Abstract
Background
Studying the insulin signaling response at the synapse is an important approach to understand molecular mechanisms involved in disease-related neurodegenerative processes.
New Method
We developed a method for studying the insulin responsiveness at the synaptic level by isolating functional synaptosomes from fresh or frozen tissue and exposing them to insulin in the presence of ATP (a critical step) to detect insulin receptor (IR) activation.
Results
We performed an ATP dose-response curve, insulin dose-response curve, and insulin response time course to optimize this method. We also demonstrated that our protocol reflects the degree of insulin responsiveness in vivo by using an animal model of known insulin resistance, AtENPP1-Tg mice.
Comparison with Existing Method(s)
This method is advantageous over other methods detecting IR in total brain homogenates due to the ability to detect IR response without confounding contributions from other cell areas and cell types also expressing IR. Furthermore, ex vivo insulin stimulation can be compared to baseline synaptosomes obtained from the same animal which improves reliability and statistical power while decreasing the number of animals required to perform individual experiments.
Conclusions
We have developed a reliable, efficient method to measure insulin-driven ex vivo phosphorylation of the synaptosomal insulin receptor that can reliably reflect the pre-existing insulin responsiveness status in the CNS of the animal. To the best of our knowledge, this is the first evidence of stimulation of isolated synaptosomes with insulin and a promising new technique to study the synaptic CNS insulin responsiveness under physiological or disease conditions.
Keywords: Synaptosomes, Insulin Responsiveness, Insulin Signaling, Insulin receptor phosphorylation
1. INTRODUCTION
Alterations of insulin signaling in neurons have been linked to many different disorders including Type 2 diabetes, inflammation, and Alzheimer’s disease (AD) (Verdile et al., 2015). Synapses are rich with insulin receptors, and insulin has been shown to be an important component for maintaining synaptic health/integrity (Zhao et al., 2008 and De Felice et al., 2009). Notably, decreased insulin receptor function increases synaptic sensitivity to the binding of and dysfunction by amyloid beta (Aβ) (De Felice et al., 2009), the toxic protein that accumulates in AD, thus contributing to the cognitive decline that characterizes this neurodegenerative disorder. Therefore, studying the insulin signaling response at the synapse is an important approach to understand molecular mechanisms involved in disease-related neurodegenerative processes and test the effectiveness of potential new treatments. With this goal in mind, we have developed a method for studying the insulin responsiveness at the synaptic level by isolating functional synaptosomes from fresh or frozen tissue and exposing them to insulin in the presence of ATP to detect insulin receptor (IR) activation.
2. MATERIALS
2.1 Reagents
Adenosine 5′-triphosphate disodium salt hydrate: Sigma-Aldrich A2383-1G
CaCl2
D(+)-Glucose
Double-distilled or double-deionized water (DDI)
0.5M EDTA stock solution
Halt Phosphatase inhibitor cocktail (100X): Thermo Scientific #1861277
HCl
HEPES
Humulin® R U-100 Regular insulin human injection, USP (rDNA origin): Eli Lilly and Company NDC 0002-8215-17 (HI-213)
KCl
MgCl2
MgSO4·7 H2O
NaCl
Na2HPO4
NaOH
Non-fat dry milk
NP-40
Odyssey Blocking buffer: LI-COR #927-40000
Pierce® BCA Reagent A and B: Thermo Scientific #23223 and 23224
Protease inhibitor cocktail(100X): Sigma-Aldrich P-8340
Sucrose: Sigma-Aldrich S9378
SynPER: Thermo Scientific #87793
1X TBS-T
Tris base
TritonX-100
Antibodies
β tubulin: Cell Signaling #2146S
Goat anti-rabbit IR Dye® 800CW: LI-COR 926-32211
IR: Cell Signaling #3025S
P-IGF-IRβ: Cell Signaling #3024L
PSD95: Cell Signaling #3409S
Equipment
Bio-Rad PowerPac™ HC
Eppendorf Centrifuge 5417R (Eppendorf, Mississauga, Canada)
Optima TLX Preparative Ultracentrifuge (Beckman Coulter, Brea, CA)
TLA110 rotar (Beckman Coulter, Brea, CA)
Whatman Protran® nitrocellulose transfer membrane
2.2 Reagent setup
Fractionation-Synaptosomal Isolation
-
0.1mM CaCl2 (1L)
Dissolve 0.0147g CaCl2 in 1 L DDI water. Store at 4 °C.
-
0.32M sucrose, 1mM MgCl2, 0.1mM CaCl2 (50mLs)
Dissolve 5.4g sucrose and 0.01g MgCl2 in about 30mLs of the 0.1mM CaCl2 solution. Level to 50mLs with the 0.1mM CaCl2 solution. Autoclave and can be stored up to 6 months at 4 °C.
-
1M sucrose, 0.1mM CaCl2 (50mLs)
Dissolve 17g sucrose in about 30mLs of the 0.1mM CaCl2 solution. Level to 50mLs with the 0.1mM CaCl2 solution. Autoclave and can be stored up to 6 months at 4 °C.
-
2M sucrose (50mLs)
Dissolve 34g sucrose in about 25mLs DDI water. May need to heat and vortex to fully dissolve. Level to 50mLs with DDI water. Autoclave and can be stored up to 6 months at 4 °C.
Insulin Stimulation
-
HEPES/Tris buffer, 87 mM
Dissolve 10.5 g of HEPES in 500 ml of water. Adjust the pH to 7.4 by adding drops of saturated Tris base. (Saturated Tris base is made by adding 40g of Tris to 100mls of DDI water.) Mix well in a bottle and leave overnight. Filter and store for up to 6 months at 4 °C.
-
HEPES-buffered Krebs-like buffer (HBK)
Prepare the following stock solutions: 308 mM NaCl (9 g of NaCl dissolved in 500 ml of water); 308 mM KCl (11.5 g in 500 ml); 154 mM MgSO4·7 H2O (1.9 g in 50 ml); 1 M CaCl(7.36 g in 50 ml); and 100 mM Na2HPO4 (0.7 g in 50 ml). All stocks should be filtered and can be stored up to 6 months at 4 °C.
Combine stocks by adding, in the following order, 121 ml of NaCl (308 mM); 4 ml of KCl (308 mM); 2 ml of MgSO4·7H2O; 312 μl of CaCl2 (1 M); 60 ml of HEPES/Tris (87 mM), pH adjusted to 7.4; and finally, 260 μl ofNa2HPO4 (100 mM). Make up to 260 ml with water and add 0.48 g D(+)-glucose. Dissolve the glucose, filter and store for 1 week at 4°C.
The final composition of this buffer is 143 mM NaCl, 4.7 mM KCl, 1.3 mM MgSO4, 1.2 mM CaCl2, 20 mM HEPES, 0.1 mM NaH2PO4 and 10 mM D-glucose (pH 7.4).
Critical: Although other physiological buffers may be used in this assay, our stimulation protocols are optimized for the HBK described here.
-
100mM ATP (2mLs)
Dissolve 0.1102g of adenosine 5′-triphosphate disodium salt hydrate (Sigma, St. Louis, MO) in 2mLs DDI water. Adjust pH to 7.3–7.5 with NaOH. Aliquot and store at −20°C.
-
1X RIPA (200mLs)
-
Add 0.877g NaCl, 0.7098g Na2PO4, 0.4mL of 0.5M EDTA stock, 1mL NP-40, and 1mL TritonX-100 and level to 100mLs with DDI water. Adjust to pH 7.2 using HCl. Dilute with equal volume DDI water for 1X. Store up to 3 months at 4°C.
Final composition is 75mM NaCl, 25mM Na2PO4, 1mM EDTA, 0.5% NP-40, and 0.5% TritonX-100.
-
Freezing Synaptosomes Prior To Insulin Stimulation
-
Sucrose/EDTA/Tris (SET) buffer
-
Dissolve 54.75g of sucrose and 303mg Tris in ~400mLs of DDI water. Add 1 mL of 0.5M EDTA disodium salt stock solution. Level off to 500mLs with DDI water. Adjust pH to 7.4 with HCl. Filter and store up to 6 months at 4°C.
Final composition is 0.32M sucrose, 1mM EDTA, 5mM Tris.
-
3. METHODS
3.1 Fractionation-Synaptosomal Isolation
-
Homogenize 60mg tissue in 180μL 0.32M sucrose and 10% protease (Sigma, St. Louis, MO) and phosphatase cocktail inhibitors (Thermo Scientific, Rockford, IL) on ice.
Can save an aliquot as homogenate.
Mix homogenate with 720μL 2M sucrose and 300μL 0.1mM CaCl2. Mix well. Transfer to 5mL ultracentrifuge tube.
Overlay carefully with ~1.8mLs 1M sucrose solution. Balance with 2nd tube.
Centrifuge in ultracentrifuge (Beckman Coulter, Brea, CA) at 127,000 RCF for 3 hours at 4°C in TLA110 rotor (Beckman Coulter, Brea, CA).
Discard myelin layer floating on top. Harvest synapse band at sucrose layers interface (Figure 1B).
Store synapse band overnight at −20°C.
Next day, transfer synapse band to ultracentrifuge tube. Centrifuge in ultracentrifuge (Beckman Coulter, Brea, CA) at 18,200 RCF for 30 minutes at 4°C in TLA110 rotor (Beckman Coulter, Brea, CA).
Pour supernatant off.
-
Resuspend pellet in 48μL HBK buffer.
Can resuspend in 1XRIPA plus 1% protease and phosphatase cocktail inhibitors to western blot without insulin stimulation
Figure 1. Synaptosomal Sucrose Fractionation.
A) A schematic showing the pre- and post-synaptic areas retained in the synaptosomal isolation. B) Synaptosomal band (white arrow) to collect at sucrose interface after first centrifugation in sucrose fractionation protocol. C) Representative Western blot detecting enrichment of the post-synaptic marker PSD95 in synaptosomal fraction versus total homogenate, indicating successful synaptosomal isolation.
3.2 Insulin Stimulation
Pipette sample resuspended in HBK buffer into desired number of aliquots.
Add ATP amount of 100mM ATP for desired final mM concentration.
Add insulin amount of U-100 insulin using U-100 insulin syringe. Dilute first if necessary.
Vortex and incubate for specified amount of time at 37°C.
Spin in centrifuge (Eppendorf, Mississauga, Canada) at 10,000g RCF for 10 minutes at 4°C.
Resuspend in HBK buffer, equal parts to original amount of aliquot.
Spin in centrifuge (Eppendorf, Mississauga, Canada) at 10,000g RCF for 10 minutes at 4°C.
Resuspend in 1X RIPA plus 1% protease (Sigma, St. Louis, MO) and phosphatase cocktail inhibitors (Thermo Scientific, Rockford, IL).
Store at −80°C.
3.3 Western-Blot Analysis
Phosphorylation extent of IR was determined using western blots with specific antibodies against the phosphorylated form. The phosphorylated form was normalized against the total amount of IR. The ratio of phosphorylated-protein over the regular protein was used to assess the extent of phosphorylation.
Briefly, the BCA method (Thermo Scientific) was used for protein estimation to prepare samples of equal protein concentration. Samples were prepared in 2-mercaptoethanol (2-ME) and boiled prior to loading. Thirty micrograms of protein was loaded with appropriate marker on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels followed by transfer to nitrocellulose transfer membrane (Whatman, Dassel, Germany) for 1 hour at 100V. The membrane was blocked using Odyssey blocking buffer (LI-COR, Lincoln, Nebraska) for 1 hour at room temperature. Primary antibody was diluted in 1X TBST and incubated at times and concentrations according to manufacturer’s recommendations. The membrane was washed twice with 1X TBST for 15 minutes each and incubated with secondary antibody diluted in 1X TBST with 3% non-fat dry milk at times and concentrations according to manufacturer’s recommendations. The membrane was again washed twice with 1X TBST for 15 minutes each.
3.4 Data Analysis
Western blots were imaged using LI-COR Odyssey infared imaging system (LI-COR, Lincoln, Nebraska), application software version 3.0.30. The density of each immunoreactive band was measured using Image J.
4. RESULTS
4.1 Synaptosomal Fractionation
Synaptosomes containing both pre- and post-synaptic components (Figure 1A) were isolated from frozen tissue using the sucrose fractionation procedure detailed above. The frozen tissue had been snap frozen on dry ice and transferred to −80°C. The final pellet was resuspended in 1X RIPA plus 1% protease inhibitor cocktail and phosphatase inhibitor cocktail to solubilize the proteins for western blot detection. This fraction resuspended in RIPA buffer will be referred to as the “synaptosomal protein fraction”. To confirm the isolation of the synaptosomes, a western blot comparing the synaptosomal protein fraction versus the homogenate was performed and probed for a post-synaptic marker, post-synaptic density 95 (PSD95) (Figure 1C). We saw an enrichment of this marker in the synaptosomal protein fraction indicating that the fractionation procedure was successful.
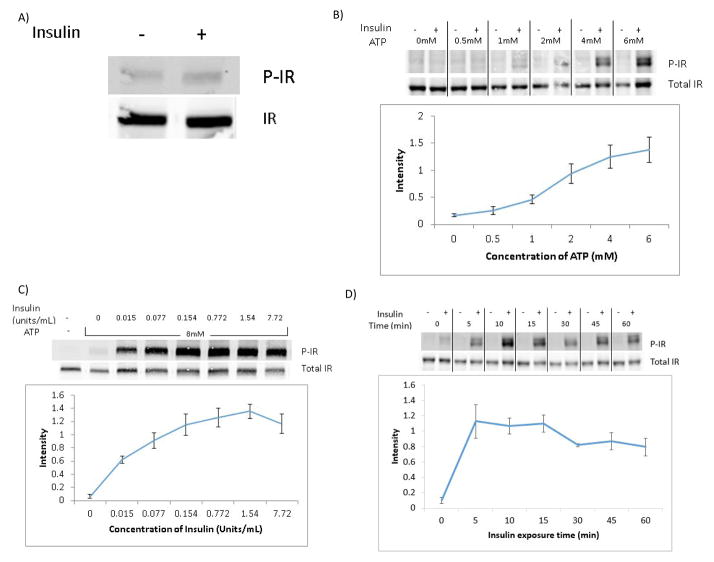
4.2 ATP Dose Response Curve
Our initial attempts at stimulating IR phosphorylation with insulin in isolated synaptosomes were unsuccessful (Figure 2A). However, we observed full insulin-driven IR phosphorylation when ATP was finally added to the incubation buffer during insulin stimulation. We then performed an ATP dose response curve on isolated synaptosomes from frozen rat brain stimulated with 1.67units/mL of insulin per sample for 15 minutes at 37°C (Figure 2B). Since ATP concentration inside the cell is between 0–10mM, we performed our concentration curve within this range. In the western blot depicted, we chose 0–6mM ATP concentration. We saw no plateau in insulin receptor activity in this concentration range as determined by Western blot.
Figure 2. Optimization of Method.
A) Western blot showing lack of IR phosphorylation in synaptosomes from frozen rat brain stimulated with insulin (2.34units/mL of insulin for 5 min at 37°C) in the absence of ATP added to the incubation buffer. B) Representative Western blot of insulin-stimulated IR phosphorylation in the presence of increasing concentrations of ATP in the incubation buffer. The line graph shows the average for quantification of the immunoreactive bands expressed as the ratio p-IR/Total IR ±SEM from 3 independent experiments. Synaptosomes from frozen rat brain tissue were stimulated with 1.67units/mL of insulin for 15 min at 37°C. C) Representative Western blot showing IR phosphorylation in response to increasing concentrations of insulin. The line graph shows the average for quantification of the immunoreactive bands expressed as the ratio p-IR/Total IR ±SEM from 3 independent experiments. Synaptosomes from frozen mouse brain were stimulated with insulin at the concentrations shown for 15 min at 37°C in the presence of 8mM ATP. D) Representative Western blot and relative average quantification line graph of IR phosphorylation ±SEM (from 3 independent experiments) at different time points after addition of 0.333units/mL of insulin in the presence of 8mM ATP in synaptosomes isolated from frozen mouse brain tissue.
4.3 Insulin Dose Response Curve
We analyzed a dose response curve of insulin receptor phosphorylation in synaptosomes isolated from frozen mouse brain and exposed to increasing concentrations of insulin for 15 minutes at 37°C in the presence of 8mM ATP using western blot analysis (Figure 2C). At the insulin concentrations used, we saw a plateaued response beginning at 0.154units/mL of insulin.
4.4 Insulin Response Time Course
An insulin-receptor activation curve of synaptosomes exposed to 0.333units/mL of insulin for varying amounts of time from 0 to 1 hour at 37°C with 8mM ATP was performed and analyzed by western blot (Figure 2D). Maximal IR phosphorylation was observed 10 to 15 minutes after addition of insulin.
4.5 Freezing Synaptosomes Prior To Insulin Stimulation
To evaluate whether storing isolated synaptosomes at −80°C would affect the insulin stimulation response, we performed a slow freeze on synaptosomes isolated by a sucrose fractionation. The sample was split into 2 aliquots. One of the aliquots was resuspended in an iso-osmotic medium, sucrose/EDTA/tris buffer (SET) (Daniel, 2012), containing 5% DMSO (vol/vol) as the final concentration before the slow-freeze was carried out. The other aliquot was resuspended in the physiological buffer used in the insulin stimulation experiments, HBK buffer, before the slow-freeze was carried out. The slow-freeze of both aliquots was performed in a polystyrene box (Fig 3A) to slow the freezing process at −80°C similar to that described for the slow freezing in Daniel et al., 2012. The samples in the slow-freeze box were placed at −80°C for 1 day and then transferred to liquid nitrogen. The samples underwent a rapid thaw before the insulin stimulation. The samples were incubated at 37°C for 5 minutes, and then the reaction was stopped by adding twice as much ice-cold buffer that the sample was resuspended in. Both samples were then pelleted at 10,000xg for 20 minutes at 4°C and resuspended in 48μL HBK buffer and the insulin stimulation performed. We found an increased phosphorylation of the IR in samples frozen in either buffer while the total IR density remained the same (Figure 3B and 3C).
Figure 3. Freezing Synaptosomes Prior To Insulin Stimulation.
A) Polystyrene box used to slow the freezing of the synaptosomes to −80°C. B) Representative Western blot detecting insulin-stimulated phosphorylation of the IR in synaptosomes that had previously been slow frozen and stored at −80°C. Synaptosomes were isolated from frozen mouse brain, resuspended in SET buffer with 5%DMSO or HBK buffer, and slow frozen prior to storage at −80°C. Frozen synaptosomes were then rapidly thawed and stimulated with 0.333 units/mL of insulin for 15 min at 37°C with 8mM ATP. C) Graph representing average for quantification of the band intensity of the Western blots ±SEM from 3 independent experiments.
4.6 Post-Mortem Interval
To determine the impact of varying post-mortem intervals (PMI) prior to tissue collection, we looked at a post-mortem interval in mouse brain tissue collection that modeled the conditions that would normally occur for collection of autopsy brain human specimens. Animals were sacrificed by CO2 overdose followed by cervical dislocation. After sacrifice, the animal was left at room temperature for 1 hour and then placed at 4°C for the remainder of the post-mortem interval. After this time, the brain was collected and placed at −80°C. We looked at a PMI of 8 hours, 12 hours, and 21 hours (Figure 4). At all three PMIs, we found no significant differences in the ratio of P-IR/IR against control where the brain was immediately collected and placed at −80°C upon sacrifice.
Figure 4. Post-Mortem Interval.
A) Representative Western blot showing insulin-stimulated phosphorylation of the IR in synaptosomes isolated from brain tissue collected from mice at varying times after sacrifice (post-mortem interval, PMI). Mice were sacrificed by CO2 asphyxiation followed by cervical dislocation and left at room temperature for 1 hour prior to being placed at 4°C for the remainder of the specified PMI times. Afterwards, brains were collected, snap frozen, and stored at −80°C. Synaptosomes were isolated from the frozen mouse brains and stimulated with 0.333 units/mL of insulin for 15 min at 37°C with 8mM ATP. B) Graph showing average for quantification of the immunoreactive bands of the Western blots ±SEM from 3 independent experiments.
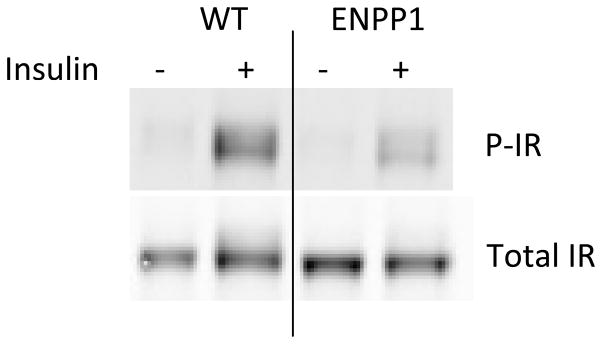
4.7 Comparison of Ex Vivo and In Vivo Synaptosomal Insulin Response
To determine if the extent of the response of the isolated synaptosomes to ex vivo insulin was consistent with the response observed after in vivo insulin stimulation, we used a transgenic mouse model of systemic insulin resistance, AtENPP1-Tg. This mouse model has also previously been shown to present with marked synaptic insulin resistance (Sallam et al, 2015). Following in vivo insulin stimulation via an intraperitoneal (IP) insulin injection and contrary to the response seen in wild-type (WT) mice, AtENPP1-Tg mice do not demonstrate increased phosphorylation of signaling elements downstream of the IR as determined by Western blot in isolated synaptosomes (Sallam et al, 2015).
To determine whether synaptosomal insulin resistance of AtENPP1-Tg mice could also be detected ex vivo using our newly developed method, we isolated synaptosomes from both the WT and ENPP1 transgenic mice and performed the insulin stimulation on the isolated synaptosomes for 30 minutes at 37°C with 8mM ATP. Our results (Figure 5) show a marked reduction of insulin-driven IR phosphorylation detected in synaptosomes isolated from AtENPP1-Tg mice compared to wild-type animals, confirming the synaptosomal insulin resistance in this transgenic mouse model. This result indicates that our ex vivo stimulation protocol reflects the degree of insulin responsiveness in vivo and can reliably detect decreased responses of the synaptosomal insulin receptor in an animal model of known insulin resistance.
Figure 5. Detection of Reduced Insulin-Stimulated IR Phosphorylation in Synaptosomes Prepared From a Tg Mouse Model of Insulin Resistance.
Representative Western blot detecting insulin-driven phosphorylated IR in isolated synaptosomes from brain tissue from AtENPP1-Tg mice or WT mice. Synaptosomes were stimulated with 0.333 units/mL of insulin for 30 min at 37°C in the presence of 8mM ATP.
5 DISCUSSION/CONCLUSION
The main goal of the present study was to develop a reliable and efficient method to evaluate insulin responsiveness specifically at the synapse. This method is advantageous over other methods detecting IR in total brain homogenates due to the ability to detect IR response without confounding contributions from other cell areas (e.g. cell soma) and cell types (glia) also expressing substantial levels of IR. This unique characteristic makes this new method particularly useful in situations when insulin responsiveness at the synapses must be differentiated from other subcellular compartments. For example, it has been found that Aβ-oligomers induced an IR redistribution from the dendrites to the cell body in a hippocampal neuronal culture (De Felice et al., 2009). Considering this along with the demonstration that dendritic IR function protects against binding of Aβ-oligomers to the synapses (De Felice et al., 2009), it is of particular significance to be able to differentiate between IR activity at the synapses versus other sub-cellular areas or other non-neuronal cell types. Furthermore, contrary to in vivo administration where insulin-treated animals must be compared to vehicle-treated controls, ex vivo insulin stimulation can be compared to baseline (non-stimulated) synaptosomes prepared from frozen tissue obtained from the same animal. This unique feature improves reliability and statistical power of the measurements (having baseline and insulin-stimulated synaptosomal IR phosphorylation values from the same animal) while greatly decreasing the number of animals required to perform individual experiments.
We discovered that one critical step to achieve phosphorylation of the IR after insulin stimulation is the addition of ATP in the incubation buffer. Our initial attempts were unsuccessful until ATP was added. The need for ATP to achieve full activation of the IR is illustrated by the results shown in Figure 2A where no phosphorylation of the IR is observed unless the insulin stimulation is performed in the presence of ATP. It is not clear why ATP must be added to the stimulation buffer. One possibility is that endogenous ATP is depleted during synaptosome preparation and resident mitochondria do not efficiently replace such loss, at least not within the experimental times used here. Once the need for the addition of exogenous ATP to the stimulation buffer was established, we optimized this method by performing dose response curves to ATP (Figure 2B) and insulin (Figure 2C) as well as an insulin time course (Figure 2D) on IR activation.
Using this method coupled to Western blot analysis, we were able to detect insulin-driven phosphorylation of the insulin receptor (IR) and obtain reliable results using synaptosomes isolated from both rats and mice. Also, effective phosphorylation of the IR in isolated synaptosomes could be observed using either insulin or IGF-1 (Supplemental Figure 1), further indicating the presence of a functional IR that responds to multiple ligands as it naturally does in the CNS (Denley et al., 2007).
We have also tested an alternative fractionation method using SynPER reagent (Thermo Scientific, Rockford, IL) for synaptosomal isolation that is significantly simpler as compared to the sucrose gradient method described above and can be completed in less than 2 hours. Using this alternative method, we observed post-synaptic enrichment in the synaptosomal protein fraction versus homogenate, indicating a satisfactory synaptosomal preparation that was further confirmed by direct observation using electron microscopy (Supplementary Figure 2). When synaptosomes prepared with the SynPER reagents were stimulated with insulin, we obtained phosphorylation of the insulin receptor that was similar to that observed in synaptosomes isolated using the sucrose-gradient method (Supplementary Figure 2). We conclude that, depending on specific needs and availability, both methods can be effectively used to prepare insulin-responsive synaptosomes from frozen brain tissue.
We further examined the effect on the response of the insulin receptor of storing isolated synaptosomes frozen between the fractionation and insulin stimulation steps. The use of a sucrose-based buffer in freezing has been reported to help prevent aggregation of the synaptosomes (Daniel, 2012). Therefore, we used the sucrose-based SET buffer to resuspend one of the samples before the slow-freezing process. This sample contained 5% DMSO due to the report that some find it to be an effective cryoprotectant (Daniel, 2012). However, there are mixed reviews where others find that DMSO greatly disrupts synaptosomal plasma membranes (Dodd, 1986). The synaptosomes underwent a rapid thaw, pelleting by centrifugation, and then resuspended in HBK buffer for the insulin stimulation experiment. HBK buffer was also tested as a slow-freeze buffer since it is the physiological buffer used for the insulin stimulation experiments. Our results showed that proper stimulation of IR phosphorylation can be achieved either in freshly prepared synaptosomes or in synaptosomes that have been stored frozen after isolation, suggesting that freezing synaptosomes for later use does not affect their responsivity to insulin in terms of IR phosphorylation. However, due to our results that showed a higher response of the IR after freezing (Figure 3), it is recommended to use the same conditions in storing synaptosomes within the same experiment.
Lastly, we performed a post-mortem interval experiment to determine the impact of varying postmortem times prior to tissue collection (Figure 4). We found no difference in the extent of insulin-promoted IR phosphorylation in synaptosomes prepared from tissue obtained up to 21 hours of PMI, suggesting a remarkable resistance of the synaptosomal IR to post-mortem degradation/proteolytic events. Regardless of the involved molecular mechanism, these results indicate that quick collection and freezing of tissue, while always good practice, is not necessarily a critical step for obtaining insulin-responsive synaptosomes.
In conclusion, we have developed a reliable, efficient method to measure insulin-driven ex vivo phosphorylation of the synaptosomal insulin receptor that can reliably reflect the pre-existing insulin responsiveness status in the CNS of the animal (Figure 5). To the best of our knowledge, this is the first evidence of stimulation of isolated synaptosomes with insulin and a promising new technique to study the synaptic CNS insulin responsiveness under physiological or disease conditions.
Supplementary Material
Highlights.
Ex vivo insulin stimulation of synaptosomes allows for detection of IR activation.
We optimized the method with insulin and ATP dose and time-response curves.
Ex vivo stimulation reliably reflects the pre-existing insulin responsiveness state.
Acknowledgments
This work was supported by NIH/NIA grant 1R01AG042890 and start-up funds from the Mitchell Center for Neurodegenerative Diseases. We would like to thank Michael Woodson from the University of Texas Medical Branch at Galveston for his contribution and help to generate the electron microscopy images.
Footnotes
CONFLICT OF INTEREST
None to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Daniel JA, Malladi CS, Kettle E, McCluskey A, Robinson PJ. Analysis of synaptic vesicle endocytosis in synaptosomes by high-content screening. Nat Protoc. 2012 Jul 5;7(8):1439–55. doi: 10.1038/nprot.2012.070. [DOI] [PubMed] [Google Scholar]
- De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, Viola KL, Zhao WQ, Ferreira ST, Klein WL. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci U S A. 2009 Feb 10;106(6):1971–6. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denley A, Carroll JM, Brierley GV, Cosgrove L, Wallace J, Forbes B, Roberts CT., Jr Differential activation of insulin receptor substrates 1 and 2 by insulin-like growth factor-activated insulin receptors. Mol Cell Biol. 2007 May;27(10):3569–77. doi: 10.1128/MCB.01447-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd PR, Hardy JA, Baig EB, Kidd AM, Bird ED, Watson WE, Johnston GA. Optimization of freezing, storage, and thawing conditions for the preparation of metabolically active synaptosomes from frozen rat and human brain. Neurochem Pathol. 1986 Jun;4(3):177–98. doi: 10.1007/BF02834357. [DOI] [PubMed] [Google Scholar]
- Sallam HS, Tumurbaatar B, Zhang WR, Tuvdendorj D, Chandalia M, Tempia F, Laezza F, Taglialatela G, Abate N. Peripheral adipose tissue insulin resistance alters lipid composition and function of hippocampal synapses. J Neurochem. 2015 Apr;133(1):125–33. doi: 10.1111/jnc.13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdile G, Fuller SJ, Martins RN. The role of type 2 diabetes in neurodegeneration. Neurobiol Dis. 2015 Apr 26; doi: 10.1016/j.nbd.2015.04.008. pii: S0969-9961(15)00155-2. [DOI] [PubMed] [Google Scholar]
- Zhao WQ, De Felice FG, Fernandez S, Chen H, Lambert MP, Quon MJ, Krafft GA, Klein WL. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 2008 Jan;22(1):246–60. doi: 10.1096/fj.06-7703com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.