Abstract
Human chorionic gonadotropin (hCG) is a key autocrine/paracrine regulator of placental syncytiotrophoblast, the transport epithelium of the human placenta. Syncytiotrophoblast hCG secretion is modulated by the partial pressure of oxygen (pO2), reactive oxygen species (ROS) and potassium (K+) channels. Here we test the hypothesis that K+ channels mediate the effects of pO2 and ROS on hCG secretion. Placental villous explants from normal term pregnancies were cultured for 6 days at 6% (normoxia), 21% (hyperoxia) or 1% (hypoxia) pO2. On days 3–5, explants were treated with 5mM 4-aminopyridine (4-AP) or tetraethylammonium (TEA), blockers of pO2-sensitive voltage-gated K+ (KV) channels, or ROS (10–1000μM H2O2). hCG secretion and lactate dehydrogenase (LDH) release, a marker of necrosis, were determined daily. At day 6, hCG and LDH were measured in tissue lysate and 86Rb (K+) efflux assessed to estimate syncytiotrophoblast K+ permeability. hCG secretion and 86Rb efflux were significantly greater in explants maintained in 21% pO2 than normoxia. 4-AP/TEA inhibited hCG secretion to a greater extent at 21% than 6% and 1% pO2, and reduced 86Rb efflux at 21% but not 6% pO2. LDH release and tissue LDH/hCG were similar in 6%, 21% and 1% pO2 and unaffected by 4-AP/TEA. H2O2 stimulated 86Rb efflux and hCG secretion at normoxia but decreased 86Rb efflux, without affecting hCG secretion, at 21% pO2. 4-AP/TEA-sensitive K+ channels participate in pO2-sensitive hCG secretion from syncytiotrophoblast. ROS effects on both hCG secretion and 86Rb efflux are pO2-dependent but causal links between the two remain to be established.
Introduction
The endocrine and nutrient transport functions of the human placenta depend on appropriate maintenance of syncytiotrophoblast, a highly specialised multinucleate epithelial cell. Syncytiotrophoblast has a short life span and is renewed during pregnancy by cellular turnover. Proliferative mononucleate cytotrophoblasts exit the cell cycle, differentiate and fuse into the overlying syncytiotrophoblast and then aged syncytial nuclei are removed, possibly by apoptosis and autophagy, to complete turnover [1, 2]. In normal pregnancy these processes are highly coordinated but in pregnancies complicated by pre-eclampsia [3, 4], fetal growth restriction [4] and maternal obesity [5], an imbalance in cell turnover dysregulates syncytiotrophoblast renewal which compromises function and contributes to maternal and fetal mortality, and morbidity associated with these pregnancy complications.
Cell turnover to renew syncytiotrophoblast is maintained by several hormones including human chorionic gonadotrophin (hCG). hCG is an autocrine/paracrine regulator of syncytiotrophoblast renewal, acting via the G-protein coupled luteinizing hormone/hCG-receptor to elevate cAMP/protein kinase A and promote cytotrophoblast differentiation [6], gap junction communication and cellular fusion to form multinucleated syncytia [7]. hCG is synthesized and secreted by terminally differentiated syncytiotrophoblast and promotes continued trophoblast renewal by positive feedback. Thus appropriate regulation of hCG synthesis and secretion is essential for maintenance of syncytiotrophoblast and successful pregnancy.
Syncytiotrophoblast hCG secretion is modulated in vitro by oxygen tension (pO2) and reactive oxygen species (ROS). Lowering pO2 inhibited hCG secretion by villous explants [8] and by primary cultures of cytotrophoblasts [9] from normal term placentas. Hydrogen peroxide (H2O2) treatment of cytotrophoblasts, to generate oxidative stress, inhibited hCG secretion at high (>50μM) but markedly stimulated secretion at lower (1–50μM) concentrations [10]. Modulation of hCG secretion by these factors is likely to be of pathophysiological significance in pre-eclampsia and fetal growth restriction as altered placental pO2 and increased placental oxidative stress are associated with these conditions [11–13]. Indeed, increased levels of markers of oxidative stress are found in placental tissue from women with pre-eclampsia [14, 15] as well as elevated serum levels of H2O2 compared to normal pregnancies [16]. In addition to modulating hCG secretion, altered pO2 and elevated ROS also dysregulate syncytiotrophoblast turnover in vitro [8, 17, 18], but the underlying mechanism/s are currently unexplored.
hCG secretion by term placental trophoblast involves constitutive release [19] and Ca2+-dependent exocytosis [20]. Accordingly, the regulated component of hCG secretion is modulated by factors that influence intracellular Ca2+, including ion channels. We have previously shown that pharmacological blockade of Ca2+ entry channels [21] and voltage-gated K+ (KV) channels, inhibit hCG secretion from placental villous explants and isolated cytotrophoblasts [22]. Blocking KV channels also inhibits trophoblast fusion to form multinucleate syncytia [22] suggesting that activity of these channels is required both for hCG secretion and syncytiotrophoblast renewal.
The KV channel family comprises 11 members [23], and the expression/activity of some KV channel subunits is acutely and chronically modulated by pO2 [24, 25]. pO2-sensitive KV channels close in response to lowered pO2, raising the possibility that the reduction in hCG secretion from syncytiotrophoblast under hypoxic conditions is a result of blocking KV channels. Furthermore, long term exposure to oxidative stress (ROS) alters K+ channel expression/activity and acute exposure has direct effects on K+ channel proteins to alter their activity [26, 27]. The effects of H2O2 are diverse and depend on tissue type; H2O2 has been reported to both close [28] and open [29, 30] KV channels. As KV channels are modulated by ROS in non-placental tissue, it is plausible that ROS regulate syncytiotrophoblast hCG secretion through effects on KV channels.
Here we test the hypothesis that pO2 and/or ROS regulate hCG secretion through an effect on K+ channels. Using placental villous tissue from normal term pregnancy we compared the effect of KV channel blockers on hCG secretion and 86Rb efflux (a marker of K+ permeation through ion channels) from villous explants maintained at placental normoxia (6% pO2), with extreme hypoxia (1% pO2) and hyperoxia (21% pO2). We also investigated the effect of H2O2, used to generate ROS, on hCG secretion and 86Rb efflux at the three different pO2.
Materials and Methods
Materials
Unless otherwise stated, all chemicals were from Sigma-Aldrich (Poole, UK).
Ethics Statement
Human placentas used in this study were obtained from St. Mary’s Hospital Maternity Unit (Manchester, UK) following written informed consent as approved by the Local Research Ethics Committee (North West—Haydock Research Ethics Committee (Ref: 08/H1010/55), Central Manchester University Hospitals NHS Foundation Trust). Normal term placentas (37–42 weeks gestation) were obtained from uncomplicated pregnancies following vaginal delivery or Caesarean section. 3–14 placentas were collected depending on the type of experiment. The investigation conforms to the principles outlined in the Declaration of Helsinki.
Placental villous explant culture
Term placental villous tissue maintained in explant culture is a well characterised model [31] which has been used extensively to study the chronic effects of regulators on syncytiotrophoblast biology [8, 17, 18] and the method for culture of villous explants has been published previously [22, 31]. Briefly, chorionic villous sections (1.5cm3) were sampled, further dissected into explants (3–5mm3) and cultured at 37°C in explant culture medium (10% CMRL-1066, 100μg/ml streptomycin sulphate, 100IU/ml penicillin-G, 0.1μg/ml hydrocortisone, 0.1μg/ml retinol acetate, 0.1μg/ml insulin, 5% fetal calf serum, pH 7.2). Explants were placed onto Netwell permeable supports (70μM mesh; Corning Costar, Loughborough, UK) at the air/liquid interface and cultured in humidified incubators at 6% pO2 (with 5% CO2/balance N2; normoxic for term placenta, 40–50mmHg; assuming 1atm = 760mmHg), 21% pO2 (with 95% air/5% CO2; hyperoxia for term placenta, 160mmHg) or 1% pO2 (with 5% CO2/balance N2; hypoxia for term placenta, 7.6mmHg) for 6 days. Culture medium was replaced daily and fresh medium was pre-equilibrated (24h in advance) at each pO2 before addition to explants. On days 3–5, explants were untreated (control) or treated daily with pO2-sensitive K+ channel blockers 5mM 4-AP or 5mM TEA (these concentrations have been previously reported to produce the maximal inhibitory effect on hCG secretion without effecting tissue integrity [22]), or H2O2 (10, 100μM or 1mM).
Explant culture medium was collected daily and stored at -20°C before measuring hCG secretion and lactate dehydrogenase (LDH; released from necrotic cells and used as marker of cellular viability).
On day 6 explants were dissolved in 0.3M NaOH at 37°C for 24h to measure protein content. Otherwise explants were placed into water for 18h at room temperature to lyse for measurement of cellular hCG/LDH. The supernatant was collected and stored at -20°C, and explants were dissolved into 0.3M NaOH. These samples were used to measure protein content with Bio-Rad Protein Assay (Bio-Rad Laboratories, Hempstead, UK).
Measurement of hCG and LDH
hCG was assayed in the explant-conditioned culture medium and in villous explants lysed in water at day 6 of culture using an ELISA (DRG Diagnostics, Marburg, Germany) following the instructions of the manufacturer. hCG secretion was expressed as mIU/ml/h/mg protein.
LDH release into explant-conditioned culture medium culture medium was measured using a cytotoxicity detection kit (Roche Diagnostics, Mannheim, Germany) according to the instructions of the manufacturer. A standard curve was generated using L-Lactic dehydrogenase from rabbit muscle as an internal control. LDH release was expressed as absorbance units/mg protein/h.
86Rb efflux from placental villous explants
86Rb, a tracer of K+, permeates most K+-selective channels. It has been used to indirectly assess K+ permeability of the syncytiotrophoblast [31, 32]. 86Rb efflux was measured in placental villous explants using a technique previously described [31]. In principle, the tissue is incubated with 86Rb to achieve a stable intracellular level of isotope and then the extracellular 86Rb is removed by washing. Efflux of 86Rb into 86Rb-free buffer is measured over time and expressed either as a proportion of 86Rb in the tissue (%86Rb efflux) or as the fall in intracellular 86Rb (86Rb efflux rate constant). Specifically, fragments were incubated for 2h at 37°C in 1ml Tyrode’s buffer (135mM NaCl, 5mM KCl, 1.8mM CaCl2, 1mM MgCl2, 10mM HEPES, 5.6mM glucose, pH 7.4; osmolality 300mOsm/kgH2O) containing 4μCi/ml 86Rb (89.7μM; PerkinElmer, Waltham, MA, USA). After incubation, fragments were washed in 15ml Tyrode’s buffer twice for 5min each. Basal 86Rb efflux was then measured by changing and collecting 4ml Tyrode’s buffer every 2min for 10min at 37°C. Finally, villi were lysed in water for 18h to release intracellular non-membrane bound 86Rb which was then measured in the supernatant to give a measure of total 86Rb remaining in the tissue at the end of the experiment (86Rb in tissue). Effluxed and tissue 86Rb was measured in a beta-counter (Packard 2000, CA, USA).
The time course of percentage (%) 86Rb efflux was calculated as:
The efflux rate constant was also determined, making the assumption that, in control untreated explants, 86Rb efflux at steady state reflects the loss of 86Rb from a single compartment (syncytiotrophoblast) limited by the K+ permeability of the microvillous membrane. Consequently, the loss of 86Rb was measured by a first-order rate constant which was calculated over 16min experimental period as:
Expression of Results and Statistics
Statistical analysis was performed using GraphPad Prism version 5 software. hCG secretion and LDH release from control untreated explants were expressed as mean ± SE (n = number of placentas). Due to variability in hCG secretion between placentas [33], hCG secretion in treated explants at days 4, 5 and 6 of culture was expressed as a percentage of control (established as a 100%) and analysed with a Wilcoxon signed-rank test. A p value less than 0.05 was considered statistically significant. Data are median ± interquartile range (IQR).
%86Rb efflux from placental villous explants was expressed as mean ± SE for each time point. For all 86Rb efflux experiments, significant differences between 86Rb rate constants were assessed using least squares linear regression. A p value less than 0.05 was considered statistically significant.
Results
Effect of pO2 on hCG secretion from placental villous explants
The temporal changes in hCG secretion from term placental villous explants maintained at 21% pO2 over a 6-day culture period (Fig 1A) were similar to those previously reported [22, 31].
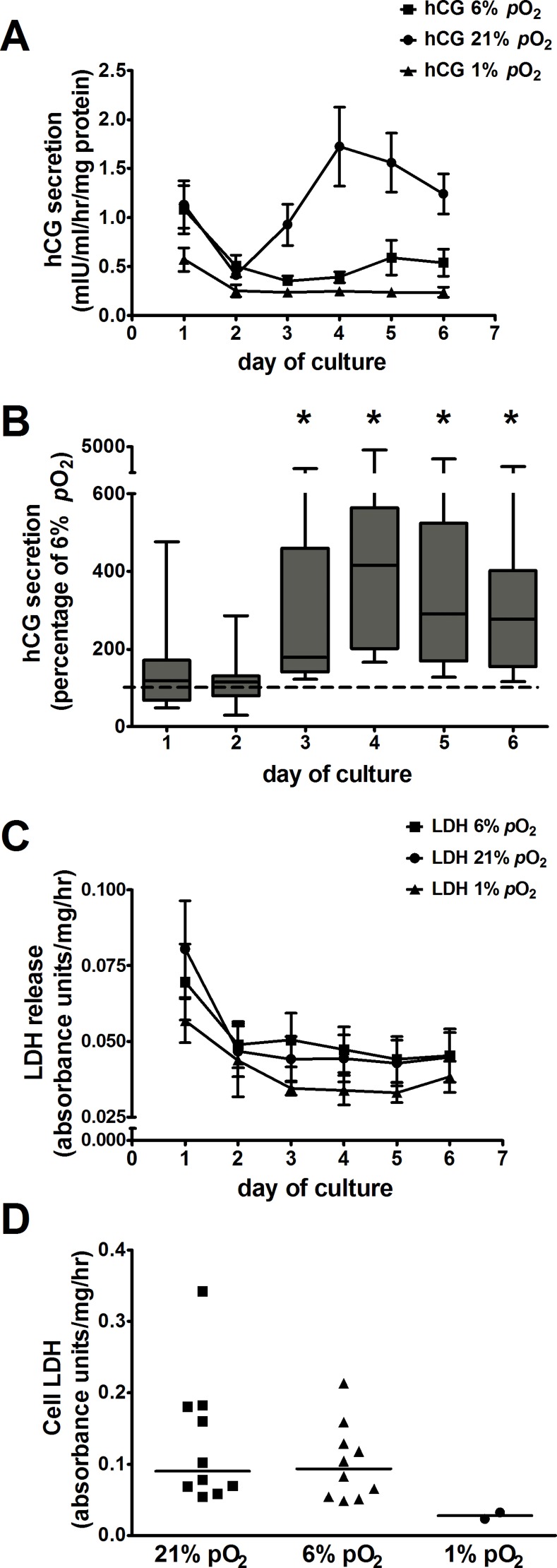
Fig 1. Effect of pO2 on hCG secretion from villous explants.
A: time course of hCG secretion from explants maintained at 21%, 6% and 1% pO2 during 6 days of culture. Values are mean ± SE; n = 14 placentas (n = 3 placentas maintained at 1% pO2). B: hCG secretion in explants maintained at 21% pO2 expressed as a percentage of hCG secretion at 6% pO2 (100%, dotted line); data are expressed as median ± IQR; n = 14 placentas, Wilcoxon signed-rank test compared to 100%, *p<0.001. C: time course of LDH release from explants maintained at 6%, 21% and 1% pO2 during 6 days of culture. Values are mean ± SE; n = 14 placentas (n = 3 placentas maintained at 1% pO2). D: cellular LDH measured at day 6 of culture in explants maintained at 6%, 21% and 1% pO2. Scatter dot plot shows line at median; n = 10 placentas (n = 2 placentas maintained at 1% pO2).
At all pO2, hCG secretion was high at day 1 and fell markedly at day 2. Afterwards, hCG secretion increased 4-fold by day 4 in 21% pO2, showed a slight gradual increase towards the end of culture in 6% pO2 but remained stable at low values at 1% pO2 (Fig 1A). Compared to hCG secretion at 6% pO2 (considered to be placental normoxia), secretion was significantly higher (4.1-fold) at 21% pO2 (Fig 1B) but not different at 1% pO2 (data not shown).
Fig 1C shows that after the first day in culture, LDH release declined in explants maintained at 6%, 21% and 1% pO2, indicating that tissue viability and cellular integrity was maintained in all pO2. However, the low LDH release at 1% pO2 might be due to reduced production of the enzyme in hypoxia as cellular LDH was ~3 times lower in 1% than either 21 or 6% pO2 (Fig 1D).
Effect of pO2-sensitive K+ channel blockers on hCG secretion from placental villous explants
Fig 2A and 2B show the effects of pO2-sensitive K+ channel blockers 4-AP and TEA respectively on hCG secretion from placental villous explants maintained at 6%, 21% and 1% pO2.
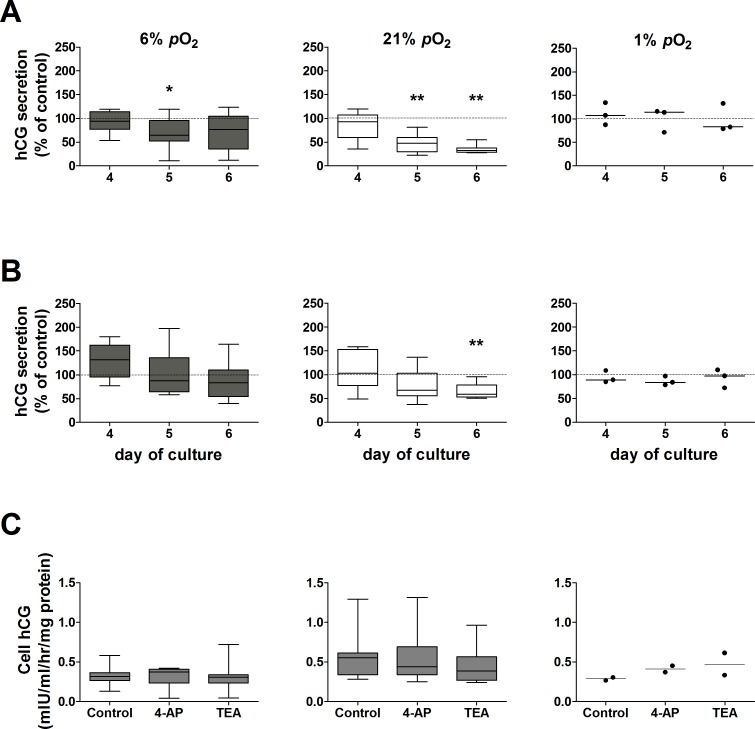
Fig 2. Effect of pO2-sensitive K+ channel blockers on hCG secretion from placental villous explants maintained at 6%, 21% or 1% pO2.
hCG secretion at days 4, 5 and 6 of culture was normalized as a percentage of hCG secretion in control untreated explants at the corresponding pO2 (dotted line, 100%); assessed by Wilcoxon signed-rank test compared to 100%. A: 4-AP (*p = 0.04, ** p = 0.008; n = 8 placentas; 1% pO2 n = 3 placentas); B: TEA (**p = 0.008, n = 8 placentas; 1% pO2 n = 3 placentas). Data are expressed as median ± IQR (line at median in 1% pO2). C: Effect of pO2-sensitive K+ channel blockers on cellular hCG from placental villous explants maintained at 6, 21% or 1% pO2. Cell hCG was measured at day 6 of culture. Median ± IQR for 6% and 21% pO2; n = 7 placentas. At 1% pO2 line represents median; n = 2 placentas.
In villous explants maintained at 6% pO2, 4-AP caused a transient decrease (35%) in hCG secretion on day 5 compared to control untreated explants (Fig 2A). In contrast, explants maintained at hyperoxia (21% pO2) showed a significant reduction in hCG secretion when treated with 4-AP at days 5 (52%) and 6 (68%) of culture (Fig 2A). This effect was completely suppressed under hypoxia (1% pO2), where hCG secretion was unaffected by 4-AP (Fig 2A).
TEA did not affect hCG secretion by explants maintained in placental normoxia (Fig 2B). On the contrary, when explants were maintained at 21% pO2, treatment with TEA caused a significant reduction in hCG secretion at day 6 of culture (41%; Fig 2B). TEA had no effect on explants maintained at 1% pO2 (Fig 2B).
LDH release from placental villous explants was not affected by treatment with 4-AP or TEA compared to their corresponding controls at the same pO2 (data not shown), indicating that tissue viability was not compromised by treatment with these pO2-sensitive K+ channel blockers.
Neither 4-AP nor TEA altered cellular hCG at any of the pO2 tested (Fig 2C), implicating an effect of these K+ channel blockers on the secretory mechanism for hCG and not hCG production.
From these data it is evident that culture of term placental villous explants for 6 days in hypoxic (1% pO2) conditions reduced hCG secretion to a low level, inhibited the temporal recovery in hCG secretion which is associated with syncytiotrophoblast regeneration/renewal at higher pO2 and reduced the cellular production of LDH and hCG. Furthermore, hCG secretion at 1% pO2 was unaffected by 4-AP and TEA. Therefore, experiments to evaluate the effect of these K+ channel blockers on 86Rb efflux were not performed at 1% pO2.
Basal 86Rb (K+) efflux from syncytiotrophoblast: chronic effect of pO2
86Rb efflux was measured at day 6 of culture in placental villous explants maintained at 6% or 21% pO2. Fig 3A shows the time course for basal %86Rb efflux at a steady state over a 16min period in explants maintained at 6% and 21% pO2. %86Rb efflux is higher from explants maintained at 21% than 6% pO2. Fig 3B shows the area under the curve for the total %86Rb efflux over 16min from explants maintained at 21% pO2 as a percent of efflux from explants at 6% pO2 (100%, dotted line). Basal %86Rb efflux was significantly higher in explants maintained for 6 days in hyperoxia (21% pO2) compared to normoxia.
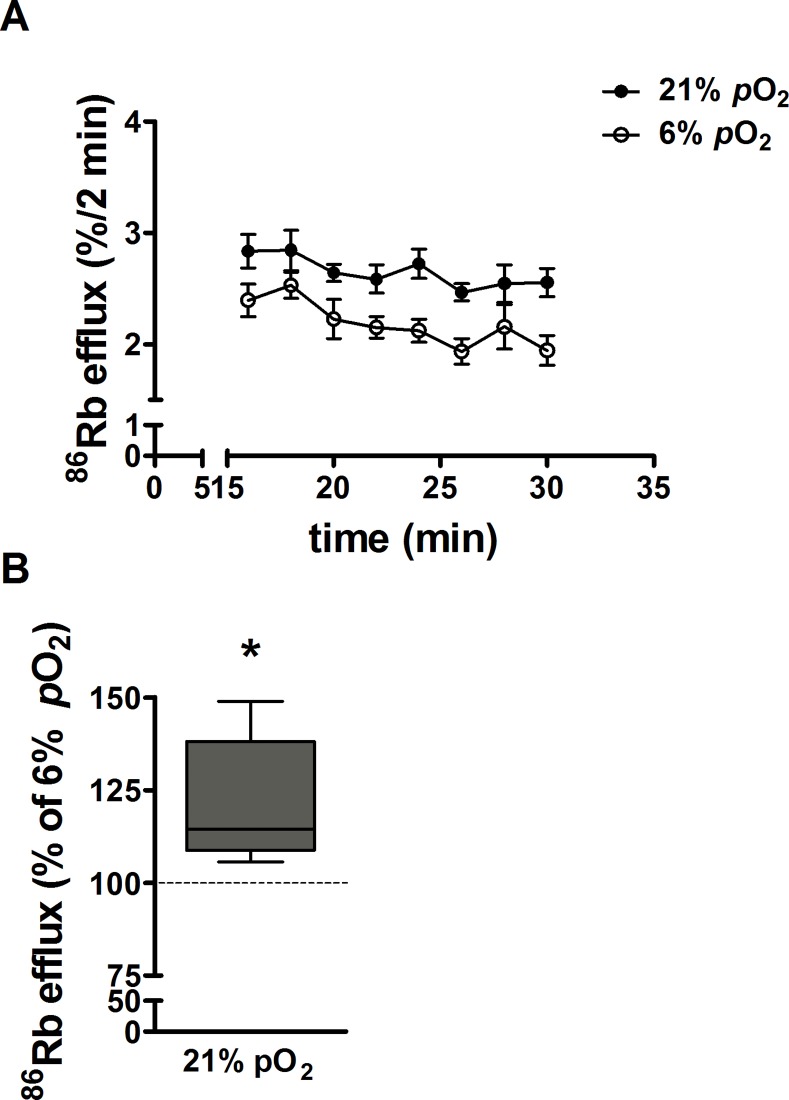
Fig 3. Effect of pO2 on syncytiotrophoblast K+ permeability.
A: Time course for the %86Rb efflux over 16min. Untreated (control) villous explants were cultured at 6% and 21% pO2 and basal 86Rb efflux was measured at day 6. Data are expressed as mean ± SE (n = 10 placentas). B: The %86Rb efflux over 16min in explants maintained at 21% was expressed as a percentage of efflux in explants maintained at 6% pO2 (placental normoxia; dotted line). Data are median ± IQR, Wilcoxon signed-rank test compared to 6% pO2, *p = 0.002; n = 10 placentas.
The 86Rb efflux rate constants calculated for untreated control explants maintained at 6% and 21% pO2 is shown in Table 1. Rate constant analysis shows that the fall in intracellular 86Rb can be described by a single exponential decline indicating that efflux is predominantly from a single tissue compartment, which we take to be the syncytiotrophoblast, in agreement with previous reports [31]. The mean rate constant for 86Rb efflux was significantly lower in explants maintained at placental normoxia (6% pO2) than hyperoxia (21% pO2) (Table 1).
Table 1. Rate constants of 86Rb efflux in control and treated placental villous explants.
| Condition | 86Rb efflux rate constant(ln 86Rb (t = x)/(t = 0)/min-1) | r2 | p value | n |
|---|---|---|---|---|
| Control 6% pO2 | -0.0111 ± 0.0003 | 0.956 | 10 | |
| Control 21% pO2 | -0.0136 ± 0.0003* | 0.954 | <0.0001 | 10 |
| Control for treatments 6% pO2 | -0.0107 ± 0.0005 | 0.948 | 4 | |
| 5mM 4-AP 6% pO2 | -0.0114 ± 0.0009† | 0.840 | 0.494 | 4 |
| 5mM TEA 6% pO2 | -0.0111 ± 0.0005† | 0.944 | 0.630 | 4 |
| 100μM H2O2 6% pO2 | -0.0119 ± 0.0003† | 0.976 | 0.048 | 4 |
| 1mM H2O2 6% pO2 | -0.0118 ± 0.0007† | 0.916 | 0.002 | 4 |
| Control for treatments 21% pO2 | -0.0144 ± 0.0006 | 0.953 | 4 | |
| 5mM 4-AP 21% pO2 | -0.0113 ± 0.0006** | 0.918 | 0.0007 | 4 |
| 5mM TEA 21% pO2 | -0.0117 ± 0.0002** | 0.987 | <0.0001 | 4 |
| 100μM H2O2 21% pO2 | -0.0122 ± 0.0004** | 0.963 | 0.004 | 4 |
| 1mM H2O2 21% pO2 | -0.0130 ± 0.0005** | 0.963 | 0.014 | 4 |
Rate constants of 86Rb efflux in control and treated placental villous explants maintained at 6% and 21% pO2 over 16 min. Data are mean ± SE; n is the number of placentas. In all conditions the r2 values, determined by linear regression, were close to 1 and significant (p<0.001), indicating a single exponential decline in intracellular 86Rb over 16 min. The p value corresponds to the following differences in the rate constants between groups: *control 6% pO2 vs control 21% pO2; †treatments at 6% pO2 vs corresponding control at 6% pO2; **treatments at 21% pO2 vs corresponding control at 21% pO2.
Long term effects of pO2: effect of pO2-sensitive K+ channel blockers on syncytiotrophoblast K+ permeability
86Rb (K+) permeability was assayed at day 6 in villous explants cultured at 6% and 21% pO2. Explants were untreated (controls) or treated from day 3 onwards with 4-AP or TEA.
The effect of the K+ channel blockers was assessed by analysing the differences between the rate constant of decline in intracellular 86Rb for each treatment compared to controls (performed in the same placentas n = 4) at the same pO2 (Table 1). The efflux rate constant was significantly reduced by 4-AP and TEA in explants maintained in hyperoxia (21% pO2) but was without effect in explants maintained in normoxia (6% pO2) (Table 1).
Effect of H2O2 on basal 86Rb (K+) permeability and hCG secretion from placental villous explants
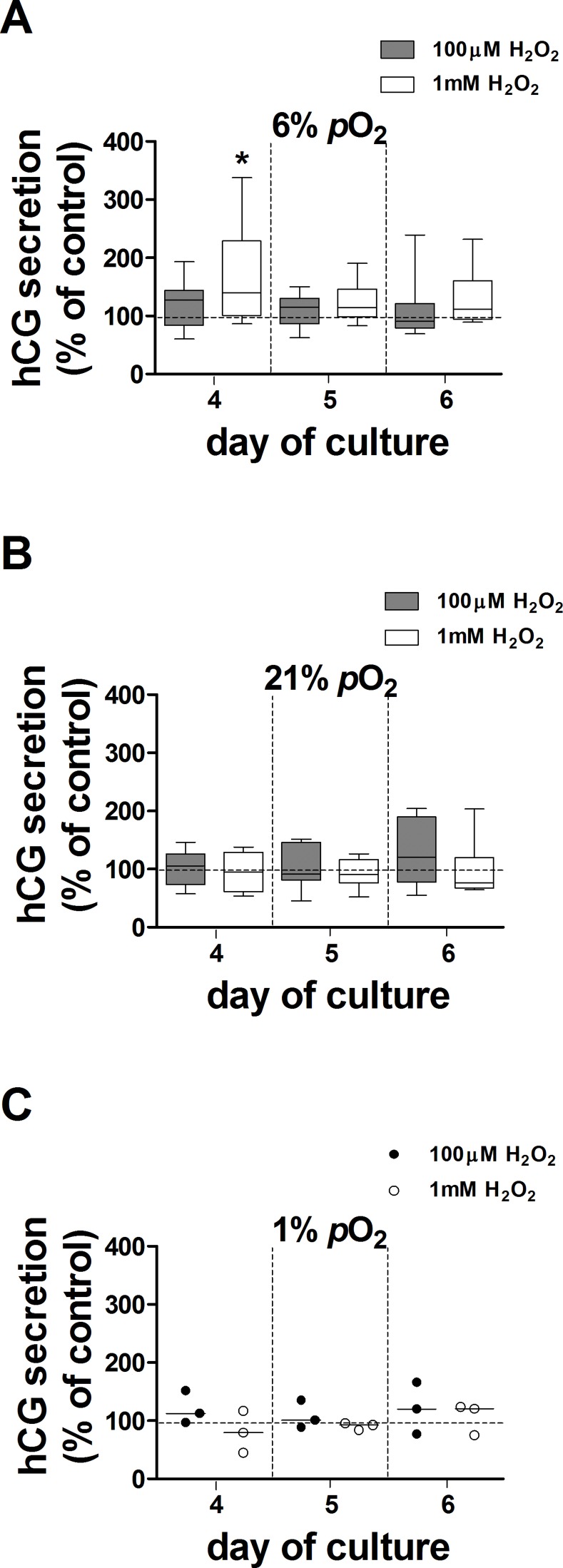
The effect of H2O2, used to generate oxidative stress, on hCG secretion was tested in explants maintained at 6%, 21% and 1% pO2 over days 3–5 of culture. There was no effect of 100μM H2O2 at 6%, 21% or 1% pO2 (Fig 4). In contrast, 1mM H2O2 transiently increased hCG secretion by 40% in explants maintained at 6% pO2 compared to controls (Fig 4A). 10μM H2O2 had no effect on hCG secretion (data not shown). Treatment with H2O2 did not affect LDH release from villous explants at any of the concentrations used (data not shown).
Fig 4. Effect of 100μM and 1mM H2O2 on hCG secretion from villous explants.
hCG secretion from H2O2-treated explants maintained at 6% (A), 21% (B) and 1% pO2 (C) at days 4, 5 and 6 of culture was expressed as a percentage of control (100%, dotted line); data are expressed as median ± IQR; n = 9 placentas for 100μM H2O2 (except n = 3 placentas at 1% pO2); n = 8 placentas for 1mM H2O2 (except n = 3 placentas in 1% pO2). Wilcoxon signed-rank test compared to 100%, *p = 0.04.
The effect of H2O2 on 86Rb efflux was measured at day 6 in explant cultures maintained at 6% and 21% pO2 (Table 1). H2O2 increased the 86Rb efflux rate constant at 100μM and 1mM compared to corresponding controls in explants maintained at 6% pO2. In contrast, treatment of villous explants with 100μM and 1mM H2O2 produced the opposite effect in 21% pO2, and significantly reduced the 86Rb rate constant (Table 1) compared to controls at the same pO2. 10μM H2O2 had no effect on basal 86Rb efflux from explants cultured at either 6% or 21% pO2 (data not shown).
Discussion
This study confirms and extends previous observations that hCG secretion from term placental trophoblast is sensitive to pO2 [8, 18] and ROS [10]. In villous explants prepared from the same placenta, hCG secretion was higher in 21% pO2, and lower in 1% pO2, than 6% pO2 (normoxic) culture conditions. Syncytiotrophoblast K+ permeability, estimated by 86Rb efflux, was higher in explants cultured in 21% than 6% pO2. In accordance with this, 4-AP and TEA, blockers of pO2-sensitive KV channels, inhibited hCG secretion and 86Rb efflux to a greater extent in 21% than 6% pO2. H2O2, used to induce oxidative stress, stimulated 86Rb efflux and transiently increased hCG secretion from explants cultured at 6% pO2 but inhibited 86Rb efflux, without affecting hCG secretion, at 21% pO2. Thus, effects of oxidative stress on hCG secretion and syncytiotrophoblast K+ permeability depend on the pO2.
hCG secretion from term placental syncytiotrophoblast is pO2-dependent
The temporal pattern of hCG secretion from term placental explants maintained at 21% pO2 for 6 days was originally described by Siman et al. [31]. Similar studies to relate hCG secretion to syncytiotrophoblast regeneration over 6 days at placental normoxia (6% pO2), and extreme hypoxia (1% pO2), have not been performed. However, in shorter term cultures (4 days), hCG secretion was lower at 6% and 1% compared to 21% pO2 and this was associated with dysregulated syncytiotrophoblast turnover such as decreased cytotrophoblast proliferation and enhanced apoptosis [18]. In the present study, the time course of hCG secretion from explants maintained at 21% pO2 coincided with previous observations [22, 31]. Secretion was significantly lower at 6% pO2, with only a small rise on day 4, and at 1% pO2 low hCG secretion at day 2 persisted for the duration of the culture. Cellular hCG was lower (1.7-fold) in explants maintained at 6% pO2 compared to 21% pO2, suggesting that pO2 regulates hCG synthesis. However, as hCG secretion at 6% pO2 was 4.1-fold lower than at 21% pO2, the hCG secretory mechanism is additionally regulated by pO2. In contrast, 1% pO2 reduced cellular hCG to the same proportion as secretion, indicating that the reduced secretion in hypoxia is predominantly due to altered synthesis. LDH release was unaffected by pO2 which could indicate that extremes of pO2 do not markedly alter tissue integrity. However, culturing villous tissue at 1% pO2 inhibited LDH synthesis and using LDH release alone as a marker of tissue viability in hypoxia might not be reliable.
Inhibition of hCG secretion by KV channel blockers is pO2-sensitive
We previously demonstrated that chronic exposure to the KV channel blockers 4-AP and TEA induced a concentration-dependent inhibition of hCG secretion in explants and cytotrophoblasts maintained at 21% pO2 [22]. In the current study, 4-AP and TEA inhibited hCG secretion from villous explants to a greater extent in 21% than 6% pO2 and had no effect on secretion at 1% pO2. As the activity and expression of KV channels can be down-regulated by hypoxia [24, 25], our results support the possibility that the lower hCG secretion at 6% compared to 21% pO2 is mediated by closure of KV channels.
Inhibition of 86Rb (K+) efflux by KV channel blockers is pO2-sensitive
Direct study of ion channels in the syncytiotrophoblast of intact placental villi using patch clamp methods is technically challenging as seals are hard to achieve [34] and the multinucleate nature of the tissue precludes whole cell recording. In this study we used 86Rb (K+) efflux to assess whether 4-AP and TEA inhibited K+ conductance in the syncytiotrophoblast and whether the inhibition was pO2-sensitive. We have previously shown that basal 86Rb efflux from placental explants cultured at 21% pO2 is inhibited by Ba2+, a broad spectrum K+ channel blocker [31], implicating K+ conductances in the microvillous, maternal facing plasma membrane of the syncytiotrophoblast.
In the current study, 86Rb efflux measured on day 6 of culture showed that basal K+ permeability was significantly higher in explants maintained in hyperoxia compared to placental normoxia, suggesting that chronic exposure to 21% pO2 over a 6-day period increases the activity and/or expression of syncytiotrophoblast K+ channels. In support of this, treatment of villous explants with 4-AP and TEA significantly reduced 86Rb efflux when the tissue was cultured at 21% but not 6% pO2, consistent with an inhibition of pO2-sensitive KV channels that are more active/more highly expressed at 21% than at 6% pO2. In addition, the inhibition of both 86Rb efflux and syncytiotrophoblast hCG secretion by 4-AP and TEA at 21% but not 6% pO2, suggests that 4-AP and TEA-sensitive KV channels mediate the stimulatory effect of higher pO2 on hCG secretion.
Effect of H2O2 on hCG secretion and 86Rb efflux
Placental oxidative stress and reduced antioxidant defences are key features of pre-eclampsia [35, 36]. In this study we explored the effects of oxidative stress (H2O2) on syncytiotrophoblast hCG secretion and whether they could be modulated through K+ channels. Previous reports showed that in vitro treatment of placental villous tissue with 1mM H2O2 caused oxidative stress which was reversed by vitamins C and E [37].
H2O2 (10μM-1mM) did not alter hCG secretion at 21% pO2 but 1mM caused a transient increase at 6% pO2. This contrasts with the concentrations of H2O2 reported to affect hCG secretion by cytotrophoblasts (inhibition at >50μM and stimulation at 1–50μM) perhaps due to differences between these in vitro preparations; in explants cellular interactions are maintained and tissue antioxidant defences are available to scavenge ROS [38].
H2O2 also had pO2-dependent effects on 86Rb efflux, with 100μM-1mM increasing syncytiotrophoblast 86Rb efflux at 6% pO2, but inhibiting efflux at 21% pO2. This is consistent with the variable effects of ROS on K+ channel activity reported on the literature [28, 30] and raises the possibility that in placental normoxia, K+ channels can be activated by H2O2. However, it is possible that the effect of H2O2 on 86Rb efflux and hCG secretion can be independent events and further work is required to determine which channels are activated by H2O2 in normoxia and whether they are involved in hCG secretion.
Mechanism of hCG secretion: Role of K+ channels
In contrast to hCG secretion in the first trimester of pregnancy [19], the mechanism of secretion by the syncytiotrophoblast at term is not fully elucidated. The present work proposes a role for 4-AP and TEA-sensitive pO2-sensitive K+ channels in regulating hCG secretion. According to the specificity and the concentration of 4-AP and TEA used, the targeted K+ channels belong mainly to the KV channel family [23]. Indeed, K+ channels belonging to other families such as ATP-sensitive K+ channels are not involved in hCG secretion [39].
KV channel mRNA is expressed by whole placental homogenate [40, 41] and immunostaining for KV1.5 and 2.1 has localized protein expression to the syncytiotrophoblast [42]. KV1.5 and 2.1 are pO2-sensitive [43] and blocked by 4-AP and TEA [44] and thus closure of these channels could underlie the lower hCG secretion from placentas maintained in 6% compared to 21% pO2.
In the normal placenta at term where villi are exposed to maternal blood at 6% pO2, pO2-sensitive KV channels could be down-regulated/closed. We have previously shown that hCG secretion at 21% pO2 is stimulated by Ca2+ entry through non-selective cation channels (NSCC; [45]). Therefore, a relatively depolarised membrane potential could minimise Ca2+ entry through NSCC and sustain basal levels of syncytiotrophoblast hCG secretion seen under normoxic conditions. Elevated K+ permeability by ROS (H2O2) in normoxia could reflect increased K+ channel activity, membrane hyperpolarization, promotion of Ca2+ entry and stimulation of hCG secretion.
In conditions such as pre-eclampsia, a pregnancy complication associated with altered pO2 [13], whilst the range of pO2 in the placental bed is unlikely to be as wide as that used in vitro in this study, current data suggest the syncytiotrophoblast could be exposed to both hypoxia [46] and/or hyperoxia [47, 48]. In the latter, pO2-sensitive KV channels could be activated, hyperpolarising the membrane potential, stimulating Ca2+ entry and promoting syncytiotrophoblast hCG secretion. In this regard it is interesting to note that maternal plasma hCG is higher in women with late onset pre-eclampsia compared to women having normal pregnancy [16], and that serum hCG levels vary depending on the severity of disease showing a several-fold increase in severe [49] but not in moderate pre-eclampsia. Consequently, pO2 changes in the placenta might differ related to the severity of disease and this could influence the regulation of hCG secretion by elevated ROS.
Several mechanistic links remain to be explored. For example, although hypoxia inhibits cytotrophoblast cell fusion and hCG secretion [9], there is insufficient evidence at present to confirm that these events are independently pO2-sensitive. Using primary cultures of placental trophoblast in vitro, Alsat et al. (1996) demonstrated that low oxygen (~9% pO2) reduced the formation of multinucleate cells (syncytialisation) and this was associated with an increase in expression of desmoplakin and e-cadherin, and a reduction in hCG secretion. While these data illustrate a clear effect of oxygenation on morphological and biochemical differentiation of cytotrophoblasts, it is unclear whether the primary effect is to reduce hCG secretion, which then inhibits fusion, or whether the primary effect of low pO2 is to inhibit fusion which prevents biochemical differentiation. Furthermore, it is also unknown whether the expression/activity of pO2-sensitive KV channels is altered either by cytotrophoblast differentiation, or pO2, per se.
The extent to which pO2-sensitive K+ channels play a role in hCG secretion in pregnancy disease has yet to be explored; dysregulation of syncytiotrophoblast K+ channel activity and/or expression through chronic exposure to altered pO2 and/or increased ROS could potentially contribute to altered trophoblast renewal and hCG secretion in pre-eclampsia.
Acknowledgments
The authors wish to thank the midwives and patients at the Maternity Unit at St. Mary’s Hospital, Manchester, UK, for their assistance.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by Tommy’s, the baby charity, CONICYT-Becas Chile 72090593 and Action Medical Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Longtine MS, Barton A, Chen B, Nelson DM. Live-cell imaging shows apoptosis initiates locally and propagates as a wave throughout syncytiotrophoblasts in primary cultures of human placental villous trophoblasts. Placenta. 2012;33(12):971–6. 10.1016/j.placenta.2012.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longtine MS, Chen B, Odibo AO, Zhong Y, Nelson DM. Caspase-mediated apoptosis of trophoblasts in term human placental villi is restricted to cytotrophoblasts and absent from the multinucleated syncytiotrophoblast. Reproduction (Cambridge, England). 2012;143(1):107–21. Epub 2011/11/03. doi: REP-11-0340 [pii] 10.1530/REP-11-0340 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim KH, Zhou Y, Janatpour M, McMaster M, Bass K, Chun SH, et al. Human cytotrophoblast differentiation/invasion is abnormal in pre-eclampsia. Am J Pathol. 1997;151(6):1809–18. Epub 1997/12/24. [PMC free article] [PubMed] [Google Scholar]
- 4.Crocker IP, Tansinda DM, Baker PN. Altered cell kinetics in cultured placental villous explants in pregnancies complicated by pre-eclampsia and intrauterine growth restriction. The Journal of pathology. 2004;204(1):11–8. . [DOI] [PubMed] [Google Scholar]
- 5.Higgins L, Mills TA, Greenwood SL, Cowley EJ, Sibley CP, Jones RL. Maternal obesity and its effect on placental cell turnover. J Matern Fetal Neonatal Med. 2012;26(8):783–8. 10.3109/14767058.2012.760539 [DOI] [PubMed] [Google Scholar]
- 6.Weedon-Fekjaer MS, Tasken K. Review: Spatiotemporal dynamics of hCG/cAMP signaling and regulation of placental function. Placenta. 2012;33 Suppl:S87–91. 10.1016/j.placenta.2011.11.003 . [DOI] [PubMed] [Google Scholar]
- 7.Cronier L, Bastide B, Herve JC, Deleze J, Malassine A. Gap junctional communication during human trophoblast differentiation: influence of human chorionic gonadotropin. Endocrinology. 1994;135(1):402–8. Epub 1994/07/01. . [DOI] [PubMed] [Google Scholar]
- 8.Crocker IP, Tansinda DM, Jones CJ, Baker PN. The influence of oxygen and tumor necrosis factor-alpha on the cellular kinetics of term placental villous explants in culture. J Histochem Cytochem. 2004;52(6):749–57. . [DOI] [PubMed] [Google Scholar]
- 9.Alsat E, Wyplosz P, Malassine A, Guibourdenche J, Porquet D, Nessmann C, et al. Hypoxia impairs cell fusion and differentiation process in human cytotrophoblast, in vitro. J Cell Physiol. 1996;168(2):346–53. Epub 1996/08/01. [pii]. . [DOI] [PubMed] [Google Scholar]
- 10.Kharfi Aris A, Leblanc S, Ouellet A, Moutquin JM. Dual action of H2O2 on placental hCG secretion: implications for oxidative stress in preeclampsia. Clinical biochemistry. 2007;40(1–2):94–7. . [DOI] [PubMed] [Google Scholar]
- 11.Poston L, Igosheva N, Mistry HD, Seed PT, Shennan AH, Rana S, et al. Role of oxidative stress and antioxidant supplementation in pregnancy disorders. Am J Clin Nutr. 2011;94(6 Suppl):1980S–5S. 10.3945/ajcn.110.001156 [DOI] [PubMed] [Google Scholar]
- 12.Myatt L, Cui X. Oxidative stress in the placenta. Histochemistry and cell biology. 2004;122(4):369–82. . [DOI] [PubMed] [Google Scholar]
- 13.Hung TH, Burton GJ. Hypoxia and reoxygenation: a possible mechanism for placental oxidative stress in preeclampsia. Taiwanese journal of obstetrics & gynecology. 2006;45(3):189–200. . [DOI] [PubMed] [Google Scholar]
- 14.Raijmakers MT, Peters WH, Steegers EA, Poston L. Amino thiols, detoxification and oxidative stress in pre-eclampsia and other disorders of pregnancy. Curr Pharm Des. 2005;11(6):711–34. Epub 2005/03/22. . [DOI] [PubMed] [Google Scholar]
- 15.Takagi Y, Nikaido T, Toki T, Kita N, Kanai M, Ashida T, et al. Levels of oxidative stress and redox-related molecules in the placenta in preeclampsia and fetal growth restriction. Virchows Arch. 2004;444(1):49–55. Epub 2003/10/24. 10.1007/s00428-003-0903-2 . [DOI] [PubMed] [Google Scholar]
- 16.Kharfi A, Giguere Y, De Grandpre P, Moutquin JM, Forest JC. Human chorionic gonadotropin (hCG) may be a marker of systemic oxidative stress in normotensive and preeclamptic term pregnancies. Clinical biochemistry. 2005;38(8):717–21. . [DOI] [PubMed] [Google Scholar]
- 17.Moll SJ, Jones CJ, Crocker IP, Baker PN, Heazell AE. Epidermal growth factor rescues trophoblast apoptosis induced by reactive oxygen species. Apoptosis. 2007;12(9):1611–22. . [DOI] [PubMed] [Google Scholar]
- 18.Heazell AE, Lacey HA, Jones CJ, Huppertz B, Baker PN, Crocker IP. Effects of oxygen on cell turnover and expression of regulators of apoptosis in human placental trophoblast. Placenta. 2008;29(2):175–86. . [DOI] [PubMed] [Google Scholar]
- 19.Yoshida Y. Secretion of human chorionic gonadotropin in early pregnancy. Medical molecular morphology. 2005;38(2):104–11. . [DOI] [PubMed] [Google Scholar]
- 20.Meuris S, Polliotti B, Robyn C, Lebrun P. Ca2+ entry through L-type voltage-sensitive Ca2+ channels stimulates the release of human chorionic gonadotrophin and placental lactogen by placental explants. Biochimica et biophysica acta. 1994;1220(2):101–6. . [DOI] [PubMed] [Google Scholar]
- 21.Long O, Clarson LH. The effect of Ca2+-permeable channel blockers on human chorionic gonadotrophin (hCG) secretion by villous fragments from term placentas. The Journal of physiology. 2002;539P:126P. [Google Scholar]
- 22.Williams JL, Fyfe GK, Sibley CP, Baker PN, Greenwood SL. K+ channel inhibition modulates the biochemical and morphological differentiation of human placental cytotrophoblast cells in vitro. American journal of physiology. 2008;295(4):R1204–13. 10.1152/ajpregu.00193.2008 [DOI] [PubMed] [Google Scholar]
- 23.Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, et al. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacological reviews. 2005;57(4):473–508. . [DOI] [PubMed] [Google Scholar]
- 24.Platoshyn O, Yu Y, Golovina VA, McDaniel SS, Krick S, Li L, et al. Chronic hypoxia decreases K(V) channel expression and function in pulmonary artery myocytes. Am J Physiol Lung Cell Mol Physiol. 2001;280(4):L801–12. Epub 2001/03/10. . [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Barneo J, del Toro R, Levitsky KL, Chiara MD, Ortega-Saenz P. Regulation of oxygen sensing by ion channels. J Appl Physiol. 2004;96(3):1187–95; discussion 70–2. Epub 2004/02/10. 10.1152/japplphysiol.00929.2003 96/3/1187 [pii]. . [DOI] [PubMed] [Google Scholar]
- 26.Kourie JI. Interaction of reactive oxygen species with ion transport mechanisms. The American journal of physiology. 1998;275(1 Pt 1):C1–24. . [DOI] [PubMed] [Google Scholar]
- 27.Gutterman DD, Miura H, Liu Y. Redox modulation of vascular tone: focus of potassium channel mechanisms of dilation. Arterioscler Thromb Vasc Biol. 2005;25(4):671–8. . [DOI] [PubMed] [Google Scholar]
- 28.Archer SL, Wu X-C, Thebaud B, Moudgil R, Hashimoto K, Michelakis ED. O2 sensing in the human ductus arteriosus: redox-sensitive K+ channels are regulated by mitochondria-derived hydrogen peroxide. Biol Chem. 2004;385(3–4):205–16. [DOI] [PubMed] [Google Scholar]
- 29.Rogers PA, Chilian WM, Bratz IN, Bryan RM Jr, Dick GM. H2O2 activates redox- and 4-aminopyridine-sensitive Kv channels in coronary vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2007;292(3):H1404–11. 10.1152/ajpheart.00696.2006 [DOI] [PubMed] [Google Scholar]
- 30.Appiah I, Milovanovic S, Radojicic R, Nikolic-Kokic A, Orescanin-Dusic Z, Slavic M, et al. Hydrogen peroxide affects contractile activity and anti-oxidant enzymes in rat uterus. Br J Pharmacol. 2009;158(8):1932–41. Epub 2009/11/18. doi: BPH490 [pii] 10.1111/j.1476-5381.2009.00490.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siman CM, Sibley CP, Jones CJ, Turner MA, Greenwood SL. The functional regeneration of syncytiotrophoblast in cultured explants of term placenta. American journal of physiology. 2001;280(4):R1116–22. . [DOI] [PubMed] [Google Scholar]
- 32.Boyd CA. Cotransport systems in the brush border membrane of the human placenta. Ciba Found Symp. 1983;95:300–26. [DOI] [PubMed] [Google Scholar]
- 33.Turner MA, Roulstone CJ, Desforges M, Cretney M, Champion E, Lacey H, et al. The extent and variability of effects of culture conditions on the secretion of human chorionic gonadotrophin and interleukin-6 by human, term placental explants in culture. Placenta. 2006;27(1):98–102. . [DOI] [PubMed] [Google Scholar]
- 34.Brown PD, Greenwood SL, Robinson J, Boyd RD. Chloride channels of high conductance in the microvillous membrane of term human placenta. Placenta. 1993;14(1):103–15. Epub 1993/01/01. . [DOI] [PubMed] [Google Scholar]
- 35.Myatt L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta. 2010;31 Suppl:S66–9. Epub 2010/01/30. doi: S0143-4004(09)00411-1 [pii] 10.1016/j.placenta.2009.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Walsh SW. Antioxidant activities and mRNA expression of superoxide dismutase, catalase, and glutathione peroxidase in normal and preeclamptic placentas. Journal of the Society for Gynecologic Investigation. 1996;3(4):179–84. . [PubMed] [Google Scholar]
- 37.Cindrova-Davies T. Gabor Than Award Lecture 2008: pre-eclampsia-from placental oxidative stress to maternal endothelial dysfunction. Placenta. 2009;30 Suppl A:S55–65. 10.1016/j.placenta.2008.11.020 [DOI] [PubMed] [Google Scholar]
- 38.Hempstock J, Bao YP, Bar-Issac M, Segaren N, Watson AL, Charnock-Jones DS, et al. Intralobular differences in antioxidant enzyme expression and activity reflect the pattern of maternal arterial bloodflow within the human placenta. Placenta. 2003;24(5):517–23. . [DOI] [PubMed] [Google Scholar]
- 39.Lybaert P, Hoofd C, Guldner D, Vegh G, Delporte C, Meuris S, et al. Detection of KATP channels subunits in human term placental explants and evaluation of their implication in human placental lactogen (hPL) and human chorionic gonadotropin (hCG) release. Placenta. 2013;34(6):467–73. 10.1016/j.placenta.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 40.Lacey H, Glazier JD, Greenwood SL, Sibley CP. Potassium channel gene expression over gestation in human placenta. The Journal of physiology. 2005;565P:PC173. [Google Scholar]
- 41.Corcoran J, Lacey H, Baker PN, Wareing M. Altered Potassium Channel Expression in the Human Placental Vasculature of Pregnancies Complicated by Fetal Growth Restriction. Hypertens Pregnancy. 2008;27(1):75–86. 10.1080/10641950701826158 [DOI] [PubMed] [Google Scholar]
- 42.Williams JL, Jones RL, Sibley CP, Baker PN, Greenwood SL. Elevated expression of oxygen-sensitive K+ channels in human placental syncytiotrophoblast of growth restricted fetuses. Reprod Sci. 2009;16(No. 3 (Suppl)):346A. [Google Scholar]
- 43.Patel AJ, Honore E. Molecular physiology of oxygen-sensitive potassium channels. Eur Respir J. 2001;18(1):221–7. . [DOI] [PubMed] [Google Scholar]
- 44.Burg ED, Remillard CV, Yuan JX. K+ channels in apoptosis. The Journal of membrane biology. 2006;209(1):3–20. . [DOI] [PubMed] [Google Scholar]
- 45.Clarson LH, Roberts VH, Hamark B, Elliott AC, Powell T. Store-operated Ca2+ entry in first trimester and term human placenta. The Journal of physiology. 2003;550(Pt 2):515–28. Epub 2003/05/27. 10.1113/jphysiol.2003.044149 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burton GJ. Oxygen, the Janus gas; its effects on human placental development and function. J Anat. 2009;215(1):27–35. Epub 2009/01/30. doi: JOA978 [pii] 10.1111/j.1469-7580.2008.00978.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kingdom JCP, Kaufmann P. Oxygen and placental villous development: Origins of fetal hypoxia. Placenta. 1997;18(8):613–21. 10.1016/S0143-4004(97)90000-X [DOI] [PubMed] [Google Scholar]
- 48.Huppertz B, Weiss G, Moser G. Trophoblast invasion and oxygenation of the placenta: measurements versus presumptions. Journal of Reproductive Immunology. 2014;101–102:74–9. 10.1016/j.jri.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 49.Hsu CD, Chan DW, Iriye B, Johnson TR, Hong SF, Repke JT. Elevated serum human chorionic gonadotropin as evidence of secretory response in severe preeclampsia. American journal of obstetrics and gynecology. 1994;170(4):1135–8. Epub 1994/04/01. doi: S0002937894005752 [pii]. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.