Abstract
A one-pot synthesis of various GABA amides has been demostrated, employing the nucleophilic addition of primary and secondary amines across the double bond of cyclopropene-3-carboxamides, followed by ring-opening of the resulting donor-acceptor cyclopropanes and subsequent in situ reduction of enamine (imine) intermediates.
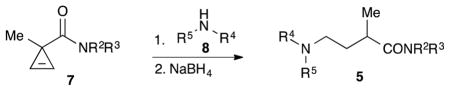
Graphical abstract

γ-Aminobutyric acid (GABA) is the chief inhibitory neurotransmitter in the mammalian central nervous system playing a principal role in reducing neuronal excitability,1 and is a species of immense importance for modern bioorganic and medicinal chemistry. This motif is omnipresent in natural products, including Bistramide A2 and cyclic oligopeptides Microsclerodermins3 and Unguisins.4 GABA derivatives are also widely used in numerous over-the-counter and prescription medicinal agents, such as Noofen (Phenibut),5 Picamilon,6 Lioresal (Baclophen), Lyrica (Pregabalin)7 or anti-arthritic drug Trocade (Cipemastat)8 (Figure 1).
Figure 1.
In our studies9 of donor-acceptor cyclopropanes (DAC),10 we investigated the possibility to access substituted GABA derivatives 5 via the ring opening of DAC 1 (Scheme 1). The propensity of DACs toward ring cleavage is proportional to polarization of the C–C bond between electron-donating (EDG) and electron-withdrawing (EWG) groups. The requisite polarization is commonly achieved through installation of strong EWGs, typically two ester groups, additionally activated by a Lewis acid (“pull” strategy), which leads to products with an “extra” carboxylate moiety at the α-carbon. In our recent report9 we proposed the possibility to employ an alternative “push” strategy by taking advantage of our formal nucleophilic substitution methodology that allows for installation of various N-moieties in the cyclopropane ring. Herein we demonstrate the proof of concept and employment of this strategy toward synthesis of GABA amide derivatives.
Scheme 1.
The above-mentioned formal nucleophilic substitution of halocyclopropanes 69 provides convenient access to various cyclopropylamine derivatives 11, including carboxamides and sulphonamides of trans-β-aminocyclopropanecarboxylic acid (β-ACC), N-cyclopropylhetaryls and N-cyclopropylanilines (Scheme 2).9,11 These reactions proceed via a base-driven nucleophilic addition across the double bond of conjugate cyclopropene 10.12,13 However, attempts to isolate hydroamination products resulting from the addition of electron-rich amine derivatives to the in situ generated, very electrophilic 1-substituted cyclopropenes 10 were unsuccessful. Small amount of water generated as by-product upon dehydrohalogenation of 6 in the presence of KOH resulted in a rapid, amine-mediated ring-opening hydration of intermediate cyclopropene 10 affording aldehyde 12 (Scheme 2).9 Furthermore, nitrogen nucleophiles do not easily add to less electrophilic, non-conjugate 3,3-disubstituted cyclopropenes 7 under conditions used for generation thereof.14 This reaction was totally suppressed by a much more facile addition of an alkoxide (employed as a base for dehydrohalogenation step), to afford cyclopropanol ether 9. Thus, employment of a stable, isolated cyclopropenes 715 was envisaged as an alternative approach to hydroamination that could be carried out under alcohol- and/or water-free conditions. It was anticipated that the addition of an electron-rich amino group would help trigger the desired bond cleavage in intermediate 1. The resulting zwitterionic intermediate 2, in the presence of a proton source, would be stabilized in a form of imine 3 (if derived from primary amine 8) or enamine 4, respectively. Species 3 or 4 can subsequently be reduced in situ to give GABA amide 5 (Scheme 1), or be employed as electrophilic or nucleophilic moiety in various imine or enamine chemistry.
Scheme 2.
Accordingly, we exposed neat N,N-diethyl-1-methylcycloprop-2-ene-1-carboxamide (7a)15 to diethylamine (8a, 3.0 equiv.) at various temperatures to monitor the ring opening. It was found that heating the mixture at 100 °C allowed for complete and clean ring cleavage. GC analysis of crude reaction mixtures showed a single product peak, attributed to enamine 4aa. Next, the crude mixture was treated with borohydride (NaBH4 or NaBH(OAc)3) in dichloromethane to afford the target amide 5aa as a single product in good yield (Table 1, entry 1). Interestingly, under similar conditions, diisopropylamine (8b) did not react at all, leaving cyclopropene 7a intact even after extended heating at 125 °C. Apparently, this bulky amine was insufficiently nucleophilic to enable the hydroamination step (entry 2). In contrast, cyclic secondary amines, such as pyrrolidine (8c), morpholine (8d), N-ethyl- (8e), and N-benzylpiperazines (8f) afforded GABA amides 5ad-5af in good yields (entries 3–6). N,N-Diisopropyl-1-methylcycloprop-2-ene-1-carboxamide (7b)15 and (1-methylcycloprop-2-en-1-yl)(pyrrolidin-1-yl)methanone (7c)15 proved to be similarly efficient as 7a with a number of secondary amines (entries 7–12). Reaction with primary amines, such as phenethylamine (8g), benzylamine (8h), and n-butylamine (8j), also proceeded uneventfully, although somewhat more sluggishly (entries 13, 14, 16–19). It was also necessary to raise the temperature to 140 °C to drive the reaction with aniline (8i) to complete conversion (entry 15).
Table 1.
Synthesis of GABA amides via reductive ring cleavage of cyclopropylamines generated in situ upon hydroamination of cyclopropenes.
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| # | 7 | 8 (equiv.) | 5 | R2 | R3 | R4 | R5 | T, °C (time, h) | Yield, %a |
| 1 | 7a | 8a (3.0) | 5aa | Et | Et | Et | Et | 100 (1) | 66 |
| 2 | 7a | 8b (3.0) | 5ab | Et | Et | i-Pr | i-Pr | 125 (3) | NR |
| 3 | 7a | 8c (3.0) | 5ac | Et | Et | -(CH2)4- | 100 (1) | 71 | |
| 4 | 7a | 8d (1.5) | 5ad | Et | Et | -(CH2)2O(CH2)2- | 100 (1) | 68 | |
| 5 | 7a | 8e (1.5) | 5ae | Et | Et | -(CH2)2N(Et)(CH2)2- | 100 (1) | 72 | |
| 7 | 7a | 8f (1.5) | 5af | Et | Et | -(CH2)2N(Bn)(CH2)2- | 100 (1) | 75 | |
| 7 | 7b | 8a (3.0) | 5ba | i-Pr | i-Pr | Et | Et | 100 (1) | 71 |
| 8 | 7b | 8c (3.0) | 5bc | i-Pr | i-Pr | -(CH2)4- | 100 (1) | 73 | |
| 9 | 7b | 8d (1.5) | 5bd | i-Pr | i-Pr | -(CH2)2O(CH2)2- | 100 (1.5) | 66 | |
| 10 | 7c | 8a (3.0) | 5ca | -(CH2)4- | Et | Et | 100 (1) | 65 | |
| 11 | 7c | 8c (3.0) | 5cc | -(CH2)4- | -(CH2)4- | 100 (1) | 73 | ||
| 12 | 7c | 8d (1.5) | 5cd | -(CH2)4- | -(CH2)2O(CH2)2- | 100 (1) | 68 | ||
| 13 | 7a | 8g (1.5) | 5ag | Et | Et | PhCH2CH2 | H | 100 (2) | 75 |
| 14 | 7a | 8h (2.0) | 5ah | Et | Et | PhCH2 | H | 115 (2) | 65 |
| 15 | 7a | 8i (1.3) | 5ai | Et | Et | Ph | H | 140 (5) | 68 |
| 16 | 7b | 8g (1.5) | 5bg | i-Pr | i-Pr | PhCH2CH2 | H | 100 (2) | 76 |
| 17 | 7b | 8h (1.3) | 5bh | i-Pr | i-Pr | PhCH2 | H | 100 (2) | 68 |
| 18 | 7b | 8j (3.0) | 5bj | i-Pr | i-Pr | n-Bu | H | 100 (1) | 76 |
| 19 | 7c | 8h (1.3) | 5ch | -(CH2)4- | PhCH2 | H | 100 (2) | 65 | |
| 20 | 7a | 8k (1.5) | 5ak | Et | Et | PhCHMe | H | 100 (3) | 65 (1:1)b |
Isolated yields of purified products are listed.
Diastereomeric ratio measured by GC of crude reaction mixture is shown in parentheses.
Finally, a possibility to induce a diastereoselective ring cleavage upon addition of chiral amines was probed by reacting cyclopropene 7a with α-phenylethylamine (8k). Unfortunately, transfer of asymmetric information from a remote stereogenic center was not efficient, and the corresponding adduct 5ak was produced as a 1:1 mixture of two diastereomers (entry 20).
Conclusions
In conclusion, we have successfully employed an alternative “push” strategy for ring opening of “push-pull” cyclopropanes generated in situ via the unassisted nucleophilic addition of electron-rich amines across the double bond of cyclopropene-3-carboxamides. This concept was utilized in efficient one-pot synthesis of GABA derivatives. Further investigations of this transformation are currently underway in our laboratories, which include (a) exploring the possibility of controlling the diastereoselectivity of small ring cleavage upon addition of chiral amines, assisted by Lewis acidic chelating agents; (b) investigating regio- and stereoselectivity of ring cleavage in 1,3,3-trisubstituted chiral cyclopropenes en route to chiral GABA derivatives possessing several contiguous stereogenic centers; (c) intercepting imine species 3 in diastereoselective reactions with C-nucleophiles. The results on these studies will be reported in due course.
Supplementary Material
Acknowledgments
This work was financed through the International Collaboration Program, supported by the Ministry of Education and Science of the Russian Federation and the Ministry of Education of Perm Krai. We are also grateful for the financial support by the Russian Foundation for Basic Research (grant #15-03-02661). Support for NMR instruments used in this project was provided by NIH Shared Instrumentation Grant #S10RR024664 and NSF Major Research Instrumentation Grant #0329648.
Footnotes
Electronic Supplementary Information (ESI) available: Experimental procedures, physico-chemical and spectral data. See DOI: 10.1039/x0xx00000x
Notes and references
- 1.(a) Sieghart W. Pharmacol Rev. 1995;47:181. [PubMed] [Google Scholar]; (b) Ben-Ari Y. Nature Rev Neurosci. 2002;3:728. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]; (c) Owens DF, Kriegstein AR. Nature Rev Neurosci. 2002;3:715. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- 2.(a) Statsuk AV, Liu D, Kozmin SA. J Am Chem Soc. 2004;126:9546. doi: 10.1021/ja046588h. [DOI] [PubMed] [Google Scholar]; (b) Crimmins MT, DeBaillie AC. J Am Chem Soc. 2006;128:4936. doi: 10.1021/ja057686l. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lowe JT, Wrona IE, Panek JS. Org Lett. 2007;9:327. doi: 10.1021/ol062957y. [DOI] [PubMed] [Google Scholar]
- 3.(a) Bewley CA, Debitus C, Faulkner DJ. J Am Chem Soc. 1994;116:7631. [Google Scholar]; (b) Zhu J, Ma D. Angew Chem, Int Ed. 2003;42:5348. doi: 10.1002/anie.200352423. [DOI] [PubMed] [Google Scholar]
- 4.Hunter L, Chung JH. J Org Chem. 2011;76:5502. doi: 10.1021/jo200813a. [DOI] [PubMed] [Google Scholar]
- 5.Lapin I. CNS Drug Rev. 2001;7:471. doi: 10.1111/j.1527-3458.2001.tb00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denisenko OV, Shandra OA, Karpov LM, Siomik LI. Neurophysiology. 2014;46:284. [Google Scholar]
- 7.(a) Taylor CP, Angelotti T, Fauman E. Epilepsy Res. 2007;73:137. doi: 10.1016/j.eplepsyres.2006.09.008. [DOI] [PubMed] [Google Scholar]; (b) Bryans JS, Wustrow DJ. Med Res Rev. 1999;19:149. doi: 10.1002/(sici)1098-1128(199903)19:2<149::aid-med3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.(a) Borkakoti N. Biochem Soc Trans. 2004;32:17. doi: 10.1042/bst0320017. [DOI] [PubMed] [Google Scholar]; (b) Hemmings FJ, Farhan M, Rowland J, Banken L, Jain R. Rheumatol. 2001;40:537. doi: 10.1093/rheumatology/40.5.537. [DOI] [PubMed] [Google Scholar]
- 9.Banning JE, Gentillon J, Ryabchuk PG, Prosser AR, Rogers A, Edwards A, Holtzen A, Babkov IA, Rubina M, Rubin M. J Org Chem. 2013;78:7601. doi: 10.1021/jo4011798. [DOI] [PubMed] [Google Scholar]
- 10.For recent reviews, see: Schneider TF, Kaschel J, Werz DB. Angew Chem, Int Ed. 2014;53:5504. doi: 10.1002/anie.201309886.de Nanteuil F, De Simone F, Frei R, Benfatti F, Serrano E, Waser J. Chem Commun. 2014;50:10912. doi: 10.1039/c4cc03194f.Liao S, Sun X, Tang Y. Acc Chem Res. 2014;47:2260. doi: 10.1021/ar800104y.Grover HK, Emmett MR, Kerr MA. Org Biomol Chem. 2015;13:655. doi: 10.1039/c4ob02117g.
- 11.(a) Ryabchuk P, Edwards A, Gerasimchuk N, Rubina M, Rubin M. Org Lett. 2013;15:6010. doi: 10.1021/ol4027792. [DOI] [PubMed] [Google Scholar]; (b) Ryabchuk P, Rubina M, Xu J, Rubin M. Org Lett. 2012;14:1752. doi: 10.1021/ol300352z. [DOI] [PubMed] [Google Scholar]; (c) Prosser AR, Banning JE, Rubina M, Rubin M. Org Lett. 2010;12:3968. doi: 10.1021/ol101228k. [DOI] [PubMed] [Google Scholar]; (d) Alnasleh BK, Sherrill WM, Rubina M, Banning J, Rubin M. J Am Chem Soc. 2009;131:6906. doi: 10.1021/ja900634m. [DOI] [PubMed] [Google Scholar]
- 12.For ring-retentive nucleophilic addition of amines to cyclo- propenes, see: Gritsenko EI, Khaliullin RR, Plemenkov VV, Faizullin EM. Zh Obshch Khim. 1988;58:2733.Franck-Neumann M, Miesch M, Kempf H. Tetrahedron. 1988;44:2933.Albert RM, Butler GB. J Org Chem. 1977;42:674.
- 13.For other examples of nucleophilic displacement of halogen in cyclopropyl halides with nitrogen-based nucleophiles, see: Kang SY, Lee SH, Seo HJ, Jung ME, Ahn K, Kim J, Lee J. Bioorg Med Chem Lett. 2008;18:2385. doi: 10.1016/j.bmcl.2008.02.061.Shavrin KN, Gvozdev VD, Nefedov OM. Russ Chem Bull. 2010;59:396.Basarab GS, Hill P, Eyermann CJ, Gowravaram M, Kack H, Osimoni E. Bioorg Med Chem Lett. 2012;22:5600. doi: 10.1016/j.bmcl.2012.07.004.Walls TH, Grindrod SC, Beraud D, Zhang L, Baheti AR, Dakshanamurthy S, Patel MK, Brown ML, MacArthur LH. Bioorg Med Chem. 2012;20:5269. doi: 10.1016/j.bmc.2012.06.042.Zhu Y, Gong Y. J Org Chem. 2015;80:490. doi: 10.1021/jo502502z.
- 14.Banning JE, Prosser AR, Alnasleh BK, Smarker J, Rubina M, Rubin M. J Org Chem. 2011;76:3968. doi: 10.1021/jo200368a. [DOI] [PubMed] [Google Scholar]
- 15.(a) Sherrill WM, Kim R, Rubin M. Synthesis. 2009:1477. [Google Scholar]; (b) Kim R, Sherrill WM, Rubin M. Tetrahedron. 2010;66:4947. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





