Abstract
Objectives:
Platelet preparations are commonly used to enhance bone and soft tissue regeneration. Considering the existing controversies on the efficacy of platelet products for tissue regeneration, more in vitro studies are required. The aim of the present study was to compare the in vitro effects of plasma rich in growth factors (PRGF) and platelet-rich fibrin (PRF) on proliferation and viability of human gingival fibroblasts (HGFs).
Materials and Methods:
Anitua’s PRGF and Choukran’s PRF were prepared according to the standard protocols. After culture periods of 24, 48 and 72 hours, proliferation of HGFs was evaluated by the methyl thiazol tetrazolium assay. Statistical analysis was performed using one-way ANOVA followed by Tukey-Kramer’s multiple comparisons and P-values<0.05 were considered statistically significant.
Results:
PRGF treatment induced statistically significant (P<0.001) proliferation of HGF cells compared to the negative control (100% viability) at 24, 48 and 72 hours in values of 123%±2.25%, 102%±2.8% and 101%±3.92%, respectively. The PRF membrane treatment of HGF cells had a statistically significant effect on cell proliferation (21%±1.73%, P<0.001) at 24 hours compared to the negative control. However, at 48 and 72 hours after treatment, PRF had a negative effect on HGF cell proliferation and caused 38% and 60% decrease in viability and proliferation compared to the negative control, respectively. The HGF cell proliferation was significantly higher in PRGF than in PRF group (P< 0.001).
Conclusion:
This study demonstrated that PRGF had a strong stimulatory effect on HGF cell viability and proliferation compared to PRF.
Keywords: Blood Platelets, Cell Proliferation, Fibroblasts
INTRODUCTION
Wound healing is a complex process involving four distinct, but overlapping phases of hemostasis, inflammation, proliferation and remodeling [1]. The unique structure of the periodontium makes periodontal regeneration a more complex process compared to the healing of other soft tissue components. It requires an interaction between the soft and hard tissues, gingival connective tissue, periodontal ligament, cementum and bone [2]. In general, studies on periodontal wound healing indicate that conventional periodontal therapy most commonly results in repair by collagenous fibrous tissue and apical migration of gingival epithelium between the gingival connective tissue and the root surface [3]. This healing process does not fully restore the form and function of the lost structures and hence does not constitute regeneration [4]. Wound healing process is stimulated and regulated by biologically active substances known as growth factors, which regulate key cellular processes such as mitogenesis, chemotaxis, cell differentiation and metabolism. In early stages of wound healing, platelets play a pivotal role in release of growth factors [5]. Platelet-rich plasma is an easily accessible autologous source of growth factors. It can have beneficial effects for soft and hard tissue healing by significantly reducing the wound healing time. The philosophy behind its use refers to the increased level of growth factors present in a well-prepared platelet-rich plasma (PRP) concentrate [6].
Whitman et al, first suggested the use of PRP [7].
Beneficial effects of PRP on tissue regeneration have been investigated for several clinical applications in oral and maxillofacial surgery [8], periodontology [9], plastic surgery [10], orthopedics [11] and treatment of chronic cutaneous ulcers [12].
Although the majority of these studies have shown excellent outcomes, many had no control groups and many were only small case studies. Additionally, some studies were unable to yield any additional benefit of PRP in tissue regeneration [13].
In 1999, Anitua introduced the concept of PRGF technology for the first time [14]. The term “PRGF” identifies exclusively 100% autologous and biocompatible formulations elaborated by a one-step centrifugation process using sodium citrate and calcium chloride as anticoagulant and activator, respectively. Plasma rich in growth factors has a moderated platelet concentration and does not contain leukocytes, with the aim of avoiding the proinflammatory effects of proteases and acid hydrolases in white blood cells [15,16]. Platelet-rich fibrin described by Choukroun et al, [17] is a second-generation platelet concentrate produced without any anticoagulants [18]. Compared with other autologous platelet concentrates, there are few references in the literature about the biological properties of PRF. Since basic studies are insufficient to support the efficacy of PRF and PRGF and because of many controversies on the effects of PRP on bone and soft tissue regeneration, more in vitro studies are still needed. The aim of the present in vitro study was to compare the effect of PRGF and PRF on proliferation of HGFs.
MATERIALS AND METHODS
Blood Collection
Blood samples were obtained from a healthy 28 year-old, nonsmoker Iranian woman, after obtaining her written informed consent. This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences.
Preparation of PRF
Twenty-seven milliliters of venous blood was collected in three dry glass tubes (9mL in each) (Blood collecting tubes®, Process, Nice, France) without any anticoagulant. According to the standard Choukroun’s protocol, tubes were immediately centrifuged at 2,700 rpm (approximately 400g) for 12 minutes. A fibrin dense clot was then obtained in the middle of the tube, between the red cells at the bottom and the liquid serum called platelet poor plasma at the top. The PRF Box (Process, Nice, France) was used to prepare standardized PRF membranes in a sterile environment (class II biological hood).
Preparation of PRGF
Fifteen milliliters of venous blood was drawn and transferred to three vacutainer tubes (Blood collecting tubes®, BTI Biotechnology Institute, Araba, Spain) containing 3.8% sodium citrate as anticoagulant (5mL in each). The tubes were centrifuged at 460g for eight minutes (PRGF® System III, BTI Biotechnology Institute®, Araba, Spain) to separate the different phases of the blood.
The 0.5 mL fractions located immediately above the buffy coat were collected from each tube and transferred to a sterile Petri dish. In order to initiate clotting and activation of platelets to release growth factors, PRGF activator® (10% calcium chloride) was added to the liquid PRGF (50 microliters per milliliter of PRGF).
The dishes were incubated at 37°C for 30 minutes until a consistent easily-handled gelatinous layer was formed.
Cell Culture and Treatment
A HGF cell line (HGF.1-PI, NCBI: C-165) was obtained from the National Cell Bank of Iran (NCBI, Pasteur Institute of Iran, Tehran).
The cells after passage four were seeded into 12 culture dishes (60mm diameter; Nunc International, NY, USA) at density of 50,000 cells per dish in complete medium consisting of Dulbecco’s modified Eagle’s medium (DMEM) + 10% fetal bovine serum (FBS).
After 24 hours, complete medium was exchanged with low-serum medium (DMEM+ 1% FBS) in order to stop proliferation (cell synchronization). Twenty-four hours later, the cells were treated as follows. Twelve groups were set and incubated as follows: three cultures were supplemented with 1% FBS (negative control group), three cultures received 10% FBS (positive control group), three received PRF membrane (test group 1) and three received PRGF gel (test group 2). After a culture period of 24, 48, and 72 hours, a culture dish of each group was removed and washed twice with sterile phosphate buffered saline (PBS) solution and HGF proliferation and viability were evaluated using the methyl thiazol tetrazolium (MTT) assay.
Cell Proliferation and Viability Assay
The MTT colorimetric assay was used to monitor cell proliferation and viability. In this method, only the mitochondria of viable cells can reduce MTT to formazan. Briefly, the MTT solution (MTT, Sigma Chemical Co., Baltimore, MD, USA) with the final concentration of 0.5mg/mL was added to each culture dish at each experimental time. The dishes were placed in an incubator at 37°C. After four hours of incubation, the medium containing MTT was removed carefully. The produced insoluble formazan, dissolved with dimethyl sulfoxide (DMSO), was added to each dish.
The culture dishes were agitated on a shaker for 10 minutes at room temperature to ensure the dissolution of formazan crystals.
Then, 100 μL of the purple solution was transferred into a well of a 96-well ELISA reader plate (Nunc International, NY, USA) (eight replicates for each treatment).
The spectrophotometric absorbance (optical density) was read at 570 nm with 620 nm as a reference using an ELISA reader (Bio-TeK Instruments Inc., Winooski, VT, USA). Triplicate experiments were performed to ensure reproducibility. Statistical analysis was performed using one-way ANOVA followed by Tukey-Kramer’s multiple comparisons and P values less than 0.05 were considered significant.
RESULTS
For the MTT assay, the viability of the negative control cultures (1% FBS) was set at 100%. The mean optical density of the test groups was divided by that of the control group and expressed as percentage of the control value. The microscopic effects of PRGF and PRF on proliferation and viability of HGF cells at 24, 48, and 72 hours after treatment are shown in Fig. 1. The data obtained from the MTT assay (Fig. 2) confirmed the microscopic results.
Fig. 1.
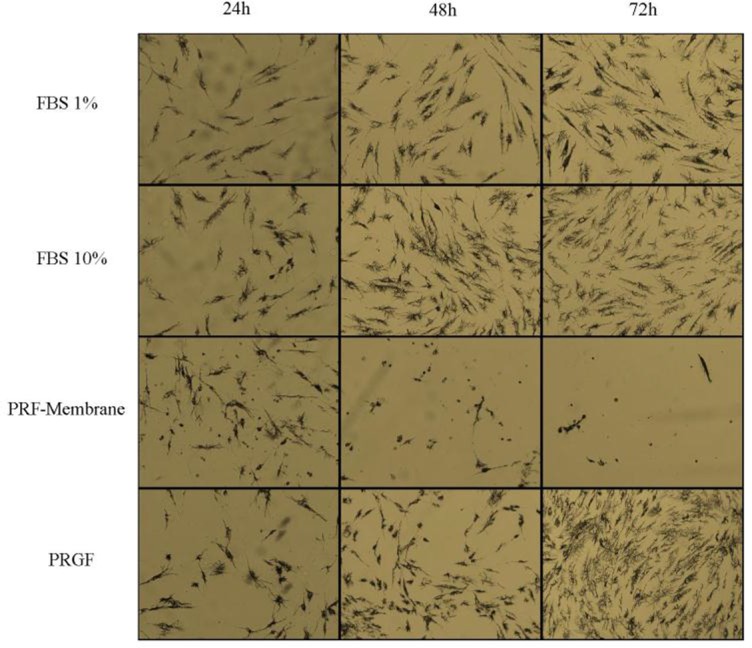
Microscopic analysis of HGF cells in four different conditions:
HGF+ medium + 1% FBS (negative control group), HGF+ medium + 10% FBS (positive control group), HGF + medium + 1% FBS + PRF-membrane (test group 1), and HGF + medium + 1% FBS + PRGF (test group 2). Assessments were done at 24, 48 and 72 hours after treatment.
Fig. 2.
The effects of PRF and PRGF on proliferation and viability of HGF cells compared to controls at 24, 48 and 72 hours after treatment. Negative control contained culture medium supplemented with 1% FBS and positive control contained culture medium supplemented with 10% FBS. The results were calculated as the viability (percent control) compared to the negative control (100% viability) and presented as mean ± SD. ***P < 0.001
According to our results, PRGF treatment induced statistically significant (P< 0.001) proliferation of HGF cells as compared with the negative control (100% viability) at all three time points; the increase in proliferation was 123% ± 2.25 at 24 hours, 102% ± 2.8 at 48 hours and 101% ± 3.92 at 72 hours. Data showed that the viability of cells treated with PRGF significantly increased as compared to the positive control (100% viability) at 24, 48 and 72 hours in values of 104, 126 and 116%, respectively. The PRF membrane treatment of HGF cells had a statically significant effect on cell proliferation (21%± 1.73, P< 0.001) at 24 hours compared to the negative control group. However, at 48 and 72 hours after treatment, PRF had a negative effect on HGF cell proliferation and caused 38% and 60% decrease in viability and proliferation compared to the negative control and 61% and 78% decrease compared to the positive control, respectively. According to this study, the highest HGF cell proliferation and viability was obtained with PRGF and the difference between the effect of PRGF and PRF on cells was statistically significant (P< 0.001).
DISCUSSION
Our results showed that both PRF and PRGF at 24 hours, and PRGF at 48 and 72 hours significantly increased HGF proliferation and viability. Furthermore, PRGF had a significantly greater proliferative effect than PRF at 48 and 72 hours after treatment. These observations are likely to be of great interest and are truly complex to interpret. Our findings were different from those of Dohan Ehrenfest et al, [19] who showed that PRF significantly induced the proliferation of gingival fibroblasts at three, seven, 14 and 21 days as compared with the control groups.
Different results may be due to individual variations in terms of platelet count and the time intervals between blood sample centrifugation, obtaining fibrin membranes and treating the cell culture.
It has been shown that the effect of PRP on cell proliferation does not necessarily improve with increasing concentration of platelets, but there is a range of optimal concentration of platelets. As shown in previous studies, platelet concentration equivalent to 2.5 times the normal amount in blood is ideal for cell proliferation [20].
The final platelet concentration of PRGF (two to three times the normal level) is the optimal concentration range of platelets [21]. Since platelets are trapped within the dense fibrin network of PRF, it is not possible to count the number of platelets in PRF [22]. Liu et al. [23] proposed that the lower proliferation was pH dependent, and that high platelet concentration resulted in pH changes that negatively affected fibroblast proliferation.
Furthermore, all other studies recruited more than one volunteer to prepare PRP or PRF. As previously reported for the transforming growth factor beta (TGF-β), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF), important variations in growth factor concentrations were detected among individuals having very similar platelet counts [24,25] and this may affect the results. In our study, PRF and PRGF were obtained from the same donor to eliminate any possible confounding effect. The inter-individual variations may explain, in part, some of the controversial results.
Platelet concentrates contain different factors with extremely diverse effects. For example, platelet granules include angiogenesis stimulators including bFGF and VEGF and angiostatic factors such as endostatin and thrombospondin-1; each having confounding effects [26]. Differences in levels of these factors may explain the variability in the obtained results. Others have shown that the mitogenic activity of platelet supernatant is not confined to its growth factors, and platelet particles and membrane fragments also play an important role in this regard.
Most experimental studies examined the effects of PRP and PRF supernatant except two [19,27]. This is a major limitation of these studies, since only growth factors and proteins found in the exudate of these products were tested and the actual effects of the product as a whole were overlooked. Platelet rich fibrin is a dense fibrin matrix with specific structure and cell content and its exudate is not the only component of this product [28].
Similarly, although PRP is a homogenous liquid product, its exudate is not its only active component. Since platelets aggregate to create a fibrin gel, which also stimulates many biological mechanisms [29,30], examining PRF and PRGF as a whole rather than their components separately is of great importance. To study the platelet product as a whole, use of large enough culture plates is essential to receive a complete piece of platelet clot or membranes, without compressing the cultivated cells. A minimum diameter of 60 mm has been suggested in previous studies to allow the interaction of cells and platelet cytokines [19,27]. In a normal healing process, the platelets first accumulate and then release growth factors [31]. There is a fibrin matrix in PRF, in which the platelets and probably the released cytokines are trapped after a certain period of time [32]. Tuan et al. [33] indicated the role of fibrin in tissue repair and demonstrated the ability of fibroblasts to reconstruct a fibrin matrix and to produce collagen. On this basis, dense PRF matrix may be effective in stimulating the wound healing process.
In the study by Dohan Ehrenfest et al, [19] primary cell cultures were used to eliminate the potential effects of leukocytes involved in the immune response on the cultured cells. However, considering the similar results of our pilot study on PRGF and PRF supernatant (data not shown), a definite conclusion on this topic cannot be drawn, since all factors and cells except for growth factors are eliminated in the supernatant.
No consensus has been reached on the inclusion of leukocytes in platelet products either. Despite emphasizing on the potential ability of leukocytes in proliferation, differentiation and immunity in some studies [34], the deleterious biological effects of matrix metalloproteinases 8 and 9 produced by neutrophils have been well described [35,36]. In addition, neutrophils may induce tissue damage by releasing reactive oxygen species in the inflammatory phase of tissue injury [37]. According to a recent study, synthesis of thrombin-induced interleukin (IL)-1β could be observed in platelet suspension containing at least one leukocyte per 105 platelets [38]. On the other hand, many studies have addressed the negative effects of IL-1β on periodontal destruction [39,40]. Nevertheless, the effects of leukocytes existing in platelet concentrates have not yet been assessed and their overall effects remain unclear. Further studies focusing on the function of these cells are required to fully determine the relationship between these components and to help develop products with specifically required components. Consequently, until confirmation of the effects of leukocytes in future studies, use of platelet products lacking leukocytes is more prudent.
In vitro experiments are helpful for assessing the biological efficacy of blood products; but these studies have limitations in mimicking the clinical conditions.
It may be unrealistic to generalize in vitro findings to in vivo situations.
The majority of studies on both Anitua’s PRGF and Choukroun’s PRF were conducted by companies that developed these products. It seems that more extensive, independent studies are required to obtain more valuable results. We studied the effects of PRF and PRGF supernatant and membrane on HGF cell line using the MTT proliferation and viability assay. It is highly recommended to design further studies on the effects of PRF and PRGF on more cell lines and primary cells with different in vitro proliferation assays and in vivo trials.
CONCLUSION
This study demonstrated that PRGF had a strong stimulatory effect on HGF cell viability and proliferation as compared with PRF.
ACKNOWLEDGMENTS
This article was approved and funded by the Dental Research Center of Shahid Beheshti University of Medical Sciences, Tehran, Iran.
REFERENCES
- 1-. Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004. January 1; 9: 283– 9. [DOI] [PubMed] [Google Scholar]
- 2-. Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976. May; 47 (5): 256– 60. [DOI] [PubMed] [Google Scholar]
- 3-. Caton J, Nyman S, Zander H. Histometric evaluation of periodontal surgery. II. Connective tissue attachment levels after four regenerative procedures. J Clin Periodontol. 1980. June; 7 (3): 224– 31. [DOI] [PubMed] [Google Scholar]
- 4-. Ivanovski S. Periodontal regeneration. Aust Dent J. 2009. September; 54 Suppl 1: S118– 28. [DOI] [PubMed] [Google Scholar]
- 5-. Garg AK. The use of platelet-rich plasma to enhance the success of bone grafts around dental implants. Dent Implantol Update. 2000. March; 11 (3): 17– 21. [PubMed] [Google Scholar]
- 6-. Arora NS, Ramanayake T, Ren YF, Romanos GE. Platelet-rich plasma: a literature review. Implant Dent. 2009. August; 18 (4): 303– 10. [DOI] [PubMed] [Google Scholar]
- 7-. Whitman DH, Berry RL, Green DM. Platelet gel: an autologous alternative to glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1997. November; 55 (11): 1294– 9. [DOI] [PubMed] [Google Scholar]
- 8-. Sarkarat F, Kalantar Motamedi MH, Jahanbani J, Sepehri D, Kahali R, Nematollahi Z. Platelet-Rich Plasma in Treatment of Zoledronic Acid-Induced Bisphosphonate-related Osteonecrosis of the Jaws. Trauma Mon. 2014. April; 19 (2): e17196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9-. Kukreja BJ, Dodwad V, Kukreja P, Ahuja S, Mehra P. A comparative evaluation of platelet-rich plasma in combination with demineralized freeze-dried bone allograft and DFDBA alone in the treatment of periodontal intrabony defects: A clinicoradiographic study. J Indian Soc Periodontol. 2014. September; 18 (5): 618– 23. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10-. Willemsen JC, van der Lei B, Vermeulen KM, Stevens HP. The effects of platelet-rich plasma on recovery time and aesthetic outcome in facial rejuvenation: preliminary retrospective observations. Aesthetic Plast Surg. 2014. October; 38 (5): 1057– 63. [DOI] [PubMed] [Google Scholar]
- 11-. Sadoghi P, Rosso C, Valderrabano V, Leithner A, Vavken P. The role of platelets in the treatment of Achilles tendon injuries. J Orthop Res. 2013. January; 31 (1): 111– 8. [DOI] [PubMed] [Google Scholar]
- 12-. Pinto JM, Pizani NS, Kang HC, Silva LA. Application of platelet-rich plasma in the treatment of chronic skin ulcer - case report. An Bras Dermatol. 2014. Jul-Aug; 89 (4): 638– 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13-. Del Fabbro M, Bortolin M, Taschieri S, Weinstein R. Is platelet concentrate advantageous for the surgical treatment of periodontal diseases? A systematic review and meta-analysis. J Periodontol. 2011. August; 82 (8): 1100– 11. [DOI] [PubMed] [Google Scholar]
- 14-. Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of sites for implants. Int J Oral Maxillofac Implants. 1999. Jul-Aug; 14 (4): 529–35. [PubMed] [Google Scholar]
- 15-. Anitua E, Sánchez M, Orive G, Andia I. Delivering growth factors for therapeutics. Trends Pharmacol Sci. 2008. January; 29 (1): 37– 41. [DOI] [PubMed] [Google Scholar]
- 16-. Anitua E, Sánchez M, Orive G, Andía I. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials. 2007. November; 28 (31): 4551– 60. [DOI] [PubMed] [Google Scholar]
- 17-. Choukroun J, Adda F, Schoeffer C, Vervelle A. PRF: An opportunity in perioimplantology (in French). Implantodontie. 2000; 42: 55– 62. [Google Scholar]
- 18-. Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006. March; 101 (3): e51– 5. [DOI] [PubMed] [Google Scholar]
- 19-. Dohan Ehrenfest DM, Diss A, Odin G, Doglioli P, Hippolyte MP, Charrier JB. In vitro effects of Choukroun's PRF (platelet-rich fibrin) on human gingival fibroblasts, dermal prekeratinocytes, preadipocytes, and maxillofacial osteoblasts in primary cultures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009. September; 108 (3): 341– 52. [DOI] [PubMed] [Google Scholar]
- 20-. Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res. 2006. April; 17 (2): 212– 9. [DOI] [PubMed] [Google Scholar]
- 21-. Lopez-Vidriero E, Goulding KA, Simon DA, Sanchez M, Johnson DH. The use of platelet-rich plasma in arthroscopy and sports medicine: optimizing the healing environment. Arthroscopy. 2010. February; 26 (2): 269– 78. [DOI] [PubMed] [Google Scholar]
- 22-. Gassling VL, Açil Y, Springer IN, Hubert N, Wiltfang J. Platelet-rich plasma and platelet-rich fibrin in human cell culture. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009. July; 108 (1): 48– 55. [DOI] [PubMed] [Google Scholar]
- 23-. Liu Y, Kalén A, Risto O, Wahlström O. Fibroblast proliferation due to exposure to a platelet concentrate in vitro is pH dependent. Wound Repair Regen. 2002. Sep-Oct; 10 (5): 336– 40. [DOI] [PubMed] [Google Scholar]
- 24-. Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002. April; 30 (2): 97– 102. [DOI] [PubMed] [Google Scholar]
- 25-. Martineau I, Lacoste E, Gagnon G. Effects of calcium and thrombin on growth factor release from platelet concentrates: kinetics and regulation of endothelial cell proliferation. Biomaterials. 2004. August; 25 (18): 4489– 502. [DOI] [PubMed] [Google Scholar]
- 26-. Italiano JE, Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008. February 1; 111 (3): 1227– 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27-. Dohan Ehrenfest DM, Doglioli P, de Peppo GM, Del Corso M, Charrier JB. Choukroun's platelet-rich fibrin (PRF) stimulates in vitro proliferation and differentiation of human oral bone mesenchymal stem cell in a dose-dependent way. Arch Oral Biol. 2010. March; 55 (3): 185– 94. [DOI] [PubMed] [Google Scholar]
- 28-. Dohan Ehrenfest DM, Del Corso M, Diss A, Mouhyi J, Charrier JB. Three-dimensional architecture and cell composition of a Choukroun's platelet-rich fibrin clot and membrane. J Periodontol. 2010. April; 81 (4): 546– 55. [DOI] [PubMed] [Google Scholar]
- 29-. Kawase T, Okuda K, Wolff LF, Yoshie H. Platelet-rich plasma-derived fibrin clot formation stimulates collagen synthesis in periodontal ligament and osteoblastic cells in vitro. J Periodontol. 2003. June; 74 (6): 858– 64. [DOI] [PubMed] [Google Scholar]
- 30-. Kawase T, Okuda K, Saito Y, Yoshie H. In vitro evidence that the biological effects of platelet-rich plasma on periodontal ligament cells is not mediated solely by constituent transforming-growth factor-beta or platelet-derived growth factor. J Periodontol. 2005. May; 76 (5): 760– 7. [DOI] [PubMed] [Google Scholar]
- 31-. Schilephake H. Bone growth factors in maxillofacial skeletal reconstruction. Int J Oral Maxillofac Surg. 2002. October; 31 (5): 469– 84. [DOI] [PubMed] [Google Scholar]
- 32-. Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005. August; 3 (8): 1894– 904. [DOI] [PubMed] [Google Scholar]
- 33-. Tuan TL, Song A, Chang S, Younai S, Nimni ME. In vitro fibroplasia: matrix contraction, cell growth, and collagen production of fibroblasts cultured in fibrin gels. Exp Cell Res. 1996. February 25; 223 (1): 127– 34. [DOI] [PubMed] [Google Scholar]
- 34-. Froum SJ, Wallace SS, Tarnow DP, Cho SC. Effect of platelet-rich plasma on bone growth and osseointegration in human maxillary sinus grafts: three bilateral case reports. Int J Periodontics Restorative Dent. 2002. February; 22 (1): 45– 53. [PubMed] [Google Scholar]
- 35-. Schnabel LV, Mohammed HO, Miller BJ, McDermott WG, Jacobson MS, Santangelo KS, et al. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J Orthop Res. 2007. February; 25 (2): 230– 40. [DOI] [PubMed] [Google Scholar]
- 36-. Anitua E, Sánchez M, Nurden AT, Nurden P, Orive G, Andía I. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006. May; 24 (5): 227– 34. [DOI] [PubMed] [Google Scholar]
- 37-. Tidball JG. Inflammatory cell response to acute muscle injury. Med Sci Sports Exerc. 1995. July; 27 (7): 1022– 32. [DOI] [PubMed] [Google Scholar]
- 38-. Pillitteri D, Bassus S, Boller K, Mahnel R, Scholz T, Westrup D, et al. Thrombin-induced interleukin 1beta synthesis in platelet suspensions: impact of contaminating leukocytes. Platelets. 2007. March; 18 (2): 119– 27. [DOI] [PubMed] [Google Scholar]
- 39-. Gustafsson A, Ito H, Asman B, Bergström K. Hyper-reactive mononuclear cells and neutrophils in chronic periodontitis. J Clin Periodontol. 2006. February; 33 (2): 126– 9. [DOI] [PubMed] [Google Scholar]
- 40-. Orozco A, Gemmell E, Bickel M, Seymour GJ. Interleukin-1beta, interleukin-12 and interleukin-18 levels in gingival fluid and serum of patients with gingivitis and periodontitis. Oral Microbiol Immunol. 2006. August; 21 (4): 256– 60. [DOI] [PubMed] [Google Scholar]



