Summary
Fibroblast growth factor 21 (FGF21) is a hormone induced by various metabolic stresses, including ketogenic and high carbohydrate diets, that regulates energy homeostasis. In humans, SNPs in and around the FGF21 gene have been associated with macronutrient preference, including carbohydrate, fat and protein intake. Here we show that FGF21 administration markedly reduces sweet and alcohol preference in mice, and sweet preference in cynomolgus monkeys. In mice, these effects require the FGF21 co-receptor β-Klotho in the central nervous system and correlate with reductions in dopamine concentrations in the nucleus accumbens. Since analogs of FGF21 are currently undergoing clinical evaluation for the treatment of obesity and type 2 diabetes, our findings raise the possibility that FGF21 administration could affect nutrient preference and other reward behaviors in humans.
Graphical Abstract
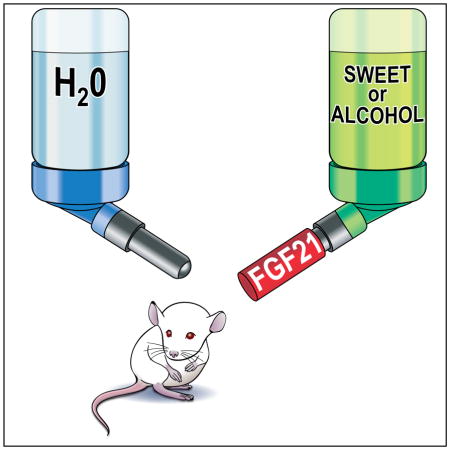
Introduction
FGF21 is induced in liver in response to fasting and other forms of metabolic stress including ketogenic and high carbohydrate diets (Badman et al., 2007; Dushay et al., 2015; Inagaki et al., 2007; Sanchez et al., 2009; Zhao et al., 2015). FGF21 in turn regulates diverse aspects of energy homeostasis, including hepatic fatty acid oxidation and ketogenesis, circadian behavior, growth and female reproduction (Owen et al., 2015). Pharmacologically, FGF21 causes weight loss and improves insulin sensitivity in obese mice, monkeys and humans (Gimeno and Moller, 2014). Long-acting analogs of FGF21 are currently in clinical trials for the treatment of obesity and type 2 diabetes.
FGF21 acts through a cell surface receptor composed of a conventional FGF receptor in complex with β-Klotho, a single-pass transmembrane protein (Owen et al., 2015). FGF21 crosses the blood-brain barrier (Hsuchou et al., 2007) and exerts many of its actions, including its effects on growth, female reproduction and weight loss, by acting on its cognate receptor in the CNS (Bookout et al., 2013; Douris et al., 2015; Liang et al., 2014; Owen et al., 2013; Owen et al., 2014; Sarruf et al., 2010). Among its central actions, FGF21 induces corticotropin-releasing factor and suppresses arginine vasopressin expression in the hypothalamus (Bookout et al., 2013; Liang et al., 2014; Owen et al., 2013; Owen et al., 2014).
In humans, SNPs in and around the FGF21 gene are associated with changes in macronutrient preference, including increases in carbohydrate consumption and decreases in fat and protein intake (Chu et al., 2013; Tanaka et al., 2013). These findings raise the possibility of additional effects of FGF21 on the brain. In this report, we examine the effect of FGF21 on sweet and alcohol preference in mice and monkeys.
Results and Discussion
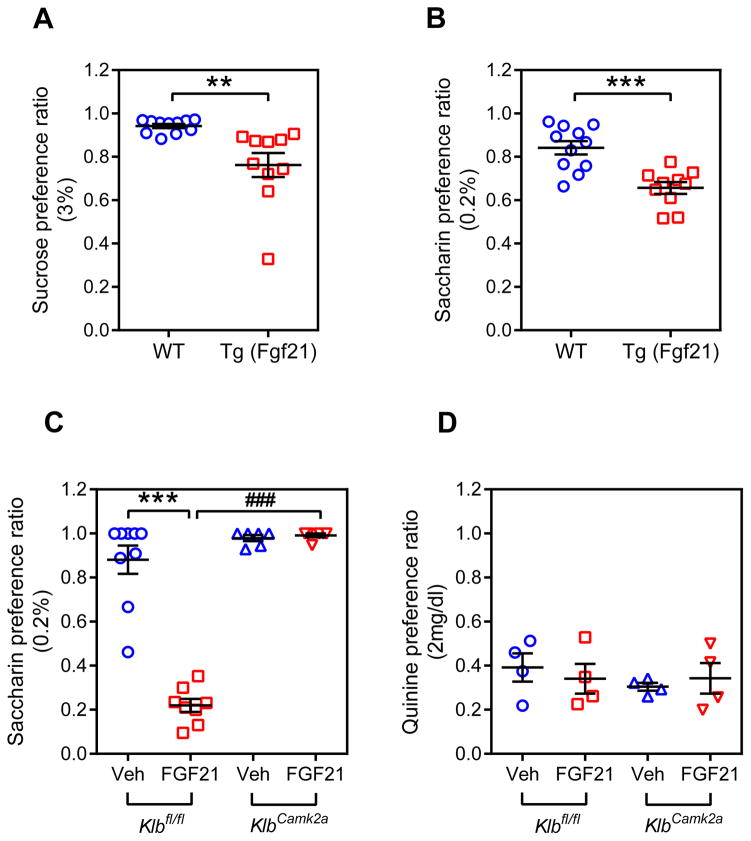
Since FGF21 is induced by carbohydrates in rodents and humans (Dushay et al., 2015; Sanchez et al., 2009), and SNPs in the FGF21 gene are associated with carbohydrate intake in humans (Chu et al., 2013; Tanaka et al., 2013), we investigated whether chronic FGF21 exposure affects sweet preference. Two-bottle preference assays with water and either 3% sucrose or 0.2% saccharin (Krishnan et al., 2007; Tordoff and Bachmanov, 2003) were performed using wild-type and Fgf21-transgenic (Tg) mice expressing supraphysiological concentrations of FGF21 (Inagaki et al., 2007). Saccharin was included to eliminate the potentially confounding effect of caloric content. As expected, wild-type mice showed a strong preference for drinking water sweetened with either sucrose or saccharin (Figure 1A and B, Table S1). Notably, the Tg(Fgf21) mice had significant decreases in both sucrose and saccharin preference (Figure 1A and B).
Figure 1. FGF21 decreases sweet preference ratio in mice by acting on the CNS.
(A) Two-bottle preference assay in wild-type (WT) and Tg(Fgf21) mice administered water vs. 3% sucrose. Representative 24 hour data from day 2 after initiating the assay are shown as the sucrose preference ratio (sucrose intake volume/total fluid intake volume). n = 10–11/group.
(B) Two-bottle preference assay in WT and Tg(Fgf21) mice administered water vs. 0.2% saccharin. Representative 24 hour data from day 2 after initiating the assay are shown. n = 10–11/group.
(C) Two-bottle preference assay with water vs. 0.2% saccharin for Klbfl/fl and KlbCamk2a mice administered either FGF21 (1 mg/kg/day) or vehicle. Representative 24 hour data from day 3 after initiating the assay are shown. n = 6–9/group.
(D) Two-bottle preference assay with water vs. 2 mg/dl quinine for Klbfl/fl and KlbCamk2a mice administered either FGF21 (1 mg/kg/day) or vehicle. n = 4/group.
Values are means ±S.E.M. *, p<0.05; ***, p<0.001; ###, p<0.001 by Student’s t-test.
See also Figure S1, Table S1 and S2.
To determine whether FGF21 acts on the CNS to regulate sweet preference, we administered recombinant FGF21 or vehicle by osmotic minipump to groups of control mice with floxed β-Klotho alleles (Klbfl/fl) or mice specifically lacking β-Klotho in the CNS (KlbCamk2a), and two-bottle saccharin preference tests were performed. FGF21 strongly suppressed saccharin preference in Klbfl/fl mice but had no effect in KlbCamk2a mice (Figure 1C, Table S2). As reported (Camporez et al., 2013), administration of recombinant FGF21 increased total fluid intake, and this effect required β-Klotho in the CNS (Table S2). In contrast, total fluid intake was unchanged in Tg(Fgf21) compared to control mice (Table S1 and S2). The reason for this difference between transgenic FGF21 overexpression and recombinant FGF21 administration on fluid intake is not known. Nevertheless, FGF21 decreased sweet preference in both contexts.
In additional two-bottle preference tests, FGF21 had no effect on preference for 1% sunflower oil (data not shown) or quinine (Figure 1D), indicating that FGF21 does not affect responses to fatty acids or bitter taste. FGF21 administration also had no effect on either tail-suspension or forced-swim tests, both standard measures of behavioral despair (Figure S1A and B). We conclude that FGF21 acts directly on the brain to regulate sweet preference without causing despair.
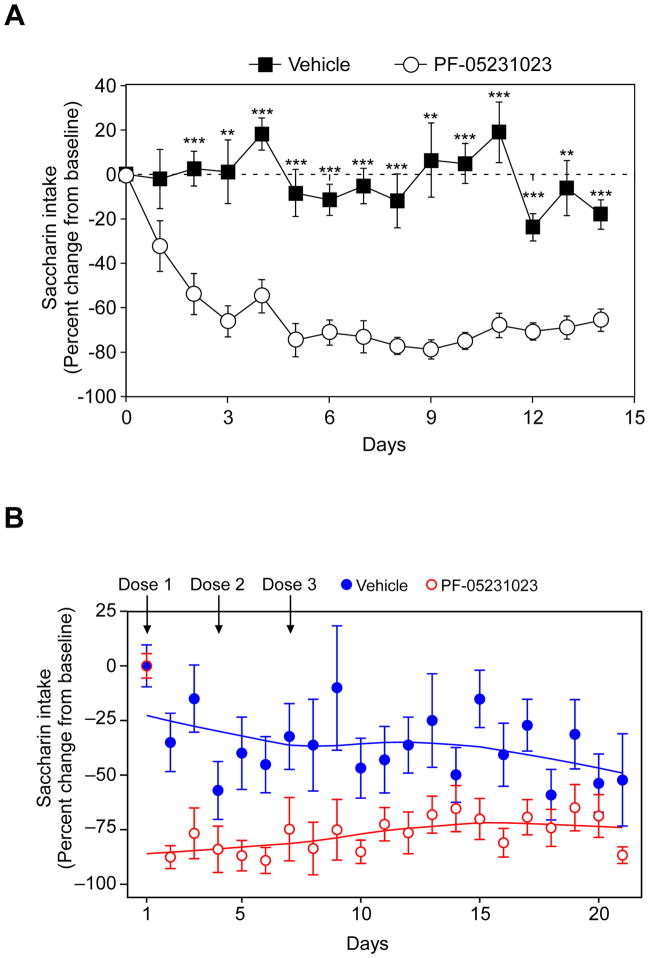
To determine whether FGF21 also affects sweet preference in primates, we analyzed saccharin preference in obese cynomolgus monkeys administered PF-05231023, a long-acting FGF21 analog consisting of two molecules of modified FGF21 linked by an antibody scaffold (Dong et al., 2015; Giragossian et al., 2015; Weng et al., 2015). We first tested this analog in mice. PF-05231023 administration decreased saccharin preference to a similar degree as native FGF21, with maximal efficacy observed 3–5 days after dosing (Figure 2A). For the monkey study, PF-05231023 or vehicle was administered on days 1, 4 and 7 of the three-week experiment. Notably, PF-05231023 administration significantly decreased saccharin preference in the monkeys (Figure 2B). The effect on saccharin intake in the monkeys was striking even within one day of receiving a single dose of the FGF21 analog, and the effect was sustained for several days after receiving the last dose. Thus, FGF21 also affects sweet preference in primates.
Figure 2. A stable FGF21 analog decreases saccharin preference in mice and monkeys.
(A) Two bottle preference assay with 0.1% saccharin in diet-induced obese mice administered either PF-05231023 (10 mg/kg) or vehicle on days 0, 3, 7 and 10.
Data are shown as the mean ± S.E.M.; n = 8/group. **p < 0.01, ***p < 0.001 versus vehicle group.
(B) Two bottle preference assay with 0.2% saccharin in obese cynomolgus monkeys administered either PF-05231023 (n=8; 10 mg/kg) or vehicle (n=7) on days 1, 4 and 7. Data are presented as mean percentage change in saccharin water intake ± S.E.M. for vehicle-treated (closed blue circles) and PF-05231023-treated (open red circles) monkeys. Solid lines are locally weighted scatterplot smoothing fits to the means of percent change. Mixed effect modeling fitted to these longitudinal data using R, version 3.1.2 (Pinheiro et al., 2013), showed a significant difference (p = 0.003) between groups. Number of days after first treatment, treatment type, and the interaction term between treatment groups and time were specified as fixed effects and monkey labels as a random effect.
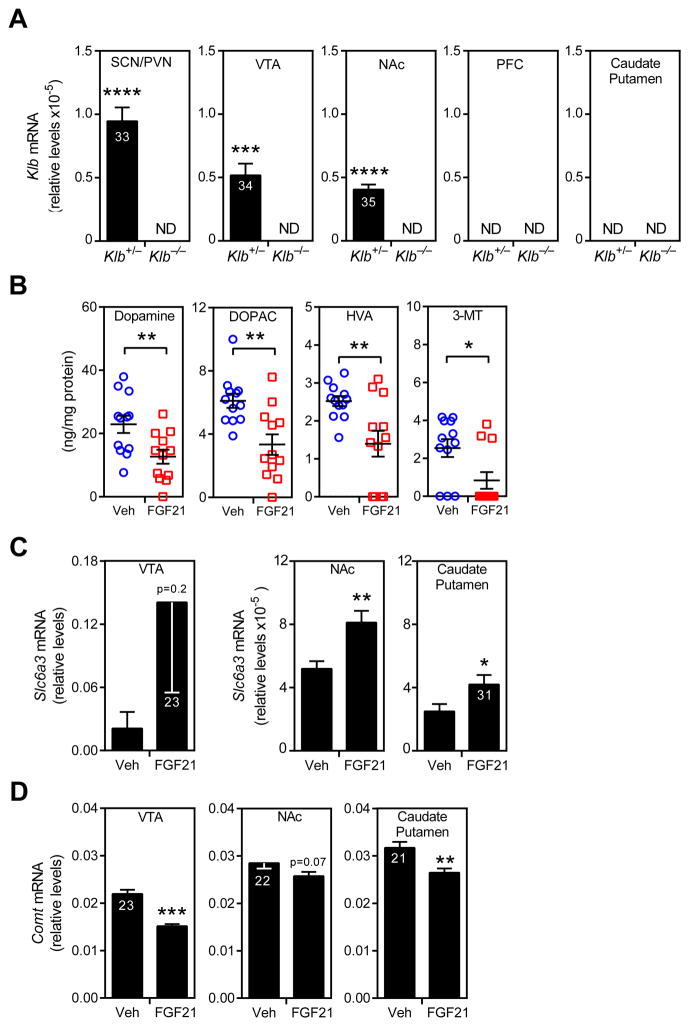
The neurotransmitter dopamine has a central role in regulating reward behavior, including sucrose and saccharin preference (Fernstrom et al., 2012). To examine whether FGF21 affects dopamine signaling, including the mesolimbic pathway, we first measured β-Klotho expression in the ventral tegmental area (VTA), nucleus accumbens (NAc), medial prefrontal cortex (PFC) and caudate putamen (CP) of Klb+/− and Klb−/− mice, with the latter mice serving as a negative control. As expected (Bookout et al., 2013; Liang et al., 2014), Klb mRNA was detected in the suprachiasmatic nucleus/paraventricular (SCN/PVN) nucleus region of the hypothalamus in Klb+/− mice by qPCR (Figure 3A). Klb mRNA was also detected in VTA and NAc in Klb+/− mice, albeit at relatively low levels, but not the PFC or CP (Figure 3A). As expected, Klb mRNA was not detected in any of the regions in the Klb−/− control mice (Figure 3A). Consistent with the qPCR data, Klb mRNA was detected by in situ hybridization in SCN and a small subset of cells in the VTA and NAc (Figure S2A). Expression of FGF receptor 1, which partners with β-Klotho to form the FGF21 receptor, was detected by qPCR in all of these brain regions (Figure S2B).
Figure 3. FGF21 affects dopamine signaling.
(A) β-Klotho (Klb) mRNA levels in the suprachiasmatic nucleus/paraventricular nucleus (SCN/PVN) region of the hypothalamus, ventral tegmental area (VTA), nucleus accumbens (NAc), medial prefrontal cortex (PFC) and caudate putamen of Klb+/− and Klb−/− mice (n = 6/group). Ct values are shown in the bars. ND, not detected.
(B) Concentrations of dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA) and 3-methoxytyramine (3-MT) in the NAc of mice administered either vehicle or FGF21 for 2 weeks by osmotic minipump (n = 12/group).
(C) mRNA levels of dopamine transporter (Slc6a3) or (D) catechol-O-methyl transferase (Comt) in VTA, NAc and caudate putamen of mice administered either vehicle or FGF21 for 2 weeks by osmotic minipump (n = 7–8/group). Ct values are shown.
Values are means ±S.E.M. *, p<0.05; **, p<0.01; ***, p<0.001 versus control group by Student’s t-test.
See also Figure S2.
The FGF21 receptor expression data led us to examine whether FGF21 affects the levels of dopamine and its metabolites in NAc, which coordinates reward behaviors. Notably, FGF21 administration for two weeks significantly decreased dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA) and 3-methoxytyramine (3-MT) concentrations (Figure 3B). FGF21 administration did not decrease dopamine, DOPAC, HVA or 3-MT concentrations in the CP (Figure S2C). FGF21 administration also caused changes in the expression of dopamine-related genes, including an increase in the dopamine transporter in the NAc and CP and a decrease in catechol-O-methyl transferase in the VTA and CP (Figure 3C and D). FGF21 had little or no effect on the mRNA levels of tyrosine hydroxylase or dopamine receptor 1 in these brain regions (Figure S2D and E) nor did it change the levels of total and phosphorylated tyrosine hydroxylase in VTA (Figure S2F). Taken together, these data suggest that FGF21 may affect sweet preference via effects on dopamine signaling. However, additional experiments will be required to confirm this possibility and to determine the mechanism whereby FGF21 reduces dopamine concentrations.
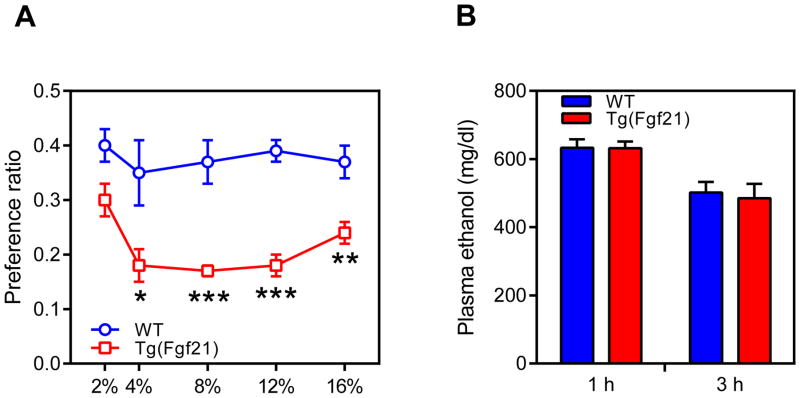
Since dopamine signaling impacts ethanol drinking behavior (Gonzales et al., 2004), we examined whether FGF21 also affects alcohol preference. Groups of wild-type and Tg(Fgf21) mice were exposed stepwise to increasing concentrations of ethanol in a two-bottle preference assay. Tg(Fgf21) mice had a decreased ethanol preference ratio at the 4%, 8%, 12% and 16% ethanol concentrations (Figure 4A, Table S3). In an ethanol bioavailability test, there was no difference between wild-type and Tg(Fgf21) mice in plasma ethanol concentrations at 1 and 3 hours after ethanol administration (Figure 4B). Thus, FGF21 suppresses ethanol preference without affecting its bioavailability.
Figure 4. FGF21 decreases alcohol preference.
(A) Ethanol preference ratio in wild-type (WT) and Tg(Fgf21) mice at the indicated ethanol concentrations (n = 9/group).
(B) Plasma ethanol concentrations in groups of WT and Tg(Fgf21) mice 1 or 3 hours after i.p. injection of ethanol (4g/kg) (n = 4–5/group).
Values are means ±S.E.M. *, p<0.05; **, p<0.01; and ***, p<0.001 versus control group by Student’s t-test.
See also Table S3.
In summary, we show that FGF21 regulates sweet and alcohol preference in mice, and sweet preference in monkeys. Since circulating levels of FGF21 increase in response to carbohydrate consumption in rodents and humans (Dushay et al., 2015; Sanchez et al., 2009; Zhao et al., 2015) and alcohol consumption in rodents (Zhao et al., 2015), this may represent a feed forward regulatory pathway for limiting consumption. In mice, the effects on sweet and alcohol preference correlate with reductions in dopamine concentrations in the NAc, which coordinates reward behavior. These results suggest a mechanistic basis for the association between SNPs in and around the FGF21 gene with macronutrient preference in humans (Chu et al., 2013; Tanaka et al., 2013). Moreover, since FGF21 is currently in clinical trials for treating obesity and type 2 diabetes, these findings suggest that additional studies are warranted to assess the effects of FGF21 on sweet and alcohol preference and other reward behavior in humans.
Methods
Mouse experiments with FGF21
All mouse experiments involving native FGF21 were approved by the Internal Animal Care and Use Committee of the University of Texas Southwestern Medical Center. Wild-type and Tg(Fgf21) mice were on a C57BL/6J background. Klbfl/fl and KlbCamk2a mice were on a mixed C57BL/6J;129/Sv background as described (Bookout et al., 2013). All experiments were performed with 2- to 4-month-old male mice. Tg(Fgf21) and Klbfl/fl mouse experiments were performed with littermates. Mice were housed on a standard 12-hour light/dark cycle and had free access to chow. Recombinant human FGF21 protein was provided by Novo Nordisk and administered by subcutaneous osmotic minipumps (Alzet) at a dose of 1 mg/kg/day. Mice were allowed to recover from minipump surgery for 1 week prior to preference tests. Mice were single caged following minipump surgery, which was conducted under isoflurane anaesthesia and 24 hour buprenorphine analgesia.
Two-bottle preference assays
For the two-bottle sucrose and saccharin preference assays, mice were acclimated to cages with 2 bottles of just water for 4 d. Mice were then given access to bottles with water and water containing 3% sucrose or 0.2% saccharin (w/v). For the quinine preference assay, mice were given access to water and water containing 2 mg/dl quinine. In each case, consumption was measured daily for at least 3 days. For ethanol preference assays, mice were given access to two bottles, one containing water and the other containing either 2, 4, 8, 12 or 16% ethanol (v/v) in water. The same mice were exposed to an ascending concentration of each ethanol concentration for 5 days. The position of the two bottles was changed every two days to exclude position effects. Water and ethanol-containing water intake were measured each day.
Mouse immobility assays
Tail-suspension and forced-swim tests were performed as described (Can et al., 2012a; Can et al., 2012b) on mice administered either FGF21 or vehicle by osmotic minipump for 7–14 days. In both tests, the experimenter was blinded to the treatment group. For the forced-swim test, mice were placed in cylindrical tanks (20 cm in diameter) filled with water (25 ± 2°C). The cylinder was filled to a depth of 12 cm to prevent the mice from using their tails to support themselves in the water. In both experiments, the cumulative time spent immobile was recorded over the course of a six minute experiment.
Ethanol clearance assays
Mice were intraperitoneally injected with ethanol (4 g/kg) in saline, and tail vein blood was drawn at regular intervals. Plasma ethanol levels were measured using the EnzyChrom™ Ethanol Assay Kit (BioAssay Systems).
Microdissection of brain regions
Mouse brains were extracted from the skull and kept under dry ice vapor for all dissections. Coronal sections (1mm thick) were cut using a brain-slicing matrix (Braintree Scientific). Medial prefrontal cortex, whole NAC (shell and core), the hypothalamic suprachiasmatic and paraventricular region, CP (striatum) and VTA were identified by gross architectural landmarks (Paxinos and Franklin, 2004). Medial prefrontal cortex and the hypothalamic suprachiasmatic and paraventricular region were dissected using a 14-gauge tissue punch. NAC, CPA and the VTA were dissected using a 16-gauge tissue punch. Tissue was homogenized by passage through a 26½-gauge syringe in RNA-STAT60.
Quantitative PCR analysis
Total RNA was isolated from tissue using RNA-STAT60 reagent and RNA was reverse-transcribed into cDNA (Invitrogen). Gene expression was measured with an Applied Biosystems 7900HT Sequence Detection System using the ΔΔCT assay and normalized to GAPDH.
In situ hybridization analysis
Brains were dissected from male C57BL/6J mice, embedded in OCT compound (Sakura) and flash frozen in cooled isopentane. Coronal sections (14–16 μm) were cut using a cryostat (Leica) followed by fixation for 15 min at 4°C with 10% neutral buffered formalin. In situ hybridization was performed using the RNAscope 2.5 brown chromogenic assay pretreatment and detection kits (Advanced Cell Diagnostics). Probes for cyclophilin B (positive control), dapB (negative control) and Klb were purchased from Advanced Cell Diagnostics. Hybridized sections were counterstained with hematoxylin, dehydrated, cleared and mounted with Ecomount (Biocare Medical). Images were taken using a Zeiss Axioscan Z1 at 40X magnification. The signal from the mRNA was highlighted using the color threshold function in Image J.
Western blot analysis
Western blot analysis was performed using antibodies for total (Cell Signaling, #2792) and Ser40 phosphorylated tyrosine hydroxylase (AbCam, #51206). Data were acquired and quantified using an ImageQuant LAS 4000 and Multi Gauge v3.1 software (Fujifilm).
Dopamine measurements
Dopamine and its metabolites were measured by HPLC by the Vanderbilt Neurochemistry Core.
Mouse and monkey experiments with PF-05231023
All animal care and experimental procedures for studies involving PF-05231023 were conducted in compliance with the US Animal Welfare Act and the ILAR Guide for the Care and Use of Laboratory Animals, 1996. The procedures used in these studies were reviewed and approved by the Pfizer Institutional Animal Care and Use Committee (AUP# GTN-2013-00793).
Male C57BL/6J mice fed a 60% high fat diet (Research Diets D12492i) for 12–14 weeks were used for the saccharin studies. Mice were singly housed with access to regular water and 0.1% saccharin (S6047, Sigma-Aldrich, St. Louis, MO) water throughout the study. Water intake was monitored using a BioDaq system (Research Diets Inc., New Brunswick, NJ). The position of sweetened and unsweetened water bottles was switched each day to eliminate positional preference. Mice were acclimatized to the cage for 10–14 days, after which they were stratified based on body weight and baseline saccharin water intake. PF-05231023 (Huang et al., 2013; Weng et al., 2015) or vehicle were administered subcutaneously twice per week for a total of 3 doses at 10 mg/kg.
Male Macaca fascicularis (cynomolgus monkeys) from Charles River Labs were single-housed under a 12-hour light/dark cycle. Monkeys were between 7–24 years of age and weighed between 7–12 kg. Monkeys were provided standard lab diet 5K91 (LabDiet, St. Louis, MO) supplemented once a day with fruits and peanuts. The two-bottle saccharin preference assay was performed as described (Tordoff and Bachmanov, 2003). The monkeys underwent a training period during which the standard water system was shut off and 2 bottles, one with water and the other with water containing 0.2% saccharin, were attached side-by-side to the cage. To determine the amount water consumed, bottles were weighed before and after filling, with the difference in weight equated to the volume consumed. The position of sweetened and unsweetened water bottles was switched each day to eliminate positional preference. Several animals in the original cohort were eliminated from the study due to water bottle damage, failure to adapt to the water bottle or if one of the allocation endpoints represented an outlier.
Fifteen monkeys were divided into 2 groups based on body weight, triglyceride and fasted glucose levels and baseline daily food and water consumption. Following a baseline period, PF-05231023 or vehicle (30 mM lactate pH 4.8, 9% Trehalose, 0.05 mg/ml EDTA, 0.1 mg/ml L-methionine, 0.5 mg/ml Tween 20) was administered intravenously to 8 and 7 animals, respectively, on days 1, 4 and 7. Dosing was performed in a blinded manner. Water consumption was monitored at least twice daily.
Statistical analyses
All data are expressed as means ± S.E.M. Statistical analysis between the two groups was performed by unpaired two-tailed Student’s t test using Excel or GraphPad Prism (GraphPad Software, Inc.), or by using R software (Fraley et al., 2012; Pinheiro et al., 2013).
Supplementary Material
Acknowledgments
We thank Birgitte Andersen (Novo Nordisk) for providing recombinant FGF21, Yang Xie for assistance with the statistical analyses, and David Self for assistance with brain dissection. This work was supported by National Institutes of Health grants R01DK067158 (S.A.K. and D.J.M.), R01CA152301 (H.T.), NNX11AC54G (H.T.), 5P50 CA70907-16 (H.T.); the Robert A. Welch Foundation (grant I-1558 to S.A.K. and grant I-1275 to D.J.M.); the Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant Number 105545/Z/14/Z to B.M.O.); the Ford Foundation Fellowship (G.H.); the German National Science Foundation (DFG) grant MU 2789/8-1 and IZKF (Project E13) (C.P.M.); and the Howard Hughes Medical Institute (D.J.M.).
Footnotes
Author Contributions
S.T., B.M.O., P.S., G.H., and Y. Zhou designed, performed and analyzed experiments; Y. Zhang, and W.T.S. performed and analyzed experiments; B.P., T.T., A.S., and B.B. performed experiments; H.T. analyzed experiments; C.P.M. designed and analyzed experiments; B.G., S.A.K., and D.J.M. designed, supervised, and analyzed experiments and wrote the paper. P.S. and G.H. contributed equally. All authors commented and approved the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, Lawrence HL, Ding X, Elmquist JK, Takahashi JS, Mangelsdorf DJ, Kliewer SA. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nature Med. 2013;19:1147–1152. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camporez JP, Jornayvaz FR, Petersen MC, Pesta D, Guigni BA, Serr J, Zhang D, Kahn M, Samuel VT, Jurczak MJ, Shulman GI. Cellular mechanisms by which FGF21 improves insulin sensitivity in male mice. Endocrinology. 2013;154:3099–3109. doi: 10.1210/en.2013-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD. The mouse forced swim test. J Vis Exp. 2012a:e3638. doi: 10.3791/3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, Dao DT, Terrillion CE, Piantadosi SC, Bhat S, Gould TD. The tail suspension test. J Vis Exp. 2012b:e3769. doi: 10.3791/3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu AY, Workalemahu T, Paynter NP, Rose LM, Giulianini F, Tanaka T, Ngwa JS, Qi Q, Curhan GC, Rimm EB, Hunter DJ, Pasquale LR, Ridker PM, Hu FB, Chasman DI, Qi L. Novel locus including FGF21 is associated with dietary macronutrient intake. Human molecular genetics. 2013;22:1895–1902. doi: 10.1093/hmg/ddt032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong JQ, Rossulek M, Somayaji VR, Baltrukonis D, Liang Y, Hudson K, Hernandez-Illas M, Calle RA. Pharmacokinetics and pharmacodynamics of PF-05231023, a novel long-acting FGF21 mimetic, in a first-in-human study. Br J Clin Pharmacol. 2015 doi: 10.1111/bcp.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douris N, Stevanovic D, Fisher FM, Cisu TI, Chee MJ, Ly Nguyen N, Zarebidaki E, Adams AC, Kharitonenkov A, Flier JS, Bartness TJ, Maratos-Flier E. Central Fibroblast Growth Factor 21 Browns White Fat via Sympathetic Action in Male Mice. Endocrinology. 2015 doi: 10.1210/en.2014-2001. en20142001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dushay JR, Toschi E, Mitten EK, Fisher FM, Herman MA, Maratos-Flier E. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Molecular metabolism. 2015;4:51–57. doi: 10.1016/j.molmet.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernstrom JD, Munger SD, Sclafani A, de Araujo IE, Roberts A, Molinary S. Mechanisms for sweetness. J Nutr. 2012;142:1134S–1141S. doi: 10.3945/jn.111.149567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno RE, Moller DE. FGF21-based pharmacotherapy--potential utility for metabolic disorders. Trends in endocrinology and metabolism: TEM. 2014;25:303–311. doi: 10.1016/j.tem.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Giragossian C, Vage C, Li J, Pelletier K, Piche-Nicholas N, Rajadhyaksha M, Liras J, Logan A, Calle RA, Weng Y. Mechanistic Investigation of the Preclinical Pharmacokinetics and Interspecies Scaling of PF-05231023, a Fibroblast Growth Factor 21-Antibody Protein Conjugate. Drug Metab Dispos. 2015;43:803–811. doi: 10.1124/dmd.114.061713. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacology & therapeutics. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Hsuchou H, Pan W, Kastin AJ. The fasting polypeptide FGF21 can enter brain from blood. Peptides. 2007;28:2382–2386. doi: 10.1016/j.peptides.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Liang Q, Zhong L, Zhang J, Wang Y, Bornstein SR, Triggle CR, Ding H, Lam KS, Xu A. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes. 2014;63:4064–4075. doi: 10.2337/db14-0541. [DOI] [PubMed] [Google Scholar]
- Owen BM, Bookout AL, Ding X, Lin VY, Atkin SD, Gautron L, Kliewer SA, Mangelsdorf DJ. FGF21 contributes to neuroendocrine control of female reproduction. Nature Med. 2013;19:1153–1156. doi: 10.1038/nm.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen BM, Ding X, Morgan DA, Coate KC, Bookout AL, Rahmouni K, Kliewer SA, Mangelsdorf DJ. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 2014;20:670–677. doi: 10.1016/j.cmet.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen BM, Mangelsdorf DJ, Kliewer SA. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends in endocrinology and metabolism: TEM. 2015;26:22–29. doi: 10.1016/j.tem.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB. The mouse brain in stereotaxic coordinates. Gulf Professional Publishing; 2004. [Google Scholar]
- Sanchez J, Palou A, Pico C. Response to carbohydrate and fat refeeding in the expression of genes involved in nutrient partitioning and metabolism: striking effects on fibroblast growth factor-21 induction. Endocrinology. 2009;150:5341–5350. doi: 10.1210/en.2009-0466. [DOI] [PubMed] [Google Scholar]
- Sarruf DA, Thaler JP, Morton GJ, German J, Fischer JD, Ogimoto K, Schwartz MW. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes. 2010;59:1817–1824. doi: 10.2337/db09-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Ngwa JS, van Rooij FJ, Zillikens MC, Wojczynski MK, Frazier-Wood AC, Houston DK, Kanoni S, Lemaitre RN, Luan J, Mikkila V, Renstrom F, Sonestedt E, Zhao JH, Chu AY, Qi L, Chasman DI, de Oliveira Otto MC, Dhurandhar EJ, Feitosa MF, Johansson I, Khaw KT, Lohman KK, Manichaikul A, McKeown NM, Mozaffarian D, Singleton A, Stirrups K, Viikari J, Ye Z, Bandinelli S, Barroso I, Deloukas P, Forouhi NG, Hofman A, Liu Y, Lyytikainen LP, North KE, Dimitriou M, Hallmans G, Kahonen M, Langenberg C, Ordovas JM, Uitterlinden AG, Hu FB, Kalafati IP, Raitakari O, Franco OH, Johnson A, Emilsson V, Schrack JA, Semba RD, Siscovick DS, Arnett DK, Borecki IB, Franks PW, Kritchevsky SB, Lehtimaki T, Loos RJ, Orho-Melander M, Rotter JI, Wareham NJ, Witteman JC, Ferrucci L, Dedoussis G, Cupples LA, Nettleton JA. Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. Am J Clin Nutr. 2013;97:1395–1402. doi: 10.3945/ajcn.112.052183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Bachmanov AA. Influence of the number of alcohol and water bottles on murine alcohol intake. Alcohol Clin Exp Res. 2003;27:600–606. doi: 10.1097/01.ALC.0000060529.30157.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Y, Chabot JR, Bernardo B, Yan Q, Zhu Y, Brenner MB, Vage C, Logan A, Calle R, Talukdar S. Pharmacokinetics (PK), pharmacodynamics (PD) and integrated PK/PD modeling of a novel long acting FGF21 clinical candidate PF-05231023 in diet-induced obese and leptin-deficient obese mice. PLoS One. 2015;10:e0119104. doi: 10.1371/journal.pone.0119104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Liu Y, Xiao J, Liu L, Chen S, Mohammadi M, McClain CJ, Li X, Feng W. FGF21 mediates alcohol-induced adipose tissue lipolysis by activation of systemic release of catecholamine in mice. Journal of lipid research. 2015;56:1481–1491. doi: 10.1194/jlr.M058610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.