Abstract
Type IV collagens are the most abundant proteins in basement membranes. Distinct genes encode each of six isoforms, α1(IV) through α6(IV), which assemble into one of three characteristic heterotrimers. Disease-causing mutations in each of the six genes are identified in humans or mice and frequently include diverse ocular pathogenesis that encompass common congenital and progressive blinding diseases, such as optic nerve hypoplasia, glaucoma, and retinal degeneration. Understanding where and when collagen IV molecules are expressed is important because it defines limits for the location and timing of primary pathogenesis. Although localization of collagen IV isoforms in developed human eyes is known, the spatial and temporal distribution of type IV collagens throughout ocular development has not been determined in humans or in mice. Here, we use isoform-specific monoclonal antibodies to systematically reveal the localization of all six collagen IV isoforms in developing mouse eyes. We found that α1(IV) and α2(IV) always co-localized and were ubiquitously expressed throughout development. α3(IV) and α4(IV) also always co-localized but in a much more spatially and temporally specific manner than α1(IV) and α2(IV). α5(IV) co-localized both with α3(IV)/α4(IV), and with α6(IV), consistent with α5(IV) involvement in two distinct heterotrimers. α5(IV) was present in all basement membranes except those of the vasculature. α6(IV) was not detected in vasculature or in Bruch's membrane, indicating that α5(IV) in Bruch's membrane is part of the α3α4α5 heterotrimer. This comprehensive analysis defines the spatial and temporal distribution of type IV collagen isoforms in the developing eye, and will contribute to understanding the mechanisms underlying collagen IV-related ocular diseases that collectively lead to blindness in millions of people worldwide.
Keywords: Type IV collagen, Basement membrane, Ocular development
1. Introduction
The role for type IV collagens in human diseases is gaining broader appreciation. The variability of diseases caused by mutations in type IV collagen genes is dictated, at least in part, by the distribution of the corresponding protein product. α1(IV) and α2(IV) are present in nearly all basement membranes in the body. Mutations in the genes encoding α1(IV) and α2(IV) were only recently identified and the full breadth of their contribution to disease remains to be discovered (Breedveld et al., 2006; de Vries et al., 2009; Favor et al., 2007; Gould et al., 2005, 2006; Plaisier et al., 2007; Sibon et al., 2007; Vahedi et al., 2007a,b). Ocular anterior dysgenesis is a highly penetrant phenotype in mice with Col4a1 or Col4a2 mutations. Mutant mice have ocular anterior segment dysgenesis (ASD) that resembles a spectrum of human phenotypes, including Axenfeld–Rieger Anomaly, which causes secondary glaucoma (Favor et al., 2007; Gould et al., 2007; Van Agtmael et al., 2005). In the posterior eye, Col4a1 mutation causes optic nerve hypoplasia (Gould et al., 2007) and retinal degeneration (D.B. Gould unpublished results).
Mutations in genes coding for the more spatially restricted α3(IV), α4(IV), and α5(IV) cause Alport syndrome. Alport syndrome is characterized by hematuria, progressive renal impairment, high-tone sensorineural hearing loss, and ocular abnormalities. Ocular abnormalities are highly, but not completely, penetrant. Approximately 70% of patients have thin lens capsules and lenticonus (bulging of the anterior lens), and 50–80% of patients have central or peripheral retinopathy appearing as whitish-yellow dots and flecks (Barker et al., 1990; Chugh et al., 1993; Colville and Savige, 1997; Jacobs et al., 1992; Streeten et al., 1987). Other ocular features including posterior polymorphous corneal dystrophy or macular holes are also reported in rare cases (Colville et al., 1997a). The vast majority of Alport syndrome is X-linked and caused by mutations in COL4A5; however, ocular features do not appear to differ between X-linked and autosomal recessive cases (attributed to COL4A3 or COL4A4 mutations) (Colville et al., 1997b; Colville and Savige, 1997). To date, mutations in COL4A6, alone, are not shown to contribute to disease but mutation of COL4A6 in combination with COL4A5 contributes to leiomyomatosis (Cochat et al., 1988; Heidet et al., 1997).
Normal vision requires precise localization and arrangement of several distinct ocular structures. Development of these structures requires coordinated interactions between embryonic tissues, all of which interact with basement membranes. Mutations of basement membrane components lead to ocular dysgenesis; however, the mechanism(s) are unknown (Favor et al., 2007; Gould et al., 2005, 2007; Semina et al., 2006; Sibon et al., 2007; Van Agtmael et al., 2005; Zenker et al., 2004, 2005). In mice, ocular development starts around mid-embryogenesis and proceeds through a series of characteristic morphological stages (Gould et al., 2004; Kaufmann, 2005). By birth, the major ocular structures are formed and ocular basement membranes are in place surrounding the cornea, lens, retina and vasculature (Fig. 1).
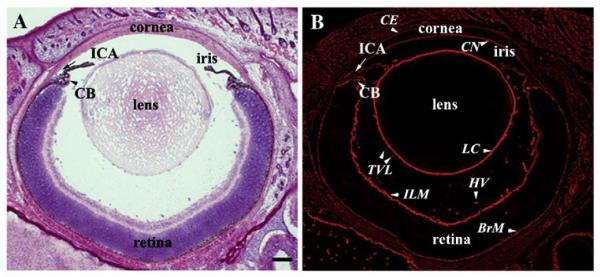
Fig. 1.
Basement membranes in the developing eye. (A) A cross-section of a P4 eye stained with Hematoxylin & Eosin showing the major ocular structures. (B) An immediate adjacent section labeled with a pan-laminin antibody revealing basement membranes in the ocular tissue. BrM: Bruch's membrane; CB: ciliary body; CE: corneal epithelium; CN: corneal endothelium; HV: hyaloid vasculature; ICA: iridocorneal angle; ILM: inner limiting membrane; LC: lens capsule; TVL: tunica vasculosa lentis. Scale bar: 100 μm.
Basement membranes are specialized structures of extracellular matrix that play essential roles in tissue development and maintenance, and type IV collagens are abundant components of all basement membranes. Type IV collagens are a family of six proteins encoded by six distinct genes, COL4A1 through COL4A6 (Hostikka et al., 1990; Hostikka and Tryggvason, 1988; Leinonen et al., 1994; Mariyama et al., 1994; Soininen et al., 1987; Zhou et al., 1994). They are arranged on chromosomes head-to-head in pairs, with COL4A1 and COL4A2 on chromosome 13, COL4A3 and COL4A4 on chromosome 2, and COL4A5 and COL4A6 on the X chromosome (Hudson et al., 1993; Kuhn, 1995). During assembly in the endoplasmic reticulum, three collagen IV peptides interact to form one of three triple-helical heterotrimers, α1α1α2, α3α4α5, or α5α5α6 (Borza et al., 2001; Gunwar et al., 1998; Risteli et al., 1980). After secretion, heterotrimers make further, higher-order associations to form mesh-like collagen IV networks and integrate with other components, such as laminin, Nidogen/entactin and perlecan, to form basement membranes (Borza et al., 2001, 2002; Timpl et al., 1981). Distribution and abundance of collagen IV heterotrimers is tissue specific and varies with developmental stages (Hasegawa et al., 2007; Nakano et al., 2007; Saito et al., 2000; Urabe et al., 2002).
To better understand the mechanism(s) of collagen IV-related ocular diseases, we sought to determine the timing and distribution of all six collagen IV isoforms in the developing mouse eye. The timing and location of initial protein expression defines limits for timing and location of primary pathogenesis. To date, only a single study examined the distribution of type IV collagens in the developing human or mouse eye and it focused exclusively on the lens capsule (Kelley et al., 2002). Other reports have studied type IV collagens in the developed human eye but the distribution and timing of expression during development is currently unknown (Chen et al., 2003; Kabosova et al., 2007; Schlotzer-Schrehardt et al., 2007). By only studying already-developed and adult organs, one could overlook important biological and disease-related processes. Equally important as stage-specific differences are species-specific differences. Distribution differences between humans and mice will be important to identify and define as mouse models are increasingly used to understand collagen IV-related human ocular diseases. Here, we define the localization of collagen IV isoforms in the developing mouse eye.
2. Results
2.1. Differential distribution of type IV collagens in the developing eye
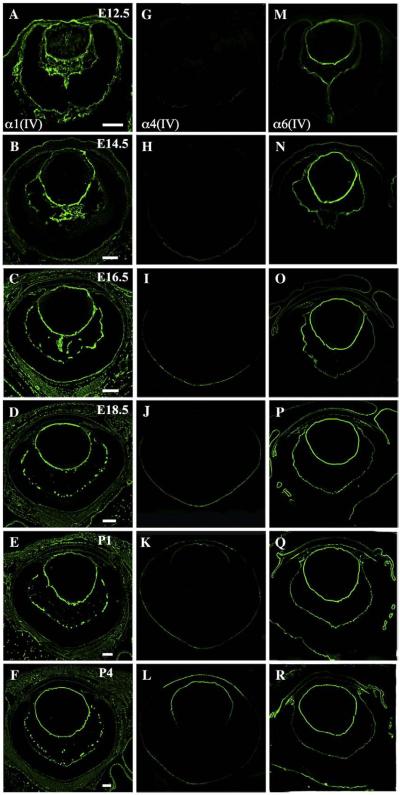
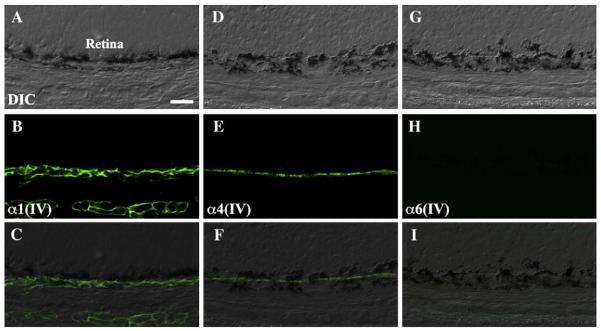
To examine the distributions of all six collagen IV isoforms in developing eyes, we labeled eyes from various embryonic and postnatal stages with monoclonal antibodies against each isoform (Sado et al., 1995). We found that α1(IV) always co-localized with α2(IV) and that α3(IV) always co-localized with α4(IV), whereas α5(IV) colocalized both with α3(IV)/α4(IV) and with α6(IV) (Table 1). This is consistent with the existence of three types of heterotrimers, α1α1α2, α3α4α5 and α5α5α6. Data from α1(IV), α4(IV) and α6 (IV) were chosen to represent the three heterotrimers respectively (Fig. 2).
Table 1.
Summary of type IV collagen isoform distribution in the developing mouse eye.
| Col IV Isoform |
Age | Ocular basement membranes |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conjunctiva | Corneal epithelium |
Corneal endothelium |
Ciliary body & ICA |
Lens capsule |
ILM | Hyaloid vessels |
Bruch's membrane |
|||
| RPE | Choroicapillaris | |||||||||
| α1/α2(IV) | E12.5 | N/A | ++ | N/A | N/A | ++ | ++ | ++ | ++ | ++ |
| E14.5 | N/A | + | + | N/A | +++ | ++ | +++ | ++ | ++ | |
| E16.5 | N/A | + | + | + | +++ | + | +++ | ++ | ++ | |
| E18.5 | + | + | + | + | +++ | + | +++ | ++ | ++ | |
| P1 | ++ | ++ | ++ | ++ | +++ | + | +++ | ++ | ++ | |
| P4 | ++ | ++ | ++ | ++ | +++ | + | +++ | ++ | ++ | |
| α3/α4(IV) | E12.5 | N/A | − | N/A | N/A | − | − | − | ± | − |
| E14.5 | N/A | − | − | N/A | − | − | − | +a | − | |
| E16.5 | N/A | − | − | − | − | − | − | + | − | |
| E18.5 | − | − | ± | − | ± | − | − | ++ | − | |
| P1 | − | − | ++ | − | ++b | − | − | ++ | − | |
| P4 | − | − | +++ | − | +++ | − | − | ++ | − | |
| α5(IV) | E12.5 | N/A | − | N/A | N/A | ++ | ++ | − | +b | − |
| E14.5 | N/A | + | ± | N/A | ++ | ++ | − | + | − | |
| E16.5 | N/A | + | + | ++ | +++ | ++ | − | ++ | − | |
| E18.5 | ++ | ++ | ++ | +++ | +++ | ++ | − | ++ | − | |
| P1 | ++ | ++ | +++ | +++ | +++ | ++ | − | ++ | − | |
| P4 | ++ | ++ | +++ | +++ | +++ | ++ | − | ++ | − | |
| α6(IV) | E12.5 | N/A | − | N/A | N/A | ++ | ++ | − | +b | − |
| E14.5 | N/A | + | ± | N/A | +++ | ++ | − | +b | − | |
| E16.5 | N/A | + | + | ++ | +++ | ++ | − | − | − | |
| E18.5 | + | + | + | ++ | +++ | ++ | − | − | − | |
| P1 | ++ | ++ | ++ | +++ | +++ | ++ | − | − | − | |
| P4 | ++ | ++ | ++ | +++ | +++ | ++ | − | − | − | |
Relative intensity of the fluorescent signal between tissues for each antibody is indicated as:
−, negative; ±, weak positive; +, positive; ++, strong positive; +++, strongest positive. ICA: iridocorneal angle; ILM: inner limiting membrane; N/A: not available; RPE: retinal pigment epithelium.
Expressed only at the posterior pole.
Expressed only at the anterior pole.
Fig. 2.
Differential distribution of type IV collagens in the developing mouse eye. Ocular distribution of collagen IV isoforms was determined using monoclonal antibodies to each isoform at advancing stages of development (the age for each row is indicated in the upper right corner of panels A–F). α1(IV) and α2(IV) always co-localized, were ubiquitously present, and are represented by α1(IV) labeling (A–F). α3(IV) and α4(IV) always co-localized, had developmentally dynamic expression, and are represented by α4(IV) labeling (G–L). α5(IV) co-localized both with α3/α4(IV) and with α6(IV) (M–R). Together α1(IV), α4(IV) and α6(IV) represent the α1α1α2, α3α4α5 and α5α5α6 heterotrimers respectively. A summary of the data can be found in Table 1. Scale bars: E12.5–E14.5: 200 μm; E16.5–P4: 100 μm.
α1(IV) and α2(IV) were ubiquitously expressed in all ocular basement membranes at all developmental stages tested, with the strongest labeling intensity detected in the lens capsule. α1(IV) and α2(IV) were already present at E12.5 and labeled the entire lens capsule and tunica vasculosa lentis (the vascular network immediately adjacent to the posterior surface of the lens capsule) throughout development (Figs. 2 and 3). Both the epithelium and endothelium of the cornea were labeled, as were the pigmented and non-pigmented epithelia of the ciliary body and the pigmented epithelia of the iris (Fig. 4). In the iridocorneal angle, α1(IV) and α2(IV) were detected in the episcleral vein and the developing trabecular meshwork. In the vitreous and inner retina, α1(IV) and α2(IV) antibodies strongly and consistently labeled the hyaloid vasculature whereas the labeling intensity of the inner limiting membrane was weaker and decreased after E16.5 (Figs. 2 and 5). Bruch's membrane is an organized extracellular matrix structure outside the retina, with basement membranes secreted from the RPE (retinal pigment epithelium) and the vascular endothelial cells of the choriocapillaris, which define the innermost and outermost layers respectively. α1(IV) and α2(IV) both exhibited network-like labeling in Bruch's membrane, consistent with the endothelial basement membrane of the choriocapillaris (Fig. 6). The intimate association of the RPE and choroidal basement membranes prevented unequivocal determination of labeling in the RPE basement membranes. However, high magnification confocal microscopy revealed complete co-localization with laminin, suggesting that α1(IV) and α2 (IV) were expressed both in RPE and choroidal basement membranes (data not shown). Overall, α1(IV) and α2(IV) always co-localized and were present in all basement membranes of the developing eye.
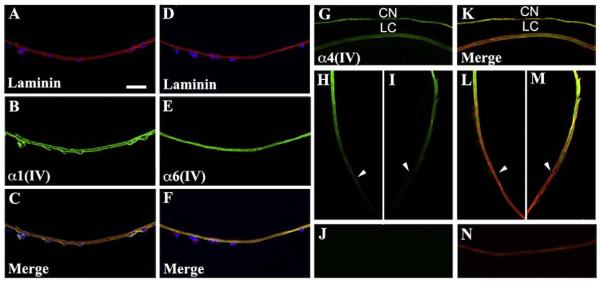
Fig. 3.
Collagen IV distribution in the lens. All three collagen IV heterotrimers were detected in the postnatal lens capsule. (A and D) At P4, the posterior lens capsule was labeled with laminin (red). Vascular endothelial cells of the tunica vasculosa lentis labeled with DAPI (blue), and their basement membranes labeled with laminin. (B) α1(IV) antibody (green) labeled both the lens capsule and the tunica vasculosa lentis and co-localized with laminin (yellow signal in C). (E) α6(IV) antibody labeled the lens capsule but did not co-localize with laminin in the tunica vasculosa lentis (F). α4(IV) antibody revealed spatially restricted localization to the anterior lens capsule (G and K) but not the posterior lens capsule (J and N) with a transition near the equatorial zone (arrowheads) of the lens (H, I, L, and M). CN: Corneal endothelium; LC: Lens capsule. Scale bar: 50 μm.
Fig. 4.
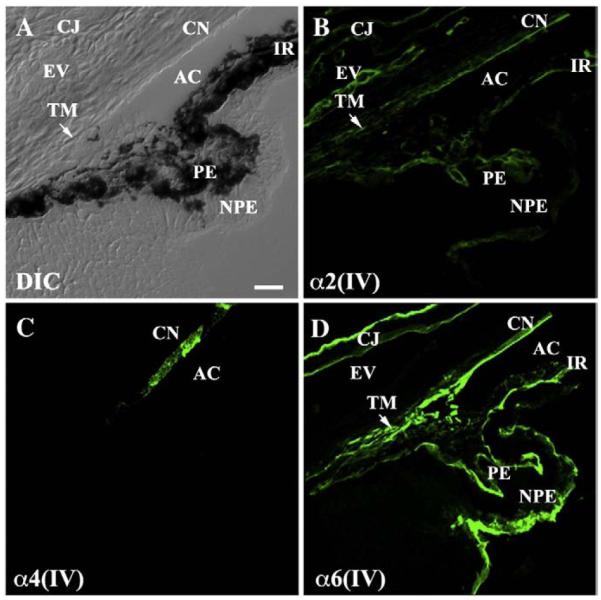
Collagen IV distribution in the anterior segment. (A) A differential interference contrast image (DIC) shows anatomical structures of the iridocorneal angle at P4. (B) Labeling with a α2(IV) antibody was positive in all basal lamina of the anterior segment and in the differentiating trabecular meshwork of the iridocorneal angle. (C) In contrast, α4(IV) labeling was restricted only to the corneal endothelium. (D) α6(IV) labeling was nearly ubiquitous and strongly labeled the developing trabecular meshwork and all basal lamina, with the exception of the episcleral vessel. AC: anterior chamber; CJ: conjunctiva; CN: corneal endothelium; EV: episcleral vessel; IR: iris; NPE: non-pigmented epithelium of the ciliary body; PE: pigmented epithelium of the ciliary body; TM: trabecular meshwork. Scale bar: 20 μm.
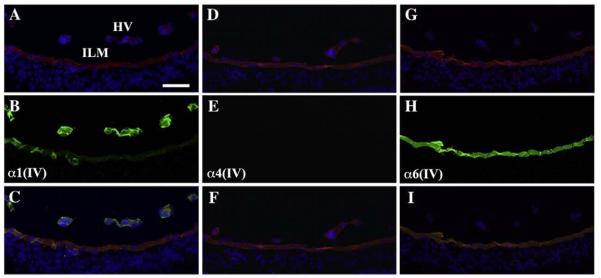
Fig. 5.
Collagen IV distribution in the inner retina. (A, D, G) Laminin labeling (red) of the inner retina at P4 revealed basement membranes of the hyaloid vasculature (HV) and inner limiting membrane (ILM). (B) α1(IV) antibody strongly labeled the hyaloid vasculature in the inner retina and weakly labeled the inner limiting membrane (overlay with laminin in C). (E) α4(IV) was absent from both hyaloid vasculature and inner limiting membrane (overlay with laminin in F). (H) α6(IV) was present only in the inner limiting membrane (overlay with laminin in I). Nuclei were stained with DAPI (blue). Scale bar: 50 μm.
Fig. 6.
Collagen IV distribution in the outer retina. (A, D, G) DIC images of eyes from P4 mice. (B) α1(IV) labeled Bruch's membrane in a network-like pattern consistent with the basement membranes of the choroidal vasculature. (E) α4(IV) labeled Bruch's membrane in an even, continuous linear pattern consistent with the basement membrane of the retinal-pigmented epithelium. (H) α6(IV) labeling was negative in the outer retina. Merged images (C, F, and I) are shown in the bottom panels. Scale bar: 50 μm.
α3(IV) and α4(IV) had the most restricted distribution and underwent the most dynamic changes during ocular development. They were first detected at E14.5 as a faint labeling of Bruch's membrane at the posterior pole of the eye (Fig. 2). By E18.5, the intensity of the signal had increased and the signal spread peripherally to include all of Bruch's membrane, implying an increase in both the level and distribution of α3(IV) and α4(IV) expression. Labeling in Bruch's membrane was unlike the network-like labeling of the choriocapillaris detected with α1(IV) and α2(IV) but instead was a single, tight line indicating the RPE basement membrane. Similar to the posterior pole of the eye, a central-to-peripheral expansion of labeling was detected in the lens capsule. Labeling was first detected in the anterior pole of the lens capsule but then spread peripherally in early postnatal eyes (Fig. 3). By P4, the entire anterior and peripheral lens was positive for α3(IV) and α4(IV). Postnatal labeling was also detected in the corneal endothelium. In summary, localization of α3(IV) and α4(IV) was temporally dynamic and relatively restricted compared to other type IV collagen isoforms.
α5(IV) and α6(IV) were present in most ocular basement membranes. α5(IV) co-localized both with α3(IV)/α4(IV) and with α6(IV) and contributes to two different heterotrimers, α3α4α5 and α5α5α6. Therefore, only α6(IV) labeling is specific to the α5α5α6 heterotrimer. α5(IV) and α6(IV) were already detected in the entire lens capsule and the inner limiting membrane at E12.5 and labeling remained throughout development. Corneal epithelium was labeled at E14.5 followed by corneal endothelial labeling at E16.5 (Fig. 2). Relatively intense labeling was detected adjacent to the pigmented and non-pigmented epithelia of the ciliary body and throughout the iridocorneal angle with the exception of the episcleral vein, which was labeled by α1(IV) and α2(IV) (Fig. 4). The vitreous and inner retina revealed further notable differences between α5α5α6 compared to α1α1α2. The inner limiting membrane labeled relatively stronger with α5(IV) and α6(IV); however, no labeling was observed in the hyaloid vasculature or the tunica vasculosa lentis for either α5(IV) or α6(IV) indicating that only the α1α1α2 heterotrimer is present there (Figs. 3 and 5). A second notable difference was observed in the RPE contribution of Bruch's membrane. After transient detection of α6(IV) in the anterior and peripheral Bruch's membrane at E12.5 and E14.5, α5(IV), but not α6(IV), was detected in the basal RPE indicating that α5(IV) in Bruch's membrane is part of the α3α4α5 heterotrimer. Neither α5(IV) nor α6(IV) was present in the choriocapillaris (Fig. 6). Taken together, α5(IV) was detected in all basement membranes except for the episcleral vein, hyaloid vasculature, tunica vasculosa lentis and choriocapillaris indicating that α1α1α2 is the only heterotrimer localized to the vasculature.
3. Discussion
Our data demonstrate the distribution pattern of all six collagen IV isoforms. Localization of these isoforms is consistent with formation of the three heterotrimers: α1α1α2, α3α4α5 and α5α5α6 and the results are summarized in Table 1. Different heterotrimers are proposed to have different physical characteristics. For example, compared to α1α1α2 heterotrimers, α3α4α5 heterotrimers have a greater number of inter- and intra-molecular cross-links. As a result, α3α4α5 heterotrimers are predicted to be stronger and more resistant to proteolysis, while α1α1α2 heterotrimers are predicted to be more elastic and susceptible to physical or proteolytic breakdown. Others have suggested that isoform switching reflects a tissue's changing demands for mechanical strength or elasticity (Kabosova et al., 2007; Kelley et al., 2002). While potentially correct, it is important to consider that the physical properties of the basement membranes might not be entirely dictated by the collagen network and contributions of other basement membrane proteins should be considered. Additionally, differential isoform usage may reflect not only physical properties but also other important biological properties; for example, cell-matrix interactions or differential sequestration/release of growth factors during key developmental windows. Comprehensive studies of basement membrane composition, physical properties (Candiello et al., 2007) and intracellular signaling through development, especially during times of isoform changes, will be important for understanding the significance of these events.
To better understand which cell types and basement membranes might be responsible for the pathology in collagen IV-related diseases, we paid particular attention to which heterotrimers localized to sites of pathogenesis. The lens capsule is a specialized basement membrane enveloping the entire lens, and its components are produced and secreted by lens epithelial cells. Mice with mutation of Col4a1 or Col4a2 have lens abnormalities including cataracts and lens vacuoles (Favor et al., 2007; Gould et al., 2007; Van Agtmael et al., 2005). Similarly, Alport syndrome patients with mutations in COL4A3, COL4A4, or COL4A5 are frequently reported with cataracts, and ruptured or weakened lens capsule that results in lenticonus (Arnott et al., 1966; Colville and Savige, 1997). Consistent with these phenotypes, all three heterotrimers are detected in the developing lens.
In addition to these cell autonomous defects, the lens is important for normal development of the ocular anterior segment. Dysgenesis of the anterior segment, and specifically the ocular drainage structures in the iridocorneal angle strongly predispose to elevated intraocular pressure and glaucoma. Glaucoma is one of leading causes of blindness and is often associated with elevated intraocular pressure. Col4a1 mutant mice have anterior segment dysgenesis and elevated intraocular pressure (Gould et al., 2007), which model human developmental glaucoma. Recently a COL4A1 mutation was identified in a family with anterior segment dysgenesis (Axenfeld–Reiger Anomaly) and glaucoma (Sibon et al., 2007). One possibility is that anterior segment dysgenesis is secondary to primary lens defects. Co14a1 mutation causes endoplasmic reticulum stress in lens epithelial cells (Gould et al., 2007), which might influence production or secretion of growth factors. Alternatively, structural differences in mutant lens capsules could lead to aberrant sequestration or release of important signaling molecules (Neptune et al., 2003; Wang et al., 2008). Absence of anterior segment dysgenesis and glaucoma in Alport patients might suggest that the timing of the lens defects is important, the mechanism is different (either functional defects in lens epithelium or structural defects of lens capsule) or that the lens is not the primary site of pathogenesis. The presence of α1α1α2, but not α3α4α5 heterotrimer, in the developing iridocorneal angle might suggest that iridocorneal angle, and not the lens, is a primary site of pathogenesis. Understanding the location of the primary insult will greatly facilitate identification of the underlying cellular mechanism. We have developed a Col4a1 conditional mutant allele that will enable us to test the effect of the mutant protein when expressed in a tissue specific manner.
In the retina, developmental or progressive loss of retinal ganglion cells can contribute to optic nerve hypoplasia or glaucoma respectively. The inner limiting membrane is adjacent to retinal ganglion cells and is important for retinal ganglion cell survival during development (Halfter et al., 2005). Col4a1 mutant mice have inner limiting membrane abnormalities, and increased loss of retinal ganglion cells during development appears to contribute to optic nerve hypoplasia (D. J. Dilworth manuscript in preparation). Although currently untested, inner limiting membrane abnormalities could also give rise to retinal ganglion cell death in response to elevated intraocular pressure, and thus predispose to glaucoma. The signal intensity of α1α1α2 in the inner limiting membrane decreased with age and α5α5α6 appeared to be the predominant heterotrimer postnatally (Fig. 2). This suggests that α1α1α2 might be important during development but that α5α5α6 might be more important in the mature inner limiting membrane.
Bruch's membrane is an important anatomical structure for retinal diseases and has two distinct basement membranes. The innermost layer of Bruch's membrane is a basal lamina secreted by the RPE cells and the outermost layer is a basal lamina secreted by the vascular endothelium of the choriocapillaris. Similar to the distribution in human Bruch's membrane (Chen et al., 2003), α1α1α2 is the only heterotrimer present in the choroidal vasculature, and α3α4α5 molecules are present in the RPE basement membrane. Most Alport syndrome patients have dot-and-fleck retinopathy, however, little is known about the pathogenic mechanisms. Depending on genetic context, Col4a1 mutant mice have clinical, histological and molecular similarities to human patients with age-related macular degeneration (D. B. Gould manuscript in preparation). Macular degeneration is the leading cause of blindness in the elderly. Understanding both the cellular mechanism(s) and the relative contribution of RPE versus choroidal vasculature to diseases could provide important insights into prevention and treatment of this common cause of blindness.
Here, we examined the differential localization of type IV collagens during ocular development with special attention to sites relevant for human ocular diseases. Understanding the timing and localization of different isoforms will help elucidate mechanism(s) of ocular diseases caused by collagen IV mutations. Using this comprehensive and detailed analysis as a template, we will further refine the primary site (s) of Col4a1-related ocular pathology using tissue specific expression of a conditional mutant allele. These studies have direct relevance to several developmental and progressive diseases including optic nerve hypoplasia, glaucoma, and macular degeneration that collectively contribute to vision loss in millions of patients worldwide.
4. Experimental procedures
4.1. Tissue preparation
All animals used in this study were inbred derivations of C57BL/6J mice obtained from the Jackson Laboratory (Bar Harbor, ME). All experiments conducted were in accordance with the Institutional Animal Care and Use Committee guidelines. Two reference points were used to determine developmental stage: for embryonic samples, the time when a vaginal plug was observed was designated as embryonic day 0.5 (E0.5); for postnatal samples, the date of birth was designated as postnatal day 0 (P0). Embryos at E12.5, E14.5, E16.5 and E18.5 were harvested from timed pregnant females. For both embryonic and postnatal samples, heads were quickly excised, washed 3 times in cold PBS and incubated in 4% sucrose for 4 h. Samples were then embedded in OCT compound (Sakura Finetek USA Inc. Torrance, CA) and frozen with dry-ice/ethanol bath and stored at −20 °C. All tissue sections were obtained by cryosectioning in the horizontal plane at a thickness of 8–10 μm using a Leica CM1900 cryostat (Rankin Biomedical Corp. Holly, MI).
4.2. Hematoxylin & eosin staining
Cryosections were fixed in 100% ethanol for 10 min, incubated in hematoxylin (Richard–Allan Scientific, Kalamazoo, MI) for 2 min. Samples were then washed sequentially in H2O for 30 s, Clarifier I (Richard–Allan Scientific, Kalamazoo, MI) for 1 min, H2O for 1 min, Bluing Reagent (Richard–Allan Scientific, Kalamazoo, MI) for 1 min, H2O for 1 min and finally 95% ethanol for 30 s The sections were counterstained with eosin Y (Fisher Scientific, Fair Lawn, NJ) for 2.5 min, washed two times each in 100% ethanol and xylene, and then cover slipped using Permount mounting media (Fisher Scientific, Fair Lawn, NJ). Images were obtained using an AxioImager M1 microscope equipped with an AxioCam ICc3 color digital camera and AxioVision software (Zeiss, Germany). Post-acquisition images were processed in Photoshop CS3 (Adobe).
4.3. Immunohistochemistry
Cryosections were fixed in acetone for 10 min, rinsed briefly with TT buffer (50 mM Tris–HCl, pH 7.4, 0.1% Tween 20) and then subjected to two different antigen retrieval methods. All antibodies gave consistent labeling with both antigen retrieval methods and so we proceeded with the method that gave the best signal-to-noise ratio for each antibody. For retrieval of α1(IV), α2(IV) and α5(IV) antigens, sections were incubated in acid solution (0.1 M KCl/HCl, pH1.5) for 10 min. For retrieval of α3(IV), α4(IV) and α6(IV) antigens, sections were incubated for 20 min in 6 M urea containing 0.1 M glycine (pH3.5). After antigen retrieval, sections were washed 3 times in TT buffer, incubated for 1 h in blocking buffer (10% normal goat serum and 2 mg/ml bovine serum albumin in Tris buffer), and then incubated for 2 h in TT buffer containing rabbit anti-laminin antibody (dilution 1:2000, Abcam Inc, MA) and/or collagen α(IV) chain-specific antibody: H11 (dilution 1:200), H22 (dilution 1:20), H31 (dilution 1:50), RH42 (dilution 1:100), H53 (dilution 1:100), B66 (dilution 1:50) (Shigei Medical Research Institute, Japan). After washing three times with TT buffer, samples were incubated in a dark, humid chamber for 40 min with appropriate secondary antibodies: Alexa Fluor 594 anti-rabbit IgG for laminin or Alexa Fluor 488 anti-rat IgG for collagen IV antibodies (dilution 1:2000, Molecular Probes, OR). Samples were washed three times with TT buffer and cover slipped with Vectashield Hardset mounting media with DAPI (Vector Laboratories Inc. CA). Images were captured using either a LSM5 PASCAL confocal microscope with Neofluor objective lens, or an AxioImager M1 microscope equipped with an AxioCam MRm digital camera and AxioVision software (Zeiss, Germany). Post-acquisition images were processed in Photoshop CS3 (Adobe).
4.4. Antibodies
Monoclonal antibodies against each of the six collagen IV isoforms were purchased from Shigei Medical Research Institute (Japan) and have been described previously (Ninomiya et al., 1995). Briefly, rats or mice were immunized with synthetic peptides to collagen IV NCI or the triple helical domain. Lymphocytes from the immunized animals were harvested and fused with mouse myeloma cells. Supernatants from hybridoma cultures were screened with ELISA and indirect immunofluorescence. We tested ten antibodies: one each for α1(IV) and α3(IV) and two each for α2(IV), α4(IV), α5(IV) and α6(IV). Where two antibodies were available for a protein, data were always consistent and we chose the antibody that gave the strongest signal-to-noise ratio in our hands. We chose rat antibodies H11, H22, H31, RH42, H53 and B66 for α1(IV) through α6(IV) respectively.
Acknowledgements
We would like to thank Dr. Geoffrey Lambright for advice and assistance with confocal microscopy and Dr. Richard Libby for critical review of the manuscript. This work was supported by grants from That Man May See (X.B. and D.B.G.), Alberta Heritage Foundation for Medical Research (D.J.D.), the Heart and Stroke Foundation of Canada (D.J.D.), the Larry L. Hillblom Foundation (Y.C.W. and D.B.G.), a core grant from the National Eye Institute (EY02162) and a Research To Prevent Blindness Unrestricted Grant.
Abbreviations
- BM
basement membrane
- DIC
differential interference contrast
- ILM
inner limiting membrane
- ICA
iridocorneal angle
- RPE
retinal pigment epithelium
References
- Arnott EJ, Crawfurd MD, Toghill PJ. Anterior lenticonus and Alport's syndrome. Br. J. Ophthalmol. 1966;50(7):390–403. doi: 10.1136/bjo.50.7.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, Gregory MC, Skolnick MH, Atkin CL, Tryggvason K. Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science. 1990;248(4960):1224–1227. doi: 10.1126/science.2349482. [DOI] [PubMed] [Google Scholar]
- Borza DB, Bondar O, Ninomiya Y, Sado Y, Naito I, Todd P, Hudson BG. The NC1 domain of collagen IV encodes a novel network composed of the alpha 1, alpha 2, alpha 5, and alpha 6 chains in smooth muscle basement membranes. J. Biol. Chem. 2001;276(30):28532–28540. doi: 10.1074/jbc.M103690200. [DOI] [PubMed] [Google Scholar]
- Borza DB, Bondar O, Todd P, Sundaramoorthy M, Sado Y, Ninomiya Y, Hudson BG. Quaternary organization of the goodpasture autoantigen, the alpha 3(IV) collagen chain. Sequestration of two cryptic autoepitopes by intrapromoter interactions with the alpha4 and alpha5 NC1 domains. J. Biol. Chem. 2002;277(42):40075–40083. doi: 10.1074/jbc.M207769200. [DOI] [PubMed] [Google Scholar]
- Breedveld G, de Coo IF, Lequin MH, Arts WF, Heutink P, Gould DB, John SW, Oostra B, Mancini GM. Novel mutations in three families confirm a major role of COL4A1 in hereditary porencephaly. J. Med. Genet. 2006;43(6):490–495. doi: 10.1136/jmg.2005.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candiello J, Balasubramani M, Schreiber EM, Cole GJ, Mayer U, Halfter W, Lin H. Biomechanical properties of native basement membranes. FEBS J. 2007;274(11):2897–2908. doi: 10.1111/j.1742-4658.2007.05823.x. [DOI] [PubMed] [Google Scholar]
- Chen L, Miyamura N, Ninomiya Y, Handa JT. Distribution of the collagen IV isoforms in human Bruch's membrane. Br. J. Ophthalmol. 2003;87(2):212–215. doi: 10.1136/bjo.87.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh KS, Sakhuja V, Agarwal A, Jha V, Joshi K, Datta BN, Gupta A, Gupta KL. Hereditary nephritis (Alport's syndrome)—clinical profile and inheritance in 28 kindreds. Nephrol. Dial. Transplant. 1993;8(8):690–695. doi: 10.1093/ndt/8.8.690. [DOI] [PubMed] [Google Scholar]
- Cochat P, Guibaud P, Garcia Torres R, Roussel B, Guarner V, Larbre F. Diffuse leiomyomatosis in Alport syndrome. J. Pediatr. 1988;113(2):339–343. doi: 10.1016/s0022-3476(88)80280-4. [DOI] [PubMed] [Google Scholar]
- Colville DJ, Savige J. Alport syndrome. A review of the ocular manifestations. Ophthalmic Genet. 1997;18(4):161–173. doi: 10.3109/13816819709041431. [DOI] [PubMed] [Google Scholar]
- Colville D, Savige J, Branley P, Wilson D. Ocular abnormalities in thin basement membrane disease. Br. J. Ophthalmol. 1997a;81(5):373–377. doi: 10.1136/bjo.81.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colville D, Savige J, Morfis M, Ellis J, Kerr P, Agar J, Fasset R. Ocular manifestations of autosomal recessive Alport syndrome. Ophthalmic Genet. 1997b;18(3):119–128. doi: 10.3109/13816819709057125. [DOI] [PubMed] [Google Scholar]
- de Vries LS, Koopman C, Groenendaal F, Van Schooneveld M, Verheijen FW, Verbeek E, Witkamp TD, van der Worp HB, Mancini G. COL4A1 mutation in two preterm siblings with antenatal onset of parenchymal hemorrhage. Ann. Neurol. 2009;65(1):12–18. doi: 10.1002/ana.21525. [DOI] [PubMed] [Google Scholar]
- Favor J, Gloeckner CJ, Janik D, Klempt M, Neuhauser-Klaus A, Pretsch W, Schmahl W, Quintanilla-Fend L. Type IV procollagen missense mutations associated with defects of the eye, vascular stability, the brain, kidney function and embryonic or postnatal viability in the mouse, Mus musculus: an extension of the Col4a1 allelic series and the identification of the first two Col4a2 mutant alleles. Genetics. 2007;175(2):725–736. doi: 10.1534/genetics.106.064733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould DB, Smith RS, John SW. Anterior segment development relevant to glaucoma. Int. J. Dev. Biol. 2004;48(8–9):1015–1029. doi: 10.1387/ijdb.041865dg. [DOI] [PubMed] [Google Scholar]
- Gould DB, Phalan FC, Breedveld GJ, van Mil SE, Smith RS, Schimenti JC, Aguglia U, van der Knaap MS, Heutink P, John SW. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science. 2005;308(5725):1167–1171. doi: 10.1126/science.1109418. [DOI] [PubMed] [Google Scholar]
- Gould DB, Phalan FC, van Mil SE, Sundberg JP, Vahedi K, Massin P, Bousser MG, Heutink P, Miner JH, Tournier-Lasserve E, John SW. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N. Engl. J. Med. 2006;354(14):1489–1496. doi: 10.1056/NEJMoa053727. [DOI] [PubMed] [Google Scholar]
- Gould DB, Marchant JK, Savinova OV, Smith RS, John SW. Col4a1 mutation causes endoplasmic reticulum stress and genetically modifiable ocular dysgenesis. Hum. Mol. Genet. 2007;16(7):798–807. doi: 10.1093/hmg/ddm024. [DOI] [PubMed] [Google Scholar]
- Gunwar S, Ballester F, Noelken ME, Sado Y, Ninomiya Y, Hudson BG. Glomerular basement membrane. Identification of a novel disulfide-cross-linked network of alpha3, alpha4, and alpha5 chains of type IV collagen and its implications for the pathogenesis of Alport syndrome. J. Biol. Chem. 1998;273(15):8767–8775. doi: 10.1074/jbc.273.15.8767. [DOI] [PubMed] [Google Scholar]
- Halfter W, Willem M, Mayer U. Basement membrane-dependent survival of retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 2005;46(3):1000–1009. doi: 10.1167/iovs.04-1185. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Naito I, Nakano K, Momota R, Nishida K, Taguchi T, Sado Y, Ninomiya Y, Ohtsuka A. The distributions of type IV collagen alpha chains in basement membranes of human epidermis and skin appendages. Arch. Histol. Cytol. 2007;70(4):255–265. doi: 10.1679/aohc.70.255. [DOI] [PubMed] [Google Scholar]
- Heidet L, Cai Y, Sado Y, Ninomiya Y, Thorner P, Guicharnaud L, Boye E, Chauvet V, Solal LC, Beziau A, Torres RG, Antignac C, Gubler MC. Diffuse leiomyomatosis associated with X-linked Alport syndrome: extracellular matrix study using immunohistochemistry and in situ hybridization. Lab. Invest. 1997;76(2):233–243. [PubMed] [Google Scholar]
- Hostikka SL, Eddy RL, Byers MG, Hoyhtya M, Shows TB, Tryggvason K. Identification of a distinct type IV collagen alpha chain with restricted kidney distribution and assignment of its gene to the locus of X chromosome-linked Alport syndrome. Proc. Natl. Acad. Sci. U. S. A. 1990;87(4):1606–1610. doi: 10.1073/pnas.87.4.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostikka SL, Tryggvason K. The complete primary structure of the alpha 2 chain of human type IV collagen and comparison with the alpha 1(IV) chain. J. Biol. Chem. 1988;263(36):19488–19493. [PubMed] [Google Scholar]
- Hudson BG, Reeders ST, Tryggvason K. Type IV collagen: structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J. Biol. Chem. 1993;268(35):26033–26036. [PubMed] [Google Scholar]
- Jacobs M, Jeffrey B, Kriss A, Taylor D, Sa G, Barratt TM. Ophthalmologic assessment of young patients with Alport syndrome. Ophthalmology. 1992;99(7):1039–1044. doi: 10.1016/s0161-6420(92)31853-6. [DOI] [PubMed] [Google Scholar]
- Kabosova A, Azar DT, Bannikov GA, Campbell KP, Durbeej M, Ghohestani RF, Jones JC, Kenney MC, Koch M, Ninomiya Y, Patton BL, Paulsson M, Sado Y, Sage EH, Sasaki T, Sorokin LM, Steiner-Champliaud MF, Sun TT, Sundarraj N, Timpl R, Virtanen I, Ljubimov AV. Compositional differences between infant and adult human corneal basement membranes. Invest. Ophthalmol. Vis. Sci. 2007;48(11):4989–4999. doi: 10.1167/iovs.07-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann MH. The Atlas of Mouse Development. Elsevier Academic Press; London, UK: 2005. [Google Scholar]
- Kelley PB, Sado Y, Duncan MK. Collagen IV in the developing lens capsule. Matrix Biol. 2002;21(5):415–423. doi: 10.1016/s0945-053x(02)00014-8. [DOI] [PubMed] [Google Scholar]
- Kuhn K. Basement membrane (type IV) collagen. Matrix Biol. 1995;14(6):439–445. doi: 10.1016/0945-053x(95)90001-2. [DOI] [PubMed] [Google Scholar]
- Leinonen A, Mariyama M, Mochizuki T, Tryggvason K, Reeders ST. Complete primary structure of the human type IV collagen alpha 4(IV) chain. Comparison with structure and expression of the other alpha (IV) chains. J. Biol. Chem. 1994;269(42):26172–26177. [PubMed] [Google Scholar]
- Mariyama M, Leinonen A, Mochizuki T, Tryggvason K, Reeders ST. Complete primary structure of the human alpha 3(IV) collagen chain. Coexpression of the alpha 3(IV) and alpha 4(IV) collagen chains in human tissues. J. Biol. Chem. 1994;269(37):23013–23017. [PubMed] [Google Scholar]
- Nakano K, Naito I, Momota R, Sado Y, Hasegawa H, Ninomiya Y, Ohtsuka A. The distribution of type IV collagen alpha chains in the mouse ovary and its correlation with follicular development. Arch. Histol. Cytol. 2007;70(4):243–253. doi: 10.1679/aohc.70.243. [DOI] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003;33(3):407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Kagawa M, Iyama K, Naito I, Kishiro Y, Seyer JM, Sugimoto M, Oohashi T, Sado Y. Differential expression of two basement membrane collagen genes, COL4A6 and COL4A5, demonstrated by immunofluorescence staining using peptide-specific monoclonal antibodies. J. Cell Biol. 1995;130(5):1219–1229. doi: 10.1083/jcb.130.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaisier E, Gribouval O, Alamowitch S, Mougenot B, Prost C, Verpont MC, Marro B, Desmettre T, Cohen SY, Roullet E, Dracon M, Fardeau M, VanAgtmael T, Kerjaschki D, Antignac C, Ronco P. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N. Engl. J. Med. 2007;357(26):2687–2695. doi: 10.1056/NEJMoa071906. [DOI] [PubMed] [Google Scholar]
- Risteli J, Schuppan D, Glanville RW, Timpl R. Immunochemical distinction between two different chains of type IV collagen. Biochem. J. 1980;191(2):517–522. doi: 10.1042/bj1910517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sado Y, Kagawa M, Kishiro Y, Sugihara K, Naito I, Seyer JM, Sugimoto M, Oohashi T, Ninomiya Y. Establishment by the rat lymph node method of epitope-defined monoclonal antibodies recognizing the six different alpha chains of human type IV collagen. Histochem. Cell Biol. 1995;104(4):267–275. doi: 10.1007/BF01464322. [DOI] [PubMed] [Google Scholar]
- Saito K, Naito I, Seki T, Oohashi T, Kimura E, Momota R, Kishiro Y, Sado Y, Yoshioka H, Ninomiya Y. Differential expression of mouse alpha5(IV) and alpha6(IV) collagen genes in epithelial basement membranes. J. Biochem. 2000;128(3):427–434. doi: 10.1093/oxfordjournals.jbchem.a022770. [DOI] [PubMed] [Google Scholar]
- Schlotzer-Schrehardt U, Dietrich T, Saito K, Sorokin L, Sasaki T, Paulsson M, Kruse FE. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp. Eye Res. 2007;85(6):845–860. doi: 10.1016/j.exer.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Semina EV, Bosenko DV, Zinkevich NC, Soules KA, Hyde DR, Vihtelic TS, Willer GB, Gregg RG, Link BA. Mutations in laminin alpha 1 result in complex, lens-independent ocular phenotypes in zebrafish. Dev. Biol. 2006;299(1):63–77. doi: 10.1016/j.ydbio.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Sibon I, Coupry I, Menegon P, Bouchet JP, Gorry P, Burgelin I, Calvas P, Orignac I, Dousset V, Lacombe D, Orgogozo JM, Arveiler B, Goizet C. COL4A1 mutation in Axenfeld–Rieger anomaly with leukoencephalopathy and stroke. Ann. Neurol. 2007;62(2):177–184. doi: 10.1002/ana.21191. [DOI] [PubMed] [Google Scholar]
- Soininen R, Haka-Risku T, Prockop DJ, Tryggvason K. Complete primary structure of the alpha 1-chain of human basement membrane (type IV) collagen. FEBS Lett. 1987;225(1–2):188–194. doi: 10.1016/0014-5793(87)81155-9. [DOI] [PubMed] [Google Scholar]
- Streeten BW, Robinson MR, Wallace R, Jones DB. Lens capsule abnormalities in Alport's syndrome. Arch. Ophthalmol. 1987;105(12):1693–1697. doi: 10.1001/archopht.1987.01060120091033. [DOI] [PubMed] [Google Scholar]
- Timpl R, Wiedemann H, van Delden V, Furthmayr H, Kuhn K. A network model for the organization of type IV collagen molecules in basement membranes. Eur. J. Biochem. 1981;120(2):203–211. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- Urabe N, Naito I, Saito K, Yonezawa T, Sado Y, Yoshioka H, Kusachi S, Tsuji T, Ohtsuka A, Taguchi T, Murakami T, Ninomiya Y. Basement membrane type IV collagen molecules in the choroid plexus, pia mater and capillaries in the mouse brain. Arch. Histol. Cytol. 2002;65(2):133–143. doi: 10.1679/aohc.65.133. [DOI] [PubMed] [Google Scholar]
- Vahedi K, Boukobza M, Massin P, Gould DB, Tournier-Lasserve E, Bousser MG. Clinical and brain MRI follow-up study of a family with COL4A1 mutation. Neurology. 2007a;69(16):1564–1568. doi: 10.1212/01.wnl.0000295994.46586.e7. [DOI] [PubMed] [Google Scholar]
- Vahedi K, Kubis N, Boukobza M, Arnoult M, Massin P, Tournier-Lasserve E, Bousser MG. Col4a1 mutation in a patient with sporadic, recurrent intracerebral hemorrhage. Stroke. 2007b;38(5):1461–1464. doi: 10.1161/STROKEAHA.106.475194. [DOI] [PubMed] [Google Scholar]
- Van Agtmael T, Schlotzer-Schrehardt U, McKie L, Brownstein DG, Lee AW, Cross SH, Sado Y, Mullins JJ, Poschl E, Jackson IJ. Dominant mutations of Col4a1 result in basement membrane defects which lead to anterior segment dysgenesis and glomerulopathy. Hum. Mol. Genet. 2005;14(21):3161–3168. doi: 10.1093/hmg/ddi348. [DOI] [PubMed] [Google Scholar]
- Wang X, Harris RE, Bayston LJ, Ashe HL. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455(7209):72–77. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- Zenker M, Aigner T, Wendler O, Tralau T, Muntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wuhl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dotsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A. Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum. Mol. Genet. 2004;13(21):2625–2632. doi: 10.1093/hmg/ddh284. [DOI] [PubMed] [Google Scholar]
- Zenker M, Pierson M, Jonveaux P, Reis A. Demonstration of two novel LAMB2 mutations in the original Pierson syndrome family reported 42 years ago. Am. J. Med. Genet. A. 2005;138(1):73–74. doi: 10.1002/ajmg.a.30894. [DOI] [PubMed] [Google Scholar]
- Zhou J, Ding M, Zhao Z, Reeders ST. Complete primary structure of the sixth chain of human basement membrane collagen, alpha 6(IV). Isolation of the cDNAs for alpha 6(IV) and comparison with five other type IV collagen chains. J. Biol. Chem. 1994;269(18):13193–13199. [PubMed] [Google Scholar]