostic criteria for sepsis [2]. However, CRP level can be increased by other factors such as cardiovascular disease, chronic obstructive pulmonary disease and obstructive sleep apnea syndrome [4–7]. Furthermore, the increase in CRP levels by 2 SD is commonly seen in a considerable number of patients admitted to the ICU. Hence, hemogram parameters which are inexpensive laboratory tests can be helpful for diagnosis of sepsis. Although WBCC was indicated as a sepsis criteria in the last guideline, some studies have demonstrated that it has low sensitivity and specificity for sepsis diagnosis [8,9]. Neutrophil count (NeuC) and eosinophil count (EoC) were used as a predictor of sepsis in the early 1990s [9–13]. EoC and lymphocyte count (LymC) were known to decrease in acute stress disorders such as trauma or infection [14,15]. Thus, in some studies, Eoc, LymC and neutrophil-lymphocyte count ratio (NLCR) were used as indicators for sepsis diagnosis [8,9,16,17]. The changes of CRP levels and hemogram parameters and their combinations may help to distinguish sepsis from non-sepsis SIRS at the ICU admission. The aim of this study was to investigate the CRP and hemogram parameters as an indicator of sepsis diagnosis.
Materials and Methods
Study design
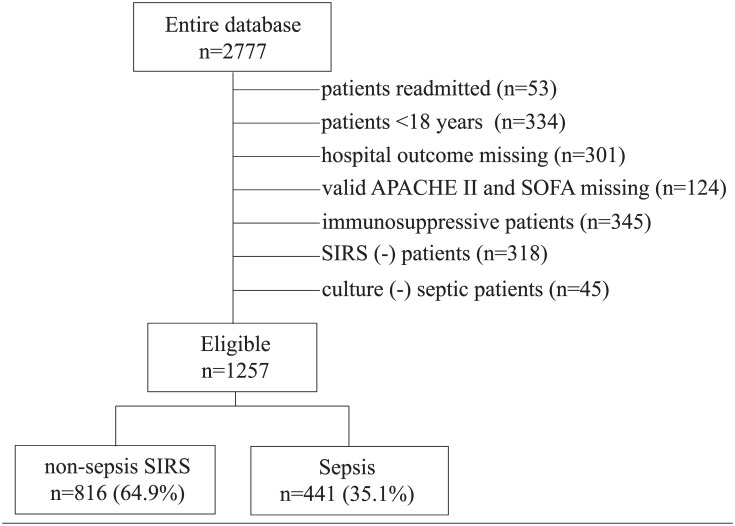
A total of 2777 medical and surgical patients admitted to the ICU’s of Acibadem International Hospital and Ataşehir Memorial Hospital between 1 January 2006 and 31 December 2013 were evaluated retrospectively. The study protocol was approved by the Acibadem University Medical Faculty Ethics Committee. Informed consent was not required because of the retrospective nature of the study. In the process of evaluating files of patients, the personal details of these patients were not recorded. The patients were diagnosed as SIRS (-), non-sepsis SIRS and sepsis at the ICU admission. SIRS and sepsis were defined in accordance with 1992 Sepsis Guideline [1]. The patients who were under 18 years old, re-admitted, diagnosed with hematological disease, on corticosteroid and immunosuppressive therapy, SIRS (-), culture negative, undocumented laboratory values and outcomes were excluded. The eligible patients were divided into 2 groups namely non-sepsis SIRS and sepsis (Fig 1).
Fig 1. Study flowchart.
Abbreviations: APACHE II, Acute Physiology And Chronic Health Evaluation; SIRS, systemic inflammatory response syndrome; SOFA, Sequential Organ Failure Assessment.
Database
The patients’ age, gender, APACHE II (Acute Physiology And Chronic Health Evaluation) and SOFA (Sequential Organ Failure Assessment) scores, diagnosis (medical, elective and emergency surgery), length of ICU stay, mortality, CRP (mg dL-1), WBCC (x103 μL-1), NeuC (x103 μL-1), LymC (x103 μL-1), NLCR, EoC (μL-1), platelet count (PLTC) (x103 ul-1), mean platelet volume (MPV) (fL) were recorded. All laboratory values were obtained from the Acibadem Inernational Hospital and Ataşehir Memorial Hospital databases.
Sepsis Definition
Sepsis was defined in accordance with 1992 sepsis guideline [1]. The patients who had at least two SIRS criterias (WBCC>12.000 or <4000 or >10% immature form; temperature>38.3°C or <36.0°C; respiratory rate >25 or PaCO2<32 mmHg; heart rate >90) on ICU admission and positive culture were considered to be sepsis. CRP was not used as a criterion in the diagnosis of sepsis.
Laboratory measurements
Evaluated blood samples had been taken at ICU admission prior to any medical treatment. In both hospitals, all blood samples taken for hemogram parameters were stored in the tubes in which ethylene diamine tetra acetic acid was used as anticoagulant and the measurements were carried out with Sysmex hematology analyzer (Sysmex XT-2000i, Kobe, Japan). WBCC, NeuC, LymC and EoC were measured by the application of semiconductor flow cytometry method; PLTC was measured with hydrodynamic focusing DC detection and semiconductor laser flow cytometry method and MPV was measured with the use of PLT-particle-size distribution method. The blood samples taken for CRP was stored in vacuumed tubes in which silica gel was used. CRP was measured with a Cobas Integra (Roche Diagnostics, Mannheim, Germany) device by applying the immunoturbidimetry method.
Cultures
The patients’ cultures (bloodstream, respiratory secretion, urine, cerebrospinal fluid) which had been taken at the ICU admission before antibiotics were administered were recorded. Colony counts 100000 CFU mL-1 or more were accepted as positive culture. The type of microorganisms were recorded as gram-negative bacteria, gram-positive bacteria, fungi and multiple microorganisms. There was no viremia in any patients.
Statistical analysis
The stastistical analysis was perfomed using the Wizard Pro Version 1.7.20 (154). All variables in the database were summarized using descriptive statistics. Categorical data were described with number (percentage) and analyzed with chi-square test. Sepsis and non-sepsis SIRS groups and survival and non-survival groups were compared with Mann Whitney U test due to non-normal distribution patterns. Results were given as percentage and median (interquartiles). Effects of parameters to estimate sepsis were evaluated with multivariate logistic regression model. Logistic regression analysis model included age, APACHE II and SOFA scores, diagnosis at ICU admission, CRP, WBCC, LymC, NLCR, NeuC, PLTC. Cut-off values for sepsis were determined by using the received operation curve (ROC) analysis. Type 1 error level was set as 5%. Correlation test was used for correlation between parameters and given as r2 value.
Results
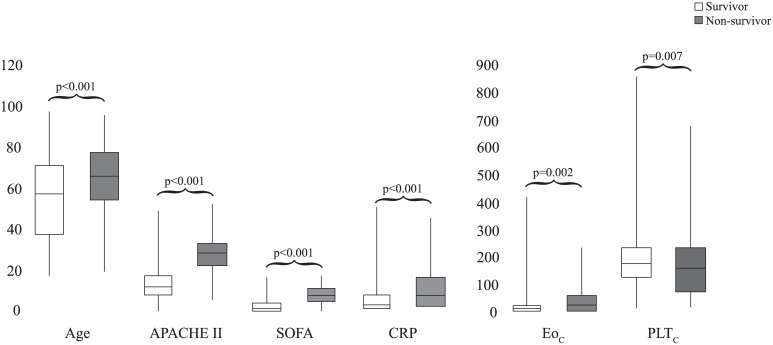
1257 patients were included in the study. Non-sepsis SIRS group consisted of 816 (64.9%), sepsis group consisted of 441 (35.1%) patients (Fig 1). In the sepsis group; age, APACHE II and SOFA scores, length of ICU stay, mortality, CRP and NLCR were significantly higher than non-sepsis SIRS group (p<0.001 for each). WBCC, NeuC, LymC and PLT were significantly lower in sepsis group than non-sepsis SIRS group (p = 0.003 p = 0.005 p<0.001 p = 0.01 respectively). Gender, Neu%, EoC and MPV were similar in both groups (p = 0.906 p = 0.312 p = 0.176 p = 0.733 respectively). Gram-negative microorganisms were most common in the sepsis group (28.1%). Cut off values of CRP, LymC, NeuC, NLCR and PLTC for sepsis were ≥4.0, <0.45, ≥10.0, ≥14.2 and <150 (Table 1). In non-survivor patients, age, APACHE II and SOFA scores, CRP and EoC were significantly higher; PLTC was significantly lower than survivor patients. (p<0.001 p<0.001 p<0.001 p<0.001 p = 0.002 and p = 0.007) (Fig 2).
Table 1. Demografic data and clinicall outcome.
| non-sepsis SIRS (n = 816) | Sepsis (n = 441) | p | |
|---|---|---|---|
| Age, years, | 55 (37–69) | 63 (51–76) | <0.001 |
| Male, n (%) | 482 (59.1) | 262 (59.4) | 0.906 |
| APACHE II | 9 (6–13) | 18 (14–25) | <0.001 |
| SOFA | 1 (0–2) | 4 (1–7) | <0.001 |
| Diagnosis | <0.001 | ||
| Elective surgery, n (%) | 540 (66.3) | 76 (17.2) | <0.001 |
| Medical diseases, n (%) | 240 (29.4) | 354 (80.3) | <0.001 |
| Emergency surgery, n (%) | 36 (4.3) | 11 (2.5) | <0.001 |
| Microorganisms n (%) | 0 (0.0) | 124 (28.1) | <0.001 |
| Gram-negative | 0 (0.0) | 64 (14.5) | <0.001 |
| Gram-positive | 0 (0.0) | 86 (19.5) | <0.001 |
| Fungi | 0 (0.0) | 167 (37.9) | <0.001 |
| Multiple organism | <0.001 | ||
| Length of ICU stay, days | 1 (1–2) | 4 (2–10) | <0.001 |
| Mortality, n(%) | 25 (3.1) | 104 (23.6) | <0.001 |
| CRP, (<0.5)a, (≥4.0)b | 2.0 (0.5–6.1) | 5.6 (1.6–13.9) | <0.001 |
| WBCC, (3.98–10.04)a | 11.27 (8.18–15.05) | 10.04 (7.1–14.62) | 0.003 |
| NeuC, (1.56–6.13)a, (≥10.0)b | 9.29 (6.55–12.7) | 8.27 (5.58–12.73) | 0.005 |
| Neu (%) | 85 (80–88) | 85 (79–90) | 0.312 |
| LymC, (1.18–3.74)a, (<0.45)b | 0.93 (0.62–1.36) | 0.71 (0.44–1.16) | <0.001 |
| NLCR, (≥14.2)b | 10 (6.7–14.5) | 11.5 (7.2–18.6) | <0.001 |
| EoC, (40–360)a | 10 (0–40) | 10 (0–30) | 0.176 |
| PLT, (182–369)a, (<150)b | 190 (133–242) | 171 (101–256) | 0.01 |
| MPV, (9.4–12.4)a | 10.1 (9.4–10.7) | 10 (9.3–10.8) | 0.733 |
a normal values for hemogram parameters.
b cut off values for likelihood of sepsis.
Results were given as percentage and median (interquartiles). Mann-Whitney U and chi-square tests were used for analysis. P<0.05 was accepted for statistically significant. Abbreviations: APACHE II, Acute Physiology And Chronic Health Evaluation; CRP, C-reactive protein; EoC, eosinophil count; MPV, mean platelet volume; NeuC, neutrophil count; NLCR, neutrophil-lymphocyte count ratio; LymC, lymphocyte count; PLTC, platelet count; SOFA, Sequential Organ Failure Assessment; WBCC, white blood cell count
Fig 2. Comparison of survivor and non-survivor patients.
Abbreviations: APACHE II, Acute Physiology And Chronic Health Evaluation; CRP, c-reactive protein; EoC, eosinophil count; PLTC, platelet count; SOFA, Sequential Organ Failure Assessment.
In the multivariate analysis, the likelihood of sepsis was increased 3.2 (2.2–4.6), 1.7 (1.2–2.4), 1.6 (1.2–2.1), 2.3 (1.4–3.8), 1.5 (1.1–2.1) times by the APACHE II≥13, SOFA score≥4, CRP≥4.0, LymC<0.45 and PLTC<150 respectively (p<0.001 p = 0.007 p = 0.004 p<0.001 p = 0.027) (Table 2). The likelihood of sepsis was increased 18.1 (8.4–38.7) times by the combination of CRP≥4.0, lymC<0.45 and PLTC<150 (p<0.001) (Fig 3).
Table 2. Multivariate logistic regression model for sepsis.
| OR (95% CI) | p | |
|---|---|---|
| Age | 1.001 (0.993–1.009) | 0.776 |
| Medical disease | 5.3 (3.7–7.7) | <0.001 |
| APACHE II≥13 | 3.2 (2.2–4.6) | <0.001 |
| SOFA score≥4 | 1.7 (1.2–2.4) | 0.007 |
| CRP≥4.0 | 1.6 (1.2–2.1) | 0.004 |
| WBCC<4.0 | 1.2 (0.6–2.4) | 0.577 |
| WBCC>12.0 | (0.5–1.04) | 0.083 |
| NeuC≥10 | 1.1 (0.8–1.6) | 0.630 |
| LymC<0.45 | 2.3 (1.4–3.8) | <0.001 |
| NLCR≥14.2 | 1.4 (0.9–2.1) | 0.142 |
| PLTC<150 | 1.5 (1.1–2.1) | 0.027 |
Abbreviations: 95% CI, 95% confidence interval; OR, odds ratio
Fig 3. Combinations of CRP and hemogram parameters for likelihood of sepsis.
Abbreviations: CRP, c-reactive protein; EoC, eosinophil count; PLTC, platelet count. #, p<0.001.
Discussion
The present study shows that CRP≥4.0, LymC<0.45 and PLTC<150 at the ICU admission can be helpful in identifying sepsis. While WBCC, NeuC, EoC and Neu% do not have any contribution towards distinguishing sepsis from non-sepsis SIRS, combinations of CRP, LymC and PLTC can be used to determining sepsis at the ICU admission.
CRP values over 0.5 mg dL-1 are shown to be related to the infection-induced inflammatory response [18]. Increase in CRP levels by 2 SD was also defined as sepsis criteria in 2012 sepsis guideline [2]. In the present study, 868 (69.1%) patients had got an increase in CRP levels by 2 SD at the ICU admission. However, 362 (41.7%) of them was diagnosed as sepsis. In 97 (21.7%) of septic patients, cardiovascular diseases and COPD were determined. Their median CRP level was 8. Furthermore, there was a poor positive correlation between CRP and each of age and sepsis (r2 = 0.04 and r2 = 0.09). Cardiovascular diseases and COPD are generally determined in overaged and it can be a reason for that correlation. 506 patients had got an increase in CRP levels by 2 SD but they were not diagnosed as sepsis. 344 (68%) of them was elective and emergency surgery patients. That results show that CRP is an inflammatory marker and it can be affected from many inflammatory clinical status. There are studies showing that procalcitonin (PCT) is a valuable marker compared to CRP as an indicator of infection. However, there are studies supporting the opposite findings [19–22].
Although CRP was related with sepsis and mortality in our patients, we are of the opinion that a combined evaluation of CRP and other hemogram parameters would increase the efficiency in diagnosing sepsis (Figs 2 and 3 and Table 2).
While <4000 or >12000 WBCC, was described as SIRS criterion in 1992 guideline, it was among the inflammatory variables of sepsis in 2012 guideline. [1,2]. Kim et al. did not indicate any difference in WBCC of sepsis and non-sepsis groups [8]. However, de Jagger et al., showed that AUC value (0.53) of WBCC for infection was not a more reliable marker than other hemogram parameters [9]. In our study, we found out that in sepsis group, WBCC was significantly lower than non-sepsis SIRS group. We believe that this difference isn’t very important since median values of WBCC for both groups are in normal range. Additionally, we didn’t find any relationship between WBCC and each of CRP, sepsis and mortality. Although WBCC is a diagnostic criteria for sepsis, we assume that WBCC at the ICU admission is far from being an important marker in diagnosing sepsis.
In endotoxemia, it is known that NeuC increases while LymC decreases in the circulation [23]. Hawkins et al. showed resistant B and T lymphopenia in gram-positive bacteraemia [24]. We indicated that there was no difference between CRP, NeuC and LymC values of gram-negative and gram-positive groups. It was stated that NLCR was an indicator of infection [25]. de Jagger et al. argued that LymC was a good indicator for infection and they indicated that NLCR had higher AUC value for mortality but did not have significant importance in the multivariate analysis [9,26]. Although Terradas et al. detected NLCR increase in sepsis and did not evaluate the effect of NeuC and LymC on this ratio [17]. In the present study, while NeuC and LmyC were significantly decrease, NLCR was also significantly increase in sepsis group. In this respect, the reason of increased NLCR in sepsis group can be a greater decrease in LymC than NeuC. In multivariate analysis, the likelihood of sepsis was increased by only LymC<0.45 (Table 2). We indicated that the likelihood of sepsis was increased by increased CRP with lymphopenia (Fig 3). For this reason, we strongly believe that LymC can be more helpful than NeuC and NLCR for diagnosis of sepsis.
In acute infection, it is known that eosinopenia develops due to peripheral sequestration and suppression of mature eosinophil production and secretion from bone marrow [27]. Acute stress-related endogenous corticosteroid production or exogenous corticosteroid use may cause eosinopenia, as well [8]. In order to make a correct interpretation of EoC, we excluded the patients on corticosteroid and other immunosuppressive agents. Terradas et al. indicated that increased EoC was an indicator of recovery and EoC<50 was an indicator of bacteraemia [17]. Abidi et al. made the same conclusion for EoC<40 [16]. However, there was no information about the patients on corticosteroid who were excluded in these two studies. On the other hand, Kim et al. excluded patients with corticosteroid therapy in pediatric patient group and showed that EoC<15 increased the rate of mortality 2.96-fold [8]. Yet, they did not find out significant relationship between infection and EoC. We found out similar EoC values in both groups (Table 1). Even in non-survivor patients, EoC was significantly higher than survivor patients (Fig 2). We can speculate that increased EoC in non-survivor patients may be due to relative adrenal insufficiency. Therefore, EoC was also far from being an important marker in diagnosing sepsis.
PLTC was identified as a diagnostic criteria for sepsis in the last guideline [2]. In present study, we also found out that PLTC related with sepsis and mortality (Tables 1 and 2 and Fig 2).
Conclusions
CRP≥4.0, LymC<0.45 and PLTC<150 can be used as indicators to distinguish sepsis from non-sepsis SIRS. Thus, the combinations of these markers can be more helpful to predict sepsis at the ICU admission. Even WBCC, NeuC, Neu%, NLCR and EoC are far from being the indicators to distinguish sepsis from non-sepsis SIRS.
Supporting Information
(XLS)
Acknowledgments
The authors thank Henry Uwem Tyron for his editorial contribution; and Mehmet Berktas, MD, for his support in statistical analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101:1644–55. [DOI] [PubMed] [Google Scholar]
- 2.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock:2012. Crit Care Med 2013; 41:580–637. 10.1097/CCM.0b013e31827e83af [DOI] [PubMed] [Google Scholar]
- 3.Sessler CN, Shepherd W. New concept in sepsis. Curr Opin Crit Care 2002; 8:465–472. [DOI] [PubMed] [Google Scholar]
- 4.Ageawal A, Gang TB, Rusinol AE. Recognition functions of pentameric C-reactive protein in cardiovascular disease. Mediators Inflamm 2014; 2014:319215 10.1155/2014/319215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouloukaki I, Mermigkis C, Kallergis EM, Moniaki V, Mauroudi E, Schiza SE. Obstructive sleep apnea syndrome and cardiovascular disease: The influence of C-reactive protein. World J Exp Med 2015; 5:77–83. 10.5493/wjem.v5.i2.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito I, Maruyama K, Eguchi E. C-reactive protein and cardiovascular disease in East asians: a systematic review. Clin Med Insights Cardiol 2015; 8:35–42. 10.4137/CMC.S17066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw JG, Vaughan A, Dent AG, O’Hare PE, Goh F, Bowman RV, et al. Biomarkers of proghression of chronic obstructive pulmonary disease (COPD). J Thorac Dis 2014; 6:1532–47. 10.3978/j.issn.2072-1439.2014.11.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YH, Park HB, Kim MJ, Kim HS, Lee HS, Han YK, et al. Prognostic usefulness of eosinopenia in the pediatric intensive care unit. J Korean Med Sci 2013; 28:114–119. 10.3346/jkms.2013.28.1.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jager CP, van Wijk PT, Mathoera RB, de Jongh-Leuvenink J, van der Poll T, Wever PC. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit Care 2010; 14:R192 10.1186/cc9309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simon CE. A manual Clinical Diagnosis. London; 1922. [Google Scholar]
- 11.Gil H, Magy N, Mauny F, Dupond JL. Value of eosinopenia in inflammatory disorders: an “old” marker revisited. Rev Med Interne 2003; 24:431–5. [DOI] [PubMed] [Google Scholar]
- 12.Setterberg MJ, Newman W, Potti A, Smego RA Jr. Utility of eosinophil count as predictor of bacteremia. Clin Infect Dis 2004; 38:460–1. [DOI] [PubMed] [Google Scholar]
- 13.Wibrow BA, Ho KM, Flexman JP, Keil AD, Kohrs D. Eosinopenia as a diagnostic marker of bloodstream infection in hospitalized paediatric and adult patients: a case control study. Anaesth Intensive Care 2011; 39:224–30. [DOI] [PubMed] [Google Scholar]
- 14.Munck A, Náray-Fejes-Tóth A. Glucocorticoids and stress: permissive and suppressive actions. Ann N Y Acad Sci 1994; 746:115–130. [DOI] [PubMed] [Google Scholar]
- 15.Barnes PJ, Adcock I. Anti-inflammatory actions of steroids: molecular mechanisms. Trends Pharmacol Sci 1993; 14:436–441. [DOI] [PubMed] [Google Scholar]
- 16.Abidi K, Khoudri I, Belayachi J, Madani N, Zekraoui A, Zeggwagh AA, et al. Eosinopenia is a reliable marker of sepsis on admission to medical intensive care units. Crit Care 2008; 12:R59 10.1186/cc6883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terradas R, Grau S, Blanch J, Riu M, Saballs P, Castells X, et al. Eosinophil count and neutrophil-lymphocyte count ratio as prognostic markers in patients with bacteriemia: a retrospective cohort study. PLoS One 2012; 7:e42860 10.1371/journal.pone.0042860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Povoa P, Almeida E, Moreira P, Fernandes A, Mealha R, Aragao A, et al. C-reactive protein as an indicator of sepsis. Internsive Care Med 1998; 24:1052–6. [DOI] [PubMed] [Google Scholar]
- 19.Castelli GP, Pognani C, Cita M, Stuani A, Sgarbi L, Paladini R. C-reactive protein, white blood cells and SOFA score in ICU: diagnosis and monitoring of sepsis. Minerva Anestesiol 2006; 72:69–80. [PubMed] [Google Scholar]
- 20.Meisner M, Tschaikowsky K, Palmaers T, Schmidt J. Comparison of procalcitonin (PCT) and C-reactive protein (CRP) plasma concentrations at different SOFA scores during the course of sepsis and MODS. Crit Care 1999; 3:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller B, Becker KL, Schachinger H, Rickenbacher PR, Huber PR, Zimmerli W, et al. Ritz R. Calcitonin precursors are realible markers of sepsis in a medical intensive care unit. Crit Care Med 2000; 28:977–83. [DOI] [PubMed] [Google Scholar]
- 22.Luzzani A, Polati E, Dorizzi R, Rungatscher A, Pavan R, Merlini A. Comparison of procalcitonin and C-reactive protein as markers of sepsis. Crit Care Med 2003; 31:1737–41. [DOI] [PubMed] [Google Scholar]
- 23.Jilma B, Blann A, Pernerstorfer T, Stohlawetz P, Eichler HG, Vondrovec B, et al. Regulation of adhesion molecules during human endotoxemia. No acute effects of aspirin. Am J Respir Crit Care Med 1999; 159: 857–863. [DOI] [PubMed] [Google Scholar]
- 24.Hawkins CA, Collignon P, Adams DN, Bowden FJ, Cook MC. Profound lymphopenia and bacteraemia. Intern Med J 2006; 36:385–388. [DOI] [PubMed] [Google Scholar]
- 25.Zahorec R. Ratio of neutrophil to lymphocyte counts-rapid and simple parameter of systemic inflammatiion and stress in critically ill. Bratisl Lek Listy 2001; 102:5–14. [PubMed] [Google Scholar]
- 26.de Jager CP, Wever PC, Gemen EFA, Kusters R, van Gageldonk-Lafeber AB, van der Poll T, et al. The Neutrophil-Lymphocyte Count Ratio in Patients with Community-Acquired Pneumonia. PLoS One 2012; 7(10): e46561 10.1371/journal.pone.0046561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bass DA. Behavior of eosinophil leukocytes in acute inflammation. II. Eosinophil dynamics during acute inflammation. J Clin Invest 1975; 56:870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.