Abstract
Purpose
Multidrug resistance (MDR) is a major obstacle to the successful treatment of osteosarcoma with chemotherapy. Effectiveness of cancer therapy correlates with the ability to induce a p53-dependent apoptotic response. p53 is a tumor suppressor gene that is mutated in 22% of osteosarcomas. While impaired p53 has been implicated in the oncogenesis of osteosarcoma, it is unclear whether overexpression of wild type p53 can increase chemosensitivity in MDR osteosarcoma cells.
Methods
We transfected a plasmid encoding the wild type p53 gene to MDR osteosarcoma cell lines, which have different p53 statuses, U-2OSR2 with wild type p53 (Wt-p53) and KHOSR2 with mutant p53 (Mt-p53), and determined the effect of p53 overexpression on chemosensitivities.
Results
Both of the U-2OSR2 and KHOSR2 cell lines displayed similar trends in p53 induced drug sensitivities. However, it seems that the impact of p53 overexpression is different based on the differential intrinsic p53 status in these cell lines. In the KHOSR2 cell line (Mt-p53), overexpression of p53 up-regulates the expression of pro-apoptotic protein p21 and Bax, while in the U-2OSR2 cell line (Wt-p53), overexpression of p53 down-regulates IGF-1r expression significantly.
Conclusions
These results demonstrated that tansfection of wild type p53 increases chemosensitivity through inhibiting either IGF-1r or through increasing the expression of pro-apoptotic proteins p21 and Bax in human MDR osteosarcoma cell lines.
Keywords: Osteosarcoma, p53, MDR, Apoptosis
Introduction
Osteosarcoma is the most common malignant primary bone tumor characterized by malignant osteoid and osteoblastic differentiation, and commonly develops in children, adolescents, and young adults. At present, effective chemotherapeutic agents, routinely including high-dose methotrexate (MTX), cisplatin (DDP), and doxorubicin (ADM) combined with advanced surgical technology, is the main therapeutic method of osteosarcoma. The use of chemotherapy in treating osteosarcoma has improved the patient 5-year survival rate from 20% to 70% in the past 30 years. Unfortunately, the remaining 30% of patients eventually develop multidrug resistance (MDR). Moreover, the long-term survival of patients with relapse is less than 20% [1–3]. MDR has been identified as one of the mechanisms for the progression of resistance to not only the related drugs to which the patient has been exposed, but also to multiple other types of structurally and functionally unrelated drugs [4]. MDR is a major obstacle to the successful treatment of osteosarcoma with chemotherapy. The MDR phenotype has been associated with many dysregulated genes, such as MDR1, MRP, and LRP [5]. Other genes related to MDR include those involved in alterations in the drug target through mutation, DNA repair, and reduced uptake of the drug [6–8]. p53 is a tumor suppressor gene that is mutated in 22% of osteosarcomas and frequently undergoes rearrangement, resulting in loss of its expression. Few genes have been more extensively studied in tumor biology than p53. p53 is involved in multiple cellular processes, including growth arrest, senescence, apoptosis, and DNA repair. Recent evidence has also shown the important roles of p53 in drug chemosensitivity and drug resistance [9,10].
Most chemotherapeutic drugs cause DNA damage and induce apoptosis in tumor cells. p53 mutations have been shown to result in impaired apoptosis and DNA repair mechanisms. Effectiveness of cancer therapy correlates with the ability to induce a p53-dependent apoptotic response [11,12]. While impaired p53 has been implicated in the oncogenesis of osteosarcoma, it is unclear whether overexpression of wild type p53 can increase chemosensitivity in MDR osteosarcoma cells. In this study, we used a Lipofectamine LTX Plus-mediated transfection to overexpress a plasmid encoding wild type p53 gene in MDR osteosarcoma cell lines, and determined the effect of p53 overexpression on chemosensitivities.
Materials and methods
Cell Lines, cell culture, and drugs
Human osteosarcoma cell lines U-2OS and KHOS were purchased from the American Type Tissue Collection (Rockville, MD). Dr. Efstathios S. Gonos (National Health Research Foundation, Athens, Greece) kindly provided the human osteosarcoma multidrug resistant cell lines KHOSR2 and U-2OSR2 [13,14]. All cell lines were cultured in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 100-units/ml penicillin, and 100µg/ml streptomycin (Invitrogen). Cells were incubated at 37°C in 5% CO2-95% air atmosphere and passaged when near confluent monolayers were achieved using 2% trypsin-EDTA solution. Doxorubicin, cisplatin, and Taxol (paclitaxel) were obtained as unused residual clinical material at the Massachusetts General Hospital. Cells were free of mycoplasma contamination as tested by MycoAlert ® Mycoplasma Detection Kit from Cambrex (Rockland, ME).
Transfection of p53
In this study, the wild type p53 and green fluorescent protein (GFP) fusion protein expression vector pGFP-p53 was purchased from Addgene (Cambridge, MA, USA). This pGFP-p53 was initially constructed with human wild type p53 and cloned upstream of GFP (EGFP) in the pEGFP/N1 vector (Clontech, CA, USA). The GFP tag in this vector has been shown to not alter the functions of the wild type or mutant p53 in several previous studies [15–18]. U-2OSR2 (wild type p53, Wt-p53) and KHOSR2 (mutant p53, Mt-p53) cells were plated in 6-well plates or 96-well plates and cultured overnight. Before transfection, the cell growth medium was replaced with serum-free RPMI 1640, cultured for 3 hours, and then transfected with pGFP-p53 by Lipofectamine LTX Plus (Invitrogen) according to the manufacturer's protocols. A control transfection of the empty vector was also used. The expression of pGFP-p53 in the transfected cells was examined using fluorescence microscopy (Nikon Corp.) equipped with a SPOT RT digital camera (Diagnostic Instruments, Inc.).
Cell proliferation assay
Cell proliferation was determined by MTT assay. Briefly, 2 × 103 KHOSR2 or U-2OSR2 cells per well were plated in 96-well plates in culture medium (RPMI1640 supplemented with 10% FBS and penicillin/streptomycin). 1, 2, 3, and 4 days after transfection, cells were stained with MTT (Sigma, St Louis, MO, USA) at 37°C for 4 hours. The resulting formazan product was dissolved with acid-isopropanol and the absorbance at a wavelength of 490 nm (A490) was read on a SPECTRAmax Microplate Spectrophotometer (Molecular Devices, Sunnyvale, CA, USA) The absorbance values were normalized by assigning the value of the control line in the medium without drug to 1.0 and the value of the no cell control to 0. Experiments were done in triplicate. Cell growth curves were calculated with use of GraphPad PRISM 4 software (GraphPad Software, San Diego, CA, USA)
Cytotoxicity MTT assay
The drug cytotoxicities of Taxol, cisplatin, and doxorubicin were assessed in vitro using the MTT (obtained from Sigma-Aldrich, St. Louis, MO, USA) assay as previously described [14]. Briefly, 2 × 103 cells per well were plated in 96-well plates during this process. The second day after transfection, cytotoxic drugs were added. Cytotoxic drug dilutions were freshly prepared. The plates were incubated for another 5 or 6 days after chemotherapy drug was added. Drugs at the concentrations utilized in the MTT assay were performed in the absence of cells to verify no change in absorbance. After culturing for 7 days, 50 µl of MTT (5 mg/ml in PBS) was added to each well and incubated for 4 hours. After dissolving the resulting formazan product with acid-isopropanol, the absorbance was read at a wavelength of 490 nm (A490) on a SPECTRAmax Microplate Spectrophotometer (Molecular Devices). The concentration of drug causing 50% cell death (IC50 of the drug) was determined from a plot of % survival versus cytotoxic drug concentration. The absorbance values were normalized assigning the value of the parent line in media without drug to 1.0 and the value of the no-cell control to 0. Further experiments were performed in duplicate. Response curves were fitted using GraphPad PRISM 4 software (GraphPad Software).
Western blotting
Bax, p21(CIP1/WAF1), BcL-XL, PARP, IGF-1r, Pgp, and p53 proteins were analyzed in total cell lysates. Western blotting analysis was performed as previously described [14,19]. Protein lysates were harvested from osteosarcoma cells by using 1×RIPA Lysis Buffer (Upstate Biotechnology, Charlottesville, VA, USA), and the concentrations were determined using Protein Assay Reagent (Bio-Rad Laboratories, Hercules, CA, USA) and a spectrophotometer (Beckman DU-640, Beckman Instruments, Inc., Columbia, MD, USA). 40 µg of total protein was run on a Nu-Page 4–12% Bis–Tris Gel (Invitrogen) and transferred to a pure nitrocellulose membrane (Bio-Rad Laboratories). Primary antibodies directed against Bax, p21, BcL-XL, PARP, IGF-1r, p53, and actin, which were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA, USA), were incubated at 1:1000 dilution in Tris-buffered saline, pH 7.4, with 0.1% Tween-20 at 4°C overnight. After washing three times with TBST, they were further incubated with respective secondary antibodies (LI-COR Biosciences, Lincoln, NE, USA) in a dilution of 1:20,000 for 1 hour at room temperature. The membranes were washed again with TBST and rinsed with PBS. Finally, membranes were scanned using Odyssey CLx equipment (LI-COR Biosciences). Densitometric analysis of Western blot results was performed with Image J as described in the software’s User Guide. The relative expression of Pgp was normalized to its respective actin expression.
Statistical data analysis
Values shown are representative of triplicate determinations in two or more experiments. Treatment effects were evaluated using a two-sided Student’s t-test (GraphPadPRISM 4 software). Errors are SD of averaged results and p<0.05 values were accepted as a significant difference between means.
Results
Transfection of wild type p53 inhibits cell proliferation and induces apoptosis
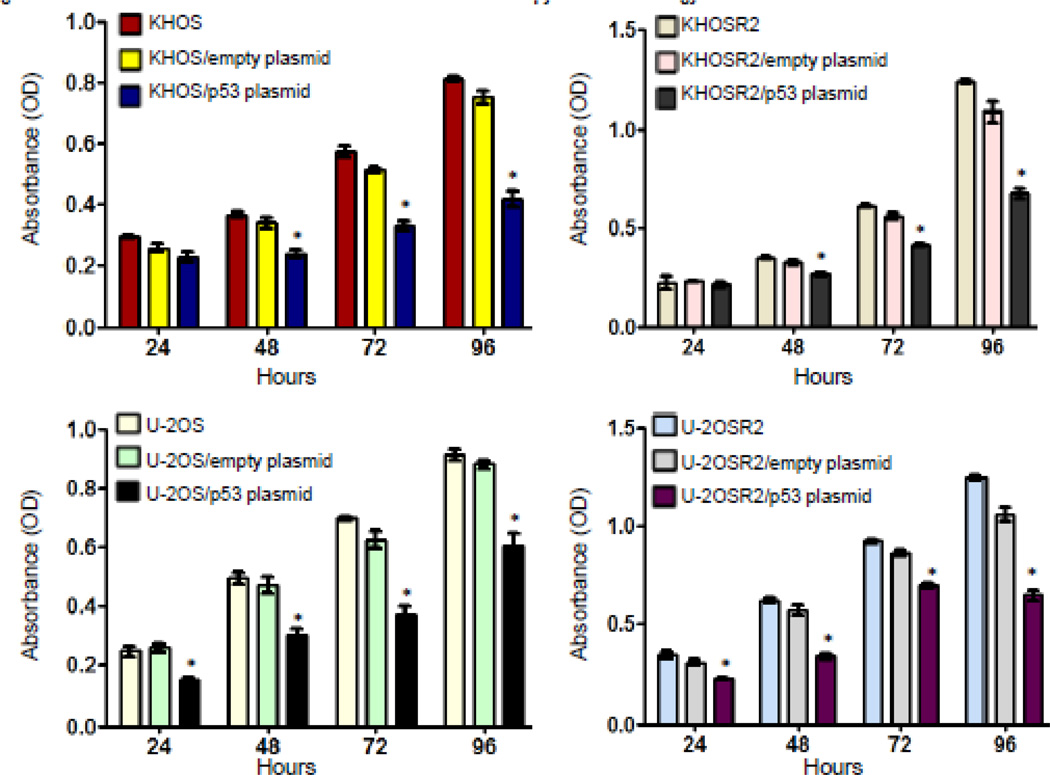
To determine the effect of overexpression of the wild type p53 gene on cellular proliferation and apoptosis, drug sensitive and resistant osteosarcoma cell lines were transfected with a p53 expression vector. Different time points (24, 48, 72 and 96 hours) were selected to detect the effect of p53 transfection on osteosarcoma cells. The results showed that after transfection with wild type p53 gene, all four of the different osteosarcoma cell lines, KHOS, KHOSR2, U-2OS, and U-2OSR2, exhibited growth inhibition and apoptosis. In each osteosarcoma cell line, transfection with the wild type p53 plasmid could remarkably inhibit cell proliferation (p<0.05), while transfection with empty plasmid showed almost no effect on cell growth (Figure 1). KHOSR2 and U-2OSR2 are both MDR osteosarcoma cell lines, which derive from KHOS and U-2OS osteosarcoma cell lines, respectively. Each has a different p53 status: U-2OS/U-2OSR2 with wild type p53, and KHOS/ KHOSR2 with mutant p53. KHOS/ KHOSR2 retain loss of function p53 mutation (nucleotide sequence: 467G>C; protein sequence: R156P) [13,20,21]. We found that, in spite of different p53 statuses, transfection of p53 resulted in significant cell growth arrest and induced apoptosis in both KHOS/KHOSR2 and U-2OS/U-2OSR2 paired osteosarcoma drug sensitive and resistant cell lines (Figure 1).
Fig. 1.
p53 transfection inhibits osteosarcoma cell proliferation. The wild type p53 and green fluorescent protein (GFP) fusion protein expression vector pGFP-p53 was transfected into U-2OS/U-2OSR2 (wild type p53, Wt-p53) and KHOS/KHOSR2 (mutant p53, Mt-p53) cells. Cells were plated in 96-well plates and cultured. Cell proliferation was determined by MTT assay. Results were calculated from five (n=5) repeated experiments with triplicate wells to reflect STD/SEM.
Transfection of wild type p53 increases chemosensitivity in human MDR osteosarcoma cell lines
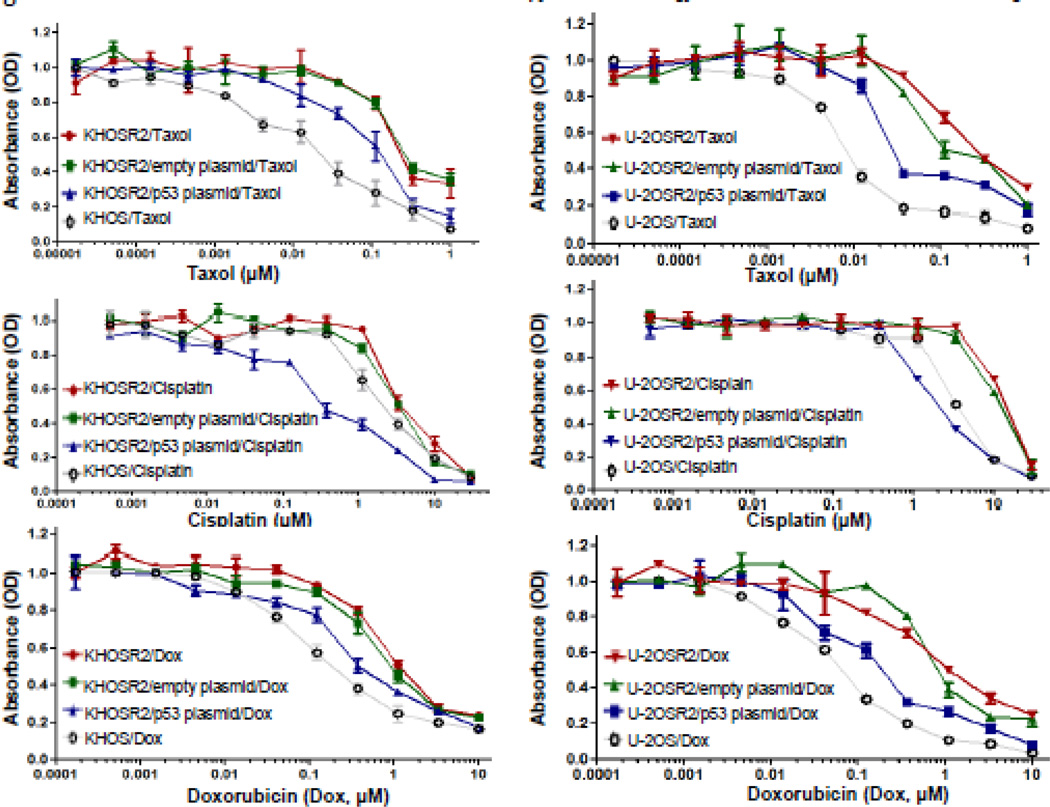
The chemosensitivities of Taxol, cisplatin, and doxorubicin in KHOSR2 and U-2OSR2 transfected wild type p53 were assessed by MTT assay. The parental drug sensitive KHOS and U-2OS cell lines were used as controls for comparison. The results showed that KHOSR2 and U-2OSR2 were MDR and resistant to Taxol, cisplatin, and doxorubicin as compared with KHOS and U-2OS. Transfection of wild type p53 gene showed significant reversal of drug resistance in both KHOSR2 and U-2OSR2 to Taxol, cisplatin, and doxorubicin (p<0.05). Both U-2OSR2 and KHOSR2 cell lines displayed similar trends in p53 induced drug sensitivities (Figure 2, Table 1). Overexpression of p53 increased the cisplatin sensitivity in KHOSR2 and U-2OSR2 even more than in KHOS and U-2OS cell lines (Figure 2, Table 1). Transfection of Wt-p53 significantly increased Taxol, cisplatin, and doxorubicin sensitivity in MDR osteosarcoma cell lines, despite of their different p53 statuses.
Fig. 2.
Overexpression of p53 increases Taxol, cisplatin, and doxorubicin sensitivity. The drug cytotoxicity of Taxol, cisplatin, and doxorubicin was assessed in vitro using the MTT assay. 2 × 103 cells per well were plated in 96-well plates during this process. The second day after transfection, cytotoxic drugs were added. After culturing for 7 days, 50 µl of MTT (5 mg/ml in PBS) was added to each well and incubated for 4 hours. After dissolving the resulting formazan product with acid-isopropanol, the absorbance (A490) was read on a SPECTRAmax Microplate Spectrophotometer at a wavelength of 490 nm. Results were calculated from five (n=5) repeated experiments with triplicate wells to reflect STD/SEM.
Table 1.
Effect of transfection of p53 on the drug sensitivities in osteosarcoma MDR cell lines
| Cell line | p53 status | Taxol (IC50) | Cisplatin (IC50) | Doxorubicin (IC50) |
|---|---|---|---|---|
| KHOSR2 (n=5) | Mt | 0.1559* | 3.468 | 0.709 |
| KHOSR2/empty plasmid (n=5) | Mt | 0.1504 | 3.12 | 0.614 |
| KHOSR2/p53 plasmid (n=5) | Mt +Wt | 0.097 | 0.673 | 0.343 |
| KHOSR2 (n=5) | Mt | 0.026 | 2.157 | 0.117 |
| U-2OSR2(n=5) | Wt | 0.1477 | 12.58 | 0.746 |
| U-2OSR2/empty plasmid (n=5) | Wt | 0.116 | 13.07 | 0.597 |
| U-2OSR2/p53 plasmid (n=5) | Wt + Mt | 0.019 | 1.908 | 0.133 |
| U-2OS (n=5) | Wt | 0.007 | 3.313 | 0.053 |
µM
Differential expression of pro-apoptotic proteins p21, Bax, and IGF-1r in p53 transfected MDR osteosarcoma cell lines
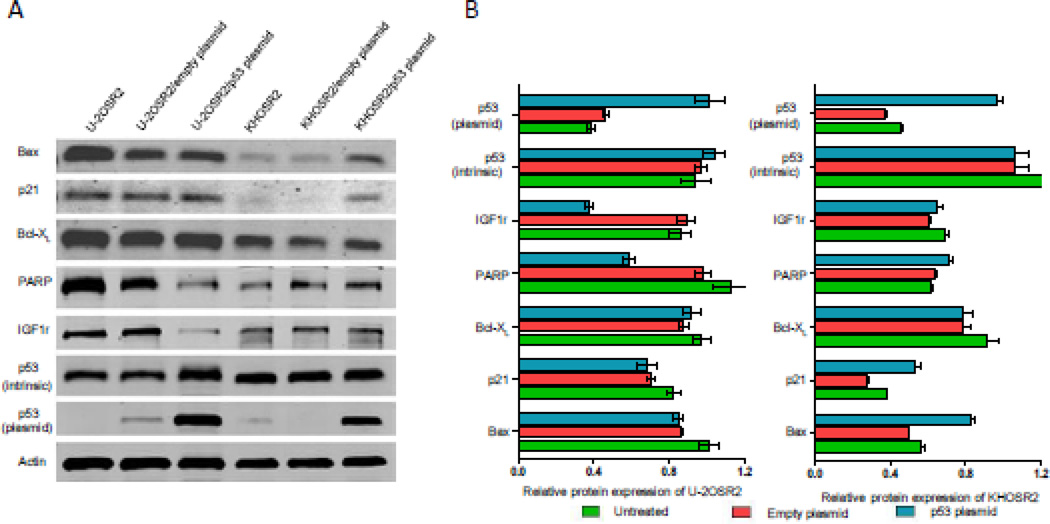
Bax, p21, BcL-XL, PARP, IGF-1r, Pgp, and p53 proteins were analyzed in total cell lysates of transfected cells. After transfection of p53, we observed a marked overexpression of exogenous p53 protein in both KHOSR2 and U-2OSR2 MDR cell lines, indicating a successful transfection of the p53 gene. Due to the differential intrinsic p53 statuses in these MDR cell lines, overexpression of p53 had different effects. In the KHOSR2 cell line with intrinsic mutant p53, overexpression of p53 up-regulated the expression of pro-apoptotic protein p21 and Bax, while in the U-2OSR2 cell line with intrinsic wild type p53, overexpression of p53 significantly down-regulated IGF-1r expression (Figure 3). In both of these Pgp overexpressed MDR osteosarcoma cell lines, overexpression Wt-p53 had no effect on Pgp and phosphorylated p53 expression (data were not shown),
Fig. 3.
Differential expression of either IGF-1r or pro-apoptotic proteins p21, Bax in p53 transfected MDR osteosarcoma cell lines U-2OSR2 and KHOSR2. A: Bax, p21(CIP1/WAF1), BcL-XL, PARP, IGF-1r, Pgp, and p53 proteins were analyzed in total cell lysates. Protein lysates were harvested from osteosarcoma cells. 40 µg of total protein was run on a Nu-Page 4–12% Bis–Tris Gel (Invitrogen) and transferred to a pure nitrocellulose membrane (Bio-Rad Laboratories). Primary antibodies directed against Bax, p21, BcL-XL, PARP, IGF-1r, p53, and actin were incubated at 1:1000 dilution in Tris-buffered saline, pH 7.4, with 0.1% Tween-20 at 4°C overnight. After washing three times with TBST, they were further incubated with respective secondary antibodies (LI-COR Biosciences, Lincoln, NE, USA) in a dilution of 1:20,000 for 1 hour at room temperature. The membranes were washed again with TBST and rinsed with PBS. Finally, membranes were scanned using Odyssey CLx equipment (LI-COR Biosciences). B: Western blots from A were analyzed by densitometry as described in Materials and methods.
Discussion
The present study demonstrated that transfection of wild type p53 inhibits osteosarcoma cell growth and proliferation and induces apoptosis. Overexpression of p53 increases chemosensitivities to Taxol, cisplatin, and doxorubicin in human MDR osteosarcoma cell lines. We show by western blotting that different Pgp independent mechanisms may be responsible for p53-based drug sensitivities. In the MDR cell line U-2OSR2 with intrinsic wild type p53, the increased chemosensitivities may be due to the inhibition of IGF-1r expression by exogenous overexpression of p53; however, in the MDR cell line KHOSR2 with intrinsic mutant p53, the increased chemosensitivities may be due to up-regulated expression of pro-apoptotic protein p21 and Bax.
MDR is a phenomenon in which the tumor cells develop resistance to chemotherapeutic drugs that may exhibit completely different structures and mechanisms of action [19]. Many studies have investigated strategies on how to reduce the MDR of the tumor cells [22,23]. IGF signaling is regulated by many factors, which are typically altered in cancer and has been proven to lead to MDR [14,24]. For example, glioblastoma cells highly express IGF-1 [25]. Breast cancer can increase IGF ligand expression (IGF-2, IGF-2), which is related to malignant resistance and transformation [26]. IGF-1r protein and mRNA levels have been found to be more highly expressed in colorectal cancer MDR cell lines than in their parental lines [27]. IGF-1r has also been found to be overexpressed in other types of MDR tumors [26,11]. Inhibition of IGF-1r can increase the chemosensitivities in both drug sensitive and resistant tumor cells [4,26]. Moreover, the IGF binding proteins (IGFBPs) have been shown to be up-regulated and play a critical role in tumor cell growth and motility [28]; [29]. Six IGFBPs have been identified and found to be expressed in cancer tissues, but only IGFBP-3 and IGFBP-5 have acid-labile subunit (ALS) binding sites, which can form a trimeric complex of IGF/IGF-BP/ALS[29]. Interestingly, the IGFBP-3 gene is a target of the p53 gene [30]. p53 combines two elements on IGFBP-3 gene, which play roles in the induction of apoptosis by p53 [30,31].
The development of MDR is a significant reason for failure of chemotherapy efficacy in cancer treatment [3,5,22,23]. One mechanism by which tumor cells develop resistance to cytotoxic agents is related to the resistance of chemotherapeutic drug induced apoptosis, which is activated by a variety of pro-apoptosis genes[32,33]. The apoptosis deficiency is considered to be a major cause of MDR in human osteosarcoma. It has been confirmed that DNA damage and cellular stress factors involve activation of tumor suppressor p53. p53 induces transcriptional activation of pro-apoptotic genes such as Bax, Puma, and Fax. DNA damage agent-induced apoptosis is dependent on the p53 status in tumor cells [34,35]. After extensive and persistent damage to DNA, cells may undergo mitochondria-mediated apoptotic death. The exact molecular mechanisms of cytotoxic drug resistance have not been completely elucidated. However, it is generally accepted that multiple mechanisms may be responsible for the MDR phenotype exhibited in different tumor cell types [36],[37]. In our study, cisplatin sensitivity in KHOSR2 and U-2OSR2 MDR cell lines were significantly increased after p53 transfection, with greater increases seen in KHOSR2 cell lines. Because of the mutant p53 status in KHOSR2 cells, these data suggest chemotherapeutic drugs, such as cisplatin, and p53 may act together to amplify pro-apoptotic signaling by inhibiting both mitochondrial and DNA replication functions, leading to synthetic lethal effects in these MDR cells. The underlying mechanism for these observations may be mediated through Bax and p21 proteins, whose deficiencies are frequently seen in many drug resistant tumors [38–40]. Loss of Bax has been shown to lead to complete resistance to cisplatin [41,42]. p21 (CIP1/WAF1) was initially identified as a potent cyclin-dependent kinase (CDK) inhibitor (CKI). The p21 protein binds to and inhibits the activity of CDK1, CDK2, and CDK4/6 complexes, and thus functions as a regulator of cell cycle progression at G1 and S phases [43]. In addition to growth arrest, p21 can mediate cellular senescence and drug resistance [44,45]. Several studies have shown that p21 can inhibit both proliferating cell nuclear antigens and CDKs, leading to subsequent cell cycle arrest in G1-, G2-, or S-phase [46–49]. These results support the hypothesis that MDR osteosarcoma would potentially show an improved response to a p53/chemotherapy drug combination treatment.
Several studies have reported that mutant p53 may gain some new functions in the induction of drug resistance in cancer cells. Knock in mutant p53 in mice shows a gain functions in vivo [50,51]. Expression of multidrug resistance gene 1 (MDR1) in drug resistant cells is highly associated with p53 mutations [52,53]. Transfection of mutant p53 (p53-V143A) into human ovarian carcinoma A2780 cells induced significant resistance to radiation, cisplatin, doxorubicin, and cytarabine [54]. Knockdown of mutant p53 decreased the resistance of human colon cancer HT29 cells to doxorubicin, cisplatin, and etoposide [55]. In this study, we also found gain-of-function mechanisms induced by overexpression of p53 in osteosarcoma drug resistant cell lines KHOSR2 and U-2OSR2. The results showed that overexpression of p53 increased chemosensitivity in these drug resistant cells by up-regulating the expression of pro-apoptotic protein p21 and Bax in KHOSR2, and down-regulating IGF-1r expression in U-2OSR2, respectively.
In conclusion, our study identifies that transfection of wild type p53 increases chemosensitivity through inhibiting either IGF-1r or through increasing the expression of pro-apoptotic proteins p21 and Bax in human MDR osteosarcoma cell lines. Although other mechanisms may also exist in MDR osteosarcoma, our current study provides a potential strategy toward the reversal or prevention of the MDR in osteosarcoma. Considering that the development of MDR is a major obstacle in the treatment of osteosarcoma, further preclinical investigations, including animal experiments, are warranted to test whether observations in this study in vitro can also effectively reverse MDR in osteosarcoma mouse models in vivo.
Acknowledgements
This work was supported in part by grants from the Gattegno and Wechsler funds, the Stanton Foundation. Dr. Duan is supported, in part, through a grant from Sarcoma Foundation of America (SFA), a grant from National Cancer Institute (NCI)/National Institutes of Health (NIH), UO1, CA151452-01, a pilot grant from Sarcoma SPORE/NIH, and a grant from an Academic Enrichment Fund of MGH Orthopedic Surgery. The authors thank Yan Gao for assistance with densitometric analysis of Western blot results by Image J.
Footnotes
DISCLOSURES: None
Ethical Responsibilities of Authors and Compliance with Ethical Standards: The authors declare that they have no conflict of interest.
References
- 1.Chou AJ, Gorlick R. Chemotherapy resistance in osteosarcoma: current challenges and future directions. Expert review of anticancer therapy. 2006;6(7):1075–1085. doi: 10.1586/14737140.6.7.1075. [DOI] [PubMed] [Google Scholar]
- 2.Chou AJ, Merola PR, Wexler LH, Gorlick RG, Vyas YM, Healey JH, LaQuaglia MP, Huvos AG, Meyers PA. Treatment of osteosarcoma at first recurrence after contemporary therapy: the Memorial Sloan-Kettering Cancer Center experience. Cancer. 2005;104(10):2214–2221. doi: 10.1002/cncr.21417. [DOI] [PubMed] [Google Scholar]
- 3.Hawkins DS, Arndt CA. Pattern of disease recurrence and prognostic factors in patients with osteosarcoma treated with contemporary chemotherapy. Cancer. 2003;98(11):2447–2456. doi: 10.1002/cncr.11799. [DOI] [PubMed] [Google Scholar]
- 4.Kartner N, Riordan JR, Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983;221(4617):1285–1288. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Tomas R. Multidrug resistance: retrospect and prospects in anti-cancer drug treatment. Current medicinal chemistry. 2006;13(16):1859–1876. doi: 10.2174/092986706777585077. [DOI] [PubMed] [Google Scholar]
- 6.Ross DD. Novel mechanisms of drug resistance in leukemia. Leukemia. 2000;14(3):467–473. doi: 10.1038/sj.leu.2401694. [DOI] [PubMed] [Google Scholar]
- 7.Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer treatment reviews. 2012;38(7):904–910. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Susa M, Milane L, Amiji MM, Hornicek FJ, Duan Z. Nanoparticles: a promising modality in the treatment of sarcomas. Pharmaceutical research. 2011;28(2):260–272. doi: 10.1007/s11095-010-0173-z. [DOI] [PubMed] [Google Scholar]
- 9.Duffy MJ, Synnott NC, McGowan PM, Crown J, O'Connor D, Gallagher WM. p53 as a target for the treatment of cancer. Cancer treatment reviews. 2014;40(10):1153–1160. doi: 10.1016/j.ctrv.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Ozaki T, Nakagawara A. p53: the attractive tumor suppressor in the cancer research field. Journal of biomedicine & biotechnology. 2011;2011:603925. doi: 10.1155/2011/603925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(23):8204–8209. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selvarajah J, Nathawat K, Moumen A, Ashcroft M, Carroll VA. Chemotherapy-mediated p53-dependent DNA damage response in clear cell renal cell carcinoma: role of the mTORC1/2 and hypoxia-inducible factor pathways. Cell death & disease. 2013;4:e865. doi: 10.1038/cddis.2013.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lourda M, Trougakos IP, Gonos ES. Development of resistance to chemotherapeutic drugs in human osteosarcoma cell lines largely depends on up-regulation of Clusterin/Apolipoprotein J. International journal of cancer Journal international du cancer. 2007;120(3):611–622. doi: 10.1002/ijc.22327. [DOI] [PubMed] [Google Scholar]
- 14.Duan Z, Choy E, Harmon D, Yang C, Ryu K, Schwab J, Mankin H, Hornicek FJ. Insulin-like growth factor-I receptor tyrosine kinase inhibitor cyclolignan picropodophyllin inhibits proliferation and induces apoptosis in multidrug resistant osteosarcoma cell lines. Molecular cancer therapeutics. 2009;8(8):2122–2130. doi: 10.1158/1535-7163.MCT-09-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyd SD, Tsai KY, Jacks T. An intact HDM2 RING-finger domain is required for nuclear exclusion of p53. Nature cell biology. 2000;2(9):563–568. doi: 10.1038/35023500. [DOI] [PubMed] [Google Scholar]
- 16.Castro ME, Leal JF, Lleonart ME, Ramon YCS, Carnero A. Loss-of-function genetic screening identifies a cluster of ribosomal proteins regulating p53 function. Carcinogenesis. 2008;29(7):1343–1350. doi: 10.1093/carcin/bgm302. [DOI] [PubMed] [Google Scholar]
- 17.Mossalam M, Matissek KJ, Okal A, Constance JE, Lim CS. Direct induction of apoptosis using an optimal mitochondrially targeted p53. Molecular pharmaceutics. 2012;9(5):1449–1458. doi: 10.1021/mp3000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han L, Zhao J, Liu J, Duan XL, Li LH, Wei XF, Wei Y, Liang XJ. A universal gene carrier platform for treatment of human prostatic carcinoma by p53 transfection. Biomaterials. 2014;35(9):3110–3120. doi: 10.1016/j.biomaterials.2013.12.048. [DOI] [PubMed] [Google Scholar]
- 19.Duan Z, Li X, Huang H, Yuan W, Zheng SL, Liu X, Zhang Z, Choy E, Harmon D, Mankin H, Hornicek F. Synthesis and evaluation of (2-(4-methoxyphenyl)-4-quinolinyl)(2-piperidinyl)methanol (NSC23925) isomers to reverse multidrug resistance in cancer. Journal of medicinal chemistry. 2012;55(7):3113–3121. doi: 10.1021/jm300117u. [DOI] [PubMed] [Google Scholar]
- 20.David-Pfeuty T, Chakrani F, Ory K, Nouvian-Dooghe Y. Cell cycle-dependent regulation of nuclear p53 traffic occurs in one subclass of human tumor cells and in untransformed cells. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 1996;7(9):1211–1225. [PubMed] [Google Scholar]
- 21.Ganjavi H, Gee M, Narendran A, Parkinson N, Krishnamoorthy M, Freedman MH, Malkin D. Adenovirus-mediated p53 gene therapy in osteosarcoma cell lines: sensitization to cisplatin and doxorubicin. Cancer gene therapy. 2006;13(4):415–419. doi: 10.1038/sj.cgt.7700909. [DOI] [PubMed] [Google Scholar]
- 22.Yusuf RZ, Duan Z, Lamendola DE, Penson RT, Seiden MV. Paclitaxel resistance: molecular mechanisms and pharmacologic manipulation. Current cancer drug targets. 2003;3(1):1–19. doi: 10.2174/1568009033333754. [DOI] [PubMed] [Google Scholar]
- 23.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nature reviews Drug discovery. 2006;5(3):219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 24.Tognon CE, Sorensen PH. Targeting the insulin-like growth factor 1 receptor (IGF1R) signaling pathway for cancer therapy. Expert opinion on therapeutic targets. 2012;16(1):33–48. doi: 10.1517/14728222.2011.638626. [DOI] [PubMed] [Google Scholar]
- 25.Pan Y, Trojan J, Guo Y, Anthony DD. Rescue of MHC-1 antigen processing machinery by down-regulation in expression of IGF-1 in human glioblastoma cells. PloS one. 2013;8(3):e58428. doi: 10.1371/journal.pone.0058428. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Davison Z, de Blacquiere GE, Westley BR, May FE. Insulin-like growth factor-dependent proliferation and survival of triple-negative breast cancer cells: implications for therapy. Neoplasia. 2011;13(6):504–515. doi: 10.1593/neo.101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li R, Pourpak A, Morris SW. Inhibition of the insulin-like growth factor-1 receptor (IGF1R) tyrosine kinase as a novel cancer therapy approach. Journal of medicinal chemistry. 2009;52(16):4981–5004. doi: 10.1021/jm9002395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baxter RC. IGF binding proteins in cancer: mechanistic and clinical insights. Nature reviews Cancer. 2014;14(5):329–341. doi: 10.1038/nrc3720. [DOI] [PubMed] [Google Scholar]
- 29.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nature reviews Cancer. 2008;8(12):915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 30.Ho L, Stojanovski A, Whetstone H, Wei QX, Mau E, Wunder JS, Alman B. Gli2 and p53 cooperate to regulate IGFBP-3- mediated chondrocyte apoptosis in the progression from benign to malignant cartilage tumors. Cancer cell. 2009;16(2):126–136. doi: 10.1016/j.ccr.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Shen L, Dean NM, Glazer RI. Induction of p53-dependent, insulin-like growth factor-binding protein-3-mediated apoptosis in glioblastoma multiforme cells by a protein kinase Calpha antisense oligonucleotide. Molecular pharmacology. 1999;55(2):396–402. doi: 10.1124/mol.55.2.396. [DOI] [PubMed] [Google Scholar]
- 32.Gimenez-Bonafe P, Tortosa A, Perez-Tomas R. Overcoming drug resistance by enhancing apoptosis of tumor cells. Current cancer drug targets. 2009;9(3):320–340. doi: 10.2174/156800909788166600. [DOI] [PubMed] [Google Scholar]
- 33.Ketley NJ, Allen PD, Kelsey SM, Newland AC. Mechanisms of resistance to apoptosis in human AML blasts: the role of differentiation-induced perturbations of cell-cycle checkpoints. Leukemia. 2000;14(4):620–628. doi: 10.1038/sj.leu.2401715. [DOI] [PubMed] [Google Scholar]
- 34.Le Bras M, Rouy I, Brenner C. The modulation of inter-organelle cross-talk to control apoptosis. Med Chem. 2006;2(1):1–12. doi: 10.2174/157340606775197787. [DOI] [PubMed] [Google Scholar]
- 35.Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends in molecular medicine. 2006;12(9):440–450. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31(15):1869–1883. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 37.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nature reviews Drug discovery. 2005;4(4):307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 38.Miao ZH, Tong LJ, Zhang JS, Han JX, Ding J. Characterization of salvicine-resistant lung adenocarcinoma A549/SAL cell line. International journal of cancer Journal international du cancer. 2004;110(5):627–632. doi: 10.1002/ijc.20026. [DOI] [PubMed] [Google Scholar]
- 39.Mayer F, Stoop H, Scheffer GL, Scheper R, Oosterhuis JW, Looijenga LH, Bokemeyer C. Molecular determinants of treatment response in human germ cell tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9(2):767–773. [PubMed] [Google Scholar]
- 40.Kim CW, Lu JN, Go SI, Jung JH, Yi SM, Jeong JH, Hah YS, Han MS, Park JW, Lee WS, Min YJ. p53 restoration can overcome cisplatin resistance through inhibition of Akt as well as induction of Bax. International journal of oncology. 2013;43(5):1495–1502. doi: 10.3892/ijo.2013.2070. [DOI] [PubMed] [Google Scholar]
- 41.Cho HJ, Kim JK, Kim KD, Yoon HK, Cho MY, Park YP, Jeon JH, Lee ES, Byun SS, Lim HM, Song EY, Lim JS, Yoon DY, Lee HG, Choe YK. Upregulation of Bcl-2 is associated with cisplatin-resistance via inhibition of Bax translocation in human bladder cancer cells. Cancer letters. 2006;237(1):56–66. doi: 10.1016/j.canlet.2005.05.039. [DOI] [PubMed] [Google Scholar]
- 42.Sugimoto C, Fujieda S, Seki M, Sunaga H, Fan GK, Tsuzuki H, Borner C, Saito H, Matsukawa S. Apoptosis-promoting gene (bax) transfer potentiates sensitivity of squamous cell carcinoma to cisplatin in vitro and in vivo. International journal of cancer Journal international du cancer. 1999;82(6):860–867. doi: 10.1002/(sici)1097-0215(19990909)82:6<860::aid-ijc15>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 43.Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer research. 2005;65(10):3980–3985. doi: 10.1158/0008-5472.CAN-04-3995. [DOI] [PubMed] [Google Scholar]
- 44.Mirzayans R, Andrais B, Scott A, Wang YW, Murray D. Ionizing radiation-induced responses in human cells with differing TP53 status. International journal of molecular sciences. 2013;14(11):22409–22435. doi: 10.3390/ijms141122409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu S, Bishop WR, Liu M. Differential effects of cell cycle regulatory protein p21(WAF1/Cip1) on apoptosis and sensitivity to cancer chemotherapy. Drug resistance updates : reviews and commentaries in antimicrobial and anticancer chemotherapy. 2003;6(4):183–195. doi: 10.1016/s1368-7646(03)00044-x. [DOI] [PubMed] [Google Scholar]
- 46.Waga S, Hannon GJ, Beach D, Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994;369(6481):574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- 47.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 48.Niculescu AB, 3rd, Chen X, Smeets M, Hengst L, Prives C, Reed SI. Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Molecular and cellular biology. 1998;18(1):629–643. doi: 10.1128/mcb.18.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogryzko VV, Wong P, Howard BH. WAF1 retards S-phase progression primarily by inhibition of cyclin-dependent kinases. Molecular and cellular biology. 1997;17(8):4877–4882. doi: 10.1128/mcb.17.8.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM, Valentin-Vega YA, Terzian T, Caldwell LC, Strong LC, El-Naggar AK, Lozano G. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell. 2004;119(6):861–872. doi: 10.1016/j.cell.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Olive KP, Tuveson DA, Ruhe ZC, Yin B, Willis NA, Bronson RT, Crowley D, Jacks T. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell. 2004;119(6):847–860. doi: 10.1016/j.cell.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Linn SC, Honkoop AH, Hoekman K, van der Valk P, Pinedo HM, Giaccone G. p53 and P-glycoprotein are often co-expressed and are associated with poor prognosis in breast cancer. British journal of cancer. 1996;74(1):63–68. doi: 10.1038/bjc.1996.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sampath J, Sun D, Kidd VJ, Grenet J, Gandhi A, Shapiro LH, Wang Q, Zambetti GP, Schuetz JD. Mutant p53 cooperates with ETS and selectively up-regulates human MDR1 not MRP1. The Journal of biological chemistry. 2001;276(42):39359–39367. doi: 10.1074/jbc.M103429200. [DOI] [PubMed] [Google Scholar]
- 54.Vasey PA, Jones NA, Jenkins S, Dive C, Brown R. Cisplatin, camptothecin, and taxol sensitivities of cells with p53-associated multidrug resistance. Molecular pharmacology. 1996;50(6):1536–1540. [PubMed] [Google Scholar]
- 55.Bossi G, Lapi E, Strano S, Rinaldo C, Blandino G, Sacchi A. Mutant p53 gain of function: reduction of tumor malignancy of human cancer cell lines through abrogation of mutant p53 expression. Oncogene. 2006;25(2):304–309. doi: 10.1038/sj.onc.1209026. [DOI] [PubMed] [Google Scholar]