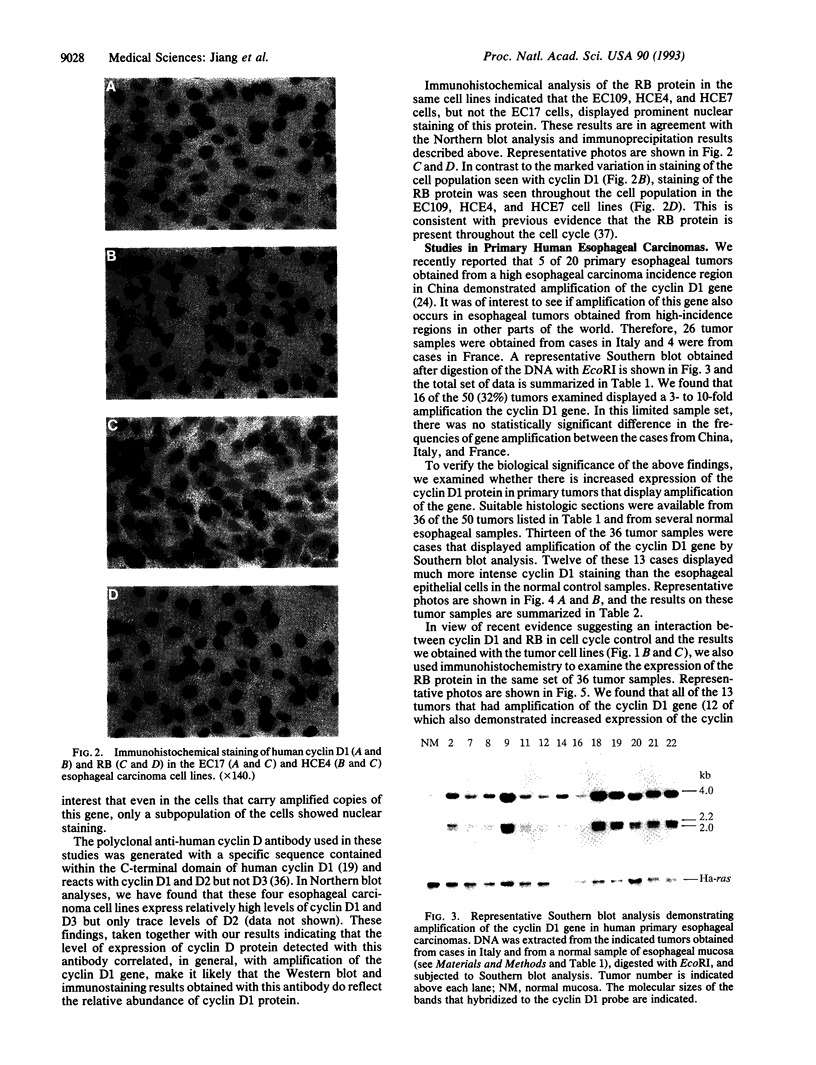
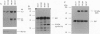Abstract
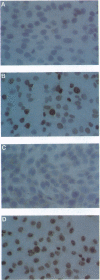
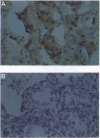
We have examined DNA from four human esophageal carcinoma cell lines and 50 primary esophageal carcinomas obtained from China, Italy, and France for amplification of the cyclin D1 gene. We also examined 36 of these 50 carcinomas for expression of the cyclin D1 and retinoblastoma (RB) proteins by immunohistochemistry. We found a 3- to 10-fold amplification of the cyclin D1 gene in 16 of the 50 (32%) tumors and in two of the four cell lines. Cyclin D1 protein was overexpressed in 12 of 13 tumors and the two cell lines that showed gene amplification when compared to normal controls. Studies on RB protein expression indicated that 6 of the 36 (17%) tumor samples examined and one cell line did not show detectable expression of this protein. The tumors and cell lines that had cyclin D1 gene amplification and overexpression exhibited normal levels of expression of RB protein. By contrast, the tumors and cell line that did not appear to express the RB protein did not show amplification of the cyclin D1 gene and expressed only low levels of the cyclin D1 protein (P = 0.03). These results suggest that the inhibitory effect of RB on cell cycle progression can be abrogated during tumor development either by loss of expression of the RB gene or by increased expression of the cyclin D1 gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks-Schlegel S. P., Quintero J. Growth and differentiation of human esophageal carcinoma cell lines. Cancer Res. 1986 Jan;46(1):250–258. [PubMed] [Google Scholar]
- Bennett W. P., Hollstein M. C., He A., Zhu S. M., Resau J. H., Trump B. F., Metcalf R. A., Welsh J. A., Midgley C., Lane D. P. Archival analysis of p53 genetic and protein alterations in Chinese esophageal cancer. Oncogene. 1991 Oct;6(10):1779–1784. [PubMed] [Google Scholar]
- Boynton R. F., Blount P. L., Yin J., Brown V. L., Huang Y., Tong Y., McDaniel T., Newkirk C., Resau J. H., Raskind W. H. Loss of heterozygosity involving the APC and MCC genetic loci occurs in the majority of human esophageal cancers. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3385–3388. doi: 10.1073/pnas.89.8.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton R. F., Huang Y., Blount P. L., Reid B. J., Raskind W. H., Haggitt R. C., Newkirk C., Resau J. H., Yin J., McDaniel T. Frequent loss of heterozygosity at the retinoblastoma locus in human esophageal cancers. Cancer Res. 1991 Oct 15;51(20):5766–5769. [PubMed] [Google Scholar]
- Day N. E. Some aspects of the epidemiology of esophageal cancer. Cancer Res. 1975 Nov;35(11 Pt 2):3304–3307. [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Lynch D., Furukawa Y., Griffin J., Piwnica-Worms H., Huang C. M., Livingston D. M. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell. 1989 Sep 22;58(6):1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- Dowdy S. F., Hinds P. W., Louie K., Reed S. I., Arnold A., Weinberg R. A. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. 1993 May 7;73(3):499–511. doi: 10.1016/0092-8674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Sluss H. K., Sherr C. J., Matsushime H., Kato J., Livingston D. M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993 May 7;73(3):487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- Goodrich D. W., Lee W. H. Abrogation by c-myc of G1 phase arrest induced by RB protein but not by p53. Nature. 1992 Nov 12;360(6400):177–179. doi: 10.1038/360177a0. [DOI] [PubMed] [Google Scholar]
- Hall F. L., Williams R. T., Wu L., Wu F., Carbonaro-Hall D. A., Harper J. W., Warburton D. Two potentially oncogenic cyclins, cyclin A and cyclin D1, share common properties of subunit configuration, tyrosine phosphorylation and physical association with the Rb protein. Oncogene. 1993 May;8(5):1377–1384. [PubMed] [Google Scholar]
- Hinds P. W., Mittnacht S., Dulic V., Arnold A., Reed S. I., Weinberg R. A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992 Sep 18;70(6):993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- Hollstein M. C., Metcalf R. A., Welsh J. A., Montesano R., Harris C. C. Frequent mutation of the p53 gene in human esophageal cancer. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9958–9961. doi: 10.1073/pnas.87.24.9958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstein M. C., Peri L., Mandard A. M., Welsh J. A., Montesano R., Metcalf R. A., Bak M., Harris C. C. Genetic analysis of human esophageal tumors from two high incidence geographic areas: frequent p53 base substitutions and absence of ras mutations. Cancer Res. 1991 Aug 1;51(15):4102–4106. [PubMed] [Google Scholar]
- Hollstein M. C., Smits A. M., Galiana C., Yamasaki H., Bos J. L., Mandard A., Partensky C., Montesano R. Amplification of epidermal growth factor receptor gene but no evidence of ras mutations in primary human esophageal cancers. Cancer Res. 1988 Sep 15;48(18):5119–5123. [PubMed] [Google Scholar]
- Horowitz J. M., Park S. H., Bogenmann E., Cheng J. C., Yandell D. W., Kaye F. J., Minna J. D., Dryja T. P., Weinberg R. A. Frequent inactivation of the retinoblastoma anti-oncogene is restricted to a subset of human tumor cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2775–2779. doi: 10.1073/pnas.87.7.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. Cell. 1991 Sep 20;66(6):1071–1074. doi: 10.1016/0092-8674(91)90028-w. [DOI] [PubMed] [Google Scholar]
- Jiang W., Kahn S. M., Guillem J. G., Lu S. H., Weinstein I. B. Rapid detection of ras oncogenes in human tumors: applications to colon, esophageal, and gastric cancer. Oncogene. 1989 Jul;4(7):923–928. [PubMed] [Google Scholar]
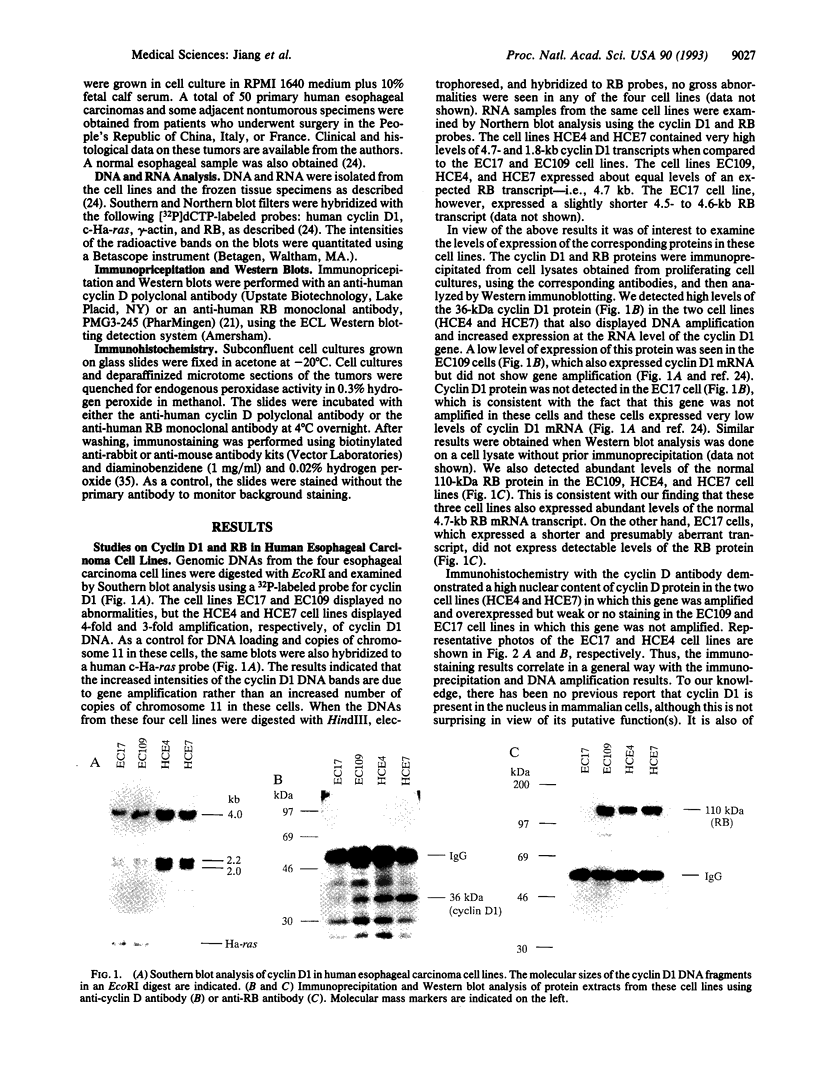
- Jiang W., Kahn S. M., Tomita N., Zhang Y. J., Lu S. H., Weinstein I. B. Amplification and expression of the human cyclin D gene in esophageal cancer. Cancer Res. 1992 May 15;52(10):2980–2983. [PubMed] [Google Scholar]
- Kato J., Matsushime H., Hiebert S. W., Ewen M. E., Sherr C. J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993 Mar;7(3):331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- Kitagawa Y., Ueda M., Ando N., Shinozawa Y., Shimizu N., Abe O. Significance of int-2/hst-1 coamplification as a prognostic factor in patients with esophageal squamous carcinoma. Cancer Res. 1991 Mar 1;51(5):1504–1508. [PubMed] [Google Scholar]
- Lammie G. A., Peters G. Chromosome 11q13 abnormalities in human cancer. Cancer Cells. 1991 Nov;3(11):413–420. [PubMed] [Google Scholar]
- Lees J. A., Buchkovich K. J., Marshak D. R., Anderson C. W., Harlow E. The retinoblastoma protein is phosphorylated on multiple sites by human cdc2. EMBO J. 1991 Dec;10(13):4279–4290. doi: 10.1002/j.1460-2075.1991.tb05006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D. J., Dulić V., Reed S. I. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991 Sep 20;66(6):1197–1206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- Lin B. T., Gruenwald S., Morla A. O., Lee W. H., Wang J. Y. Retinoblastoma cancer suppressor gene product is a substrate of the cell cycle regulator cdc2 kinase. EMBO J. 1991 Apr;10(4):857–864. doi: 10.1002/j.1460-2075.1991.tb08018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S. H., Hsieh L. L., Luo F. C., Weinstein I. B. Amplification of the EGF receptor and c-myc genes in human esophageal cancers. Int J Cancer. 1988 Oct 15;42(4):502–505. doi: 10.1002/ijc.2910420406. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Roussel M. F., Ashmun R. A., Sherr C. J. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991 May 17;65(4):701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- Mittnacht S., Weinberg R. A. G1/S phosphorylation of the retinoblastoma protein is associated with an altered affinity for the nuclear compartment. Cell. 1991 May 3;65(3):381–393. doi: 10.1016/0092-8674(91)90456-9. [DOI] [PubMed] [Google Scholar]
- Motokura T., Bloom T., Kim H. G., Jüppner H., Ruderman J. V., Kronenberg H. M., Arnold A. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature. 1991 Apr 11;350(6318):512–515. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- Pan Q. Q. Studies on esophageal cancer cells in vitro. Proc Chin Acad Med Sci Peking Union Med Coll. 1989;4(1):52–57. [PubMed] [Google Scholar]
- Parkin D. M., Lärä E., Muir C. S. Estimates of the worldwide frequency of sixteen major cancers in 1980. Int J Cancer. 1988 Feb 15;41(2):184–197. doi: 10.1002/ijc.2910410205. [DOI] [PubMed] [Google Scholar]
- Pines J. Cyclins: wheels within wheels. Cell Growth Differ. 1991 Jun;2(6):305–310. [PubMed] [Google Scholar]
- Rosenberg C. L., Kim H. G., Shows T. B., Kronenberg H. M., Arnold A. Rearrangement and overexpression of D11S287E, a candidate oncogene on chromosome 11q13 in benign parathyroid tumors. Oncogene. 1991 Mar;6(3):449–453. [PubMed] [Google Scholar]
- Rosenberg C. L., Wong E., Petty E. M., Bale A. E., Tsujimoto Y., Harris N. L., Arnold A. PRAD1, a candidate BCL1 oncogene: mapping and expression in centrocytic lymphoma. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9638–9642. doi: 10.1073/pnas.88.21.9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuuring E., Verhoeven E., Mooi W. J., Michalides R. J. Identification and cloning of two overexpressed genes, U21B31/PRAD1 and EMS1, within the amplified chromosome 11q13 region in human carcinomas. Oncogene. 1992 Feb;7(2):355–361. [PubMed] [Google Scholar]
- Shirodkar S., Ewen M., DeCaprio J. A., Morgan J., Livingston D. M., Chittenden T. The transcription factor E2F interacts with the retinoblastoma product and a p107-cyclin A complex in a cell cycle-regulated manner. Cell. 1992 Jan 10;68(1):157–166. doi: 10.1016/0092-8674(92)90214-w. [DOI] [PubMed] [Google Scholar]
- Tsuda T., Tahara E., Kajiyama G., Sakamoto H., Terada M., Sugimura T. High incidence of coamplification of hst-1 and int-2 genes in human esophageal carcinomas. Cancer Res. 1989 Oct 15;49(20):5505–5508. [PubMed] [Google Scholar]
- Victor T., Du Toit R., Jordaan A. M., Bester A. J., van Helden P. D. No evidence for point mutations in codons 12, 13, and 61 of the ras gene in a high-incidence area for esophageal and gastric cancers. Cancer Res. 1990 Aug 15;50(16):4911–4914. [PubMed] [Google Scholar]
- Wagata T., Ishizaki K., Imamura M., Shimada Y., Ikenaga M., Tobe T. Deletion of 17p and amplification of the int-2 gene in esophageal carcinomas. Cancer Res. 1991 Apr 15;51(8):2113–2117. [PubMed] [Google Scholar]
- Weinberg R. A. Tumor suppressor genes. Science. 1991 Nov 22;254(5035):1138–1146. doi: 10.1126/science.1659741. [DOI] [PubMed] [Google Scholar]
- Weinstein I. B. The origins of human cancer: molecular mechanisms of carcinogenesis and their implications for cancer prevention and treatment--twenty-seventh G.H.A. Clowes memorial award lecture. Cancer Res. 1988 Aug 1;48(15):4135–4143. [PubMed] [Google Scholar]
- Whang-Peng J., Banks-Schlegel S. P., Lee E. C. Cytogenetic studies of esophageal carcinoma cell lines. Cancer Genet Cytogenet. 1990 Mar;45(1):101–120. doi: 10.1016/0165-4608(90)90073-j. [DOI] [PubMed] [Google Scholar]
- Withers D. A., Harvey R. C., Faust J. B., Melnyk O., Carey K., Meeker T. C. Characterization of a candidate bcl-1 gene. Mol Cell Biol. 1991 Oct;11(10):4846–4853. doi: 10.1128/mcb.11.10.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Connolly T., Futcher B., Beach D. Human D-type cyclin. Cell. 1991 May 17;65(4):691–699. doi: 10.1016/0092-8674(91)90100-d. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Zhang H., Beach D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell. 1992 Oct 30;71(3):505–514. doi: 10.1016/0092-8674(92)90518-h. [DOI] [PubMed] [Google Scholar]
- Yang C. S. Research on esophageal cancer in China: a review. Cancer Res. 1980 Aug;40(8 Pt 1):2633–2644. [PubMed] [Google Scholar]