Abstract
The rate of photosynthesis (A) of plants exposed to water deficit is a function of stomatal (gs) and mesophyll (gm) conductance determining the availability of CO2 at the site of carboxylation within the chloroplast. Mesophyll conductance often represents the greatest impediment to photosynthetic uptake of CO2, and a crucial determinant of the photosynthetic effects of drought. Abscisic acid (ABA) plays a fundamental role in signalling and co-ordination of plant responses to drought; however, the effect of ABA on gm is not well-defined. Rose, cherry, olive and poplar were exposed to exogenous ABA and their leaf gas exchange parameters recorded over a four hour period. Application with ABA induced reductions in values of A, gs and gm in all four species. Reduced gm occurred within one hour of ABA treatment in three of the four analysed species; indicating that the effect of ABA on gm occurs on a shorter timescale than previously considered. These declines in gm values associated with ABA were not the result of physical changes in leaf properties due to altered turgor affecting movement of CO2, or caused by a reduction in the sub-stomatal concentration of CO2 (Ci). Increased [ABA] likely induces biochemical changes in the properties of the interface between the sub-stomatal air-space and mesophyll layer through the actions of cooporins to regulate the transport of CO2. The results of this study provide further evidence that gm is highly responsive to fluctuations in the external environment, and stress signals such as ABA induce co-ordinated modifications of both gs and gm in the regulation of photosynthesis.
Introduction
The rate of photosynthesis (A) in drought stressed plants is frequently constrained by the availability of carbon dioxide at the site of carboxylation. The concentration of CO2 within the chloroplast envelope (Cc) is determined by stomatal (gs) and mesophyll (gm) conductance [1–3]. During water deficit, levels of ABA within the leaf are often enhanced by the transport of ABA from the roots to the shoots [4] and the conversion of ‘fixed’ glycosylated ABA stored within the vacuole to ‘free’ ABA in the cytosol [5, 6]. Stomatal conductance is negatively correlated to the concentration of ABA in the xylem [7] and leaf [8]. The effect of ABA on stomatal closure may be further enhanced as apoplastic ABA concentrates at the sites of evaporation close to the stomatal pores [9], and pH changes on a cellular level facilitate the movement of ABA to the guard cells [10]. Generally, stomatal and mesophyll conductance operate in tandem, showing marked reductions to drought [2, 11, 12]. It was shown that this tight coordination was controlled in an ultradian fashion in Eucalyptus citriodora plants subjected to different degrees of water deficit [13]. However, despite transport of CO2 across the mesophyll layer representing the largest resistance step in the uptake of CO2 for photosynthesis and a critical component in the drought stress response of plants, the response of gm to ABA is not clear.
Mesophyll conductance is determined by physical [14–16] and biochemical [17, 18] factors. Any reduction in leaf turgor could alter physical resistances, such as apoplastic space and the porosity of the cell wall encountered by CO2 during transport from the sub-stomatal air-space to the chloroplast [9, 19, 20]. It is therefore necessary to distinguish between the physical influence of changes in leaf turgor experienced during drought, and any direct biochemical effects of ABA on gm. Relatively few studies have investigated the effect of ABA on gm, and have produced contrasting results; possibly due to the experimental approaches utilised to modify [ABA], either through increased endogenous production of ABA or exogenous application e. g. [21, 22], and the methods employed to measure gm e.g. [23, 24, 25]. Exogenous application of ABA to hydroponically grown soybean (Glycine max L.) and tobacco (Nicotiana tabaccum L.) resulted in reduced gs and gm values over the course of ten days [3]. Identical reductions of both gs and gm were observed in cut-leaves of drought stressed and well-watered wild-type and ABA deficient mutants of tobacco (Nicotiana plumbaginifolia) two hours after exposure to a solution containing ABA [9]. The addition of ABA to the nutrient solution of ‘sand-grown’ sunflowers (Helianthus annuus L.) also reduced canopy-level gm after three days [26]. In contrast, perlite grown sunflower plants supplied with an exogenous ABA solution showed reduced gs, but no alteration of gm after three days [25]. Furthermore, no difference was observed in gm values of wild-type and ABA insensitive mutants of Arabidopsis thaliana [27].
The disparity in responses of gm to ABA may be related to interspecific differences in drought stress physiology. For example, concomitant reductions in both gs and gm are frequently observed in response to water-deficit e.g. [2]. However, in drought tolerant plants, persistent long-term water deficit may result in an enhancement of gm in comparison to the early stages of drought, whilst gs remains low [28, 29]. One other potential explanation is the methodology employed to gauge gm. It is not possible to measure gm directly, and all approaches involve a number of assumptions and potential uncertainties [23]. Mesophyll conductance can be calculated through simultaneous measurement of leaf gas exchange and chlorophyll fluorescence parameters (the variable J method) [30, 31], determination of leaf gas exchange and carbon isotope discrimination [32, 33] or analysis of the curve of the photosynthetic response to increased [CO2] in the internal sub-stomatal air-space (Ci) [34]. It has been suggested that stomatal closure during drought stress may alter rates of photosynthetic ‘recapture’ of CO2 released during photorespiration, affecting the measurement of levels of respiration in the light (Rd) and thus the determination of gm using the variable J method [23, 35]. However, observations of gm sensitivity and insensitivity to ABA have been recorded using both the variable J method [3, 9, 25] and the carbon isotopic discrimination approach [9, 25, 26]. This may suggest that it is not possible to fully account for the disparity in gm responses to ABA due to uncertainties with the methodological approach utilised to characterise gm. Furthermore, analysis of the effects of drought on gm levels in rice (Oryza sativa) using both the variable J method and analysis of carbon isotope discrimination in recently synthesised sugars method indicated that the variable J technique was equally effective as the carbon isotopic discrimination approach in gauging gm at low levels of water availability, but crucially produced more robust results under well-watered control conditions [2].
Abscisic acid plays a fundamental role in the drought stress response of plants. Given the wide-range of gm responses to ABA reported in the literature over different timescales, we investigated gm sensitivity to exogenous ABA application in four commercially important woody species. To characterise the direct biochemical response of gm to ABA, measurements were taken over a short time period (four hours) to minimise any physical effects that may be associated with leaf water status that could affect the movement of CO2 from the sub-stomatal air-space to the chloroplast envelope. This study aimed to: i) investigate the effect of exogenous ABA on stomatal and mesophyll conductance over a four hour time-course; ii) characterise any interspecific variations in the gm response of the four species to ABA; iii) assess diffusive constraints imposed by gs and gm following ABA treatment that determine rates of photosynthesis.
Materials and Methods
Plant material, growing conditions and ABA treatment
Cherry (Prunus avium L.), black poplar (Populus nigra L.), olive (Olea europaea L.) and rose (Rosoideae rosa, hybrid tea rose “Camp David“) were grown in a greenhouse at the National Research Council, Monterotondo, Rome, Italy. The plants were approximately one year-old and grown in comparatively large 6 dm3 pots, where they did not experience root-restriction, containing a sand-perlite mixture (1:3) under natural sunlight and photoperiod from June to August. The respective daily maximum and minimum air temperatures were 38 and 20°C. To avoid any water and nutrient limitation, the saplings were watered every other day to pot water capacity and fertilised once a week, with Hoagland nutrient solution to supply nutrients at free access rates [36].
The evening prior to measurement the plants were watered to pot water capacity. The next morning branches were cut under distilled water; control treatment branches remained in distilled water, and cis-trans ABA (99% purity, Sigma) was added to the water of branches subject to ABA treatment. An ABA solution of 10−4 M concentration was used. Simultaneous measurement of gas exchange and chlorophyll fluorescence was then performed every hour over a four hour period between 08:00 and 12:00 in a well ventilated air-conditioned room at 25°C with control and ABA-treated shoots placed under a metal halide light emitting 800 μmol m-2 s-1 PPFD.
Gas exchange and fluorescence measurements
Leaf gas exchange and fluorescence parameters of the central leaf section were simultaneously measured using a LI-6400-40 leaf chamber fluorometer (Li-Cor, Inc., Nebraska, USA) equipped with a 2 cm2 cuvette. One branch for the ABA and control treatments was taken from each of four plants, with the youngest fully expanded leaf from four branches measured for each treatment. The measurements were made at a saturating photon flux density (PPFD) of 1600 μmol m-2s-1 measured using the internal quantum sensor within the leaf chamber and leaf temperature of 25°C for all four species. Leaves were exposed to a contaminant and pollutant free flux of synthetic air, composed of a mixture of nitrogen (80%), O2 (20%) and CO2 (385 ppm). The relative humidity of the air flow (500 μmol s-1) was maintained at 40–50%. To reduce diffusion leaks through the chamber gasket [37], a supplementary external chamber gasket composed of the same polymer foam was added to create an interspace between the two gaskets (i.e. a double-gasket design with a 5 mm space separating the internal and external gaskets). Then the CO2 and H2O gradients between the in-chamber air and pre-chamber air were minimized by feeding the IRGA exhaust air into the interspace between the chamber and the pre-chamber gaskets [38].
The variable J method has proven to be effective in determining gm in both well-watered and drought stressed rice varieties [2], we therefore chose this approach to assess the effect of ABA on gm in the four plant species. Mesophyll conductance was calculated using the variable J method involving simultaneous measurements of gas-exchange and chlorophyll fluorescence parameters as described by Harley et al. (30) and Loreto et al. (31) (Eqs 1 and 2):
| (1) |
where the electron transport rate (JF) is calculated from fluorescence [39]:
| (2) |
where Fm is the fluorescence maximum and the partitioning factor (β) between photosystems I and II was considered to be 0.5 and leaf absorbance (α) (0.85) [40].
The CO2 compensation point to photorespiration (Γ*) was measured on individual attached leaves of intact plants by increasing Ci at four different levels of photosynthetically active radiation [41, 42]. Levels of respiration in the light (Rd) were analysed using the Kok method [43, 44]; and respiration in the dark (Rn) was measured by switching off the light in the cuvette, when CO2 release from the leaf had become stable for approximately five to ten minutes this was recorded and considered to represent Rn [45]. Values of Γ*, Rd and Rn used in the calculation of gm utilising the variable J method are given in Table 1. Total conductance to CO2 (gtot) was calculated as:
| (3) |
Table 1. Values of the CO2 compensation point to photorespiration (Γ*), respiration in the light (Rd) and respiration in the dark (Rn) used to calculate gm levels of the four plant species using the variable J method [30, 31].
± indicates one standard deviation.
| Species | Rn (μmol m-2s-1) | Rd (μmol m-2s-1) | Γ* (μmol mol-1) |
|---|---|---|---|
| Cherry | 1.97 ± 0.28 | 1.40 ± 0.13 | 46.6 ± 2.77 |
| Olive | 1.72 ± 0.10 | 1.28 ± 0.09 | 58.4 ± 2.59 |
| Poplar | 1.80 ± 0.09 | 1.06 ± 0.12 | 45.5 ± 3.72 |
| Rose | 1.94 ± 0.20 | 1.24 ± 0.10 | 55.3 ± 3.59 |
Leaf water status
Immediately following the gas exchange measurements, each leaf was detached and weighed to determine leaf fresh mass (FM). The leaves were then placed in a plastic bag and with the cut-end submerged in distilled water and allowed to rehydrate in darkness at 5°C for 18 hours. After rehydration the leaves were dried using paper towels to remove any water on their surfaces, and then the leaves were weighed to determine the saturated mass (SM). Leaves were then dried at 80°C for 48 hours to measure dry mass (DM). The relative water content (RWC) of each leaf was then calculated as follows [46]:
| (4) |
Statistical analyses
Statistical analyses were performed using SPSS 20 (IBM, New York, USA). A one-way ANOVA with LSD post-hoc test was used to assess differences in variance between samples subjected to control conditions and ABA treatment. A significant difference between treatments was assumed to occur at a P-value <0.05. Linear regression was used to investigate potential relationships between conductance to CO2 and A in the four plant species under control conditions and ABA treatment.
Results
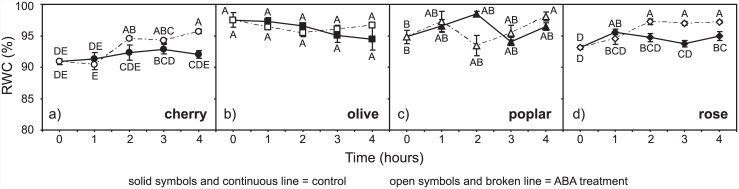
Following abscission of the cuttings, the RWC of the leaves was recorded at each hourly measurement interval to assess any alteration in leaf water content that may have influenced gm via a change in turgor. The RWC of control cherry leaves remained within the range of 88.7–94.1% throughout the measurement period (Fig 1a). However, after two hours the mean RWC of leaves exposed to exogenous ABA increased from 90.9% to 95.7%; a significant difference between the RWC values of the control and ABA treated leaves only became apparent after four hours of exposure (one-way ANOVA LSD post-hoc test, F1,6 = 25.954; P = 0.00223). The RWC of rose leaves exhibited a similar pattern, with the mean RWC of ABA treated leaves exhibiting an increase from 94.6% to 97.2% after two hours. However, RWC parameters of control leaves also showed a slight increase during the measurement period over a range of 92.2 to 96.7%. Statistically significant differences were observed in the RWC values of control and ABA treated rose leaves after two hours (one-way ANOVA LSD post-hoc test, F1,6 = 9.416; P = 0.0220) and this persisted for the remainder of the measurement period (Fig 1d). The RWC of poplar leaves showed no significant decrease between the control and ABA treatments, although RWC rose slightly by 1.6–3.4% over the four hour measurement period (Fig 1c). The RWC values of olive leaves displayed no significant change during the four hour measurement period, and no significant treatment effect associated with the application of ABA (Fig 1b).
Fig 1. Relative water content values of control and ABA treated leaves of cherry (a), olive (b), poplar (c) and rose (d) over the four hour measurement period.
Solid symbols and continuous line indicate control samples; open symbols and broken line indicate ABA treatment. Error bars indicate one standard error either side of mean. Different letters indicate significant differences between datasets based upon one-way ANOVA and LSD post-hoc test.
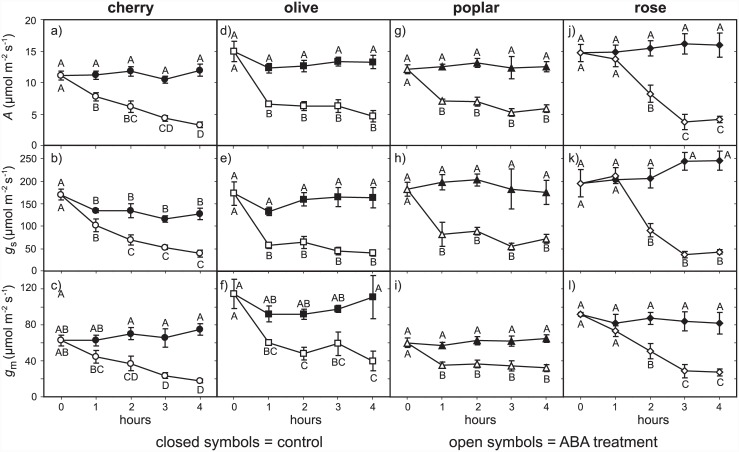
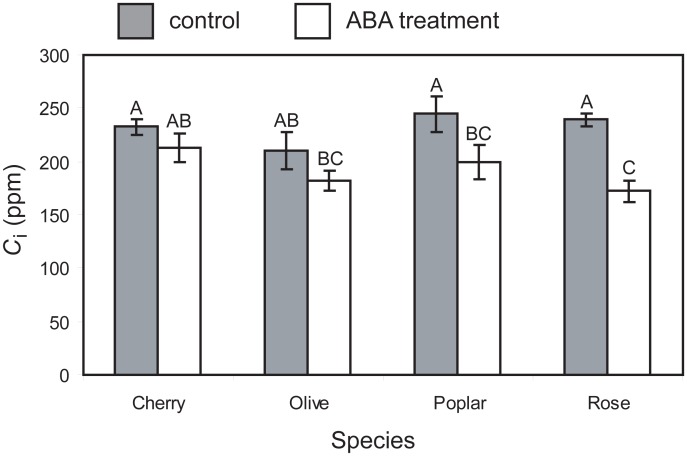
Exposure to exogenous ABA induced consistent declines in A in all four of the species studied. The control plants showed no declines in A that may have been associated with branch abscission (Fig 2). These reductions in A following exposure to ABA corresponded to identical declines in gs and gm. However, the rate of reduction in gs and gm values differed between the four species. Cherry exhibited a consistent decline in A, gs and gm throughout the measurement period following ABA treatment (Fig 2a–2c). Olive and poplar displayed rapid reductions in gs (olive: -66.7%; poplar– 54.8%) and gm (olive: -47.3%; poplar– 40.9%) in the first hour after exposure to exogenous ABA, before maintaining relatively stable gs and gm values for the remainder of the measurement period. In contrast, rose does not show a decline in levels of A, gs and gm in the first hour after ABA treatment, before exhibiting a rapid decline in the second hour (A: -44.2%; gs: -53.5%; gm: -44.5%) then stabilising in the third and fourth hour (Fig 2j–2l). The lower levels of conductance to CO2 and A following four hours of ABA treatment led to decreases in Ci of 8.4 to 28.0% in all four plant species. However, this reduced Ci was statistically significant only in poplar and rose (Fig 3).
Fig 2. Time course A, gs and gm responses of cherry (a-c), olive (d-f), poplar (g-i) and rose (j-l) cuttings to application of exogenous ABA solution.
Solid symbols indicate control samples; open symbols indicate ABA treatment. Error bars indicate one standard error either side of mean. Different letters indicate significant differences between datasets based upon one-way ANOVA and LSD post-hoc test.
Fig 3. Internal sub-stomatal concentrations of CO2 (Ci) of control and ABA treated cuttings after four hours.
Grey indicates control; white indicates ABA treatment. Error bars indicate one standard error either side of mean. * indicates significant difference between control and ABA treatment values using a one-way ANOVA (cherry, F1,6 = 1.501, P = 0.266; olive, F1,6 = 2.020, P = 0.205; poplar, F1,6 = 8.998, P = 0.0240; rose, F1,6 = 34.991, P = 0.00104). Different letters indicate significant differences between datasets based upon one-way ANOVA and LSD post-hoc test.
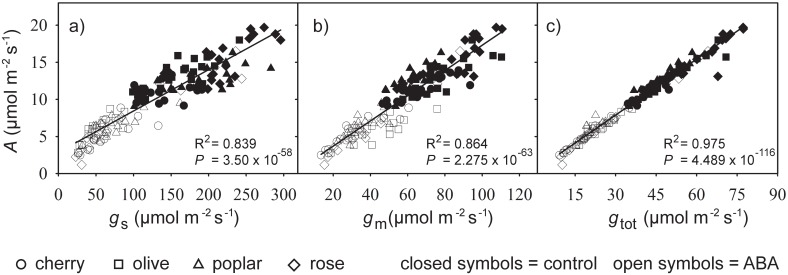
Photosynthetic rates of the four species were largely determined by conductance to CO2 (Fig 4). Under control conditions, rose generally exhibited the highest levels of A, gs and gm, while cherry displayed the lowest. However, after four hours of ABA treatment, rose and cherry showed identical photosynthetic rates and levels of conductance to CO2. The leaves treated with exogenous ABA exhibited lower levels of gs, gm and gtot that correspond to lower A. The relationship between A and conductance to CO2 was most robust when gs and gm were combined to determine gtot (Fig 4c).
Fig 4. Relationship of A to gs (a), gm (b), and gtot (c) in control (closed symbols) and ABA treated (open symbols) cuttings of cherry (circle symbol), olive (square symbol), poplar (triangle symbol) and rose (diamond symbol) after four hours of treatment.
Line indicates best fit determined by linear regression: stomatal conductance (F1,142 = 740.620; R2 = 0.839; P = 3.350 x 10–58), mesophyll conductance (F1,142 = 901.489; R2 = 0.864; P = 2.275 x 10−63), and; total conductance to CO2 (F1,142 = 5618.153; R2 = 0.975; P = 4.488 x 10−116).
Discussion
Abscisic acid plays a fundamental role in plant responses to water-deficit. The effect of ABA on gm is poorly characterised in comparison to the influence of ABA on stomatal behaviour e.g. [4, 47, 48]. This study demonstrated that exogenous ABA produced a rapid reduction in gm values within two hours of application in all of the four species studied. These declines in gm corresponded to reductions in gs, analogous to co-ordination of gs and gm observed during drought [2, 12, 49] and as circadian pattern [13]. The reductions in gs following ABA application are indicative of stomatal sensitivity to ABA and active physiological stomatal behaviour in all four of the plant species studied [50, 51]. Photosynthesis was closely related to conductance to CO2 in the control and ABA treated cuttings (Fig 4), suggesting that ABA determined A through its action upon gs and gm. The speed of response of gm, gs and A to ABA application varied between the four species, possibly due to differences in water transport e.g. [52] affecting the uptake and movement of ABA, or biochemical differences in the effect of ABA signalling between the plants e.g. [53, 54].
Mesophyll conductance is composed of physical and chemical components that determine the movement of CO2 from the internal sub-stomatal air-space to inside the chloroplast envelope [55]. Increased RWC in ABA treated cherry and rose (Fig 1), possibly due to stomatal closure reducing transpirative water-loss from the leaf, may have altered the physical properties of the leaf and thus affected gm indirectly. However, in the evening prior to abscission the plants were watered to pot capacity, and all of the leaves exhibited high RWC values that correspond to full leaf turgor [56], likely suggesting that the proportionally small percentage increases of 2–3% in RWC following ABA application did not induce significant alteration of leaf structural properties. It is noteworthy that significant differences in the RWC of control and ABA treated leaves of cherry only became apparent after two hours, whereas gm values in ABA treated leaves declined significantly within one hour (Fig 2c); suggesting that the initial declines in following ABA application were solely the result of biochemical changes, and not due to alteration of the physical properties of leaves associated with a change in foliar water status.
The effect of ABA on gm observed in this study was rapid, occurring within one hour of exposure in three of the four species studied. Short-term fluctuations in gm have also been recorded in response to [CO2] [37], salinity [1, 57], light quality [58, 59], light intensity [37] and temperature [18], and have been ascribed to the action of carbonic anhydrase and cooporins that transport CO2 across the plasma membrane [17, 60–65]. The conversion of gaseous CO2 to aqueous carbonic acid (H2CO3) represents one of the largest resistance steps encountered in photosynthetic CO2-uptake [66]. The results of this study may suggest that ABA acts to reduce the activity of cooporins involved in the transport of CO2. Cooporins belong to a group of proteins known as aquaporins, that also facilitate the movement of water across membranes [67]. As foliar [ABA] increased, the activity of aquaporins have been shown to decline during drought stress [68, 69], and induce stomatal closure through increased guard cell permeability and reduced water movement across the membrane [70]. Exogenous application of ABA to fully hydrated leaves achieved similar results [71]. However, increased [ABA] may also enhance the expression of certain aquaporins to enhance drought tolerance through increased water transport [72].
It is unclear whether alterations in gm are a by-product of the gs response, or whether the two processes are linked by co-ordinated physiological signalling [73]. Instantaneous increases in Ci applied to plants grown under optimal conditions induce reductions in gm, as the limiting effect of CO2 availability on A declines at higher Ci [37]. This would suggest that if the gm responses observed following ABA treatment in the present study were solely the result of reduced Ci due to stomatal closure, an increase in gm might be expected. However, reduced gm was recorded in all four species after application of exogenous ABA; furthermore, only two of the species exhibited significant declines in Ci after ABA treatment (Fig 3). Application of the same concentration of ABA used in this study to the roots of sunflower did not affect gm but did reduce gs. The relationship between gm and Ci in ABA treated sunflower was also identical to control plants; showing a positive correlation between gm and Ci at concentrations above 200 ppm [25]. However, detached leaves of wild-type tobacco plants when treated with exogenous ABA exhibited a positive gm—Ci relationship at sub-ambient Ci, whilst the control counterparts showed a negative gm—Ci relationship [73]. Furthermore, gm response to Ci has been shown to occur on a shorter timescale than gs response to Ci in tobacco, wheat and in both wild-type and mutant Arabidopsis thaliana that lacked the capacity for stomatal closure [74]. This may suggest that the concomitant declines in gs and gm observed in this study following ABA treatment are the result of a shared signalling mechanism regulating rates of stomatal and mesophyll conductance to CO2 [73] in addition to any subsequent effects on gm associated with stomatal closure [73–75], respiration [23] or photorespiration [24].
It is noteworthy that the effect of ABA on gm observed in the present study occurs more rapidly than previously reported e.g. [3, 9, 25, 26]. Increased foliar [ABA] following drought stress induces stomatal closure to restrict water-loss from the internal leaf [76]. It would initially appear incongruous to reduce transport of CO2 across the mesophyll layer in concert with decreased gs to minimise water-loss from the internal leaf [73]; as a high gm to gs ratio would permit the maintenance of a degree of CO2 uptake and enhanced water use efficiency during drought [27]. However, the rapid declines in gm after application with exogenous ABA may indicate a selective advantage of reduced conductance to CO2 across the mesophyll during episodes of water-deficit. A decline in the activity of cooporins responsible for the transport of CO2 may reduce energy consumption [77]. Higher cellular [78] and apoplastic [79] ABA may also be associated with changes in pH that alter membrane properties through the action of proteins such as aquaporins [80], thus affecting gm [17]. Nonetheless, it is presently unclear as to the nature of the mechanisms responsible for such rapid alterations in gm following ABA treatment or their functional significance. Further analysis of the expression of cooporin protein RNA may elucidate the biochemistry underlying this response e.g. [81]. Application with exogenous ABA induced declines in gm within one hour in three of the species studied (and within two hours in the remaining species, rose); this would suggest that ABA has a clear effect on gm, and the interaction of ABA and gm plays an important role in plant drought stress response.
Conclusion
The results of this study show consistent reductions in A, gs and gm values of all four plants following exposure to exogenous ABA. Photosynthesis in the plant species was positively related to the availability of CO2 within the chloroplast envelope (Fig 4). The observed declines in gm occurred on a shorter timescale than those reported in previous studies; suggesting that ABA serves to induce rapid reductions in gm following exposure to water deficit. These falls in gm values occurred prior to any significant alteration in RWC; suggesting that they are not associated with physical effects of increased turgor affecting the permeability of cell membranes, and are instead the result of alteration to the biochemical properties of the interface between the mesophyll and the internal sub-stomatal air-space. The reductions in gm recorded in the present study are unlikely to be the by-product of stomatal closure causing a decrease in Ci, as plants exposed to ABA exhibited reduced gm rather than increased conductance to counter lower availability of CO2 in the sub-stomatal air-space. The effect of ABA on gm is likely through the diminished activity of cooporins and carbonic anhydrase, whether these responses act on a dose-dependent basis or occur over different timescales is currently unclear. However, the findings of the present study indicate that ABA functions by inducing rapid reductions in gm that are associated with concomitant declines in gs as part of a co-ordinated gas exchange response to water deficit.
Acknowledgments
This work was supported by the Ministero dell’Istruzione, dell’Università e della Ricerca of Italy: PRIN 2010–2011 “PRO-ROOT” and Progetto Premiale 2012 “Aqua”. MH acknowledges funding from a Marie Curie IEF (2010–275626).
Data Availability
All relevant data are within the paper.
Funding Statement
This work was funded by Ministero dell’Istruzione, dell’Università e della Ricerca of Italy: PRIN 2010-2011 “PRO-ROOT” Ministero dell’Istruzione, dell’Università e della Ricerca of Italy: Progetto Premiale 2012 “Aqua”. http://www.istruzione.it/ Marie Curie IEF (2010-275626) http://ec.europa.eu/research/mariecurieactions/.
References
- 1.Centritto M, Loreto F, Chartzoulakis K. The use of low [CO2] to estimate diffusional and non-diffusional limitations of photosynthetic capacity of salt-stressed olive saplings. Plant, Cell and Environment. 2003;26(4):585–94. 10.1046/j.1365-3040.2003.00993.x [DOI] [Google Scholar]
- 2.Lauteri M, Haworth M, Serraj R, Monteverdi MC, Centritto M. Photosynthetic diffusional constraints affect yield in drought stressed rice cultivars during flowering. PloS one. 2014;9(10):e109054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flexas J, Ribas-Carbó M, Bota J, Galmés J, Henkle M, Martínez-Cañellas S, et al. Decreased Rubisco activity during water stress is not induced by decreased relative water content but related to conditions of low stomatal conductance and chloroplast CO2 concentration. New Phytol. 2006;172(1):73–82. 10.1111/j.1469-8137.2006.01794.x [DOI] [PubMed] [Google Scholar]
- 4.Davies WJ, Zhang JH. Root signals and the regulation of growth and development of plants in drying soil. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:55–76. PubMed PMID: ISI:A1991FP08300004. [Google Scholar]
- 5.Lee KH, Piao HL, Kim H-Y, Choi SM, Jiang F, Hartung W, et al. Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell. 2006;126(6):1109–20. [DOI] [PubMed] [Google Scholar]
- 6.Xu Z-J, Nakajima M, Suzuki Y, Yamaguchi I. Cloning and characterization of the abscisic acid-specific glucosyltransferase gene from adzuki bean seedlings. Plant Physiol. 2002;129(3):1285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Davies W. Changes in the concentration of ABA in xylem sap as a function of changing soil water status can account for changes in leaf conductance and growth. Plant, Cell and Environment. 1990;13(3):277–85. [Google Scholar]
- 8.Thomas DS, Eamus D. The influence of predawn leaf water potential on stomatal responses to atmospheric water content at constant Ci and on stem hydraulic conductance and foliar ABA concentrations. J Exp Bot. 1999;50(331):243–51. 10.1093/jxb/50.331.243 [DOI] [Google Scholar]
- 9.Mizokami Y, Noguchi K, Kojima M, Sakakibara H, Terashima I. Mesophyll conductance decreases in the wild type but not in an ABA-deficient mutant (aba1) of Nicotiana plumbaginifolia under drought conditions. Plant, Cell Environ. 2015;38(3):388–98. 10.1111/pce.12394 PubMed PMID: MEDLINE:24995523. [DOI] [PubMed] [Google Scholar]
- 10.Cowan IR, Raven JA, Hartung W, Farquhar GD. A possible role for abscisic acid in coupling stomatal conductance and photosynthetic carbon metabolism in leaves. Funct Plant Biol. 1982;9(4):489–98. 10.1071/PP9820489 [DOI] [Google Scholar]
- 11.Flexas J, Ribas-Carbó M, Diaz-Espejo A, Galmēs J, Medrano H. Mesophyll conductance to CO2: current knowledge and future prospects. Plant, Cell Environ. 2008;31(5):602–21. 10.1111/j.1365-3040.2007.01757.x [DOI] [PubMed] [Google Scholar]
- 12.Centritto M, Lauteri M, Monteverdi MC, Serraj R. Leaf gas exchange, carbon isotope discrimination, and grain yield in contrasting rice genotypes subjected to water deficits during the reproductive stage. J Exp Bot. 2009;60(8):2325–39. 10.1093/jxb/erp123 PubMed PMID: WOS:000266348800010. [DOI] [PubMed] [Google Scholar]
- 13.Brilli F, Tsonev T, Mahmood T, Velikova V, Loreto F, Centritto M. Ultradian variation of isoprene emission, photosynthesis, mesophyll conductance, and optimum temperature sensitivity for isoprene emission in water-stressed Eucalyptus citriodora saplings. J Exp Bot. 2013;64(2):519–28. 10.1093/jxb/ers353 PubMed PMID: WOS:000313618900012. [DOI] [PubMed] [Google Scholar]
- 14.Adachi S, Nakae T, Uchida M, Soda K, Takai T, Oi T, et al. The mesophyll anatomy enhancing CO2 diffusion is a key trait for improving rice photosynthesis. J Exp Bot. 2013;64(4):1061–72. 10.1093/jxb/ers382 PubMed PMID: WOS:000316003600021. [DOI] [PubMed] [Google Scholar]
- 15.Evans J, Caemmerer S, Setchell B, Hudson G. The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of rubisco. Funct Plant Biol. 1994;21(4):475–95. 10.1071/PP9940475 [DOI] [Google Scholar]
- 16.Tomás M, Flexas J, Copolovici L, Galmés J, Hallik L, Medrano H, et al. Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. J Exp Bot. 2013;64(8):2269–81. 10.1093/jxb/ert086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanba YT, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K, Terashima I, et al. Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant and Cell Physiology. 2004;45(5):521–9. 10.1093/pcp/pch070 [DOI] [PubMed] [Google Scholar]
- 18.Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP. Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol. 2002;130(4):1992–8. 10.1104/pp.008250 PubMed PMID: WOS:000179990100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grassi G, Magnani F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant, Cell and Environment. 2005;28(7):834–49. [Google Scholar]
- 20.Parida AK, Das AB, Mittra B. Effects of salt on growth, ion accumulation, photosynthesis and leaf anatomy of the mangrove, Bruguiera parviflora. Trees. 2004;18(2):167–74. 10.1007/s00468-003-0293-8 [DOI] [Google Scholar]
- 21.Tardieu F, Lafarge T, Simonneau T. Stomatal control by fed or endogenous xylem ABA in sunflower: interpretation of correlations between leaf water potential and stomatal conductance in anisohydric species. Plant, Cell and Environment. 1996;19(1):75–84. [Google Scholar]
- 22.Correia MJ, Pereira JS, Chaves MM, Rodrigues ML, Pacheco CA. ABA xylem concentrations determine maximum daily leaf conductance of field-grown Vitis vinifera L. plants. Plant, Cell and Environment. 1995;18(5):511–21. 10.1111/j.1365-3040.1995.tb00551.x [DOI] [Google Scholar]
- 23.Pons TL, Flexas J, von Caemmerer S, Evans JR, Genty B, Ribas-Carbo M, et al. Estimating mesophyll conductance to CO2: methodology, potential errors, and recommendations. J Exp Bot. 2009;60(8):2217–34. 10.1093/jxb/erp081 [DOI] [PubMed] [Google Scholar]
- 24.Gilbert ME, Pou A, Zwieniecki MA, Holbrook NM. On measuring the response of mesophyll conductance to carbon dioxide with the variable J method. J Exp Bot. 2012;63(1):413–25. 10.1093/jxb/err288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vrabl D, Vaskova M, Hronkova M, Flexas J, Santrucek J. Mesophyll conductance to CO2 transport estimated by two independent methods: effect of variable CO2 concentration and abscisic acid. J Exp Bot. 2009;60(8):2315–23. 10.1093/jxb/erp115 PubMed PMID: WOS:000266348800009. [DOI] [PubMed] [Google Scholar]
- 26.Schäeufele R, Santrucek J, Schnyder H. Dynamic changes of canopy-scale mesophyll conductance to CO2 diffusion of sunflower as affected by CO2 concentration and abscisic acid. Plant, cell and environment. 2011;34(1):127–36. 10.1111/j.1365-3040.2010.02230.x [DOI] [PubMed] [Google Scholar]
- 27.Flexas J, Niinemets Ü, Gallé A, Barbour M, Centritto M, Diaz-Espejo A, et al. Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water-use efficiency. Photosynthesis Res. 2013;117(1–3):45–59. 10.1007/s11120-013-9844-z [DOI] [PubMed] [Google Scholar]
- 28.Aganchich B, Wahbi S, Loreto F, Centritto M. Partial root zone drying: regulation of photosynthetic limitations and antioxidant enzymatic activities in young olive (Olea europaea) saplings. Tree Physiology. 2009;29(5):685–96. 10.1093/treephys/tpp012 PubMed PMID: WOS:000265850500006. [DOI] [PubMed] [Google Scholar]
- 29.Shi Z, Cheng R, Liu S, Sorrentino G, Centritto M. Carbon assimilation, δ13C and water relations of Elaeagnus angustifolia grown at two groundwater depths in the Minqin desert, China. Plant Biosystems. 2008;142(3):525–32. [Google Scholar]
- 30.Harley PC, Loreto F, Dimarco G, Sharkey TD. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol. 1992;98(4):1429–36. 10.1104/pp.98.4.1429 PubMed PMID: WOS:A1992HR53200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loreto F, Harley PC, Dimarco G, Sharkey TD. Estimation of mesophyll conductance to CO2 flux by three different methods. Plant Physiol. 1992;98(4):1437–43. 10.1104/pp.98.4.1437 PubMed PMID: WOS:A1992HR53200035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans JR, Sharkey TD, Berry JA, Farquhar GD. Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Aust J Plant Physiol. 1986;13(2):281–92 PubMed PMID: WOS:A1986D134400008. [Google Scholar]
- 33.Scartazza A, Lauteri M, Guido MC, Brugnoli E. Carbon isotope discrimination in leaf and stem sugars, water-use efficiency and mesophyll conductance during different developmental stages in rice subjected to drought. Aust J Plant Physiol. 1998;25(4):489–98. PubMed PMID: WOS:000074645400011. [Google Scholar]
- 34.Ethier GJ, Livingston NJ. On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar—von Caemmerer—Berry leaf photosynthesis model. Plant, Cell and Environment. 2004;27(2):137–53. 10.1111/j.1365-3040.2004.01140.x [DOI] [Google Scholar]
- 35.Tholen D, Ethier G, Genty B, Pepin S, Zhu X-G. Variable mesophyll conductance revisited: theoretical background and experimental implications. Plant, Cell and Environment. 2012;35(12):2087–103. 10.1111/j.1365-3040.2012.02538.x [DOI] [PubMed] [Google Scholar]
- 36.Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. Circular California Agricultural Experiment Station. 1950;347(2nd edit). [Google Scholar]
- 37.Flexas J, Diaz-Espejo A, Galmés J, Kaldenhoff R, Medrano H, Ribas-Carbo M. Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant, Cell and Environment. 2007;30(10):1284–98. 10.1111/j.1365-3040.2007.01700.x [DOI] [PubMed] [Google Scholar]
- 38.Rodeghiero M, Niinemets Ü, Cescatti A. Major diffusion leaks of clamp-on leaf cuvettes still unaccounted: how erroneous are the estimates of Farquhar et al. model parameters? Plant, Cell Environ. 2007;30(8):1006–22. [DOI] [PubMed] [Google Scholar]
- 39.Genty B, Briantais J-M, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta (BBA)—General Subjects. 1989;990(1):87–92. 10.1016/S0304-4165(89)80016-9 [DOI] [Google Scholar]
- 40.Laisk A, Loreto F. Determining photosynthetic parameters from leaf CO2 exchange and chlorophyll fluorescence (ribulose-1, 5-bisphosphate carboxylase/oxygenase specificity factor, dark respiration in the light, excitation distribution between photosystems, alternative electron transport rate, and mesophyll diffusion resistance. Plant Physiol. 1996;110(3):903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laisk A. Kinetics of photosynthesis and photorespiration in C3 plants. Nauka Moscow (in Russian). 1977. [Google Scholar]
- 42.Brooks A, Farquhar GD. Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Planta. 1985;165(3):397–406. 10.1007/BF00392238 [DOI] [PubMed] [Google Scholar]
- 43.Kok B. A critical consideration of the quantum yield of Chlorella photosynthesis. Enzymologia. 1948;13:1–56. [Google Scholar]
- 44.Yin X, Sun Z, Struik PC, Gu J. Evaluating a new method to estimate the rate of leaf respiration in the light by analysis of combined gas exchange and chlorophyll fluorescence measurements. J Exp Bot. 2011;62:3489–99. 10.1093/jxb/err038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haworth M, Moser G, Raschi A, Kammann C, Grünhage L, Müller C. Carbon dioxide fertilisation and supressed respiration induce enhanced spring biomass production in a mixed species temperate meadow exposed to moderate carbon dioxide enrichment. Funct Plant Biol. 2016;43:26–39. 10.1071/FP15232 [DOI] [PubMed] [Google Scholar]
- 46.Diaz-Pérez JC, Shackel KA, Sutter EG. Relative water content and water potential of tissue 1. J Exp Bot. 1995;46(1):111–8. 10.1093/jxb/46.1.111 [DOI] [Google Scholar]
- 47.Snaith P, Mansfield T. Stomatal sensitivity to abscisic acid: can it be defined? Plant, Cell and Environment. 1982;5(4):309–11. [Google Scholar]
- 48.Tardieu F, Davies WJ. Stomatal response to abscisic acid is a function of current plant water status. Plant Physiol. 1992;98(2):540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun P, Wahbi S, Tsonev T, Haworth M, Liu S, Centritto M. On the use of leaf spectral indices to assess water status and photosynthetic limitations in Olea europaea L. during water-stress and recovery. Plos One. 2014;9(8):e105165 10.1371/journal.pone.0105165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haworth M, Killi D, Materassi A, Raschi A. Co-ordination of stomatal physiological behavior and morphology with carbon dioxide determines stomatal control. Am J Bot. 2015;102(5):677–88. 10.3732/ajb.1400508 [DOI] [PubMed] [Google Scholar]
- 51.Chater C, Gray JE, Beerling DJ. Early evolutionary acquisition of stomatal control and development gene signalling networks. Curr Opin Plant Biol. 2013;16(5):638–46. 10.1016/j.pbi.2013.06.013 [DOI] [PubMed] [Google Scholar]
- 52.Gleason SM, Butler DW, Ziemińska K, Waryszak P, Westoby M. Stem xylem conductivity is key to plant water balance across Australian angiosperm species. Funct Ecol. 2012;26(2):343–52. 10.1111/j.1365-2435.2012.01962.x [DOI] [Google Scholar]
- 53.McAdam SAM, Brodribb TJ. Fern and lycophyte guard cells do not respond to endogenous abscisic acid. The Plant Cell Online. 2012;24(4):1510–21. 10.1105/tpc.112.096404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blackman P, Davies W. The effects of cytokinins and ABA on stomatal behaviour of maize and Commelina. J Exp Bot. 1983;34(12):1619–26. [Google Scholar]
- 55.Warren CR, editor Stand aside stomata, another actor deserves centre stage: the forgotten role of the internal conductance to CO2 transfer 14th International Congress of Photosynthesis; 2007 Jul 22–27; Glasgow, Scotland: Oxford University Press. [DOI] [PubMed] [Google Scholar]
- 56.Bramley H, Ehrenberger W, Zimmermann U, Palta J, Rüger S, Siddique KM. Non-invasive pressure probes magnetically clamped to leaves to monitor the water status of wheat. Plant Soil. 2013;369(1–2):257–68. 10.1007/s11104-012-1568-x [DOI] [Google Scholar]
- 57.Loreto F, Centritto M, Chartzoulakis K. Photosynthetic limitations in olive cultivars with different sensitivity to salt stress. Plant Cell and Environment. 2003;26(4):595–601. 10.1046/j.1365-3040.2003.00994.x PubMed PMID: WOS:000182009600011. [DOI] [Google Scholar]
- 58.Loreto F, Tsonev T, Centritto M. The impact of blue light on leaf mesophyll conductance. J Exp Bot. 2009;60(8):2283–90. 10.1093/jxb/erp112 PubMed PMID: WOS:000266348800006. [DOI] [PubMed] [Google Scholar]
- 59.Pallozzi E, Tsonev T, Marino G, Copolovici L, Niinemets Ü, Loreto F, et al. Isoprenoid emissions, photosynthesis and mesophyll diffusion conductance in response to blue light. Environ Exp Bot. 2013;95(0):50–8. 10.1016/j.envexpbot.2013.06.001 [DOI] [Google Scholar]
- 60.Kaldenhoff R. Mechanisms underlying CO2 diffusion in leaves. Curr Opin Plant Biol. 2012;15(3):276–81. 10.1016/j.pbi.2012.01.011 PubMed PMID: WOS:000305710300008. [DOI] [PubMed] [Google Scholar]
- 61.Terashima I, Hanba YT, Tazoe Y, Vyas P, Yano S. Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J Exp Bot. 2006;57(2):343–54. 10.1093/jxb/erj014 PubMed PMID: WOS:000234436700010. [DOI] [PubMed] [Google Scholar]
- 62.Flexas J, Ribas-Carbo M, Hanson DT, Bota J, Otto B, Cifre J, et al. Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2 in vivo. Plant J. 2006;48(3):427–39. PubMed PMID: WOS:000241240300009. [DOI] [PubMed] [Google Scholar]
- 63.Uehlein N, Sperling H, Heckwolf M, Kaldenhoff R. The Arabidopsis aquaporin PIP1:2 rules cellular CO2 uptake. Plant Cell and Environment. 2012;35(6):1077–83. 10.1111/j.1365-3040.2011.02473.x PubMed PMID: WOS:000303052500006. [DOI] [PubMed] [Google Scholar]
- 64.Heckwolf M, Pater D, Hanson DT, Kaldenhoff R. The Arabidopsis thaliana aquaporin AtPIP1;2 is a physiologically relevant CO2 transport facilitator. Plant J. 2011;67(5):795–804. 10.1111/j.1365-313X.2011.04634.x PubMed PMID: WOS:000294827700005. [DOI] [PubMed] [Google Scholar]
- 65.Terashima I, Ono K. Effects of HgCl2 on CO2 dependence of leaf photosynthesis: evidence indicating involvement of aquaporins in CO2 diffusion across the plasma membrane. Plant and Cell Physiology. 2002;43(1):70–8. [DOI] [PubMed] [Google Scholar]
- 66.Evans JR, Kaldenhoff R, Genty B, Terashima I. Resistances along the CO2 diffusion pathway inside leaves. J Exp Bot. 2009;60(8):2235–48. 10.1093/jxb/erp117 [DOI] [PubMed] [Google Scholar]
- 67.Maurel C, Verdoucq L, Luu D-T, Santoni V. Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol. 2008;59:595–624. 10.1146/annurev.arplant.59.032607.092734 [DOI] [PubMed] [Google Scholar]
- 68.Aroca R, Ferrante A, Vernieri P, Chrispeels MJ. Drought, abscisic acid and transpiration rate effects on the regulation of PIP aquaporin gene expression and abundance in Phaseolus vulgaris plants. Ann Bot. 2006;98(6):1301–10. 10.1093/aob/mcl219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu C, Wang M, Zhou L, Quan T, Xia G. Heterologous expression of the wheat aquaporin gene TaTIP2;2 compromises the abiotic stress tolerance of Arabidopsis thaliana. Plos One. 2013;8(11):e79618 10.1371/journal.pone.0079618 PubMed PMID: WOS:000326503400131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grondin A, Rodrigues O, Verdoucq L, Merlot S, Leonhardt N, Maurel C. Aquaporins contribute to ABA-triggered stomatal closure through OST1-mediated phosphorylation. Plant Cell. 2015;27(7):1945–54. 10.1105/tpc.15.00421 PubMed PMID: WOS:000359358600013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shatil-Cohen A, Attia Z, Moshelion M. Bundle-sheath cell regulation of xylem-mesophyll water transport via aquaporins under drought stress: a target of xylem-borne ABA? The Plant Journal. 2011;67(1):72–80. 10.1111/j.1365-313X.2011.04576.x [DOI] [PubMed] [Google Scholar]
- 72.Zhou S, Hu W, Deng X, Ma Z, Chen L, Huang C, et al. Overexpression of the wheat aquaporin gene, TaAQP7, enhances drought tolerance in transgenic tobacco. Plos One. 2012;7(12):e52439 10.1371/journal.pone.0052439 PubMed PMID: WOS:000312794500170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tazoe Y, Santrucek J. Superimposed behaviour of gm under ABA-induced stomata closing and low CO2. Plant, Cell and Environment. 2015;38:385–7. 10.1111/pce.12437 [DOI] [PubMed] [Google Scholar]
- 74.Tazoe Y, Von Caemmerer S, Estavillo GM, Evans JR. Using tunable diode laser spectroscopy to measure carbon isotope discrimination and mesophyll conductance to CO2 diffusion dynamically at different CO2 concentrations. Plant, Cell and Environment. 2011;34(4):580–91. 10.1111/j.1365-3040.2010.02264.x [DOI] [PubMed] [Google Scholar]
- 75.Flexas J, Bota J, Escalona JM, Sampol B, Medrano H. Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Funct Plant Biol. 2002;29(4):461–71. [DOI] [PubMed] [Google Scholar]
- 76.Trejo C, Davies WJ. Drought-induced closure of Phaseolus vulgaris L. stomata precedes leaf water deficit and any increase in xylem ABA concentration. J Exp Bot. 1991;42(12):1507–16. [Google Scholar]
- 77.Greenway H, Gibbs J. Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential processes. Funct Plant Biol. 2003;30(10):999–1036. 10.1071/PP98096 [DOI] [PubMed] [Google Scholar]
- 78.Gehring CA, Irving HR, Parish RW. Effects of auxin and abscisic acid on cytosolic calcium and pH in plant cells. Proceedings of the National Academy of Sciences. 1990;87(24):9645–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilkinson S, Davies WJ. Xylem sap pH increase: A drought signal received at the apoplastic face of the guard cell that involves the suppression of saturable abscisic acid uptake by the epidermal symplast. Plant Physiol. 1997;113(2):559–73. PubMed PMID: ISI:A1997WH57200030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu D-T, et al. Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature. 2003;425(6956):393–7. http://www.nature.com/nature/journal/v425/n6956/suppinfo/nature01853_S1.html. [DOI] [PubMed] [Google Scholar]
- 81.Uehlein N, Otto B, Hanson DT, Fischer M, McDowell N, Kaldenhoff R. Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. The Plant Cell. 2008;20(3):648–57. 10.1105/tpc.107.054023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.