Abstract
Introduction
Streptococcus pneumoniae is the commonest cause of bacteremic pneumonia among HIV-infected persons. As more countries with high HIV prevalence are implementing infant pneumococcal conjugate vaccine (PCV) programs, we aimed to describe the baseline clinical characteristics of adult invasive pneumococcal disease (IPD) in the pre-PCV era in South Africa in order to interpret potential indirect effects following vaccine use.
Methods
National, active, laboratory-based surveillance for IPD was conducted in South Africa from 1 January 2003 through 31 December 2008. At 25 enhanced surveillance (ES) hospital sites, clinical data, including HIV serostatus, were collected from IPD patients ≥ 5 years of age. We compared the clinical characteristics of individuals with IPD in those HIV-infected and -uninfected using multivariable analysis. PCV was introduced into the routine South African Expanded Program on Immunization (EPI) in 2009.
Results
In South Africa, from 2003–2008, 17 604 cases of IPD occurred amongst persons ≥ 5 years of age, with an average incidence of 7 cases per 100 000 person-years. Against a national HIV-prevalence of 18%, 89% (4190/4734) of IPD patients from ES sites were HIV-infected. IPD incidence in HIV-infected individuals is 43 times higher than in HIV-uninfected persons (52 per 100 000 vs. 1.2 per 100 000), with a peak in the HIV-infected elderly population of 237 per 100 000 persons. Most HIV-infected individuals presented with bacteremia (74%, 3 091/4 190). HIV-uninfected individuals were older; and had more chronic conditions (excluding HIV) than HIV-infected persons (39% (210/544) vs. 19% (790/4190), p<0.001). During the pre-PCV immunization era in South Africa, 71% of serotypes amongst HIV-infected persons were covered by PCV13 vs. 73% amongst HIV-uninfected persons, p = 0.4, OR 0.9 (CI 0.7–1.1).
Conclusion
Seventy to eighty-five percent of adult IPD in the pre-PCV era were vaccine serotypes and 93% of cases had recognized risk factors (including HIV-infection) for pneumococcal vaccination. These data describe the epidemiology of IPD amongst HIV-infected and -uninfected adults during the pre-PCV era and provide a robust baseline to calculate the indirect effect of PCV in future studies.
Introduction
Streptococcus pneumoniae is the most common cause of bacteremia and bacterial pneumonia among HIV-infected persons, and has been shown to occur between 30 and 100 times more frequently in HIV-infected than HIV-uninfected individuals.[1–4] Recurrent disease is seen more frequently with HIV infection, with approximately 25% of patients having a recurrence within 12 months.[1, 5, 6]
This study was conducted in South Africa, which reports a HIV prevalence of 18% amongst adults in 2008.[7] In population groups with a high HIV prevalence the epidemiological pattern of invasive pneumococcal disease (IPD) seems to change, with a peak seen in the young adult population as well as the typical but more marked peaks seen in the infant and elderly population groups.[4, 6, 8] Despite the higher rate of IPD in HIV-infected individuals the majority of studies have not shown higher case-fatality ratios amongst people co-infected with HIV.[1, 3, 6]
Some differences in IPD epidemiology have been described in HIV-infected versus HIV-uninfected individuals. Firstly, HIV-infected adults tend to be infected more commonly with pneumococcal serotypes that are typically seen in young children, and in relation to this, HIV-infected people tend to have a higher proportion of penicillin non-susceptible isolates.[3, 9–12] In contrast, serotype 1 disease tends to be proportionally less common amongst HIV-infected than HIV-uninfected persons.[3, 4, 13] Besides HIV as a stand-alone risk factor, HIV-infected individuals with IPD tend to have less underlying disease than HIV-uninfected individuals.[14]
Use of the polysaccharide pneumococcal vaccine (PPV23) in HIV-infected African adults is controversial.[5] However, studies using the 7-valent pneumococcal conjugate vaccine (PCV7) are promising, showing a 74% vaccine efficacy against vaccine serotypes in HIV-infected adults with a previous episode of IPD, albeit with subsequent waning of immunity.[15] Some countries, including South Africa, have already shown an indirect effect in the adult population following infant PCV immunization.[16–18] In South Africa and the United States of America, this decrease was seen in both HIV-infected (-25 to -59% for vaccine serotypes attributed to childhood PCV immunization) and HIV-uninfected adults (-52 to -86% for vaccine serotypes).[18, 19]
Reporting baseline clinical characteristics of IPD amongst adults in a setting with high HIV prevalence is important to elicit the indirect and long-term effects, including serotype replacement, of PCV introduction on IPD in adult populations. In this analysis we aimed to describe the baseline epidemiology of IPD in the pre-PCV era amongst HIV-infected and -uninfected persons ≥ 5 years of age using data derived from national surveillance.
Methods
GERMS-SA conducts national, active laboratory-based surveillance across South Africa in a network consisting of approximately 130 public and private microbiology laboratories. Each laboratory is responsible for sending S. pneumoniae isolates, along with demographic details of the patient to a reference laboratory (the Centre for Respiratory Diseases and Meningitis (CRDM) at the National Institute for Communicable Diseases (NICD)) where confirmatory tests, serotyping and antimicrobial susceptibility testing are performed. Data from individuals diagnosed with IPD from 1 January 2003 through to 31 December 2008 were included in this analysis. From 2003 to 2005 our surveillance network strengthened and remained relatively stable thereafter. For this reason trends in incidence by serotype were only examined from 2005 onwards. Comprehensive audits of cases identified but not reported were conducted for all the public laboratories: unreported cases ranged from 16% to 21% over the 6 years and their demographic data were included in our database.[20] PCV7 was introduced into the South African EPI in 2009, and replaced by PCV13 in 2011. There was minimal use of either PCV7 or PPV23 during the study period.
IPD was defined as identification of S. pneumoniae from any usually sterile site (cerebrospinal fluid (CSF), blood, pleural fluid, joint fluid, ascitic fluid, vitreous fluid, etc.). Multiple isolates of S. pneumoniae identified from the same person were counted as one case provided they occurred within 21 days of the first isolate. S. pneumoniae were identified by either: culture of the organism, consistent Gram stain plus detection of S. pneumoniae antigen by latex agglutination, or detection of S. pneumoniae by direct PCR (polymerase chain reaction) of the specimen. Antimicrobial disc diffusion susceptibility testing of potentially resistant strains was confirmed by broth microdilution. Antimicrobial susceptibility was reported as susceptible or non-susceptible (intermediate and resistant isolates) based on the Clinical and Laboratory Standards Institute (CLSI) interpretive criteria.[21] Serotyping of viable organisms were determined using the Quellung reaction (Statens Serum Institut, Copenhagen, Denmark). Serotypes were grouped according to those covered by PCV13 (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) and PPV23 vaccines (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, 33F and serotype 6A cross-protection was assumed). When calculating incidence of individual serotypes we assumed the age-specific proportion of serotypes with non-viable or missing pneumococcal isolates to be that of those with available isolates and imputed serotype data for 5 041 cases.
Enhanced surveillance (ES) was conducted at 25 sentinel-hospital sites situated in all 9 provinces of South Africa. Where available, consenting persons with IPD (or their legal guardians) were interviewed using a standardized questionnaire. Clinical details were retrieved from medical record review performed on cases of IPD at these hospitals. As clinical details were only available from patients presenting to ES sites, a comparison of cases from enhanced versus non-enhanced surveillance sites was done to show representativeness of data from the ES sites.
The following demographic and clinical details were collected from each person under surveillance: age/ date of birth, sex and specimen type (CSF, blood, other for any other specimen from a sterile site). Additional details were collected at ES sites: clinical syndrome (meningitis, bacteremic pneumonia, bacteremia without focus, other), Pitt bacteremia score for severity of illness (score 0 for mild, 1–3 for moderate, 4–12 for severe illness),[22] HIV status, in-hospital outcome and any underlying condition predisposing to IPD. Predisposing conditions were further classified into ACIP (Advisory Committee on Immunization Practices) conditions (asplenia/ sickle cell disease; chronic lung, renal, liver or cardiac condition; diabetes; solid organ transplant; immunotherapy; malignancy; primary immunodeficiency; CSF leak following a head injury; alcohol dependency; tobacco use) and other risk factors (cerebrovascular accident; burns).[23] Demographic, laboratory and clinical details for each patient were linked using Epi Info, version 6.04d, and later updated to Access.
Incidence of IPD was calculated using South African population denominators from Statistics-South Africa.[24] HIV-specific incidence rates for South Africa were calculated by assuming that the proportion of patients who tested positive for HIV at sentinel sites was the same in those who were not tested. HIV population denominators for 2008 were obtained from the Thembisa model 1.7 version 3.[25]
Statistical analysis was done using Stata version 11 (StataCorp Inc., College Station, Texas, USA) and p-values of ≤0.05 were considered significant throughout. Trends in incidence rates by age-category and serotype were calculated using Poisson regression. Univariate analysis of characteristics associated with IPD among HIV-infected and HIV-uninfected persons was performed using Fisher’s exact test or the Mantel–Haenszel χ2-test for categorical variables. Multivariable logistic regression models were evaluated comparing IPD cases at enhanced and non-enhanced surveillance sites, and comparing IPD cases amongst those HIV-infected and–uninfected. We started with all variables that were significant at p-value less than 0.05 on univariate analysis, and dropped non-significant factors with stepwise backward selection. All two-way interactions were evaluated.
Ethical clearance and permission to conduct laboratory-based and enhanced surveillance in South Africa for this study was obtained from the Health Research Ethics Committee (Human), University of Witwatersrand (Clearance number M02-10-42); the University of Stellenbosch Health Research Ethics Committee (Reference number N04/01/0021), the National Institute for Communicable Diseases Research Committee (Clearance number M060449); and the South African Department of Health (Reference H2/12/8). The following consent procedures were approved by the above mentioned ethics committees: Written consent was obtained from all patients (or parents/legal guardians of minors) at ES sites, prior to being interviewed, for participation in the ES program. Patients from ES sites who had already been discharged from hospital were interviewed telephonically and gave verbal consent, documented by surveillance officers on the written consent forms. Parents/ legal guardians of minors gave written consent (or verbal if the child had already been discharged from hospital) on behalf of their children <18 years of age. Patients from ES sites who were unable to provide written or verbal consent (due to severity of illness, death or lost to follow up) were still included in the study and their clinical data were collected only through medical record review. Participants not consenting to answer the enhanced surveillance questionnaire were still included in the laboratory-based surveillance program. All patient identifiers were removed prior to data analysis.
Results
Surveillance Population
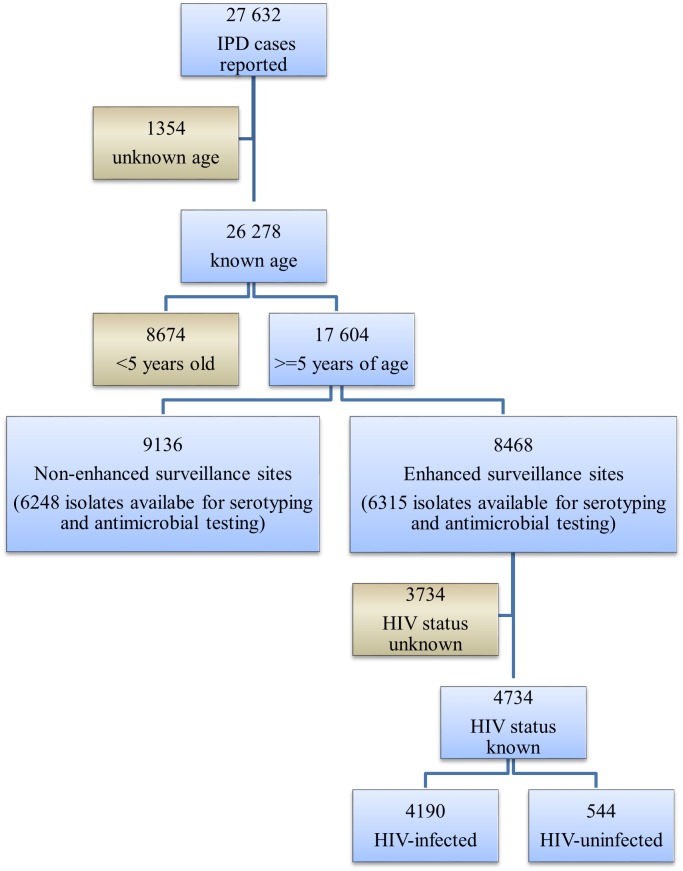
From 2003 through 2008, 27 632 individuals with IPD were reported to GERMS-SA surveillance. Ninety-five percent (26 278) had known age, and 17 604 (67%) of these were aged 5 years and older. Antimicrobial susceptibility testing and serotyping were performed on 71% (12 563/17 604) of the isolates. Forty-eight percent of individuals (8 468/17 604) were from ES sites. Medical records were found for 82% (6 960/8 468) of cases at ES sites and data on HIV-serostatus were available for 68% (4 734) of these individuals. (Fig 1)
Fig 1. Flow diagram of cases of invasive pneumococcal disease, South Africa, 2003–2008 (n = 27 632).
Incidence of invasive pneumococcal disease
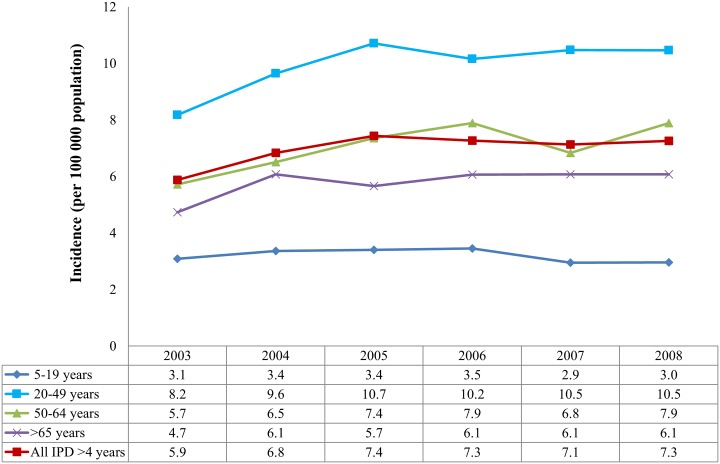
Incidence of IPD occurring in persons ≥ 5 years of age increased from 2003–2005, then remained relatively stable (7.4 to 7.3 per 100 000 population, p = 0.27; 95% CI 0.98–1.00) from 2005–2008. A small decrease in incidence was seen in the 5–19 year age group from 2005–2008 (3.4 to 3 per 100 000 population, p = 0.004, 95% CI 0.91–0.98). (Fig 2)
Fig 2. Incidence of invasive pneumococcal disease by age category and year, South Africa, 2003–2008 (n = 17 604).
Footnote: Between 2003 and 2004 there was a significant increase in disease (p<0.001), most notably in the 20–49 year age group (p<0.001), however from 2005 to 2008 disease incidence did not change significantly overall, however a small decrease was noted in the 5–19 year age group (p = 0.004).
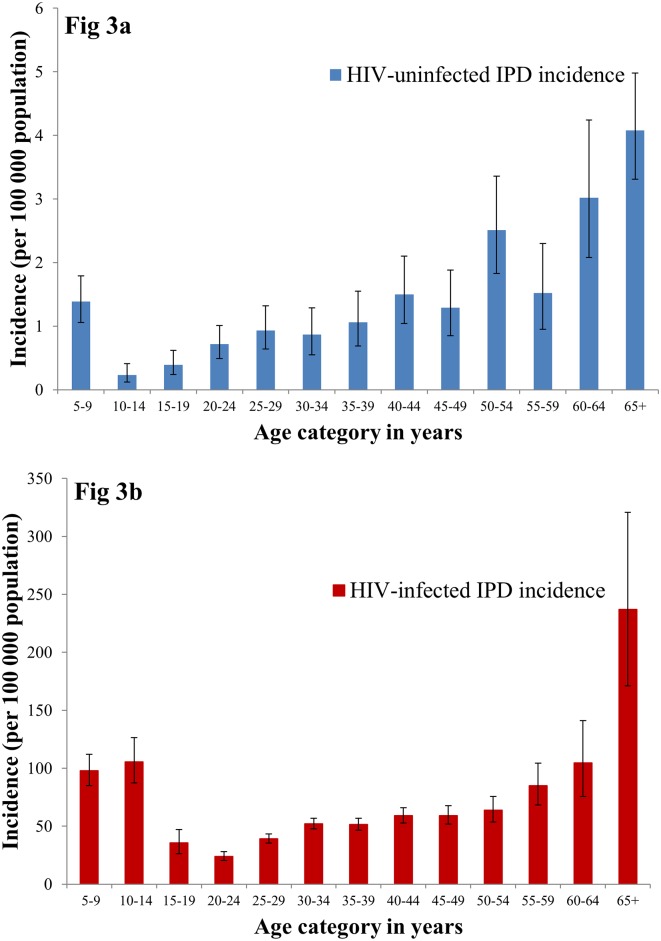
In 2008, the relative risk of IPD amongst HIV-infected individuals was 43 times greater than HIV-uninfected individuals (52 per 100 000 vs. 1.2 per 100 000, respectively). (Table 1) For HIV-uninfected individuals, incidence increased with increasing age, with a peak in those ≥ 65 years (4.1 per 100 000). (Fig 3a) However, in HIV-infected individuals incidence followed a U-shaped curve with peaks in the later childhood and elderly age categories (81.4 in 5–19 years; 46.3 in 20–49 years; 74.7 in 50–64 years; 237.2 in ≥65 years). (Fig 3b; Table 1)
Table 1. Annual incidence and relative risk of invasive pneumococcal disease amongst HIV- infected and -uninfected persons by age category, South Africa, 2008.
| Age category | Invasive pneumococcal disease incidence | Relative Risk | (95% confidence interval) | |||||
|---|---|---|---|---|---|---|---|---|
| Overall (95% confidence interval) | HIV-infected (95% confidence interval) | HIV-uninfected (95% confidence interval) | ||||||
| 5–19yrs | 3.2 | (3–3.5) | 81.4 | (73.4–90) | 0.6 | (0.5–0.8) | 127 | (101–160) |
| 20–49yrs | 10.1 | (9.7–10.5) | 46.3 | (44.3–48.4) | 1 | (0.9–1.2) | 46 | (40–54) |
| 50–64yrs | 7.9 | (7.1–8.8) | 74.7 | (66.1–84.1) | 2.3 | (1.9–2.8) | 32 | (26–41) |
| > 65yrs | 5.8 | (4.9–6.9) | 237.2 | (171–320.7) | 4.1 | (3.3–5) | 58 | (40–83) |
| All ages | 7.3 | (7.1–7.6) | 52 | (50.1–54) | 1.2 | (1.1–1.3) | 43 | (39–47) |
Fig 3. Incidence of invasive pneumococcal disease (IPD) amongst HIV-uninfected and HIV-infected persons by age category, South Africa, 2008.
Descriptive epidemiology
IPD was more common amongst females (53%, 9 096/17 375, gender unknown for 229 cases). Age ranged from 5 years through to 103 years (median 33 years, IQR 25–43). S. pneumoniae was cultured from CSF in 35% (6 128/17 604) of patients and from blood in 54% (9 537/17 604). The remaining 11% (1 939/17 604) were from either pleural fluid (1 643), peritoneal fluid (123), joint fluid (110) or pus from a deep-seated abscesses (63).
From 2005 to 2008, antimicrobial non-susceptibility of the isolates increased significantly for penicillin (range: 23–29% non-susceptible, MIC≥0.06 μg/ml (p<0.001)), erythromycin (8–11% non-susceptible, MIC≥0.25 μg/ml (p<0.001)) and cotrimoxazole (41–47% non-susceptible, MIC>0.5 μg/ml (p<0.001)) but remained constant for third generation cephalosporins (0.5% non-susceptible, MIC≥1.0 mg/ml (p = 0.028)).
Of 12 547 isolates serotyped (16 were non-typable), the commonest 10 serotypes included serotypes 1, 19A, 4, 14, 23F, 6A, 6B, 3, 8 and 19F (nine of which are included in PCV13). Thirteen patients had a mixed serotype infection. Serotype diversity increased by increasing age with 78% (1 732/2 223) of cases in the 5–19 year; 66% (5 563/8 443) in the 20–49 year; 65% (868/1 328) in the 50–64 year and 61% (337/553) in the ≥65 year age categories included in the commonest 10 serotypes.
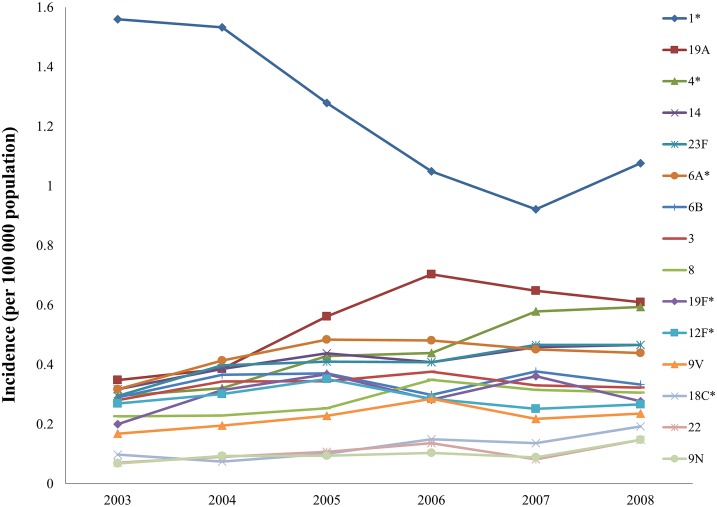
A significant decrease in serotype 1 disease incidence was seen during the surveillance period (p<0.001; 1.14 to 0.7 per 100 000 population), signifying an end to a serotype 1 epidemic that was ongoing prior to the start of the surveillance program. Other significant decreases once the surveillance program was properly established (2005–2008) were seen with serotypes 6A, 19F, 12F, 18A, 15C and 25. Serotypes 4 and 18C were the only serotypes showing significant increases in incidence over time. (Fig 4)
Fig 4. Incidence of invasive pneumococcal disease among persons 5 years and older by the commonest 15 serotypes causing disease, South Africa, 2003–2008 (n = 17 604).
Footnote: * Significant changes during the established surveillance period (2005–2008) were seen in serotype 1 (p<0.001) (down), 4 (0.02) (up), 6A (p = 0.0486) (down), 19F (p = 0.0306) (down), 12F (p = 0.0037) (down), 18C (p = 0.0368) (up). Of 17 604 cases of IPD, serotype data were missing and thus imputed for 5 041 cases.
Comparison of enhanced vs. non-enhanced surveillance sites
Significant differences between cases from enhanced (ES) versus non-enhanced surveillance sites (NESS) included specimen type (25% CSF at ES vs. 44% CSF at NESS, p<0.001, Odds Ratio (OR) 0.4 (95% CI 0.3–0.4)), cotrimoxazole non-susceptibility of the isolates (47% at ES vs. 44% at NESS, p<0.001, OR 1.1 (CI 1.1–1.2)) and percent of vaccine-preventable serotypes (77% serotypes at ES covered by PPV23 vs. 79% at NESS, p = 0.04, OR 0.9 (CI 0.8–1)). The latter two remained significant even after controlling for specimen type. (Table 2)
Table 2. Comparison of individuals with invasive pneumococcal disease identified at enhanced versus non-enhanced surveillance sites, South Africa, 2003–2008 (n = 17 604).
| Enhanced sites | Non-enhanced sites | Univariate analysis | Multivariable analysis* | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | Odds ratio (95% Confidence interval) | P-value | Odds ratio (95% Confidence interval) | P-value | |
| Total cases | 8468 | 48 | 9136 | 52 | ||||
| Age category | ||||||||
| 5–19 years | 1343 | 16 | 1609 | 18 | Reference | 0.012 | Reference | 0.152 |
| 20–49 years | 5884 | 70 | 6171 | 68 | 1.1 (1.1–1.2) | 1.1 (1–1.2) | ||
| 50–64 years | 886 | 11 | 983 | 11 | 1.1 (1–1.2) | 1.1 (0.9–1.2) | ||
| ≥65 years | 355 | 4 | 373 | 4 | 1.1 (1–1.3) | 1.0 (0.9–1.3) | ||
| Specimen type | ||||||||
| Cerebrospinal Fluid | 2106 | 25 | 4022 | 44 | Reference | <0.001 | ||
| Blood | 5683 | 67 | 3854 | 42 | 2.8 (2.6–3) | |||
| Other | 679 | 8 | 1260 | 14 | 1 (0.9–1.1) | |||
| Antimicrobial susceptibility | 6 315 | 6 248 | ||||||
| Penicillin | ||||||||
| Non-susceptible | 1582 | 25 | 1 514 | 24 | 1.0 (1–1.1) | 0.286 | ||
| Erythromycin | ||||||||
| Non-susceptible | 639 | 10 | 641 | 10 | 1 (0.9–1.1) | 0.795 | ||
| Cotrimoxazole | ||||||||
| Non-susceptible | 2 983 | 47 | 2752 | 44 | 1.1 (1.1–1.2) | <0.001 | 1.1 (1.1–1.2) | <0.001 |
| 3rd Generation Cephalosporin | ||||||||
| Non-susceptible | 34 | 1 | 30 | 1 | 1.1 (0.7–1.8) | 0.647 | ||
| Serotypes (n = 12 563) | 6 315 | 6 248 | ||||||
| PCV13** | ||||||||
| Vaccine serotypes | 4 450 | 70 | 4 469 | 72 | 0.9 (0.9–1) | 0.191 | ||
| PPV23*** | ||||||||
| Vaccine serotypes | 4 874 | 77 | 4 916 | 79 | 0.9 (0.8–1) | 0.043 | 0.9 (0.8–1) | 0.035 |
Footnote:
*Multivariable analysis was done including all variables with P-values <0.05 and controlling for laboratory syndrome (which accounts for specimen type tested).
**PCV13 (Pneumococcal Conjugate Vaccine-13 valent) serotypes include: 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F.
***PPV23 (Pneumococcal Polysaccharide Vaccine-23 valent) serotypes include: 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, 33F,(6A cross protection is assumed).
Comparison between HIV-infected vs. HIV-uninfected individuals at ES sites
Eighty-nine percent (4 190/4 734) of people with IPD from ESS with HIV data available were HIV-infected and 37% (1 568/4 190) of these HIV-infected patients were newly diagnosed during this episode of IPD.
On multivariable analysis, age between 20 and 49 years, female sex, bacteremic pneumonia and a cotrimoxazole non-susceptible isolate were significantly more common among HIV-infected than HIV-uninfected IPD patients. HIV-uninfected patients had better survival to discharge, and more underlying conditions (apart from HIV infection) predisposing them to IPD than HIV-infected patients. There was no difference between HIV-infected and–uninfected adults regarding proportion of vaccine-preventable serotypes causing IPD (71% serotypes amongst HIV-infected persons covered by PCV13 vs. 73% amongst HIV-uninfected persons, p = 0.4, OR 0.9 (CI 0.7–1.1) and 84% serotypes amongst HIV-infected persons covered by PPV23 vs. 86% amongst HIV-uninfected persons, p = 0.3, OR 0.9 (CI 0.6–1.1)). (Table 3)
Table 3. Comparison of HIV-infected and HIV-uninfected patients (5-years and older) with invasive pneumococcal disease, South Africa, 2003–2008 (n = 4 734).
| HIV-infected | HIV-uninfected | Univariate analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | Odds Ratio (95% confidence interval) | P-value | Odds Ratio (95% confidence interval) | P-value | |
| Total cases | 4 190 | 89 | 544 | 12 | ||||
| Age category (n = 4 734) | ||||||||
| 5–19 years | 567 | 14 | 140 | 26 | Reference | <0.001 | Reference | <0.001 |
| 20–49 years | 3 271 | 78 | 274 | 50 | 3 (2.4–3.7) | 2.9 (2.2–3.9) | ||
| 50–64 years | 313 | 8 | 85 | 16 | 0.9 (0.7–1.2) | 0.8 (0.6–1.2) | ||
| ≥65 years | 39 | 1 | 45 | 8 | 0.2 (0.1–0.3) | 0.3 (0.1–0.5) | ||
| Sex (n = 4 729) | ||||||||
| Female | 2 337 | 56 | 224 | 41 | 1.8 (1.5–2.2) | <0.001 | 1.5 (1.2–1.9) | <0.001 |
| Specimen type (n = 4 734) | ||||||||
| Cerebrospinal Fluid | 875 | 21 | 112 | 21 | Reference | 0.013 | ||
| Blood | 3 091 | 74 | 386 | 71 | 1 (0.8–1.3) | |||
| Other | 224 | 5 | 46 | 9 | 0.6 (0.4–0.9) | |||
| Clinical syndrome (n = 4 711) | ||||||||
| Meningitis | 1 112 | 27 | 148 | 27 | Reference | <0.001 | Reference | <0.001 |
| Bacteremic pneumonia | 2 653 | 64 | 294 | 54 | 1.2 (1–1.5) | 1.3 (1–1.7) | ||
| Bacteremia without focus | 170 | 4 | 47 | 9 | 0.5 (0.3–0.7) | 0.5 (0.3–0.8) | ||
| Other | 235 | 6 | 52 | 10 | 0.6 (0.4–0.9) | 0.6 (0.4–0.9) | ||
| Severity of illness (Pitt bacteremia score) (n = 4 155) | ||||||||
| 0 | 1 490 | 41 | 214 | 45 | Reference | Reference | 0.1 | |
| 1–3 | 1 815 | 49 | 200 | 42 | 1.3 (1.1–1.6) | 0.009 | 1.2 (0.9–1.6) | |
| 4–12 | 375 | 10 | 61 | 13 | 0.9 (0.7–1.2) | 0.8 (0.5–1.2) | ||
| Predisposing condition (n = 4 734) | ||||||||
| Yes | 790 | 19 | 210 | 39 | 0.4 (0.3–0.4) | <0.001 | 0.4 (0.3–0.5) | <0.001 |
| Predisposing condition specified | ||||||||
| - ACIP condition# | 770 | 97 | 206 | 98 | Reference | 0.599 | ||
| - Other risk factor | 20 | 3 | 4 | 2 | 1.3 (0.5–4) | |||
| Recurrences of IPD (n = 4 734) | ||||||||
| Yes | 224 | 5 | 36 | 7 | 0.1 (0.6–1.2) | 0.221 | ||
| Outcome (n = 4 710) | ||||||||
| Died in hospital | 1 138 | 27 | 107 | 20 | 1.9 (1.4–2.5) | <0.001 | 2 (1.4–2.7) | <0.001 |
| Antimicrobial susceptibility (n = 3 639) | 3 205 | 434 | ||||||
| Penicillin^ | ||||||||
| Non-susceptible | 926 | 29 | 84 | 19 | 1.7 (1.3–2.2) | <0.001 | ||
| Erythromycin | ||||||||
| Non-susceptible | 359 | 11 | 37 | 9 | 1.4 (1–1.9) | 0.093 | ||
| Cotrimoxazole | ||||||||
| Non-susceptible | 1 653 | 52 | 157 | 36 | 1.9 (1.5–2.3) | <0.001 | 1.9 (1.5–2.4) | <0.001 |
| 3rd Generation Cephalosporin | ||||||||
| Non-susceptible | 20 | 1 | 2 | 1 | 1.4 (0.3–5.8) | 0.681 | ||
| Serotypes (n = 3 639) | 3205 | 434 | ||||||
| PCV13* | ||||||||
| Vaccine types | 2272 | 71 | 317 | 73 | 0.9 (0.7–1.1) | 0.353 | ||
| PPV23** | ||||||||
| Vaccine types | 2685 | 84 | 372 | 86 | 0.9 (0.6–1.1) | 0.301 | ||
Footnote:
#ACIP (Advisory Committee on Immunization Practices) condition includes: asplenia; sickle cell anaemia; chronic lung, renal, liver or cardiac disease; diabetes; solid organ transplant; primary immunodeficiency syndromes; immunomodulation therapy; malignancies; head injuries with CSF leak; alcohol dependency and smoking.
^Penicillin non-susceptibility was associated with cotrimoxazole non-susceptibility thus it was not included in the final multivariate model.
*PCV13 (Pneumococcal Conjugate Vaccine-13 valent) serotypes include: 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F.
**PPV23 (Pneumococcal Polysaccharide Vaccine-23 valent) serotypes include: 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, 33F,(6A cross protection is assumed).
Discussion
In South Africa during the pre-vaccine era, 70% of IPD in adults and older children was caused by serotypes in PCV13 and 85% by PPV23 serotypes. IPD incidence in HIV-infected individuals was 43 times higher than in HIV-uninfected persons (52 per 100 000 vs.1 per 100 000), with HIV-infected adults ≥65 years at highest risk (IPD incidence of 237 per 100 000, 95% CI 171–321). IPD and HIV-coinfection was associated with more bacteremia (with or without pneumonia) and higher in-hospital case fatality than HIV-uninfected cases.
HIV-infection is a major risk factor for IPD, with 90% of IPD amongst South African older children and adults occurring in the 18% of South Africans who are HIV infected.[7, 24] By 2008, HAART coverage amongst HIV-infected South African adults eligible for treatment was 40% [26], this has increased to 79% in recent years.[27] Yet in South Africa, one study showed only a 7% decrease in adult IPD attributable to the increased HAART coverage during this same period.[18] Although HAART reduces the risk of IPD in HIV-infected persons, there seems to be an incomplete restoration of the immune system, necessitating additional measures for preventing IPD in this high risk group. [28–36]
Sex, age and underlying medical conditions are established risk factors for IPD and its more severe outcomes. [37–39] Globally IPD occurs more frequently in males than females however we found that in persons with IPD HIV-infection was associated with female sex. In South Africa, HIV prevalence in females is higher (11.9% in females versus 9.2% in males for 2008) which may in part account for the disparity.[7, 40]
From age 65 years, IPD incidence in both HIV-infected and uninfected persons increases exponentially, however in this age category the incidence amongst HIV-infected persons is 58 times higher than in HIV-uninfected individuals (237 vs. 4 cases per 100 000 population respectively). With the rollout of ARVs, this high-risk category of elderly HIV-infected persons is likely to grow. Similarly, there is a peak of disease in HIV-infected teenagers, which is in stark contrast to their HIV-uninfected peers (81 vs. 0.6 cases per 100 000 population respectively, relative risk of 127). Many of these HIV-infected teens are potentially long term survivors of vertical HIV-transmission, with potential added risk factor of chronic lung disease, whilst a smaller proportion may be recently infected.[41] The HIV-infected teens and elderly are at an even higher risk with the potential development of chronic diseases which further predispose to IPD.
The highest burden of IPD in South African persons five years and older is in the young to middle-aged adult population. The majority (88%) of disease occurred amongst HIV-infected persons; however, 43% of HIV-uninfected persons had other underlying conditions predisposing them to IPD. In our study, as seen in other studies, vaccine serotypes occur at high rates in older children and adults, with 70% of disease caused by PCV13 serotypes and 85% by PPV23 serotypes.[3, 6, 42, 43] This was similar for both HIV-infected and uninfected individuals, despite the individual serotype diversity seen within each group. Although South Africa follows recommendations to vaccinate high risk individuals with PPV23 (including HIV-infected individuals), vaccine uptake is extremely low and no patient in our study had received PPV23.[44, 45]
In South Africa, we recently demonstrated an indirect effect on all serotype IPD incidence, following PCV7 vaccination of infants, of 34% in the 25–44 year age category.[18] However, this indirect effect was less evident amongst those in their early teens and in the elderly (>65 years) (6% reduction in 10–14 years and 14% reduction in 65+ age group)—two groups with high incidences of IPD amongst those HIV-infected. Since 2011, PCV13 has been used in the EPI, and it is expected that the indirect effects of PCV13 on additional serotypes will further decrease the incidence of IPD in older children and adults. However, in HIV-infected populations additional preventative interventions could be considered such as direct vaccination of selected groups with pneumococcal vaccine, increased uptake of the seasonal influenza vaccine and increased use of ART.[46–49]
Previous studies in South Africa reported that HIV-coinfected persons were more likely to be infected with penicillin non-susceptible pneumococci, however when including cotrimoxazole in our multivariate model this finding fell away. Cotrimoxazole non-susceptibility remained a significant finding in HIV-coinfected patients on multivariable analysis; this may be due to the high use of cotrimoxazole prophylaxis in preventing opportunistic infections in HIV-infected patients in South Africa.[50]
One important limitation of surveillance studies is that IPD incidence may be underestimated.[51] Our case definition relies on the specimen taking practices of clinicians and the health seeking behavior of the patients, which have both been found to vary greatly across our study population.[52, 53] In many of the rural areas reporting of blood culture results are often delayed, therefore if patients do seek medical care, clinicians often treat those with a suspected bacteremia empirically without laboratory investigations to confirm their diagnosis. These cases would not have been reflected in this study and are therefore excluded from our incidence calculations. A study during the same time period confined to a single site in South Africa with a relatively well-defined population and adequate blood culturing practices, reported IPD incidence in adults of 58 per 100 000 population.[28] Therefore the true incidence of IPD in South African adults and older children likely lies above the 7 per 100 000 reflected here. An assumption was made for 29% (5 041/17 604) of IPD isolates that distribution of serotypes by age-category and province was similar amongst isolates that were available and those that were missing when calculating incidence by serotype. In addition, these missing isolates could influence the distribution of antimicrobial susceptibility data. HIV testing was not done routinely for surveillance purposes and patient HIV serostatus was only available at ES sites—and only for 68% of the patients with medical records available. Only a few parameters were available for comparing cases from ES versus non-enhanced sites, and although the main differences were found in specimen type and cotrimoxazole non-susceptibility there may be more unmeasured differences which we were unable to account for which could have influenced our model comparing HIV-infected to HIV-uninfected patients with IPD. ASSA2003 denominators for HIV-infected individuals tended to underestimate the number of HIV-infected individuals in the teenage and elderly populations, therefore we used a modified version of the ASSA2003 model, Thembisa 1.7v3, which accounts for the survival benefits of the rollout of HAART especially amongst the teenagers and elderly.[25, 54] This gave more plausible results for IPD incidence amongst the teenager and elderly HIV-infected population but may still be an underestimate of true IPD incidence.
In summary, 70–85% of adult IPD in the pre-PCV era were vaccine serotypes and 93% of cases had recognized risk factors (including HIV-infection) for pneumococcal vaccination. These data describe the epidemiology of IPD amongst HIV-infected and -uninfected adults during the pre-PCV era and provide a robust baseline to calculate the indirect effect of PCV in future studies.
Acknowledgments
We would like to thank all clinical and laboratory staff throughout South Africa for submitting case reports and isolates for the GERMS-SA national surveillance programme, as well as all the patients who agreed to provide further clinical details.
The 2005 to 2008 members of the GERMS-SA are: Principle investigator: Dr Vanessa Quan email: vanessaq@nicd.ac.za; Sandeep Vasaikar (Eastern Cape); Nolan Janse van Rensberg, André Möller, Peter Smith, Anne-Marie Pretorius (Free State); Khatija Ahmed, Anwar Hoosen, Ruth Lekalakala, Pyu Pyu Sein, Charles Feldman, Alan Karstaedt, Olga Perovic, Jeannette Wadula, Mike Dove, Kathy Lindeque, Linda Meyer, Gerhard Weldhagen (Gauteng); Stan Harvey, Pieter Jooste (Northern Cape); Danie Cilliers (North West Province); Wim Sturm, Trusha Vanmali, Prathna Bhola, Prashini Moodley, Sindiswe Sithole, Halima Dawood (KwaZulu Natal); Ken Hamese (Limpopo); Keith Bauer, Greta Hoyland, Jacob Lebudi, Charles Mutanda (Mpumalanga); Rena Hoffmann, Siseko Martin, Lynne Liebowitz, Elizabeth Wasserman, Andrew Whitelaw (Western Cape); Adrian Brink, Inge Zietsman, Maria Botha, Xoliswa Poswa, Mark da Silva, Suzy Budavari (Ampath laboratories); Claire Heney, Juanita Smit (Lancet laboratories); Marthinus Senekal (Pathcare laboratories); Cheryl Cohen, Mireille Cheyip, Leigh Dini, Linda de Gouveia, John Frean, Susan Gould, Karen Keddy, Kerrigan McCarthy, Susan Meiring, Elizabeth Prentice, Vanessa Quan, Jeffrey Ramalivhana, Arvinda Sooka, Anne von Gottberg and Nelesh Govender (National Institute for Communicable Diseases/ NHLS).
Data Availability
Data are available from corresponding author or the GERMS-SA Principal Investigator, Dr. Vanessa Quan, vanessaq@nicd.ac.za, as this is from the GERMS-SA surveillance database and data contains information that could compromise patient confidentiality. Data will be made available on request to those who meet criteria for access to confidential data.
Funding Statement
Development of this publication was partially funded by the National Health Laboratory Service/National Institute for Communicable Disease and by the HHS Centers for Disease Control and Prevention (CDC), National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (NCHHSTP), Global AIDS Program (GAP) Cooperative Agreement U62/PSO022901. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of CDC.
References
- 1.Janoff EN, Breiman RF, Daley CL, Hopewell PC. Pneumococcal disease during HIV infection. Epidemiologic, clinical, and immunologic perspectives. Ann Intern Med. 1992;117(4):314–24. [DOI] [PubMed] [Google Scholar]
- 2.Gilks CF, Ojoo SA, Ojoo JC, Brindle RJ, Paul J, Batchelor BI, et al. Invasive pneumococcal disease in a cohort of predominantly HIV-1 infected female sex-workers in Nairobi, Kenya. Lancet. 1996;347(9003):718–23. [DOI] [PubMed] [Google Scholar]
- 3.Jones N, Huebner R, Khoosal M, Crewe-Brown H, Klugman K. The impact of HIV on Streptococcus pneumoniae bacteraemia in a South African population. AIDS. 1998;12(16):2177–84. [DOI] [PubMed] [Google Scholar]
- 4.Feikin DR, Jagero G, Aura B, Bigogo GM, Oundo J, Beall BW, et al. High rate of pneumococcal bacteremia in a prospective cohort of older children and adults in an area of high HIV prevalence in rural western Kenya. BMC Infect Dis. 2010;10:186 10.1186/1471-2334-10-186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.French N, Nakiyingi J, Carpenter LM, Lugada E, Watera C, Moi K, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet. 2000;355(9221):2106–11. [DOI] [PubMed] [Google Scholar]
- 6.Nuorti JP, Butler JC, Gelling L, Kool JL, Reingold AL, Vugia DJ. Epidemiologic relation between HIV and invasive pneumococcal disease in San Francisco County, California. Ann Intern Med. 2000;132(3):182–90. [DOI] [PubMed] [Google Scholar]
- 7.ASSA2003 AIDS and demographic model [Internet]. 2005. Available from: http://aids.actuarialsociety.org.za/ASSA2003-model-3165.htm.
- 8.Yin Z, Rice BD, Waight P, Miller E, George R, Brown AE, et al. Invasive pneumococcal disease among HIV-positive individuals, 2000–2009. AIDS. 2012;26(1):87–94. 10.1097/QAD.0b013e32834dcf27 . [DOI] [PubMed] [Google Scholar]
- 9.Hibbs JR, Douglas JM Jr., Judson FN, McGill WL, Rietmeijer CA, Janoff EN. Prevalence of human immunodeficiency virus infection, mortality rate, and serogroup distribution among patients with pneumococcal bacteremia at Denver General Hospital, 1984–1994. Clin Infect Dis. 1997;25(2):195–9. [DOI] [PubMed] [Google Scholar]
- 10.Karstaedt AS, Khoosal M, Crewe-Brown HH. Pneumococcal bacteremia in adults in Soweto, South Africa, during the course of a decade. Clin Infect Dis. 2001;33(5):610–4. [DOI] [PubMed] [Google Scholar]
- 11.Crewe-Brown HH, Karstaedt AS, Saunders GL, Khoosal M, Jones N, Wasas A, et al. Streptococcus pneumoniae blood culture isolates from patients with and without human immunodeficiency virus infection: alterations in penicillin susceptibilities and in serogroups or serotypes. Clin Infect Dis. 1997;25(5):1165–72. [DOI] [PubMed] [Google Scholar]
- 12.Crowther-Gibson P, Cohen C, Klugman KP, de GL, von GA. Risk factors for multidrug-resistant invasive pneumococcal disease in South Africa, a setting with high HIV prevalence, in the prevaccine era from 2003 to 2008. Antimicrob Agents Chemother. 2012;56(10):5088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Von Mollendorf C, Cohen C, Tempia S, Meiring S, De Gouveia L, Quan V, et al. Epidemiology of serotype 1 invasive pneumococcal disease in all ages in South Africa, 2003–2013 Emerg Infect Dis. 2016;22(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgos J, Penaranda M, Payeras A, Villoslada A, Curran A, Garau M, et al. Invasive pneumococcal disease in HIV-infected adults: clinical changes after the introduction of the pneumococcal conjugate vaccine in children. J Acquir Immune Defic Syndr. 2012;59(1):31–8. 10.1097/QAI.0b013e31823d0f5f [DOI] [PubMed] [Google Scholar]
- 15.French N, Gordon SB, Mwalukomo T, White SA, Mwafulirwa G, Longwe H, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med. 2010;362(9):812–22. 10.1056/NEJMoa0903029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348(18):1737–46. [DOI] [PubMed] [Google Scholar]
- 17.Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41. 10.1086/648593 [DOI] [PubMed] [Google Scholar]
- 18.von Gottberg A, de Gouveia L, Tempia S, Quan V, Meiring S, von Mollendorf C, et al. Effects of vaccination on invasive pneumococcal disease in South Africa. N Engl J Med. 2014;371(20):1889–99. 10.1056/NEJMoa1401914 . [DOI] [PubMed] [Google Scholar]
- 19.Cohen AL, Harrison LH, Farley MM, Reingold AL, Hadler J, Schaffner W, et al. Prevention of invasive pneumococcal disease among HIV-infected adults in the era of childhood pneumococcal immunization. AIDS. 2010;24(14):2253–62. 10.1097/QAD.0b013e32833d46fd [DOI] [PubMed] [Google Scholar]
- 20.GERMS-SA Annual Report 2008 [Internet]. 2009. Available from: http://nicd.ac.za/?page=germs-sa&id=97.
- 21.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing In: Wayne PA, editor. Eighteenth informational supplement: CLSI; 2008. [Google Scholar]
- 22.Paterson DL, Hujer KM, Hujer AM, Yeiser B, Bonomo MD, Rice LB, et al. Extended-spectrum beta-lactamases in Klebsiella pneumoniae bloodstream isolates from seven countries: dominance and widespread prevalence of SHV- and CTX-M-type beta-lactamases. Antimicrob Agent Chemother. 2003;47(11):3554–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC). Use of PCV13 and PPV23 vaccines for adults with immunocompromising conditions. MMWR RecommRep. 2012;61(40):816–9. [PubMed] [Google Scholar]
- 24.Mid-year population estimates, South Africa, 2008 [Internet]. 2008. Available from: http://www.statssa.gov.za/publications/P0302/P03033010.pdf.
- 25.Johnson LF, Rehle TM, Jooste S, Bekker LG. Rates of HIV testing and diagnosis in South Africa: successes and challenges. AIDS. 2015;29(11):1401–9. 10.1097/QAD.0000000000000721 . [DOI] [PubMed] [Google Scholar]
- 26.Adam MA, Johnson LF. Estimation of adult antiretroviral treatment coverage in South Africa. S Afr Med J. 2009;99(9):661–7. . [PubMed] [Google Scholar]
- 27.Johnson LF. Access to antiretroviral treatment in South Africa, 2004–2011. South Afr J HIV Med. 2012;13(1):22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nunes MC, von Gottberg A, de Gouveia L, Cohen C, Kuwanda L, Karstaedt AS, et al. Persistent high burden of invasive pneumococcal disease in South African HIV-infected adults in the era of an antiretroviral treatment program. PloS one. 2011;6(11):e27929 10.1371/journal.pone.0027929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palella FJ Jr., Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–60. 10.1056/NEJM199803263381301 . [DOI] [PubMed] [Google Scholar]
- 30.Everett DB, Mukaka M, Denis B, Gordon SB, Carrol ED, van Oosterhout JJ, et al. Ten years of surveillance for invasive Streptococcus pneumoniae during the era of antiretroviral scale-up and cotrimoxazole prophylaxis in Malawi. PloS one. 2011;6(3):e17765 10.1371/journal.pone.0017765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayanja BN, Todd J, Hughes P, Van der Paal L, Mugisha JO, Atuhumuza E, et al. Septicaemia in a population-based HIV clinical cohort in rural Uganda, 1996–2007: incidence, aetiology, antimicrobial drug resistance and impact of antiretroviral therapy. Trop Med Int Health. 2010;15(6):697–705. 10.1111/j.1365-3156.2010.02528.x . [DOI] [PubMed] [Google Scholar]
- 32.Heffernan RT, Barrett NL, Gallagher KM, Hadler JL, Harrison LH, Reingold AL, et al. Declining incidence of invasive Streptococcus pneumoniae infections among persons with AIDS in an era of highly active antiretroviral therapy, 1995–2000. J Infect Dis. 2005;191(12):2038–45. 10.1086/430356 . [DOI] [PubMed] [Google Scholar]
- 33.Glennie SJ, Sepako E, Mzinza D, Harawa V, Miles DJ, Jambo KC, et al. Impaired CD4 T cell memory response to Streptococcus pneumoniae precedes CD4 T cell depletion in HIV-infected Malawian adults. PloS one. 2011;6(9):e25610 10.1371/journal.pone.0025610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sepako E, Glennie SJ, Jambo KC, Mzinza D, Iwajomo OH, Banda D, et al. Incomplete recovery of pneumococcal CD4 T cell immunity after initiation of antiretroviral therapy in HIV-infected malawian adults. PloS one. 2014;9(6):e100640 10.1371/journal.pone.0100640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jordano Q, Falco V, Almirante B, Planes AM, del Valle O, Ribera E, et al. Invasive pneumococcal disease in patients infected with HIV: still a threat in the era of highly active antiretroviral therapy. Clin Infect Dis. 2004;38(11):1623–8. 10.1086/420933 . [DOI] [PubMed] [Google Scholar]
- 36.Siemieniuk RA, Gregson DB, Gill MJ. The persisting burden of invasive pneumococcal disease in HIV patients: an observational cohort study. BMC Infect Dis. 2011;11:314 10.1186/1471-2334-11-314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feikin DR, Schuchat A, Kolczak M, Barrett NL, Harrison LH, Lefkowitz L, et al. Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995–1997. Am J Public Health. 2000;90(2):223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moroney JF, Fiore AE, Harrison LH, Patterson JE, Farley MM, Jorgensen JH, et al. Clinical outcomes of bacteremic pneumococcal pneumonia in the era of antibiotic resistance. Clin Infect Dis. 2001;33(6):797–805. 10.1086/322623 . [DOI] [PubMed] [Google Scholar]
- 39.Yu VL, Chiou CC, Feldman C, Ortqvist A, Rello J, Morris AJ, et al. An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administered, and clinical outcome. Clin Infect Dis. 2003;37(2):230–7. 10.1086/377534 . [DOI] [PubMed] [Google Scholar]
- 40.Buie KA, Klugman KP, von G, A., Perovic O, Karstaedt A, Crewe-Brown HH, et al. Gender as a risk factor for both antibiotic resistance and infection with pediatric serogroups/serotypes, in HIV-infected and -uninfected adults with pneumococcal bacteremia. J Infect Dis. 2004;189(11):1996–2000. [DOI] [PubMed] [Google Scholar]
- 41.Ferrand RA, Desai SR, Hopkins C, Elston CM, Copley SJ, Nathoo K, et al. Chronic lung disease in adolescents with delayed diagnosis of vertically acquired HIV infection. Clin Infect Dis. 2012;55(1):145–52. 10.1093/cid/cis271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dworkin MS, Ward JW, Hanson DL, Jones JL, Kaplan JE. Pneumococcal disease among human immunodeficiency virus-infected persons: incidence, risk factors, and impact of vaccination. Clin Infect Dis. 2001;32(5):794–800. [DOI] [PubMed] [Google Scholar]
- 43.Feikin DR, Klugman KP, Facklam RR, Zell ER, Schuchat A, Whitney CG. Increased prevalence of pediatric pneumococcal serotypes in elderly adults. Clin Infect Dis. 2005;41(4):481–7. [DOI] [PubMed] [Google Scholar]
- 44.SAMA-SA Pulmonology Society Working Group. Adult pneumococcal vaccination guideline S Afr Med J. 1999;89(11 Suppl):1222–30. . [PubMed] [Google Scholar]
- 45.Souter J. An update on pneumococcal vaccination in children and adults. S Afr Pharma J. 2014;81(2):15–8. [Google Scholar]
- 46.Burgos J, Larrosa MN, Martinez A, Belmonte J, Gonzalez-Lopez J, Rello J, et al. Impact of influenza season and environmental factors on the clinical presentation and outcome of invasive pneumococcal disease. Eur J Clin Microbiol Infect Dis. 2015;34(1):177–86. 10.1007/s10096-014-2221-9 . [DOI] [PubMed] [Google Scholar]
- 47.Bhorat AE, Madhi SA, Laudat F, Sundaraiyer V, Gurtman A, Jansen KU, et al. Immunogenicity and safety of the 13-valent pneumococcal conjugate vaccine in HIV-infected individuals naive to pneumococcal vaccination. AIDS. 2015;29(11):1345–54. 10.1097/QAD.0000000000000689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ranieri R, Veronelli A, Santambrogio C, Pontiroli AE. Impact of influenza vaccine on response to vaccination with pneumococcal vaccine in HIV patients. AIDS Res Hum Retroviruses. 2005;21(5):407–9. 10.1089/aid.2005.21.407 . [DOI] [PubMed] [Google Scholar]
- 49.Atashili J, Kalilani L, Adimora AA. Efficacy and clinical effectiveness of influenza vaccines in HIV-infected individuals: a meta-analysis. BMC Infect Dis. 2006;6:138 10.1186/1471-2334-6-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soeters HM, von Gottberg A, Cohen C, Quan V, Klugman KP. Trimethoprim-sulfamethoxazole prophylaxis and antibiotic nonsusceptibility in invasive pneumococcal disease. Antimicrob Agent Chemother. 2012;56(3):1602–5. 10.1128/AAC.05813-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bar-Zeev N, Mtunthama N, Gordon SB, Mwafulirwa G, French N. Minimum incidence of adult invasive pneumococcal disease in Blantyre, Malawi an urban African setting: a hospital based prospective cohort study. PloS one. 2015;10(6):e0128738 10.1371/journal.pone.0128738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheyip M, Cohen C, von Gottberg A, Govender N, Keddy K, GERMS-SA. Collection rates for blood culture and cerebrospinal fluid specimens and estimated burden of disease due to invasive respiratory, meningeal and enteric bacterial pathogens in South Africa. University of the Witwatersrand School of Public Health Annual Research Day; Johannesburg, South Africa2007.
- 53.Wong KK, von Mollendorf C, Martinson NA, Norris SA, Tempia S, Walaza S, et al. Healthcare utilization for common infectious disease syndromes in Soweto and Klerksdorp, South Africa. BMC Infect Dis. 2015;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Initial observations on the comparison of the 2005 HSRC household HIV prevalence and behaviour survey amongst estimates from the ASSA2003 AIDS and Demographic model [Internet]. 2006. Available from: www.actuarialsociety.co.za/applications/cms/documents/file_build.asp?id=100000119.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from corresponding author or the GERMS-SA Principal Investigator, Dr. Vanessa Quan, vanessaq@nicd.ac.za, as this is from the GERMS-SA surveillance database and data contains information that could compromise patient confidentiality. Data will be made available on request to those who meet criteria for access to confidential data.