Abstract
Programmed cell death protein 1 (PD-1) and its ligands PD-L1 and PD-L2 play critical roles in maintaining an immunosuppressive tumor microenvironment. The purpose of the present study was to assess expression of PD-1, PD-L1, and PD-L2 in mouse prostate tumors. A total of 33 mouse prostate tumors derived from Pten-null mice were examined using immunohistochemical staining for PD-1, PD-L1, and PD-L2. The animals were either with interleukin-17 receptor c (Il-17rc) wild-type or knockout genotype, or fed with regular diet or high-fat diet to 30 weeks of age. We found that Il-17rc wild-type mouse prostate tumors had significantly higher levels of PD-1, PD-L1, and PD-L2 than Il-17rc knockout mouse prostate tumors. High-fat diet-induced obese mice had significantly higher levels of PD-1, PD-L1, and PD-L2 in their prostate tumors than lean mice fed with regular diet. Increased expression of PD-1, PD-L1, and PD-L2 was associated with increased number of invasive prostate tumors formed in the Il-17rc wild-type and obese mice compared to the Il-17rc knockout and lean mice, respectively. Our findings suggest that expression of PD-1, PD-L1, and PD-L2 may enhance development of mouse prostate cancer through creating an immunosuppressive tumor microenvironment.
Keywords: Prostate cancer, PD-1, PD-L1, PD-L2, IL-17, obesity
Introduction
Prostate cancer remains to be the most common cancer and the second leading cause of cancer-related deaths in American men. The American Cancer Society estimated that approximately 220,800 new cases of prostate cancer would be diagnosed and 27,540 deaths due to prostate cancer would occur in the United States in 2015 [1]. Although prostate cancer is frequently curable in its early stage by surgical or radiation therapies, many patients with locally advanced or metastatic diseases still have no cure [2,3]. It has been recognized that prostate cancer is a disease involving complex interactions between the neoplastic epithelial compartment and the surrounding stromal compartment in which the cancer cells live. The tumor microenvironment in which the cancer cells evolve towards an aggressive phenotype is highly heterogeneous, as it is composed of different cell populations such as endothelial cells, fibroblasts, and infiltrating inflammatory cells (such as macrophages, dendritic cells, neutrophils, and lymphocytes) [4]. The infiltrating inflammatory cells appear ineffective to initiate antitumor immune responses due to presence of immunosuppressive factors such as transforming growth factor β (TGF-β) and interleukin-10 (IL-10) [5]. Enhancing antitumor immune responses can prolong patient’s survival time, which has been demonstrated by Provenge® (sipuleucel-T) in the treatment of metastatic castration-resistant prostate cancer (CRPC) and Yervoy® (ipilimumab) in the treatment of metastatic melanoma [6,7].
Programmed cell death protein 1 (PD-1) and the programmed cell death protein 1 ligand 1 and 2 (PD-L1 and PD-L2) form an immunosuppressive checkpoint. PD-1 was originally identified in search of programmed cell death genes [8]. PD-1 gene encodes a protein with 288 amino acids, which is activated during programmed cell death. However, PD-1 is found to be a negative regulator of immune responses because its mutation causes development of autoimmune lupus-like arthritis and glomerulonephritis [9,10]. PD-L1 was later identified [11], which is identical to B7-H1 [12]. A second PD-1 ligand PD-L2 was found to be identical to B7-DC [13,14]. PD-1 is found to be mainly expressed on the cellular surface of activated T cells [15,16]. PD-L1 is expressed by tumor cells and tumor-infiltrating immune cells (macrophages, dendritic cells, and T cells) [17]. Both PD-L1 and PD-L2 mRNAs are found in human heart, placenta, spleen, lymph node, and thymus tissues. In addition, PD-L2 mRNA, but not PD-L1 mRNA, is found in human lung, liver, smooth muscle, and pancreas tissues [13]. In a cohort of 824 non-small cell lung cancer (NSCLC), 23.2% of patients showed ≥ 50% of tumor cells positive for PD-L1, 37.6% of patients showed with 1 to 49% of tumor cells positive for PD-L1, and 39.2% of patients showed with <1% of tumor cells positive for PD-L1 [18]. In the tumor microenvironment, the PD-1-PD-L1/L2 axis is up-regulated, resulting in immune evasion of the tumor cells [13,19]. It has become clear that binding of PD-1 by PD-L1 and PD-L2 inhibits T cell receptor-mediated lymphocyte proliferation and cytokine secretion, thus suppressing immune responses [20]. Therefore, the antibodies against PD-1, PD-L1, and PD-L2 can block the immune evasion and induce tumor regression. The objective response rate to pembrolizumab (anti-PD-1 antibody, also called lambrolizumab or MK-3475) treatment is positively correlated with the percentage of tumor cells with membranous PD-L1 staining [18]. Subsequent clinical trials showed that anti-PD-1 antibody (lambrolizumab) had a response rate of approximately 38% in melanoma patients with or without prior ipilimumab treatment [21]. A combination of anti-PD-1 antibody (nivolumab) and ipilimumab showed a 53% objective response in the patients with advanced melanoma [22]. A phase III trial showed that pembrolizumab had a significantly better response rate (approximately 33%) than ipilimumab (11.9%, P<0.001) in the treatment of advanced melanoma [23]. Therefore, the United States Food and Drug Administration (FDA) has approved pembrolizumab (Keytruda®, Merck & Co., Inc., Whitehouse Station, NJ, USA) for the treatment of patients with unresectable or metastatic melanoma and disease progression following ipilimumab and, if B-Rapidly Accelerated Fibrosarcoma (BRAF) V600 mutation positive, a BRAF inhibitor. FDA also approved nivolumab (Opdivo®, Bristol-Myers Squibb Company, Princeton, NJ, USA) for the treatment of patients with unresectable or metastatic melanoma and disease progression following ipilimumab and, if BRAF V600 mutation positive, a BRAF inhibitor, and for the treatment of patients with metastatic squamous NSCLC with progression on or after platinum-based chemotherapy. Anti-PD-L1 antibody (MPDL3280A, Genentech, Roche, South San Francisco, CA, USA) produced responsive rates of 13% to 26% in solid tumors [17]. Thus, FDA granted MPDL3280A a breakthrough therapy designation for the treatment of PD-L1–positive NSCLC that has progressed during or after platinum-based chemotherapy, as well as a targeted therapy for patients with epidermal growth factor receptor (EGFR)-positive or anaplastic lymphoma kinase (ALK)-positive tumors. MPDL3280A is undergoing phase II and III trials to obtain FDA approval [24].
Recently, Martin et al. [25] reported that human prostate cancer cell lines PC3 and DU145 constitutively express PD-L1 and interferon-γ induces PD-L1 expression in PC3, DU145, VCaP, CWR22Rv1, and E006AA cell lines. However, LNCaP and LAPC-4 cell lines do not express PD-L1, nor can interferon-γ induce PD-L1 expression in these cell lines. On the other hand, bicalutamide does not induce PD-L1 expression in any cell lines [25]. In 20 whole-mount human primary prostate cancer samples, three (15%) were found positive for PD-L1 staining (defined as 5% cells with membrane staining) [25]. In another recent study, Bishop et al. [26] reported that PD-L1/2+ dendritic cell frequency in the blood was increased in CRPC patients who did not respond to Enzalutamide treatment, compared to the responders or untreated patients. PD-L1 expression in LNCaP-derived Enzalutamide-resistant prostate cancer cell lines (prostate-specific antigen-negative) was significantly increased, compared to the Enzalutamide-sensitive cell lines [26]. Xenografted Enzalutamide-resistant PSA- prostate cancer cells increased the frequency of PD-L1/2+ dendritic cells in the blood, but decreased the frequency of PD-L1/2+ dendritic cells in the tumors [26]. Together, these findings suggest that PD-L1 expression may be increased in a subpopulation of CRPC. However, so far the PD-1/PD-L1/2 axis has not been investigated in any mouse models of spontaneous prostate cancer. The purpose of the present study was to assess expression of PD-1, PD-L1, and PD-L2 in the prostate tumors from Pten-null mice.
Material and method
Animals
Animal study was approved by the Animal Care and Use Committee of Tulane University. Il-17rc and Pten double knockout mice were described previously [27]. Briefly, Il-17rc traditional knockout mice were crossbred with Pten conditional knockout mice [crossbreeding between Pten loxp/loxp mice (strain C;129S4-Pten tm1Hwu/J) and PB-Cre4 mice (strain B6.Cg-Tg(Pbsn-cre)4Prb)] [27,28]. Il-17rc wild-type (n=8) and Il-17rc knockout (n=8) male mice were fed with regular diet to the age of 30 weeks. A total of sixteen prostates were obtained from Il-17rc wild-type (n=8) and Il-17rc knockout (n=8) male mice, which were collected from our previous study [27]. In a separate study starting at 3 weeks of age, Il-17rc wild-type male mice with Pten conditional knockout genetic background were either fed with regular diet (13.2% calories by fat, cat# 5053, LabDiet, Brentwood, MO) or high-fat diet (60% calories by fat, cat# D12492, Research Diets, New Brunswick, NJ) to the age of 30 weeks. A total of 17 mouse prostates were obtained from lean mice (n=9, fed with regular diet) and obese mice (n=8, fed with high-fat diet).
Immunohistochemical staining
Mouse prostate tumors were fixed in 10% formalin, embedded in paraffin, and cut into 4-μm thick tissue sections. The tissue sections were baked at 60°C for 60 min, deparaffinized in xylene, and rehydrated through graded ethanol solutions to water. The antigens were retrieved by heating the tissue sections in 0.01 M ethylenediamine tetra acetic acid buffer at 95°C for 5 min and then cooling down to room temperature in 20 min. Endogenous peroxidase activity was blocked in 0.3% H2O2 for 5 min. Non-specific binding was blocked with 1.5% normal serum (VECTASTAIN Elite ABC Kit, Vector Laboratories, Burlingame, CA, USA). The tissue sections were incubated with primary antibodies in a humid chamber at 4°C overnight. Rabbit anti-PD-L1 polyclonal antibodies (Abcam, Cambridge, MA, USA; catalogue# ab58810, used at a dilution of 1:400), rabbit anti-PD-L2 polyclonal antibodies (Sigma-Aldrich, St. Louis, MO, USA; catalogue# SAB3500395-100UG, used at a dilution of 1:600), and rabbit anti-CD279 (PD-1) affinity-purified and validated polyclonal antibodies (Fisher Scientific, Pittsburgh, PA, USA; catalogue# PIPA520351, used at a dilution of 1:400) were used as the primary antibodies. After being washed 3 times in phosphate buffered saline, the tissue sections were incubated with secondary antibodies (VECTASTAIN Elite ABC Kit, Vector Laboratories, Burlingame, CA, USA) for 2 hours. The color was developed using 3,3’-diaminobenzidine (DAB) substrate kit (Vector Laboratories, Burlingame, CA, USA) following the manufacturer’s protocol. The sections were then counter stained with hematoxylin. The tissue sections previously stained positively were used as positive controls and the tissue sections stained with non-immune serum served as negative controls. Positive staining showed brown particles at the cytoplasmic membrane and/or in the cytoplasm. Under a microscope, five representative high-power fields (x400 magnification) per tissue section were randomly selected and evaluated by an investigator (S.Y.) who was blinded to the grouping of animals. We used a two-score system based on a proportion score and an intensity score described by Allred et al. [29]. The proportion scores represented the portion of positive staining: 0, none; 1, less than one-hundredth; 2, one-hundredth to one-tenth; 3, one-tenth to one-third; 4, one-third to two-thirds; and 5, greater than two thirds. The intensity scores represented the estimated average staining intensity of positive staining: 0, none; 1, weak; 2, intermediate; and 3, strong. The overall scores (Allred scores) were the sum of the proportion score and intensity score of each sample (range, 0-8).
Statistical analysis
The median, lower quartile (defined as the middle number between the smallest number and the median), and upper quartile (defined as the middle value between the median and the highest value) of Allred scores per group were presented. Statistical analysis was performed using Mann-Whitney U test. P<0.05 was considered as statistical significant.
Results
The expression levels of PD-1, PD-L1, and PD-L2 are higher in Il-17rc wild-type mouse prostates than Il-17rc knockout mouse prostates
Il-17rc wild-type and knockout mouse prostates developed prostate tumors due to Pten knockout as previously described [27]. There were more inflammatory cells infiltrating the prostatic stroma in Il-17rc wild-type mouse prostates than Il-17rc knockout mouse prostates [27]. We found that PD-1 was expressed mainly in the infiltrating inflammatory cells, rather than in the neoplastic epithelial cells (Figure 1). There were significantly more PD-1-positive cells in Il-17rc wild-type mouse prostates than Il-17rc knockout mouse prostates (Table 1). On the other hand, PD-L1 and PD-L2 were expressed mainly in the neoplastic epithelial cells, rather than in the infiltrating inflammatory cells (Figure 1). There were significantly more PD-L1-positive and PD-L2-positive cells in Il-17rc wild-type mouse prostates than Il-17rc knockout mouse prostates (Table 1).
Figure 1.
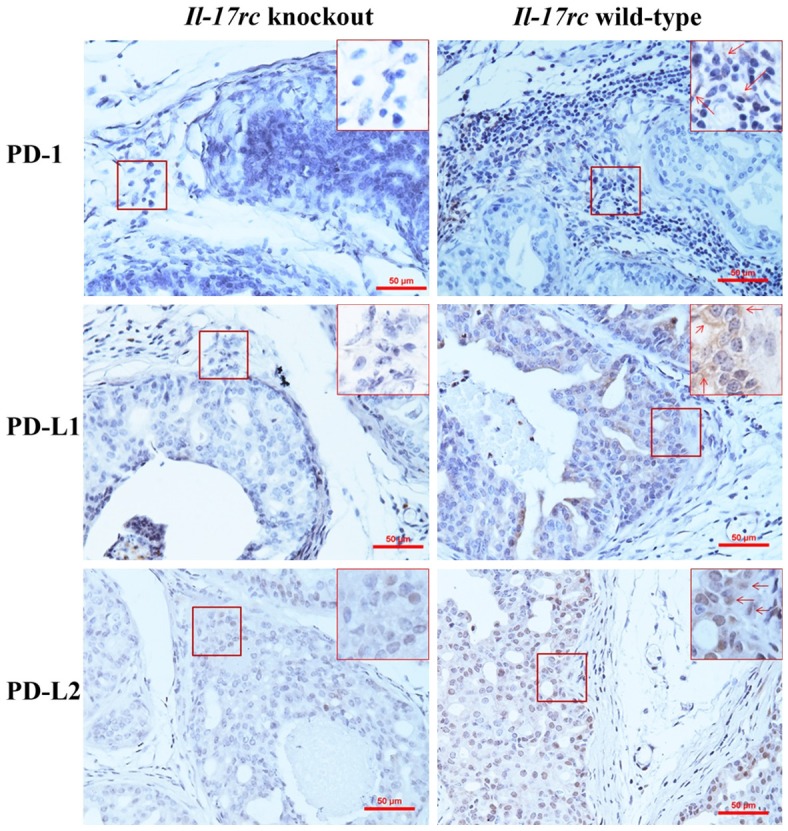
Representative photomicrographs of immunohistochemical staining of Il-17rc wild-type and knockout mouse prostate tumors. Original magnification, x200. In highlighted frames, original magnification, x400; arrows indicate the positively stained cells.
Table 1.
PD-1, PD-L1, and PD-L2 expression in the prostate tumors of Pten-null mice with or without Il-17rc knockout
| Protein | Group | Lower quartile | Median | Upper quartile | P value |
|---|---|---|---|---|---|
| PD-1 | Il-17rc wild-type (n=8) | 5.1 | 6.0 | 6.8 | 0.043 |
| Il-17rc knockout (n=8) | 2.0 | 3.0 | 5.8 | ||
| PD-L1 | Il-17rc wild-type (n=8) | 5.3 | 6.0 | 6.8 | 0.001 |
| Il-17rc knockout (n=8) | 2.0 | 3.8 | 4.0 | ||
| PD-L2 | Il-17rc wild-type (n=8) | 6.0 | 6.0 | 6.0 | 0.002 |
| Il-17rc knockout (n=8) | 1.6 | 2.8 | 4.8 |
The expression levels of PD-1, PD-L1, and PD-L2 are higher in obese mouse prostates than lean mouse prostates
Il-17rc wild-type and Pten-null mice were fed with high-fat diet to 30 weeks of age and became obviously obese. The body weight of the obese mice was approximately 52% more than the mice fed with regular diet (named as lean mice), and invasive (or microinvasive) prostate adenocarcinomas were significantly more in obese mice (42% of the prostatic glands) than lean mice (23% of the prostatic glands) (Sen Liu, Qiuyang Zhang, and Zongbing You, unpublished data). Again, we found that PD-1 was expressed mainly in the infiltrating inflammatory cells, rather than in the neoplastic epithelial cells (Figure 2). There were significantly more PD-1-positive cells in obese mouse prostates than lean mouse prostates (Table 2). In contrast, PD-L1 and PD-L2 were expressed mainly in the neoplastic epithelial cells, rather than in the infiltrating inflammatory cells (Figure 2). There were significantly more PD-L1-positive and PD-L2-positive cells in obese mouse prostates than lean mouse prostates (Table 2).
Figure 2.
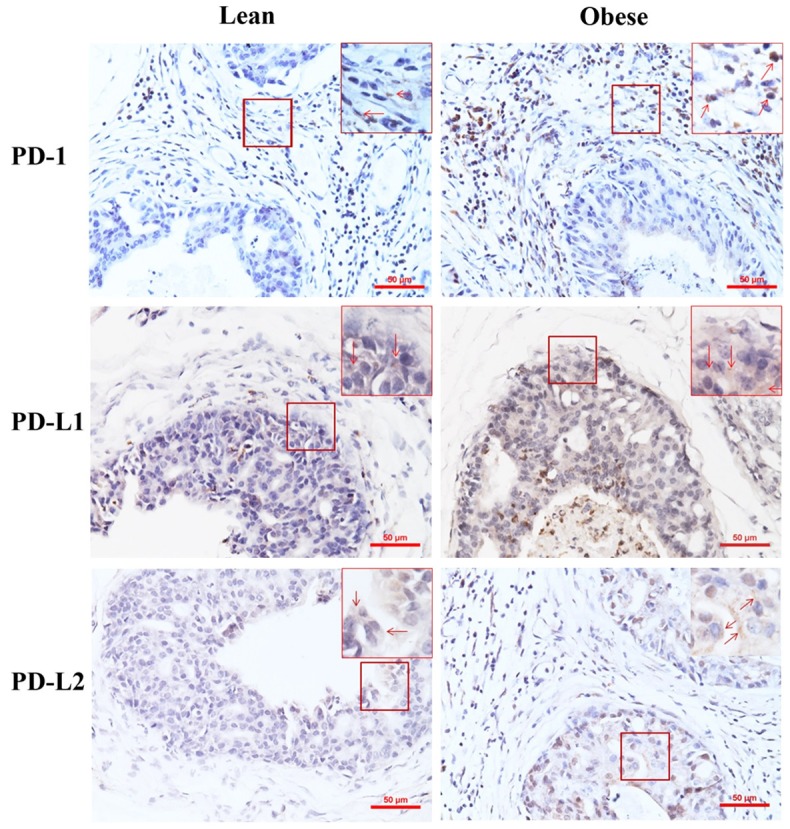
Representative photomicrographs of immunohistochemical staining of lean and obese mouse prostate tumors. Original magnification, x200. In highlighted frames, original magnification, x400; arrows indicate the positively stained cells.
Table 2.
PD-1, PD-L1, and PD-L2 expression in the prostate tumors of Pten-null mice with or without high-fat diet-induced obesity
| Protein | Group | Lower quartile | Median | Upper quartile | P value |
|---|---|---|---|---|---|
| PD-1 | Obese (n=8) | 5.0 | 5.0 | 6.6 | 0.011 |
| Lean (n=9) | 2.0 | 4.0 | 5.0 | ||
| PD-L1 | Obese (n=8) | 5.8 | 6.0 | 6.9 | 0.002 |
| Lean (n=9) | 1.0 | 3.0 | 4.5 | ||
| PD-L2 | Obese (n=8) | 6.0 | 6.5 | 7.0 | 0.002 |
| Lean (n=9) | 1.0 | 2.0 | 4.8 |
Discussion
Only two CRPC cases were included in the Phase I trial, in which both cases were negative for PD-L1 and did not respond to anti-PD-1 treatment [30]. Ongoing clinical trials are using anti-PD-1 antibody or a combination of anti-PD-1 antibody and other therapies to treat CRPC [31,32]. Only a few studies have been published regarding PD-1/PD-L1/2 axis in human prostate cancer [25,26]. At least a 15% positive rate was reported in human primary prostate cancer [25]. It is possible that some non-responders to certain therapies may present with increased PD-L1 expression [26]. Therefore, it is worth investigating PD-1/PD-L1/2 axis in prostate cancer.
In the present study, we found that the expression levels of PD-1, PD-L1, and PD-L2 are higher in Il-17rc wild-type mouse prostates than Il-17rc knockout mouse prostates. We have previously reported that Il-17rc wild-type mice developed more invasive prostate adenocarcinomas than Il-17rc knockout mice in Pten-null background [27]. This suggests that increased PD-1/PD-L1/2 expression may enhance immunosuppression in the tumor microenvironment, thus promoting prostate cancer formation. Similarly, we found that the expression levels of PD-1, PD-L1, and PD-L2 are higher in obese mouse prostates than lean mouse prostates, which coincidentally matches the increased prostate cancer formation in obese mice compared to lean mice. Taken together, these results suggest that expression of PD-1, PD-L1, and PD-L2 may enhance development of mouse prostate cancer through creating an immunosuppressive tumor microenvironment. A previous study proposed a notion that loss of Pten is potentially associated with PD-L1 expression [33]. However, a recent study provided evidence to nullify that notion; rather, it proposed that acute inflammation driven by androgen ablation may increase PD-L1 expression [25]. Characterization of PD-1/PD-L1/2 expression in this Pten-null mouse model of prostate cancer may provide a rationale to use this mouse model in future studies of PD-1/PD-L1/2 in cancer immunology and immunotherapy.
Acknowledgements
Z.Y. was supported partially by National Institutes of Health (R01CA174714 and P20GM103518), Department of Defense (W81XWH-14-1-0050, W81XWH-14-1-0149, W81XWH-14-1-0458, and W81XWH-15-1-0444), the Developmental Fund of Tulane Cancer Center (TCC), Louisiana Cancer Research Consortium (LCRC) Fund, and Tulane’s Institute of Integrated Engineering for Health and Medicine (TI2EHM). S.Y. was supported by the Service Center for Experts and Scholars of Hebei Province, China to pursue research in the USA. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Defense.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Albertsen P. Predicting survival for men with clinically localized prostate cancer: what do we need in contemporary practice? Cancer. 2008;112:1–3. doi: 10.1002/cncr.23107. [DOI] [PubMed] [Google Scholar]
- 3.So A, Gleave M, Hurtado-Col A, Nelson C. Mechanisms of the development of androgen independence in prostate cancer. World J Urol. 2005;23:1–9. doi: 10.1007/s00345-004-0473-1. [DOI] [PubMed] [Google Scholar]
- 4.Chiarugi P, Paoli P, Cirri P. Tumor microenvironment and metabolism in prostate cancer. Semin Oncol. 2014;41:267–280. doi: 10.1053/j.seminoncol.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Corn PG. The tumor microenvironment in prostate cancer: elucidating molecular pathways for therapy development. Cancer Manag Res. 2012;4:183–193. doi: 10.2147/CMAR.S32839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 7.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int Immunol. 1998;10:1563–1572. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- 10.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 11.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 13.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 14.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 17.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 19.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 20.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 21.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 24.Cha E, Wallin J, Kowanetz M. PD-L1 inhibition with MPDL3280A for solid tumors. Semin Oncol. 2015;42:484–487. doi: 10.1053/j.seminoncol.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Martin AM, Nirschl TR, Nirschl CJ, Francica BJ, Kochel CM, van Bokhoven A, Meeker AK, Lucia MS, Anders RA, DeMarzo AM, Drake CG. Paucity of PD-L1 expression in prostate cancer: innate and adaptive immune resistance. Prostate Cancer Prostatic Dis. 2015;18:325–332. doi: 10.1038/pcan.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bishop JL, Sio A, Angeles A, Roberts ME, Azad AA, Chi KN, Zoubeidi A. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget. 2015;6:234–242. doi: 10.18632/oncotarget.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Q, Liu S, Ge D, Xue Y, Xiong Z, Abdel-Mageed AB, Myers L, Hill SM, Rowan BG, Sartor O, Melamed J, Chen Z, You Z. Interleukin-17 promotes formation and growth of prostate adenocarcinoma in mouse models. Cancer Res. 2012;72:2589–2599. doi: 10.1158/0008-5472.CAN-11-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, Liu X, Wu H. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 29.Allred DC, Clark GM, Elledge R, Fuqua SA, Brown RW, Chamness GC, Osborne CK, McGuire WL. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer. J Natl Cancer Inst. 1993;85:200–206. doi: 10.1093/jnci/85.3.200. [DOI] [PubMed] [Google Scholar]
- 30.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dolan DE, Gupta S. PD-1 pathway inhibitors: changing the landscape of cancer immunotherapy. Cancer Control. 2014;21:231–237. doi: 10.1177/107327481402100308. [DOI] [PubMed] [Google Scholar]
- 32.Simons JW. Prostate cancer immunotherapy: beyond immunity to curability. Cancer Immunol Res. 2014;2:1034–1043. doi: 10.1158/2326-6066.CIR-14-0174. [DOI] [PubMed] [Google Scholar]
- 33.Crane CA, Panner A, Murray JC, Wilson SP, Xu H, Chen L, Simko JP, Waldman FM, Pieper RO, Parsa AT. PI(3) kinase is associated with a mechanism of immunoresistance in breast and prostate cancer. Oncogene. 2009;28:306–312. doi: 10.1038/onc.2008.384. [DOI] [PMC free article] [PubMed] [Google Scholar]


