Abstract
Background
Triptans are commonly prescribed for migraine, a pain condition that is highly prevalent in women of childbearing age. No prior studies have investigated associations between exposure to triptans during fetal life and risk of externalizing and internalizing behaviors in children.
Methods
This study was set in the Norwegian Mother and Child Cohort study, a prospective birth cohort. 41,173 live, singleton births without major malformations present at 36-month post-partum follow-up were included in this study; 396 used a triptan during pregnancy, 798 used a triptan prior to pregnancy only, 3291 reported migraine without triptan use, and 36,688 reported no history of migraine or triptan use. Marginal structural models were used to analyze the association between timing of triptan exposure and neurodevelopmental outcome.
Results
Children exposed to triptans during pregnancy had a 1.39-fold increased risk of externalizing behaviors compared to those whose mothers used triptans prior to pregnancy only [95% CI: 0.97 to 1.97], a 1.36-fold increased risk compared to the unmedicated migraine group [95% CI: 1.02 to 1.81], and a 1.41- fold increased risk compared to the population comparison group [95% CI: 1.08 to 1.85]. The greatest risk was associated with first trimester exposure [RR: 1.77, 95% CI: 0.98, 3.14]. Risk differences were small, ranging from 3–6%.
Conclusions
This study found an increased risk of clinically-relevant externalizing behaviors in children with prenatal exposure to triptans, and this risk was highest for first trimester exposure. Absolute risks were small, and the results may be due to confounding by underlying migraine severity.
Introduction
Migraine affects between 18% and 22% of women and is most common in women of childbearing age.1 Triptans are among the most common prescription medications given for acute relief of migraine: in a prior study of triptan use among pregnant Norwegian women, triptans were used by 0.8% of the total population2 and by 25% of women with migraine.3 Studies of the safety of triptans during pregnancy have exclusively focused on outcomes occurring during pregnancy or in the immediate post-partum period, and a recent meta-analysis found that triptan exposure was associated with an increased risk of spontaneous abortion but not other outcomes.4
While less frequently examined in studies of medication safety in pregnancy, neurodevelopmental problems in childhood are a substantial source of morbidity in the population and an important public health concern.5 Triptans are serotonin 5-HT agonists that cross the placenta and blood-brain barrier,6 and may bind to 5-HT1B/D receptors which are found in fetal brain.7 While no studies exist in humans, animal studies suggest that manipulation of 5-HT1B/D receptors in the developing brain results in alterations of thalamocortical projections,8 and in turn, human studies have shown a link between attention-deficit/hyperactivity disorder (ADHD) and thalamocortical activation,9 indicating a biologically-plausible pathway through which triptans might exert a teratogenic effect on neurodevelopment. We sought to quantify the association of prenatal triptan exposure and behavioral problems in three-year-old children.
Methods
Study Sample
The Norwegian Institute of Public Health recruited participants into the Norwegian Mother and Child Cohort Study (MoBa)10 prior to routine ultrasound appointments (pregnancy weeks 13–17). Between 1999 and 2008, 108,841 women consented with 84.8% completing 6-month post-partum and and 60.2% completing 36-month post-partum.11 This analysis was granted an exemption from the University of Massachusetts Medical School Institutional Review Board. Data were taken from the quality-ensured Data Version 6, released by MoBa in 2012 and includes all children born before 2009 for whom the age 3 years questionnaire was received by May 4, 2011; these data were linked to the Medical Birth Registry of Norway (MBRN) using participants’ 11-digit personal identification numbers.
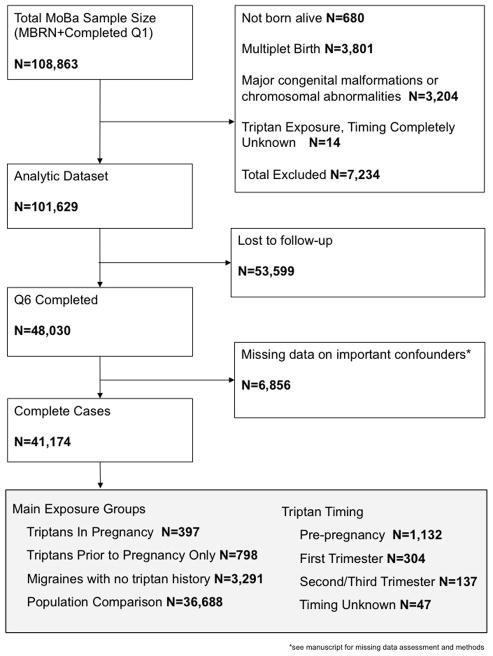
Because this study focused on infant neurodevelopment, we excluded infants not born alive (N=680), infants born with major congenital malformations or chromosomal abnormalities (N=3,204), and multiplet births (N=3,801). We excluded women who reported triptan exposure but did not report whether exposure occurred prior to or during pregnancy (N=14). Because missing data were low (<5%), we excluded 6,856 women with missing data on confounders. The initial study sample was 101,644 women. At the 36-month follow-up, 41,173 (40.5%) participants had complete data on the main outcome measure (Figure 1).
Figure 1.
Ascertainment of triptan exposure
Medication information was gathered from two prenatal (Q1, Q3) and one postpartum questionnaire (Q4); this information was reported before the outcome was known. Women indicated when they had taken a medication (during the six months before pregnancy, weeks 0–4, 5–8, 9–12, and/or 13 or later for Q1, during weeks 13–16, 17–20, 21–24, 25–28, and/or week 29 or later for Q3, and from week 30 until birth for Q4). Women who indicated multiple medications (e.g., sumatriptan and acetaminophen) were assumed to have been exposed to all listed medications in all time periods. The World Health Organization Anatomical Therapeutic Chemical (ATC) Classification System12 code N02CC identified triptans (sumatriptan, naratriptan, zolmitriptan, rizatriptan, almotriptan, eletriptan, and frovatriptan). No information was available on formulation or dose. Timing of exposure was collapsed into trimester categories (pre-pregnancy, first trimester, second/third trimester, use during pregnancy with unknown timing). Comparisons between self-reported medication exposure in the MoBa study and exposure ascertained via prescription redemption in the Prescription Drug Registry show acceptable sensitivity and high specificity,13 suggesting that women may forget medication use, but that any reported use is unlikely to be a false positive.
Ascertainment of outcome
The Child Behavior Checklist (CBCL), a validated, parent-reported measure of child behavior widely used in both clinical and research practice, was used to define neurodevelopmental outcomes at three years. A shortened version, validated in a Norwegian population,14 was used in MoBa; the majority of reporters were mothers. The externalizing (including the “attention problems” and “aggressive behavior” subscales) and internalizing (including the “emotionally reactive,” “anxious/depressed,” and “somatic complaints” subscales) domains were used; these domains have been shown to predict later psychopathology in children and adolescents.15,16 Standardized z-scores were computed for the study sample, and children with z-scores ≥ 1.5 (equivalent to a T-score of 65 or greater) were classified as having clinically significant behavior problems. This categorization is recommended by the developers of the CBCL instrument.17
Potential confounders and mediators
Confounders were identified through literature review and selected through iterative use of directed acyclic graphs (dagitty.net). Maternal age, pre-pregnancy BMI (underweight or <18.5 kg/m2, normal weight or 18.5–25 kg/m2, or overweight, >25 kg/m2 according to WHO guidelines), education (primary or secondary vs university or higher), marital status (married or cohabiting vs other), parity (multiparous vs. primiparous) were all ascertained by self-report on Q1. Smoking (ever during pregnancy vs. not during pregnancy) and alcohol use (ever during pregnancy vs. not during pregnancy) were ascertained by combining information from self-report and linkage to the Medical Birth Registry of Norway (MBRN). Maternal symptoms of depression and anxiety were assessed using the Symptom Checklist 5 (SCL-5) at Q1 and Symptom Checklist 8 (SCL-8) at Q3, two short versions of the SCL-25.18 The SCL-5 and SCL-8 are highly correlated with the SCL-25 and has been widely used in surveys of mental health. The SCL-8 is administered via self-report at Q1 and Q3. Standardized z-scores were computed at each time point, and an average SCL score was used in the models.
Because migraine and depression have significant shared genetic susceptibility,19 and migraineurs who take triptans also use other medications, some of which have been associated with neurodevelopmental problems in children,20–23 obtaining unbiased estimates of the effect of prenatal triptan exposure on neurodevelopment requires consideration of underlying disease severity and concomitant medication use. Women were asked about history of migraine at Q1 only, and were classified as having migraine headache if they reported having migraines or had taken migraine medications. Other medications considered as potential confounders were psychotropic medications, including antidepressants, benzodiazepines, and antiepileptics. All co-medications were categorized both as ever vs. never used in pregnancy and according to timing (pre-pregnancy, first trimester, second/third trimester). Because acetaminophen has been linked to child neurodevelopment in this cohort,20 we considered acetaminophen exposure as a three-category variable: no trimesters exposed, one trimester exposed, or two or more trimesters exposed.
Statistical methods
First, we estimated the prevalence of triptan use overall and by trimester and evaluated descriptive statistics by triptan use to better understand the population and factors that may confound the triptan-neurodevelopmental relationship, including prevalence of comedication in triptan users and non-users throughout pregnancy. Two modeling approaches were applied: multivariable-adjusted Poisson models and marginal structural models. We conducted sensitivity analyses to understand the possible impact of residual confounding by acetaminophen use and unmeasured confounding by migraine severity. To address the former concern, we repeated our main analyses in a subgroup of women who did not report acetaminophen exposure during pregnancy (n=22581). To address confounding by migraine severity which was not measured in MoBa, we used probabilistic bias analysis to quantify the possible bias introduced by migraine severity, using methods developed by Lash, Fox, and Fink.24 We used prevalence estimates for severe migraine ranging from 0.20 to 0.40,25 and assumed that severe migraine was twice as prevalent in triptan users as in women who discontinued triptan use before pregnancy. We allowed the association between migraine severity and behavioral problems to vary between 1.25 and 4.00, and conducted 10,000 simulations for each combination of bias parameters.
Poisson Models
Modified Poisson models provided risk ratios and risk difference estimates for the association between triptan exposure and behavioral problems in children prenatally exposed to triptans compared to two disease comparison groups (children born to women who (a) discontinued triptans prior to pregnancy or (b) reported migraine without triptan history) and a population comparison group. We calculated 95% confidence intervals (CI) using robust standard errors. Models were adjusted for maternal age, pre-pregnancy BMI, parity, marital status, education, co-medication use (NSAIDs, acetaminophen, opioids, and antidepressants), depression/anxiety symptoms, smoking, and alcohol use. We examined the risk of triptan exposure for prenatal, first trimester, and second/third trimester exposure within a subsample of women who reported a history of migraine headache.
Marginal Structural Models
Triptan medications are taken in response to an oncoming migraine attack, and as such their use will change over time during pregnancy: a migraineur may take a triptan only prior to pregnancy, during the first trimester, during later trimesters, or at multiple times, and her use of triptans may be confounded by other factors, such as concomitant medication use, which may change over time . For example, first trimester triptan exposure might be associated with second trimester acetaminophen use, which in turn could be associated with third trimester triptan use (Supplemental Figure 1). In this case, acetaminophen is both a confounder and a mediator: if acetaminophen is associated with increased risk of behavioral problems, then failing to adjust for acetaminophen use will cause an overestimate of the effects of triptan exposure, but adjusting for acetaminophen use will result in a bias of the effect estimate towards the null. The MSM approach has the effect of achieving balance of confounders within strata of exposure; if the confounders are also mediators (see causal diagram Supplemental Figure 1 for illustration), this approach may reduce bias induced by inappropriate control for an effect on the causal pathway. To adjust for time-varying confounders that are also mediators, we performed a marginal structural model analysis using methods first described by Robins & Hernan.26,27 We constructed stabilized inverse probability of treatment weights (IPTWs) via logistic regression,28 in which exposure at each time point (pre-pregnancy, first trimester, second/third trimester) was the dependent variable and predictors in the model included baseline confounders (maternal age, pre-pregnancy BMI, education, marital status), time-invariant confounders (smoking and alcohol use during pregnancy, severity of depressive symptoms in pregnancy), time-varying confounders (other medication use) and triptan history. The MSM approach produces unbiased effect estimates if the assumptions of consistency, exchangeability and positivity are met, although the reduction in bias comes with an increase in variance.28 While not formally testable, we took steps to evaluate whether the assumptions of exchangeability and positivity were met. To assess positivity, which requires that all individuals in the sample have a non-zero probability of exposure, we limited MSM analyses to women with a reported history of migraine, reasoning that triptan medications are only prescribed for migraine headache, and inclusion of non-migraineurs could result in structural zeroes. To examine the impact of unmeasured confounding by migraine severity, we compared concomitant medication use rates of women who took triptans during pregnancy compared to women who discontinued use of triptans, or those who had no history of triptan use, and conducted sensitivity analyses to assess the impact of unmeasured confounding.
After constructing stabilized weights, we fit a weighted Poisson model with robust standard errors to account for clustering induced by weighting.
Results
Prevalence of triptan use during pregnancy was 1.1% and 3.0% prior to pregnancy, with 10.9% reporting a history of migraine prior to pregnancy or up to the 13th week of pregnancy (Q1). Among those women who reported triptan use, 94.6% used triptans prior to pregnancy, 25.6% used triptans in the first trimester, and 10.6% reported use in the second or third trimesters. Women were further classified into four exposure groups: triptan exposure during pregnancy (N=396), six months prior to pregnancy but not during pregnancy (N=798), history of migraine without triptan use (N=3,291), and a population comparison group in which no history of migraine or triptan use was reported (N=36,688). Among women with a history of triptan use, women who used triptans during pregnancy were slightly older and were more likely to be multiparous. In addition, they had higher rates of pre-pregnancy opioids, antidepressant, and benzodiazepine use, and were more likely to use opioids, acetaminophen, NSAIDs, antidepressants, and benzodiazepines during pregnancy, compared to women who discontinued triptan use during pregnancy. Women who took triptans during pregnancy had higher rates of pre-eclampsia. Infants prenatally exposed to triptans were more likely to be born preterm and to have a five-minute Apgar score below seven (Table 1).
Table 1.
Maternal characteristics, medication use, and immediate birth outcomes of 41,173 included pregnancies
| History of triptan use | No triptan history | |||
|---|---|---|---|---|
| During pregnancy N=396 | Pre-pregnancy Only N=798 | Migraine N=3,291 | N=36,688 | |
| Age in years (Mean, SD) | 30.8(4.4) | 30.5(4.5) | 30.3(4.5) | 30.4(4.4) |
| BMI (kg/m2) | ||||
| <18.5 | 2.81 | 2.4 | 3.7 | 2.6 |
| 18.5–25 | 59.6 | 59.9 | 61.8 | 66.9 |
| >25 | 37.6 | 37.2 | 34.5 | 30.5 |
| Multiparous | 49.2 | 45.9 | 53.5 | 53.4 |
| Married or cohabitating | 95.5 | 98.0 | 97.0 | 97.6 |
| Mother Education | ||||
| Primary or secondary | 31.1 | 31.8 | 37.0 | 31.0 |
| University or higher | 68.9 | 68.2 | 63.0 | 69.0 |
| Smoking during pregnancy | 11.4 | 11.7 | 13.1 | 11.1 |
| Alcohol during pregnancy | 20.5 | 15.0 | 15.0 | 17.1 |
| Folate Supplementation | 60.9 | 62.2 | 57.8 | 59.2 |
| Multivitamin Supplementation | 37.9 | 43.7 | 38.9 | 37.0 |
| Migraine Preventive Therapy | 1.8 | 1.8 | 0.6 | 0.0 |
| Opioids | ||||
| Pre-pregnancy | 8.1 | 5.3 | 5.1 | 1.3 |
| In pregnancy | 12.9 | 4.6 | 4.9 | 1.4 |
| Acetaminophen | ||||
| Pre-pregnancy | 46.5 | 47.9 | 44.8 | 25.4 |
| In pregnancy | 76.8 | 70.1 | 63.7 | 42.6 |
| NSAIDs | ||||
| Pre-pregnancy | 22.0 | 25.9 | 23.3 | 9.9 |
| In pregnancy | 22.5 | 11.2 | 12.6 | 5.9 |
| Anti-convulsants | ||||
| Pre-pregnancy | 0.5 | 0.5 | 0.2 | 0.1 |
| In pregnancy | 0.3 | 0.3 | 0.3 | 0.2 |
| Antidepressants | ||||
| Pre-pregnancy | 5.3 | 4.6 | 3.8 | 2.2 |
| In pregnancy | 2.0 | 1.1 | 1.6 | 0.9 |
| Benzodiazepines | ||||
| Pre-pregnancy | 1.8 | 1.4 | 0.9 | 0.5 |
| In pregnancy | 1.8 | 0.5 | 0.5 | 0.4 |
| Maternal depressive/anxiety symptoms1 (Mean, SD) | 0.2(1.7) | 0.0(1.8) | 0.2(1.9) | −0.2(1.6) |
| Pre-eclampsia | 7.8 | 4.3 | 4.0 | 3.5 |
| Small for Gestational Age | 6.6 | 6.3 | 6.5 | 6.2 |
| Apgar 5 (<7) | 1.5 | 1.0 | 0.7 | 0.9 |
| Preterm | 4.0 | 4.6 | 4.8 | 4.6 |
| Low Birth Weight | 2.0 | 2.3 | 2.6 | 2.4 |
| Externalizing Problems (z ≥ 1.5) | 11.6 | 8.3 | 9.1 | 7.7 |
| Internalizing Problems (z ≥ 1.5) | 6.8 | 8.6 | 7.7 | 6.2 |
Figures shown are percent of column total with the exception of maternal age and depressive/anxiety symptom severity, presented as mean(standard deviation)
Sumatriptan was by far the most commonly used medication, followed by zolmitriptan and rizatriptan (Table 2). The rank order of medication did not vary by time of use during pregnancy.
Table 2.
Specific triptan medications used before and during pregnancy
| Pre-pregnancy N=1,131 | First Trimester N=304 | Second/third Trimester N=137 | Pregnancy Use (TU)2 N=46 | |
|---|---|---|---|---|
| Sumatriptan | 44.31 | 47.2 | 50.4 | 47.8 |
| Rizatriptan | 25.8 | 23.4 | 27.8 | 23.9 |
| Zolmitriptan | 17.3 | 16.8 | 13.9 | 26.1 |
| Eletriptan | 11.8 | 11.2 | 6.6 | 4.3 |
| Naratriptan | 2.5 | 3.3 | 5.8 | 2.2 |
| Almotriptan | 2.6 | 2.6 | 1.5 | 6.5 |
Column percentages sum to greater than 100% due to use of multiple triptans during the study period. Number of triptan users diverge from those shown on Table 1: of 1131 pre-pregnancy users, 798 used only prior to pregnancy and 333 used both before and during pregnancy.
“Pregnancy Use TU” refers to women who used a triptan in pregnancy but did not provide enough information to determine timing; these women were included in the main group analyses and excluded from timing models.
Externalizing behaviors
Externalizing symptoms at or above a clinical cutoff of T≥65 were present in 11.6% of children exposed to triptans in utero, compared to 8.3% in children whose mothers use triptans prior to pregnancy only, 9.1% in children whose mothers reported migraine with no triptan use, and 7.7% in a population comparison group (Supplemental Figure 2A). Table 3 shows that children whose mothers used triptans during pregnancy had a 36% increased risk of externalizing behaviors compared to a population comparison group [RRadj: 1.36, 95% CI: 1.04 to 1.79]. We observed similar risks when comparing children with prenatal triptan exposure to children whose mothers reported migraines but no triptan use [RRadj: 1.33, 95% CI: 1.00 to 1.78] and to children whose mothers used triptans prior to pregnancy only [RRadj: 1.36, 95% CI: 0.96 to 1.94]. The risk of externalizing problems was highest in children exposed to triptans in the first trimester (13.2%) and lowest in women reporting use in 2nd and 3rd trimester (8.0%) (Supplemental Figure 2B). Examining risks of externalizing behavior associated with windows of exposure to triptans among women with a history of migraine (N=4,439), first trimester exposure was associated with a 77% increased risk in multivariable-adjusted Poisson models [95% CI: 1.23 to 3.56], while no increased risk was observed for pre-pregnancy exposure. Estimates of effect from marginal structural models were similar, although 95% confidence intervals were considerably wider: first trimester exposure was associated with a 75% increased risk of externalizing symptoms in the clinical range [RRMSM: 1.75, 95% CI: 0.98 to 3.14], while pre-pregnancy [RRMSM: 0.99, 95% CI: 0.77 to 1.27] and second/third trimester [RRMSM: 0.59, 95% CI: 0.23 to 1.51] exposure showed no evidence of increased risk.
Table 3.
Comparison of risk of clinically-significant externalizing behavior problems for exposure to triptans
| Among all mothers (n=41,173)
| ||||||
|---|---|---|---|---|---|---|
| Total N | N with outcome | Crude Risk Ratio (95% Confidence Interval) | Adjusted Risk Ratio1 (95% Confidence Interval) | Crude Risk Difference(95% Confidence Interval) | Adjusted Risk Difference1(95% Confidence Interval) | |
| Triptans in pregnancy | 396 | 46 | 1.40 [0.98, 2.00] | 1.36 [0.96, 1.94] | 0.03 [0.00, 0.07] | 0.02 [−0.01, 0.06] |
| Vs.Triptans pre-pregnancy only | 798 | 66 | Referent | Referent | Referent | Referent |
| Triptans in pregnancy | 396 | 46 | 1.32 [0.98, 1.77] | 1.33 [1.00, 1.78] | 0.03 [0.00, 0.06] | 0.02 [−0.01, 0.05] |
| Vs. Migraine with no triptan use | 3,291 | 289 | Referent | Referent | Referent | Referent |
| Triptans in pregnancy | 396 | 46 | 1.50 [1.14, 1.98] | 1.36 [1.04, 1.79] | 0.04 [0.01, 0.07] | 0.02 [−0.01, 0.05] |
| Vs. Population (no triptan use or migraine) | 36,688 | 2,828 | Referent | Referent | Referent | Referent |
|
| ||||||
| Among mothers with migraine (n=4,439) | ||||||
|
| ||||||
| Total N | N with outcome | Crude Risk Ratio (95% Confidence Interval) | Marginal Structural Model Risk Ratio2 (95% Confidence Interval) | Crude Risk Difference(95% Confidence Interval) | Marginal Structural Model Risk Difference2 (95% Confidence Interval) | |
|
| ||||||
| Pre- pregnancy triptan use | 1,085 | 101 | 0.94 [0.73, 1.20] | 0.99 [0.77, 1.27] | −0.02 [−0.05, 0.01] | −0.02 [−0.05, 0.00] |
| Vs. No pre-pregnancy use | 3,354 | 297 | Referent | Referent | Referent | Referent |
| Triptans in 1st trimester | 304 | 40 | 1.77 [1.23, 2.56] | 1.75 [0.98, 3.14] | 0.06 [0.01, 0.10] | 0.06 [−0.02, 0.15] |
| Vs. No triptan use in 1st trimester | 4,135 | 358 | Referent | Referent | Referent | Referent |
| Triptans in 2nd /3rd trimester | 137 | 11 | 0.64 [0.34, 1.19] | 0.59 [0.23, 1.51] | −0.02 [−0.07, 0.02] | −0.02 [−0.11, 0.07] |
| Vs. No triptan use in 2nd/3rd trimester | 4,302 | 387 | Referent | Referent | Referent | Referent |
Models adjusted for maternal age, pre-pregnancy BMI, parity, marital status, education, cigarette and alcohol use, comedication use (NSAIDs, acetaminophen, opioids, antidepressants), depressive and anxiety symptoms
Marginal structural models weighted with stabilized inverse probability of treatment weights, constructed at each time point using baseline confounders, time-invariant confounders, and medication history.
The absolute risk for first trimester triptan exposure was 6% [95% CI: −0.02 to 0.15], equivalent to a number needed to harm of 17.
Internalizing behaviors
Fewer children exhibited clinically significant internalizing symptoms than externalizing symptoms (Supplemental Figures 2A and 2B). Comparing children born to women who used triptans during pregnancy to those whose mothers used triptans prior to pregnancy only [RRadj: 0.78, 95% CI: 0.51 to 1.21], those with migraine but no triptan use [RRadj: 0.89, 95% CI: 0.61 to 1.30], and a population comparison group [RRadj: 1.02, 95% CI: 0.71 to 1.47] revealed no increased risks of internalizing behavior problems in the clinical range; absolute risks were zero or near zero for all comparisons (Table 4). Estimates of the effect of triptan exposure at specific times revealed inconsistencies between multivariable-adjusted Poisson models and marginal structural models (Supplemental Table 1). Multivariable models showed an increased risk of internalizing symptoms associated with pre-pregnancy exposure [RRadj: 1.27, 95% CI: 1.01 to 1.59] but not during pregnancy; MSM estimates showed no association between pre-pregnancy exposure and internalizing behaviors [RRMSM: 1.04, 95% CI: 0.80 to 1.35]. Absolute risks were near zero, with 95% confidence intervals that included the null.
Table 4.
Comparison of risk of clinically-significant internalizing behavior problems for exposure to triptans
| Among all mothers (n=41,173)
| ||||||
|---|---|---|---|---|---|---|
| Total N | N with outcome | Crude Risk Ratio (95% Confidence Interval) | Adjusted Risk Ratio1 (95% Confidence Interval) | Crude Risk Difference (95% Confidence Interval) | Adjusted Risk Difference1 (95% Confidence Interval) | |
| Triptans in pregnancy | 396 | 27 | 0.79 [0.51, 1.21) | 0.78 [0.51, 1.19] | −0.02 [−0.05, 0.01] | −0.02 [−0.05, 0.01] |
| Vs.Triptans pre-pregnancy only | 798 | 69 | Referent | Referent | Referent | Referent |
| Triptans in pregnancy | 396 | 27 | 0.88 [0.60, 1.29) | 0.89 [0.61, 1.30] | −0.01 [−0.04, 0.02] | −0.01 [−0.03, 0.01] |
| Vs. Migraine with no triptan use | 3,291 | 255 | Referent | Referent | Referent | Referent |
| Triptans in pregnancy | 396 | 27 | 1.09 [0.78, 1.58) | 1.02 [0.71, 1.47] | 0.01 [−0.02, 0.03] | 0.00 [−0.03, 0.02] |
| Vs. Population (no triptan use or migraine) | 36,688 | 2284 | Referent | Referent | Referent | Referent |
|
| ||||||
| Among mothers with migraine (n=4,439) | ||||||
|
| ||||||
| Total N | N with outcome | Crude Risk Ratio (95% Confidence Interval) | Marginal Structural Model Risk Ratio2 (95% Confidence Interval) | Crude Risk Difference (95% Confidence Interval) | Marginal Structural Model Risk Difference2 (95% Confidence Interval) | |
|
| ||||||
| Pre- pregnancy triptan use | 1,085 | 86 | 1.32 [1.04, 1.66] | 1.04 [0.80, 1.35] | 0.02 [0.00, 0.04] | 0.00 [−0.02, 0.03] |
| Vs.no pre-pregnancy | 3,354 | 260 | Referent | Referent | Referent | Referent |
| Triptans in 1st trimester | 304 | 20 | 0.90 [0.54, 1.48] | 1.27 [0.57, 2.82] | −0.01 [−0.04, 0.03] | 0.02 [−0.06, 0.10] |
| Vs. No triptan use in 1st trimester | 4,135 | 326 | Referent | Referent | Referent | Referent |
| Triptans in 2nd /3rd trimester | 137 | 7 | 0.69 [0.32, 1.50] | 0.70 [0.16, 3.14] | −0.02 [−0.07, 0.02] | −0.02 [−0.11, 0.07] |
| Vs. No triptan use in 2nd/3rd trimester | 4,302 | 339 | Referent | Referent | Referent | Referent |
Models adjusted for maternal age, pre-pregnancy BMI, parity, marital status, education, cigarette and alcohol use, comedication use (NSAIDs, acetaminophen, opioids, antidepressants), depressive and anxiety symptoms.
Marginal structural models weighted with stabilized inverse probability of treatment weights, constructed at each time point using baseline confounders, time-invariant confounders, and medication history.
Sensitivity Analysis
An analysis of women who did not use acetaminophen during pregnancy revealed similar point estimates to the main set of analyses; however, the smaller sample size, which included only nine triptan-exposed children with externalizing behavior problems and six with internalizing problems, resulted in wider confidence intervals that included the null (results not shown). Probabilistic bias analysis showed that, using bias parameters that we considered reasonable based on existing literature (an association between migraine severity and externalizing symptoms of RR=1.50, prevalence of severe migraine of 40% in triptan users and 20% in triptan discontinuers), the association between triptan use and externalizing symptoms was slightly reduced [RR: 1.40 vs. bias-corrected RR: 1.29]. To completely explain the increased risk associated with triptan use, the association between migraine severity and externalizing behavior would have had to have been at least 3.0 (Supplemental Table 2).
Comments
We observed a consistent, near-40% increased risk of externalizing behavior problems in the clinical range among children born to mothers who used triptans during pregnancy, compared to two disease comparison groups and a population comparison group. The absolute risks were small and associated primarily with first trimester triptan use. No increased risk of internalizing symptoms was noted.
The increasing prevalence of neurodevelopmental and psychiatric disorders in children29,30 poses a serious public health challenge. Identifying antecedents of these disorders is vital both for understanding the pathophysiologic basis of disease, and providing opportunities for intervention. Externalizing disorders in very young children have high diagnostic stability into school age,31,32 and are predictive of major mental illness later in life. Risk factors for externalizing symptoms, therefore, can give important insights into the origins of diseases that result in substantial impairment throughout the life course.
In studies of the risks of prenatal exposure to medication, confounding by indication is a concern, especially when the indication for which the medication was prescribed is heritable33 and suspected to have direct effects on fetal development,34 as is the case with migraine. In this study, women who take a medication during pregnancy may have a more severe course of migraine than those who discontinue triptans before pregnancy. Lacking information on migraine severity (unavailable in the MoBa study), we identified multiple comparison groups: a group of women who discontinued triptan use prior to pregnancy, a group with migraine headache but no history of triptan use, and a population comparison group with no migraine or triptan history. If these groups are proxies for migraine severity, and if the observed effects are due to migraine severity, we would expect to see the highest risk of behavior problems in the women who used triptans in pregnancy, and progressively lower risks in triptan discontinuers and women with migraine but no triptan history. No such relationship was observed for externalizing symptoms; rather, we noted a modest but consistent elevated risk for prenatal triptan exposure compared to all other comparison groups, and no elevated risk for externalizing behaviors in the migraine groups that did not report triptan use. The results of probabilistic bias analysis suggest that there would need to be a substantial association between migraine severity and externalizing behaviors (RR>3.0) to fully explain the observed association between triptan exposure and externalizing symptoms.
This study is the first to examine risks of neurodevelopmental problems associated with prenatal triptan exposure, and has several important strengths. The study is based in a large, prospective birth cohort in which extensive medication data were available, including information on use of over-the-counter medications such as acetaminophen. Increased risks of externalizing and/or ADHD-like symptoms in children prenatally exposed to acetaminophen20,21 and antidepressants have been shown,22 making it particularly important to appropriately adjust for these potential confounders. We used causal inference methodology to quantify the risks associated with prenatal exposure to medications. As the brain develops throughout the entire pregnancy and onwards, the entire pregnancy is considered a vulnerable period. Consequently, when studying neurodevelopmental effects of prenatal exposure to medications, carefully modeling both time-varying exposure and confounding is necessary to avoid biased effect estimates. These innovations are particularly important in light of the high rates of concomitant medication use observed among women who took a triptan during pregnancy.
Although nearly one in four women with migraine reported using triptans either prior to or during pregnancy, the overall risk of migraine was lower than expected (10%, versus population estimates of 18–22%35,1). While the MoBa sample includes nearly 40% of births in Norway during the study period, MoBa participants are healthier than the general population.10 Women with more severe migraine and higher rates of triptan use may not have participated in this study, limiting its generalizability. Despite the large sample size, the low prevalence of triptan use limited our ability to examine effects of specific triptans, dose or formulation. While it is reassuring that few women require triptan therapy during pregnancy, replication of these findings in populations with higher rates of medication use is necessary. Confounding by indication may be present, although sensitivity analyses suggest this would have to be an extremely strong effect to fully account for the observed risks. Additionally, loss to follow-up may have introduced selection bias. Our results should be interpreted cautiously, with these strengths and limitations in mind.
Prenatal exposure to triptan medications was associated with a modest increased risk of externalizing behaviors in three-year-old children, with the most pronounced risk associated with exposure in the first trimester. These finding should be interpreted with caution and replicated in other birth cohorts before conclusions about causation are drawn.
Supplementary Material
Acknowledgments
The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and the Ministry of Education and Research, NIH/NIEHS (contract no N01-ES-75558), NIH/NINDS (grant no.1 UO1 NS 047537-01 and grant no.2 UO1 NS 047537-06A1). We are grateful to all the participating families in Norway who take part in this on-going cohort study.
Footnotes
We provide several supplemental tables and figures, which will aid in interpreting our findings in the appropriate context. Supplemental Table 1 includes results from the regression-adjusted multivariable models as well as the unadjusted and marginal structural models, to allow readers to compare the results obtained from all methods used. Supplemental Table 2 contains results from a probabilistic bias analysis, in which we assess the potential impact of unmeasured confounding by migraine severity. Supplemental Figure 1 is a possible causal model used to illustrate the time-varying exposure and confounding that may be present in our study. Supplemental Figure 2 provides information about the prevalence of behavioral problems in our sample, in order to contextualize the magnitude of the problem.
References
- 1.MacGregor EA. Headache in pregnancy. Neurol Cinics. 2012;30(3):835–66. doi: 10.1016/j.ncl.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Nezvalová-Henriksen K, Spigset O, Nordeng H. Triptan safety during pregnancy: A Norwegian population registry study. Eur J Epidemiol. 2013;28:759–769. doi: 10.1007/s10654-013-9831-x. [DOI] [PubMed] [Google Scholar]
- 3.Nezvalova-Henriksen K, Spigset O, Nordeng H. Maternal characteristics and migraine pharmacotherapy during pregnancy: cross-sectional analysis of data from a large cohort study. Cephalalgia. 2009;29(12):1267–1276. doi: 10.1111/j.1468-2982.2009.01869.x. [DOI] [PubMed] [Google Scholar]
- 4.Marchenko A, Etwel F, Olutunfese O, Nickel C, Koren G, Nulman I. Pregnancy Outcome Following Prenatal Exposure to Triptan Medications: A Meta-Analysis. Headache J Head Face Pain. 2015 doi: 10.1111/head.12500. n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 5.Sices L, Harman JS, Kelleher KJ. Health-care use and expenditures for children in special education with special health-care needs: is dual classification a marker for high use? Public Health Rep. 2007;122(4):531–40. doi: 10.1177/003335490712200415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tfelt-Hansen PC. Does sumatriptan cross the blood-brain barrier in animals and man? J Headache Pain. 2010;11(1):5–12. doi: 10.1007/s10194-009-0170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonaventure P, Schotte A, Cras P, Leysen JE. Autoradiographic mapping of 5-HT1B- and 5-HT1D receptors in human brain using [3H]alniditan, a new radioligand. Receptors Channels. 1997;5(3–4):225–30. [PubMed] [Google Scholar]
- 8.Bonnin A, Torii M, Wang L, Rakic P, Levitt P. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci. 2007;10(5):588–597. doi: 10.1038/nn1896. [DOI] [PubMed] [Google Scholar]
- 9.Clerkin SM, Schulz KP, Berwid OG, et al. Thalamo-cortical activation and connectivity during response preparation in adults with persistent and remitted ADHD. Am J Psychiatry. 2013;170(September):1011–1019. doi: 10.1176/appi.ajp.2013.12070880. [DOI] [PubMed] [Google Scholar]
- 10.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35(5):1146–50. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 11.Stoltenberg C, Schjølberg S, Bresnahan M, et al. The Autism Birth Cohort (ABC): a paradigm for gene-environment-timing research. Mol Psychiatry. 2010;15(7):676–680. doi: 10.1038/mp.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Classifications: The anatomical-therapeutic chemical classification system with defined daily doses (ATC/DDD) World Heal Organ. 2012 Available at: www.who.int/classifications/atcddd/en.
- 13.Skurtveit S, Selmer R, Tverdal A, Furu K, Nystad W, Handal M. Drug exposure: inclusion of dispensed drugs before pregnancy may lead to underestimation of risk associations. J Clin Epidemiol. 2013;66(9):964–72. doi: 10.1016/j.jclinepi.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Nøvik TS. Validity of the Child Behaviour Checklist in a Norwegian sample. Eur Child Adolesc Psychiatry. 1999;8(4):247–54. doi: 10.1007/s007870050098. [DOI] [PubMed] [Google Scholar]
- 15.Petty CR, Rosenbaum JF, Hirshfeld-Becker DR, et al. The Child Behavior Checklist broad-band scales predict subsequent psychopathology: a five-year follow-up. J Anxiety Disord. 2008;22(3):532–539. doi: 10.1016/j.janxdis.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathyssek CM, Olino TM, Verhulst FC, van Oort FVA. Childhood internalizing and externalizing problems predict the onset of clinical panic attacks over adolescence: the TRAILS study. PLoS One. 2012;7(12):e51564. doi: 10.1371/journal.pone.0051564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms & Profiles. Burlington, VT: 2000. [Google Scholar]
- 18.Fink P, Ørnbøl E, Hansen MS, Søndergaard L, De Jonge P. Detecting mental disorders in general hospitals by the SCL-8 scale. J Psychosom Res. 2004;56(3):371–375. doi: 10.1016/S0022-3999(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 19.Marino E, Fanny B, Lorenzi C, et al. Genetic bases of comorbidity between mood disorders and migraine: possible role of serotonin transporter gene. Neurol Sci. 2010;31(3):387–91. doi: 10.1007/s10072-009-0183-y. [DOI] [PubMed] [Google Scholar]
- 20.Brandlistuen RE, Ystrom E, Nulman I, Koren G, Nordeng H. Prenatal paracetamol exposure and child neurodevelopment: a sibling-controlled cohort study. Int J Epidemiol. 2013;42:1702–13. doi: 10.1093/ije/dyt183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liew Z, Ritz B, Rebordosa C, Lee P-C, Olsen J. Acetaminophen Use During Pregnancy, Behavioral Problems, and Hyperkinetic Disorders. JAMA Pediatr. 2014;168(4):313–320. doi: 10.1001/jamapediatrics.2013.4914. [DOI] [PubMed] [Google Scholar]
- 22.Oberlander TF, Reebye P, Misri S, Papsdorf M, Kim J, Grunau RE. Externalizing and attentional behaviors in children of depressed mothers treated with a selective serotonin reuptake inhibitor antidepressant during pregnancy. Arch Pediatr Adolesc Med. 2007;161(1):22–9. doi: 10.1001/archpedi.161.1.22. [DOI] [PubMed] [Google Scholar]
- 23.Oberlander TF, Papsdorf M, Brain UM, Misri S, Ross C, Grunau RE. Prenatal effects of selective serotonin reuptake inhibitor antidepressants, serotonin transporter promoter genotype (SLC6A4), and maternal mood on child behavior at 3 years of age. Arch Pediatr Adolesc Med. 2010;164(5):444–451. doi: 10.1001/archpediatrics.2010.51. [DOI] [PubMed] [Google Scholar]
- 24.Lash TL, Fox MP, Fink AK. Applying Quantitative Bias Analysis to Epidemiologic Data. Springer; 2009. [Google Scholar]
- 25.Smitherman TA, Burch R, Sheikh H, Loder E. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53(3):427–36. doi: 10.1111/head.12074. [DOI] [PubMed] [Google Scholar]
- 26.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11(5):561–70. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Robins JM, Hernan M, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–64. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutter M. Incidence of autism spectrum disorders: changes over time and their meaning. Acta Paediatr. 2005;94(1):2–15. doi: 10.1111/j.1651-2227.2005.tb01779.x. [DOI] [PubMed] [Google Scholar]
- 30.Visser SN, Danielson ML, Bitsko RH, et al. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003–2011. J Am Acad Child Adolesc Psychiatry. 2014;53(1):34–46. e2. doi: 10.1016/j.jaac.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lavigne JV, Arend R, Rosenbaum D, Binns HJ, Christoffel KK, Gibbons RD. Psychiatric disorders with onset in the preschool years: I. Stability of diagnoses. J Am Acad Child Adolesc Psychiatry. 1998;37(12):1246–54. doi: 10.1097/00004583-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Beyer T, Postert C, Müller JM, Furniss T. Prognosis and continuity of child mental health problems from preschool to primary school: results of a four-year longitudinal study. Child Psychiatry Hum Dev. 2012;43:533–43. doi: 10.1007/s10578-012-0282-5. [DOI] [PubMed] [Google Scholar]
- 33.Cox HC, Lea RA, Bellis C, et al. Heritability and genome-wide linkage analysis of migraine in the genetic isolate of Norfolk Island. Gene. 2012;494(1):119–23. doi: 10.1016/j.gene.2011.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arruda MA, Bigal ME. Migraine and behavior in children: influence of maternal headache frequency. J Headache Pain. 2012;13(5):395–400. doi: 10.1007/s10194-012-0441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343–9. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.