Abstract
Aims
To measure the systemic retention of nicotine, propylene glycol (PG), and vegetable glycerin (VG) in electronic cigarette (e-cigarette) users, and assess the abuse liability of e-cigarettes by characterizing nicotine pharmacokinetics.
Design
E-cigarette users recruited over the Internet participated in a 1-day research ward study. Subjects took 15 puffs from their usual brand of e-cigarette. Exhaled breath was trapped in gas-washing bottles and blood was sampled before and several time after use.
Setting
San Francisco, California, USA.
Participants
Thirteen healthy, experienced adult e-cigarette users (6 females and 7 males).
Measurements
Plasma nicotine was analyzed by GC-MS/MS, and nicotine, VG, and PG in e-liquids and gas traps were analyzed by LC-MS/MS. Heart rate changes and subjective effects were assessed.
Findings
E-cigarettes delivered an average of 1.3 (0.9–1.8) mg (mean and 95% CI) of nicotine and 94% of the inhaled dose, 1.2 (0.8–1.7), was systemically retained. Average maximum plasma nicotine concentration (Cmax) was 8.4 (5.4–11.5) ng/mL and time of maximal concentration (Tmax) was 2 to 5 minutes; one participant had Tmax of 30 minutes. 89% and 92% of VG and PG, respectively, was systemically retained. Heart rate increased by an average of 8.0 bpm after 5 minutes. Withdrawal and urge to smoke decreased and the e-cigarettes were described as satisfying.
Conclusions
E-cigarettes can deliver levels of nicotine that are comparable to or higher than typical tobacco cigarettes, with similar systemic retention. Although the average maximum plasma nicotine concentration in experienced e-cigarettes users appears to be generally lower than what has been reported from tobacco cigarette use, the shape of the pharmacokinetic curve is similar, suggesting addictive potential.
Keywords: E-cigarette, nicotine pharmacokinetics, nicotine retention, vegetable glycerin, propylene glycol, addiction, abuse liability
INTRODUCTION
Electronic cigarettes (e-cigarettes) have seen a considerable increase in awareness and use among adults and youth since the product entered the U.S. market in 2007 (1, 2). Despite this rapid rise in use and concerns about safety (3), e-cigarette manufacture, marketing, and sale are currently unregulated by the U.S. Food and Drug Administration (FDA) which has jurisdiction over tobacco products under the Family Smoking Prevention and Tobacco Control Act (4). The FDA has stated that it will assert jurisdiction over e-cigarettes but the final ruling has not been finalized (5).
In assessing the individual and population harms of e-cigarettes, which are key considerations in FDA’s final ruling on e-cigarettes, it is important to understand the potential abuse liability of these devices. Like other drugs/drug delivery devices, the expected population harm of e-cigarettes is the product of their toxicity, intensity of use, and prevalence of use (6). The abuse liability of e-cigarettes, determined primarily by nicotine delivery and pharmacokinetics (7), directly impacts both intensity and prevalence of use. A greater likelihood of abuse is associated with faster nicotine delivery (shorter time to peak concentration, Tmax) and greater amount of systemic nicotine absorption reflected in higher maximum blood nicotine concentration (Cmax) and area under the concentration-time curve (AUC) (6).
Some e-cigarettes are cigarette-like (cig-a-likes) with disposable or rechargeable cartridges (1st generation), tanks (2nd generation) which operate at higher power than cig-a-likes, and others are customizable advanced personal vaporizers. Early pharmacokinetic studies of e-cigarettes reported very low plasma nicotine levels, indicating low nicotine delivery (8, 9). A few later pharmacokinetic studies reported higher plasma nicotine levels among experienced e-cigarette users (10–12) but the plasma nicotine levels after 10–15 puffs have been lower than what has been reported from smoking a tobacco cigarette (~10–30 ng/mL) (13, 14). As the technology of e-cigarettes continue to evolve, their effectiveness as nicotine delivery devices is improving, thus the need for continued pharmacokinetic monitoring to assess their potential abuse liability.
The objectives of this study were to (1) measure delivery and systemic retention of nicotine, propylene glycol (PG), and vegetable glycerin (VG) from e-cigarettes and (2) assess the potential abuse liability of e-cigarettes by characterizing nicotine pharmacokinetics in experienced users. There are only a few pharmacokinetic studies of e-cigarettes and, to the best of our knowledge, no published study has determined the dose of nicotine retained from e-cigarette use (referred to as ‘vaping’). Systemic retentions of nicotine, VG, and PG also determine the amount of these constituents exhaled into the environment, which is relevant to the question of e-cigarette secondhand aerosol exposure.
METHODS
This study was part of a pilot project to examine the clinical pharmacology of e-cigarettes and consisted of a standardized e-cigarette session, reported here, and examination of use patterns during ad libitum access, which will be reported separately.
Subjects
A convenience sample of 17 healthy adult e-cigarette users was recruited and 13 subjects participated and complete the study. Participants were recruited via Craigslist.com and flyers. They were screened for eligibility at a clinical research facility. Exclusive e-cigarette users or dual users ≤ 5 tobacco cigarettes per day, who used e-cigarettes at least once daily for 3 months or more, and had saliva cotinine levels ≥30 ng/mL were eligible. Exclusion criteria included pregnancy, use of nicotine metabolism altering medications, user of zero-nicotine e-cigarettes, chronic diseases, and active substance abuse or dependence other than marijuana. The study was approved by the Committee on Human Research at the University of California, San Francisco. Written, informed consent was obtained from each participant and all participants were financially compensated.
Experimental procedure
Subjects came to the Clinical Research Center at the San Francisco General Hospital for a 1-day pharmacokinetic study. They came to the hospital the evening before and abstained from e-cigarettes and/or other tobacco products after 10 PM. Participants were awakened at 7:00 AM and an intravenous (IV) line for blood sampling was placed in the forearm at 8:00 AM followed by a light breakfast. Baseline blood was sampled and urine collected, subjective questionnaires were administered, and three heart rate measurements were made within 10 minutes by pulse oximeter (average was used as the baseline heart rate). At approximately 9:30 AM the participants were asked to use their usual brand of e-cigarette (and usual e-liquid in tanks and rebuildable atomizer models, RBA), which were supplied by the study. Participants took 15 puffs, one every 30 seconds (standardized session). Puff duration was not standardized. Participants exhaled through their mouth after each puff into a sterile polypropylene mouthpiece which was connected to 3 gas traps connected in series with silicone tubing. Each gas trap contained 50 mL of 0.2 N hydrochloric acid and a pump maintained a flow rate of 2 L per min through the traps. After the 15 puffs, participants abstained from e-cigarette use for 4 hours. During that time, blood was sampled at 2, 5, 15, 30, 45, 60, 90, 120, and 180 minutes and heart rate was measured at 5, 10, 15, 20, and 30 minutes. Subjective questionnaires were administered between the 5th and 15th minute blood samples. E-cigarettes were weighed before and after vaping using a microbalance (0.00001 g readability).
Gas trap validation
An initial test of the efficiency of nicotine trapping by the gas traps was performed before the study. Two NJOY disposables, 1 Blu eCigs disposable, 2 V2 Cigs, and a KangerTech ProTank 2 (three different nicotine-containing flavors) were individually vaped, 1 puff every 30 seconds for a total of 30 puffs, by machine at 2 L per min. E-cigarettes were weighed before and after vaping and the nicotine concentration of the e-liquids were measured. The amount of nicotine in the three traps and mouthpiece were measured. On average, 86.2 ± 5.6% (mean ± SD) (range 76–92%) of the vaped nicotine was recovered in the three traps and mouthpiece. We performed a second test of the trapping efficiency of nicotine, VG, and PG using each subject’s e-cigarette and e-liquid on the days of the pharmacokinetic study to get study e-cigarette-specific recovery averages. Each e-cigarette was machine-vaped as described before, this time 1 puff every 30 seconds for a total of 15 puffs. Average recoveries were as follows: nicotine, 86.8 ± 9.6% (range 75.0–100%); VG, 93.0 ± 10.3% (range 73.3–100%); and, PG, 86.7 ± 10.0% (range 72.7–100). We adjusted the amount of nicotine, VG, and PG measured after participants exhaled into the traps by dividing exhaled amounts by the respective average machine-derived recoveries.
Questionnaires
The Minnesota Nicotine Withdrawal Scale (MNWS) (15), the Questionnaire for Smoking Urges (QSU-Brief) modified for e-cigarettes (16), and the Positive and Negative Affect Scales (PANAS) (17) were used to measure nicotine withdrawal, craving, and positive and negative affective states, respectively, before and after e-cigarette use. The modified Cigarette Evaluation Questionnaire (mCEQ) (18), further modified for e-cigarettes, was used to measure reward after using the e-cigarette.
Analytical chemistry
Nicotine concentration in plasma was determined by GC-MS/MS, using our published GC-MS method (19), modified for tandem mass spectrometry for improved sensitivity. Nicotine was measured in the 0.02 N HCl trap solution from each trap and mouthpiece separately (and then summed to give total exhaled amount) and in e-liquids after dilution in 0.02 N HCl by LC-MS/MS using a method modified from a previous publication (20). Briefly, 100 μL aliquots were mixed with 100 μL of 100 mM pH 8.7 ammonium formate buffer and 50 μL of nicotine-d4 internal standard. Aliquots were injected onto a Water’s BEH C18 column (3X150 mm) under isocratic conditions (50% methanol 0.01M ammonium formate pH 8.7). Detection and quantitation was achieved on a Thermo Instruments Quantiva LC-MS/MS using the 163→130, 167→134 ion transitions for natural nicotine and the deuterium-labeled internal standard, respectively. The limit of quantitation (LOQ) was 0.5 ng/mL. VG and PG were quantified as the benzoate esters using PG-d6 and VG-d5 as internal standards using a modified method based on a previous publication (21). Briefly, aliquots of 0.02 N HCl trap and diluted e-liquids were mixed with internal standard and derivatized by vortexing the samples with benzoyl chloride, aqueous sodium hydroxide, and hexane. After evaporation of the hexane phase, the samples were reconstituted in 45% methanol 45% isopropanol and 10% water and separated by gradient elution on a Waters phenyl hexyl X-Select column (3X150 mm) with a water, methanol, isopropanol 10 mM ammonium formate solvent system. The following transitions for the ammonium adducts were monitored on the Thermo Instruments Quantiva: PG-d0: 302→163, PG-d6: 308→169, VG-d0: 422→283, and VG-d5: 427→288. The LOQ was 5 ug/mL for both PG and VG.
Pharmacokinetic analysis
Pharmacokinetic parameters were estimated from plasma nicotine concentrations using Phoenix WinNonlin 6.3 (Pharsight Corporation, Mountain View, CA). Tmax, Cmax, and AUC from 0 to infinity (AUC0→∞) were estimated using a noncompartmental model and trapezoidal rule. We corrected all measures for baseline values in order to assess the changes in plasma nicotine attributed to the study e-cigarettes only. This was done by estimating the plasma nicotine concentration derived from baseline levels at each sampling time-point using the formula Ct = C0e−Kt, where Ct is the estimated plasma nicotine concentration at a time-point after baseline, C0 is the baseline plasma nicotine concentration, K is the subject’s nicotine elimination rate constant, and t is the elapsed time after baseline.
Statistical analysis
The systemic retentions of nicotine, VG, and PG were calculated as follows: retention (%) = 100 × (amount delivered − amount exhaled)/amount delivered. The amount of nicotine, VG, and PG delivered (mg) were estimated as the amount of e-liquid vaped (mg) × the concentration of nicotine, VG, and PG in the e-liquid, respectively. To analyze changes in heart rate after e-cigarette use, a repeated measures one-way ANOVA was performed. Heart rate measured at 5, 10, 15, 20, and 30 minutes were compared to baseline heart rate and we used Tukey’s method to adjust the error rate. Changes in individual items and overall scores for MNWS, QSU, and PANAS were assessed using paired t test. All analyses were carried out using SAS v. 9.4 (SAS Institute, Inc. Cary, NC, USA). Statistical tests were considered significant at α<0.05.
RESULTS
Participant characteristics are presented in Table 1. Nine participants were self-reported exclusive e-cigarette users, confirmed by their low expired carbon monoxide (CO) levels at screening (range 1–4 ppm). Average saliva cotinine levels at screening was 212 ng/mL and did not differ between self-reported exclusive e-cigarette users (217 ng/mL) and dual electronic and tobacco cigarette users (199 ng/mL) (p = 0.79). Previous research has shown that cotinine levels in e-cigarette users are similar to those of tobacco cigarette smokers (22). Two participants used 1st generation e-cigarettes, 8 used 2nd generation tank devices, and 3 used RBAs (Table 2).
TABLE 1.
Demographic characteristics of study participants, tobacco cigarette use, and e-cigarette use at screening
| Subject | Sex | Age (years) | Race | BMI | Saliva cotinine (ng/mL) | Self-reported smoking status | Expired CO (ppm) | CPD | E-cig use (months) | E-cig puffs per session | E-cig puffs per day | Reason for vaping |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 56 | White | 19.0 | 505 | No | 2 | 0 | 12 | 10 | 300 | a, b, c |
| 2 | F | 31 | White | 17.9 | 432 | No | 3 | 0 | 18 | 30 | 200 | a, b, d |
| 3 | F | 19 | Asian | 22.0 | 181 | Yes | 10 | 2 | 3 | 20 | 80 | a, d |
| 4 | M | 30 | White | 26.1 | 233 | No | 2 | 0 | 12 | 3 | 50 | b, e |
| 5 | F | 53 | White | 24.3 | 303 | Yes | 10 | 3 | 3 | 6 | 24 | b |
| 6 | F | 23 | White | 18.7 | 110 | Yes | 9 | 1 | 5 | 15 | 100 | d |
| 7 | M | 40 | White | 32.9 | 231 | No | 1 | 0 | 11 | 3 | 300 | a, b, c |
| 8 | M | 29 | Asian | 25.0 | 201 | Yes | 3 | 0 | 5 | 15 | 20 | b |
| 9 | M | 36 | White | 31.8 | 246 | No | 1 | 0 | 3 | 5 | 45 | a, b |
| 10 | M | 35 | White | 26.4 | 32 | No | 4 | 0 | 4 | 2 | 170 | a, b |
| 11 | M | 34 | Mixed | 34.0 | 36 | No | 4 | 0 | 16 | 10 | 100 | a |
| 12 | F | 58 | White | 25.9 | 127 | No | 1 | 0 | 72 | 2 | 40 | f |
| 13 | M | 55 | Black | 27.8 | 116 | No | 2 | 0 | 36 | 4 | 70 | a |
|
| ||||||||||||
| Summary | 6F | 38.4 | 25.5 | 212 | 4 Yes | 4.0 | range | 15.4 | 9.1 | 115 | See notes below | |
| 7M | (13.1) | (5.3) | (140) | 9 No | (3.4) | 0–3 | (19.3) | (8.5) | (98) | |||
Notes:
reduce health risk;
quit smoking;
save money;
cut down on tobacco cigarette;
improve athletic performance;
never smoker who enjoys vaping; standard deviation in bracket
TABLE 2.
Participants’ usual e-cigarettes and e-liquids used in the study
| Subject | E-cigarette design | E-cigarette brand | e-Liquid Brand | Flavor | VG/PG ratio | Measured VG/PG ratio | Nicotine on label (mg/mL) | Measured nicotine (ug/mg) |
|---|---|---|---|---|---|---|---|---|
| 1 | Tank | Kanger EVOD2 | Velvet Cloud Vapor | White Beard Tobacco | Kosher VG | 92/8 | 24 | 15.3 |
| 2 | Cartridge | V2 Cigs Red 18 | n/a | Regular | not labeled | 25/75 | 18 | 13.9 |
| 3 | Tank | Kanger T3D | V Smoke | Watermelon Breeze | not labeled | 71/29 | 18 | 11.4 |
| 4 | RBA | Vulcan | Space Jam | Andromeda | VG/PG | 54/46 | 6 | 5.7 |
| 5 | Tank | Kanger T3D | Jackson Vapor | Peaches & Cream | not labeled | 72/28 | 18 | 15.3 |
| 6 | Tank | Kanger EVOD | Vapor All | French Vanilla | VG/PG | 37/63 | 12 | 8.6 |
| 7 | Tank | Kanger Aerotank V2 | It Is Vapor | Vanilla Custard | VG | 76/24 | 12 | 12.1 |
| 8 | RBA | K101 | CuttWood | Monster Mellon | VG/PG | 71/29 | 6 | 5.0 |
| 9 | Cartridge | Blu E-cigarette | n/a | Classic Tobacco | VG | 100/0 | 24 | 12.6 |
| 10 | Tank | Kanger EVOD | RY4 | Tobacco Flavor | VG | 73/27 | 6 | 5.6 |
| 11 | RBA | Nimbus | Dr. Picnic’s Magic Elixir | Teddy Bear | PG/VG | 69/31 | 6 | 5.0 |
| 12 | Tank | Vapor4Life | Nicoticket | The Virus (tobacco) | VG/PG | 43/57 | 6 | 5.9 |
| 13 | Tank | Kanger Protank II | Halo Purity | Torque56 (tobacco) | PG/Glycerin | 36/64 | 6 | 5.7 |
Notes: Kanger is short for KangerTech; RBA is rebuildable atomizer (all RBAs were ‘drippers’); n/a is not applicable; VG is vegetable glycerin; PG is propylene glycol
On average, 1.3 mg of nicotine (median 1.4, range 0.4–2.6 mg) was delivered in 169 mg of vaped e-liquid (median 210 mg, range 46–463 mg) from 15 puffs (Table 3). An average of 93.8% (median 99.6%, range 49.0–99.9%) or 1.2 mg of nicotine (median 1.1 mg, range 0.4–2.4 mg) was systemically retained. On average, 84.4% (median 94.8%, range 3.9–99.3%) of the delivered VG dose and 91.7% (median 98.3%, range 46.2–100%) of the delivered PG dose were systemically retained.
TABLE 3.
Nicotine delivered and retained in the body and changes in vegetable glycerin (VG)/propylene glycol (PG) ratios in e-liquid and exhaled aerosol
| Subject | E-liquid vaped (mg) |
Nicotine in e-liquid (ug/mg) |
Nicotine delivered (mg) |
Nicotine exhaled (mg) |
% Nicotine retained |
VG delivered (mg) |
PG delivered (mg) |
VG exhaled (mg) |
PG exhaled (mg) |
%VG retained |
%PG retained |
VG/PG ratio in exhalant |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 55.2 | 15.3 | 0.84 | 0.004 | 99.6 | 44.5 | 3.96 | 1.28 | 0.07 | 97.1 | 98.3 | 95/5 |
| 2 | 117.9 | 13.9 | 1.64 | 0.002 | 99.9 | 24.6 | 74.7 | 1.14 | 0.06 | 95.3 | 99.9 | 95/5 |
| 3 | 146.2 | 11.4 | 1.66 | 0.848 | 49.0 | 86.6 | 35.1 | 83.2 | 18.9 | 3.9 | 46.2 | 81/19 |
| 4 | 463.2 | 5.7 | 2.64 | 0.293 | 88.9 | 216.1 | 182.0 | 42.5 | 24.3 | 80.3 | 86.7 | 64/36 |
| 5 | 87.0 | 15.3 | 1.33 | 0.033 | 97.5 | 56.8 | 22.0 | 7.64 | 0.60 | 86.6 | 97.3 | 93/7 |
| 6 | n/a | 8.6 | n/a | 0.001 | n/a | n/a | n/a | 0.80 | 0.0 | n/a | n/a | n/a |
| 7 | 200.4 | 12.1 | 2.43 | 0.008 | 99.7 | 131.2 | 40.3 | 3.17 | 0.15 | 97.6 | 99.6 | 96/4 |
| 8 | 328.7 | 5.0 | 1.63 | 0.115 | 93.0 | 188.8 | 78.6 | 41.0 | 6.14 | 78.3 | 92.2 | 87/13 |
| 9 | 46.3 | 12.6 | 0.58 | 0.001 | 99.8 | 37.0 | 0.0 | 0.27 | - | 99.3 | - | 100/0 |
| 10 | 106.7 | 5.6 | 0.60 | 0.002 | 99.6 | 67.3 | 25.2 | 3.89 | 0.0 | 94.2 | 100.0 | 100/0 |
| 11 | 287.0 | 5.0 | 1.45 | 0.002 | 99.8 | 181.2 | 83.3 | 3.52 | 0.0 | 98.1 | 100.0 | 100/0 |
| 12 | 71.0 | 5.9 | 0.42 | 0.001 | 99.8 | 30.0 | 40.0 | 0.75 | 0.0 | 97.5 | 100.0 | 100/0 |
| 13 | 122.5 | 5.7 | 0.70 | 0.007 | 98.9 | 38.2 | 67.1 | 5.70 | 7.32 | 85.1 | 89.1 | 44/56 |
|
| ||||||||||||
| Average | 169.4 | 9.4 | 1.33 | 0.101 | 93.8 | 91.9 | 54.4 | 15.0 | 4.79 | 84.4 | 91.7 | |
| Median | 120.2 | 8.6 | 1.39 | 0.004 | 99.6 | 62.1 | 40.2 | 3.52 | 0.11 | 94.8 | 98.3 | |
| SD | 128.1 | 4.1 | 0.73 | 0.239 | 14.5 | 69.2 | 49.0 | 25.2 | 8.32 | 26.4 | 15.9 | |
| LCI | 88.0 | 6.9 | 0.87 | −0.04 | 84.6 | 47.9 | 23.2 | −0.24 | −0.50 | 67.6 | 81.1 | |
| UCI | 250.7 | 11.9 | 1.79 | 0.250 | 103.0 | 135.8 | 85.5 | 30.2 | 10.1 | 101.2 | 102.4 | |
Notes: SD, standard deviation; LCI and UCI, lower and upper limits of 95% confidence interval; post-weight for Subject 6’s e-cigarette was not determined due to e-liquid leaking from device
Cmax was within 2 to 5 minutes for all participants except one who had a Tmax of 30 minutes (Table 4). The average plasma nicotine Cmax was 8.4 ng/mL (median 5.1 ng/mL, range 2.3–19.8 ng/mL). In addition to computing the dose of nicotine retained in the body using the gas trap method, we also estimated the nicotine dose using AUC0→∞ and the average population clearance of nicotine, 1200 mL/min (13) as follows: PK-estimated dose = AUC0→∞ × 1200. The average PK-estimated nicotine dose was 1.0 mg (median 0.9 mg, range 0.3–2.2 mg) and was not significantly different from the nicotine dose determined by the gas trap method (p=0.41).
TABLE 4.
Nicotine pharmacokinetic profiles from various electronic cigarettes
| Subject | Half-life (min) | Tmax (min) | Cmax (ng/mL) | AUC(0→∞) (ng/mL•min) | Delivered nicotine dose (mg) | Retained nicotine dose (mg) | PK-estimated nicotine dose (mg) |
|---|---|---|---|---|---|---|---|
| 1 | 148 | 2 | 6.9 | 815 | 0.84 | 0.84 | 0.98 |
| 2 | 81 | 5 | 9.1 | 1345 | 1.64 | 1.63 | 1.61 |
| 3 | 87 | 30 | 2.3 | 301 | 1.66 | 0.82 | 0.36 |
| 4 | 152 | 5 | 8.9 | 1570 | 2.64 | 2.34 | 1.88 |
| 5 | 62 | 2 | 15.5 | 682 | 1.33 | 1.30 | 0.82 |
| 6 | 94 | 2 | 3.1 | 264 | n/a | n/a | 0.32 |
| 7 | 152 | 2 | 19.8 | 1813 | 2.43 | 2.42 | 2.18 |
| 8 | 132 | 5 | 8.7 | 1336 | 1.63 | 1.52 | 1.60 |
| 9 | 140 | 5 | 4.4 | 461 | 0.58 | 0.58 | 0.55 |
| 10 | 78 | 2 | 6.1 | 332 | 0.60 | 0.60 | 0.40 |
| 11 | 114 | 2 | 13.2 | 900 | 1.45 | 1.44 | 1.08 |
| 12 | 107 | 2 | 4.7 | 411 | 0.42 | 0.42 | 0.49 |
| 13 | 36 | 2 | 6.8 | 721 | 0.70 | 0.69 | 0.87 |
|
| |||||||
| Average | 106.3 | 5.1 | 8.4 | 843 | 1.33 | 1.22 | 1.01 |
| Median | 107 | 2 | 5.1 | 721 | 1.39 | 1.07 | 0.87 |
| SD | 37.2 | 7.6 | 5.1 | 519 | 0.73 | 0.68 | 0.62 |
| LCI | 84 | 0.5 | 5.4 | 529 | 0.87 | 0.80 | 0.63 |
| UCI | 129 | 9.7 | 11.5 | 1156 | 1.79 | 1.66 | 1.39 |
Notes: SD, standard deviation; LCI and UCI, lower and upper limits of 95% confidence interval; PK-estimated nicotine dose is average population clearance of nicotine (~1200 mL/min) × AUC(0–∞)
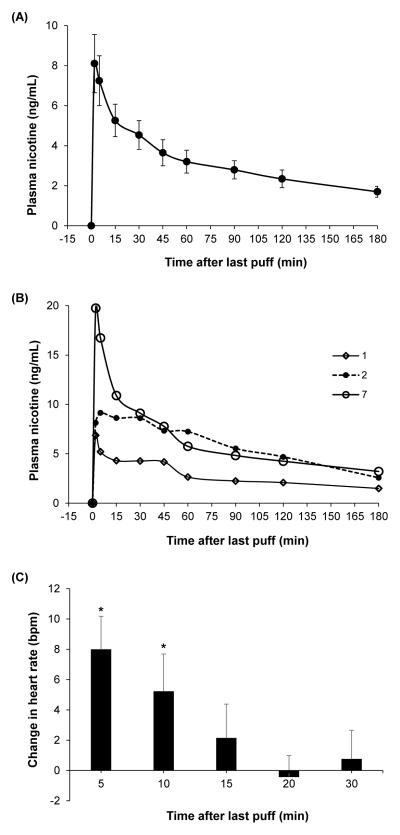
Figure 1A shows average plasma nicotine levels after e-cigarette use for all participants and Figure 1B shows plasma nicotine profiles of three subjects to illustrate the general shapes of the plasma nicotine curves observed. Compared to baseline, heart rate increased by an average of 8.0 bpm after 5 minutes (p<0.001), 5.2 bpm after 10 minutes (p=0.04), and was not significantly different after 15 minutes (Figure 1C).
FIGURE 1.
Average plasma nicotine, corrected for baseline level, (mean ± SEM) in experienced users after 15 puffs from their usual brand of e-cigarette (A); plasma nicotine profiles of three subjects (B); and average change (mean + SEM) in heart rate from baseline after e-cigarette use (C). * is significantly different from baseline (α<0.05).
The overall MNWS score decreased significantly from 7.8 to 4.8 (p=0.007) (Table 5). All individual and total QSU scores decreased significantly (p<0.05). PANAS-negative affect decreased significantly (p=0.03) while PANAS-positive affect remained unchanged. The mCEQ subscales (with maximum possible shown in [ ]) were as follows: Satisfaction [21], 16.5 ± 3.2 (mean ± SD); Reward [35], 21.3 ± 5.8; Aversion [14], 4.8 ± 2.4; Sensations [7], 4.3 ± 1.5; and, Craving Reduction [7], 5.0 (1.7).
TABLE 5.
Withdrawal and urge to use e-cigarette before and after 15 puffs
| Scale | Pre | Post | p-value |
|---|---|---|---|
| MNWS (0, none; 4, severe) | |||
| Angry, irritable, frustrated | 0.8 (1.0) | 0.2 (0.4) | 0.06 |
| Anxious, nervous | 1.0 (1.0) | 0.5 (0.8) | 0.05 |
| Depressed mood, sad | 0.2 (0.5) | 0.0 (0.0) | 0.19 |
| Desire or craving to smoke | 1.9 (1.2) | 0.7 (0.9) | 0.007 |
| Difficulty concentrating | 1.0 (1.0) | 0.6 (1.0) | 0.05 |
| Increased appetite, hungry | 0.3 (0.4) | 0.3 (0.5) | 1.00 |
| Restless | 1.0 (0.9) | 0.7 (0.9) | 0.17 |
| Impatient | 1.3 (1.1) | 0.5 (0.8) | 0.01 |
| Dizziness | 0.0 (0.0) | 0.8 (1.2) | 0.03 |
| Increased coughing | 0.1 (0.2) | 0.1 (0.3) | 1.00 |
| Nausea | 0.0 (0.0) | 0.2 (0.6) | 0.19 |
| Sorethroat | 0.2 (0.5) | 0.2 (0.4) | 0.34 |
| MNWS total score (of 48) | 7.8 (5.2) | 4.8 (4.2) | 0.007 |
| QSU (0, strongly disagree; 7, strongly agree) | |||
| Desire for e-cigarette now | 4.5 (1.7) | 2.5 (1.4) | 0.002 |
| Nothing better than to vape now | 3.9 (0.9) | 2.0 (1.0) | <0.001 |
| Would vape if possible | 5.8 (1.5) | 3.3 (2.1) | 0.002 |
| Would control thing better | 2.5 (1.5) | 1.5 (0.9) | 0.02 |
| All I want is an e-cigarette | 3.4 (1.3) | 1.6 (1.0) | <0.001 |
| Urge for e-cigarette | 4.6 (1.8) | 2.2 (1.5) | 0.002 |
| An e-cigarette would taste good | 5.1 (1.5) | 2.8 (1.6) | 0.001 |
| Would do anything for an e-cigarette | 2.4 (1.6) | 1.3 (0.6) | 0.03 |
| Vaping would make me less depressed | 2.3 (1.5) | 1.2 (0.6) | 0.02 |
| Will vape and soon as possible | 4.8 (1.8) | 3.3 (2.1) | 0.02 |
| QSU total score (of 7) | 3.2 (1.2) | 2.2 (1.1) | <0.001 |
| PANAS | |||
| PANAS positive affect score (of 50) | 26.4 (6.3) | 26.5 (6.8) | 0.99 |
| PANAS negative affect score (of 50) | 13.6 (3.7) | 11.2 (1.5) | 0.03 |
DISCUSSION
In our study, e-cigarettes delivered an average of 1.3 mg (range 0.4 to 2.6 mg) of nicotine from 15 puffs, similar to or higher than average reported yields of 0.5 to 1.5 mg nicotine per tobacco cigarette (13, 23, 24). We show, for the first time, that systemic retention of nicotine from e-cigarettes is high, averaging 94%, resulting in uptake of about 1.2 mg (0.4 to 2.4 mg) of nicotine from 15 puffs. Systemic retention of nicotine from tobacco cigarettes averages about 80 to 90% of the inhaled dose (13). Average Cmax after e-cigarette use was 8.4 ng/mL, which is lower than average levels from tobacco cigarettes but within the range of many smokers. Nicotine boost from tobacco cigarettes averages about 11 ng/mL (14, 25). We found that some e-cigarette users were able to attain tobacco cigarette-like peak plasma nicotine levels. Two subjects had Cmax (15.5 and 19.8 ng/mL) that were in the high end of the range of nicotine boost from tobacco cigarettes and another had a Cmax of 13.2 ng/mL. With one exception, Tmax was between 2 to 5 minutes. We present novel data showing VG and PG are also highly retained in the body, averaging 84% and 92%, respectively. Finally, consistent with other studies, e-cigarette use increased heart rate, and subjectively reduced withdrawal, urge to smoke, and was described as satisfying (10, 12, 26).
Nicotine from tobacco cigarettes is rapidly absorbed in the lungs, reaches the brain within seconds (27), and venous Cmax is seen within 5 to 8 minutes of initiating smoking (13). The rapid rise of blood and brain nicotine levels when delivered through the pulmonary route allows the tobacco cigarette smoker to titrate nicotine and related pharmacological effects while smoking. This makes tobacco cigarette smoking the most reinforcing form of nicotine delivery (13, 28). Except one, all participants had Cmax at 2 or 5 minutes after e-cigarette puffing, which is consistent with prior reports (10, 11, 29, 30). This indicates that nicotine from e-cigarettes is also rapidly absorbed in the lungs, allowing for nicotine titration through various user behaviors and is likely dependence-producing.
The extent to which nicotine from e-cigarettes is absorbed and the sites of absorption will affect the magnitude of nicotine’s associated effects on the user but remain important unanswered questions. Although the participants took in as much or more nicotine from 15 e-cigarette puffs, their Cmax were on average lower than available data on smoking a tobacco cigarette (~10–30 ng/mL) (13, 14). It appears then that while most of the nicotine inhaled is systemically retained, a significant amount is absorbed at sites other than the lungs. Additional sites of absorption likely include the buccal mucosa and the gastrointestinal tract following swallowing. Several subjects displayed the PK profile illustrated in Figure 1B (Subject 2), who had a plasma nicotine Cmax at 2 minutes indicative of rapid lung absorption but the persistent peak (for about 30 minutes) and slowly declining plasma nicotine resembled the PK profile of smokeless tobacco in which absorption of nicotine is primarily through the buccal cavity (13). Further, due to hepatic first pass elimination, swallowing e-cigarette aerosol would result in decreased systemic bioavailability (13), and is a likely explanation for the low Cmax in some participants.
Since e-cigarettes do not smolder like tobacco cigarettes, secondhand exposure to nicotine and toxicants in the aerosol, a public health concern, is entirely from the exhaled aerosol. Our findings of minimal nicotine, VG, and PG in the exhaled aerosol suggest that secondhand exposure to nicotine from e-cigarette aerosols would be small. However, a few studies have reported increased levels of nicotine, respirable particles, volatile organic compounds, and metals in indoor air and biomarkers of nicotine exposure in non-users after e-cigarette use (31–34). Subject 3 in our study exhaled 51% (or 0.8 mg) of the inhaled nicotine and 96% (86 mg) and 54% (19 mg) of the inhaled VG and PG, respectively, which demonstrates the possibility for generation of secondhand e-cigarette aerosol exposure by some users. It is likely that this subject inhaled the aerosol into their mouth or throat and not their lungs, resulting in a low Cmax of 2.3 ng/mL at 30 minutes and a PK profile that resembled smokeless tobacco use, indicative of poor lung absorption. Therefore, user vaping behavior is an important determinant of the extent of secondhand e-cigarette aerosol exposure.
E-cigarette use significantly increased heart rate of participants who were deprived of nicotine overnight. The observed increase of 8 bpm 5 minutes after vaping was similar to previous studies on e-cigarettes (10) but slightly lower than the 10 to 12 bpm increase following tobacco cigarette use in nicotine-deprived smokers (35, 36).
Participants reported a decrease in nicotine withdrawal which was mild to begin with. The total MNWS score before vaping was 7.8 of a possible score of 48. Mild nicotine withdrawal among e-cigarette users is consistent with users’ reports of lower dependence on e-cigarettes compared to tobacco cigarettes (37, 38). E-cigarette use reduced craving as measured by the decrease in every item of the QSU. Participants also reported above moderate scores on Satisfaction, Reward, Sensations, and Craving Reduction and low Aversion (a combination of ‘nauseous’ and ‘dizziness’ items) subscales of the mCEQ. One report suggested that these effects may be independent of nicotine and can be due to oral and tactile sensations similar to smoking (9), and also likely result from participants using their own devices. The ability of e-cigarettes to reduce urge to smoke and withdrawal symptoms and to increase reward and satisfaction, as shown in prior reports (9, 11, 39, 40), might explain high e-cigarette prevalence of use among former tobacco cigarette users (41–43).
While e-cigarette users are mainly former or current adult tobacco cigarette smokers (42, 44), the prevalence of e-cigarette use is rising among the youth (2). The ability of e-cigarettes to deliver comparable or higher amounts of nicotine compared to tobacco cigarettes raises concerns about e-cigarette use generating nicotine dependence among young people. Nicotine levels reported here are likely to cause physiologic changes in nicotinic acetylcholine receptors in the brain that would sustain nicotine addiction (45, 46). This is particularly concerning for adolescents and young adults, given that early exposure to nicotine increases the severity of future nicotine dependence (47).
Our research has some limitations. In order to exhale fully into the gas trap and not lose significant amounts of aerosol from the mouth, exhalations were slower than usual which resulted in participants holding the e-cigarette aerosol in their mouth or lung for a longer time. Longer breath-holds might facilitate greater respiratory tract retention of nicotine, VG, and PG. Breath-hold has a minimal effect on nicotine retention from tobacco cigarettes (98.0% retention with 0 second breath-hold and 99.9% with a 10 s breath-hold) (48). Although we are not aware of the effect of breath-hold on VG and PG retentions, little to no aerosol is seen when individuals ‘stealth vape’. Stealth vaping refers to using e-cigarettes where they are not allowed and includes long breath-holds. Invisibility of the exhalant may be due to lower concentrations of VG/PG in the exhalant with increased retention. Other limitations include a fixed puffing protocol of 15 puffs in a hospital research ward setting where use patterns might differ from naturalistic settings. However, we did not control puff duration, allowing users to puff as naturally as possible. The fixed puffing protocol allows for comparisons between subjects and studies. Finally, participants varied by age, BMI, smoking status, and typical e-cigarette use, which could have influenced the variability in nicotine uptake and PK. This may also be a strength of our study, given the wide variability in e-cigarette user profile and behavior in the population.
CONCLUSIONS
E-cigarettes can be highly efficient as nicotine delivery devices, delivering levels of nicotine comparable to or higher than tobacco cigarettes with similar high levels of systemic retention. The plasma nicotine Cmax after 15 puffs was on average lower than available data on smoking one tobacco cigarette, suggesting that not all nicotine inhaled and retained is being absorbed through the lungs. However, several participants had Cmax in the reported range of tobacco cigarettes. Based on pharmacokinetic considerations, e-cigarettes have the potential to produce and sustain nicotine addiction as well as to provide an effective alternative source of nicotine for tobacco cigarette users.
Acknowledgments
Funding: This study was supported by grant number 1P50CA180890 from the National Cancer Institute and Food and Drug Administration Center for Tobacco Products and P30 DA012393 from the National Institute on Drug Abuse and was carried out in part at the Clinical Research Center at San Francisco General Hospital Medical Center (NIH/NCRR UCSF-CTSI UL1 RR024131). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH) or the Food and Drug Administration (FDA).
We thank Dr. Emilio Fernandez, Jennifer Ko, and Marian Shahid for clinical coordination; Dr. Natalie Nardone for project management; and Kristina Bello and Lisa Yu for performing analytical chemistry.
Footnotes
Declarations of Interest: Dr. Neal Benowitz serves as a consultant to several pharmaceutical companies that market smoking cessation medications and has served as a paid expert witness in litigation against tobacco companies. The other authors have no conflicts to declare.
References
- 1.King BA, Patel R, Nguyen K, Dube SR. Trends in awareness and use of electronic cigarettes among US adults, 2010–2013. Nicotine Tob Res. 2015;17(2):219–27. doi: 10.1093/ntr/ntu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrazola RA, Singh T, Corey CG, Husten CG, Neff LJ, Apelberg BJ, et al. Tobacco use among middle and high school students—United States, 2011–2014. MMWR. 2015;64(14):381–5. [PMC free article] [PubMed] [Google Scholar]
- 3.Callahan-Lyon P. Electronic cigarettes: human health effects. Tob Control. 2014;23(suppl 2):ii36–ii40. doi: 10.1136/tobaccocontrol-2013-051470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Government Printing Office (GPO) Family Smoking Prevention and Tobacco Control Act. Public Law. :111–31. [Google Scholar]
- 5.Food and Drug Administration. Deeming tobacco products to be subject to the Federal Food, Drug, and Cosmetic Act, as amended by the Family Smoking Prevention and Tobacco Control Act; regulations on the sale and distribution of tobacco products and required warning statements for tobacco products. Fed Regist. 2014;79(80):23141–207. [PubMed] [Google Scholar]
- 6.Carter LP, Stitzer ML, Henningfield JE, O’Connor RJ, Cummings KM, Hatsukami DK. Abuse liability assessment of tobacco products including potential reduced exposure products. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3241–62. doi: 10.1158/1055-9965.EPI-09-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Etter J-F, Bullen C, Flouris AD, Laugesen M, Eissenberg T. Electronic nicotine delivery systems: a research agenda. Tob Control. 2011;20(3):243–8. doi: 10.1136/tc.2010.042168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1945–53. doi: 10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bullen C, McRobbie H, Thornley S, Glover M, Lin R, Laugesen M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob Control. 2010;19(2):98–103. doi: 10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- 10.Vansickel AR, Weaver MF, Eissenberg T. Clinical laboratory assessment of the abuse liability of an electronic cigarette. Addiction. 2012;107(8):1493–500. doi: 10.1111/j.1360-0443.2012.03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawkins L, Corcoran O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacol (Berl) 2014;231(2):401–7. doi: 10.1007/s00213-013-3249-8. [DOI] [PubMed] [Google Scholar]
- 12.Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Scientific reports. 2014;4(4133) doi: 10.1038/srep04133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hukkanen J, Jacob P, III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 14.Patterson F, Benowitz N, Shields P, Kaufmann V, Jepson C, Wileyto P, et al. Individual differences in nicotine intake per cigarette. Cancer Epidemiol Biomarkers Prev. 2003;12(5):468–71. [PubMed] [Google Scholar]
- 15.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 16.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nic Tob Res. 2001;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 17.Becoña E, Vázquez FL, Fuentes MaJ, del Carmen Lorenzo Ma. Anxiety, affect, depression and cigarette consumption. Pers Individ Dif. 1998;26(1):113–9. [Google Scholar]
- 18.Rose JE, Westman EC, Behm FM, Johnson MP, Goldberg JS. Blockade of Smoking Satisfaction Using the Peripheral Nicotinic Antagonist Trimethaphan. Pharmacol Biochem Behav. 1999;62(1):165–72. doi: 10.1016/s0091-3057(98)00153-1. [DOI] [PubMed] [Google Scholar]
- 19.Jacob P, 3rd, Yu L, Wilson M, Benowitz NL. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: absence of an isotope effect in the clearance of (S)-nicotine-3′,3′-d2 in humans. Biol Mass Spectrom. 1991;20(5):247–52. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- 20.Trehy ML, Ye W, Hadwiger ME, Moore TW, Allgire JF, Woodruff JT, et al. Analysis of electronic cigarette cartridges, refill solutions, and smoke for nicotine and nicotine related impurities. J Liq Chromatogr Relat Technol. 2011;34(14):1442–58. [Google Scholar]
- 21.Frieler RA, Mitteness DJ, Golovko MY, Gienger HM, Rosenberger TA. Quantitative determination of free glycerol and myo-inositol from plasma and tissue by high-performance liquid chromatography. J Chromatogr B. 2009;877(29):3667–72. doi: 10.1016/j.jchromb.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etter J-F, Bullen C. Saliva cotinine levels in users of electronic cigarettes. Eur Respir J. 2011;38(5):1219–20. doi: 10.1183/09031936.00066011. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann D, Djordjevic MV, Hoffmann I. The changing cigarette. Prev Med. 1997;26(4):427–34. doi: 10.1006/pmed.1997.0183. [DOI] [PubMed] [Google Scholar]
- 24.Armitage A, Dollery C, George C, Houseman T, Lewis P, Turner D. Absorption and metabolism of nicotine from cigarettes. BMJ. 1975;4(5992):313–6. doi: 10.1136/bmj.4.5992.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams JM, Gandhi KK, Lu S-E, Kumar S, Shen J, Foulds J, et al. Higher nicotine levels in schizophrenia compared with controls after smoking a single cigarette. Nicotine Tob Res. 2010;12(8):855–9. doi: 10.1093/ntr/ntq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nides MA, Leischow SJ, Bhatter M, Simmons M. Nicotine blood levels and short-term smoking reduction with an electronic nicotine delivery system. Am J Health Behav. 2014;38(2):265–74. doi: 10.5993/AJHB.38.2.12. [DOI] [PubMed] [Google Scholar]
- 27.Berridge MS, Apana SM, Nagano KK, Berridge CE, Leisure GP, Boswell MV. Smoking produces rapid rise of [11C] nicotine in human brain. Psychopharmacol (Berl) 2010;209(4):383–94. doi: 10.1007/s00213-010-1809-8. [DOI] [PubMed] [Google Scholar]
- 28.Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol. 1993;61(5):743. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- 29.Hajek P, Goniewicz ML, Phillips A, Smith KM, West O, McRobbie H. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine Tob Res. 2015;17(2):175–9. doi: 10.1093/ntr/ntu153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spindle TR, Breland AB, Karaoghlanian NV, Shihadeh AL, Eissenberg T. Preliminary results of an examination of electronic cigarette user puff topography: The effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nicotine Tob Res. 2015;17(2):142–9. doi: 10.1093/ntr/ntu186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ballbè M, Martínez-Sánchez JM, Sureda X, Fu M, Pérez-Ortuño R, Pascual JA, et al. Cigarettes vs. e-cigarettes: Passive exposure at home measured by means of airborne marker and biomarkers. Environ Res. 2014;135:76–80. doi: 10.1016/j.envres.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Schober W, Szendrei K, Matzen W, Osiander-Fuchs H, Heitmann D, Schettgen T, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217(6):628–37. doi: 10.1016/j.ijheh.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Schripp T, Markewitz D, Uhde E, Salthammer T. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23(1):25–31. doi: 10.1111/j.1600-0668.2012.00792.x. [DOI] [PubMed] [Google Scholar]
- 34.Czogala J, Goniewicz ML, Fidelus B, Zielinska-Danch W, Travers MJ, Sobczak A. Secondhand exposure to vapors from electronic cigarettes. Nicotine Tob Res. 2014;16(6):655–62. doi: 10.1093/ntr/ntt203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benowitz NL, Porchet H, Sheiner L, Jacob P. Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin Pharmacol Ther. 1988;44(1):23–8. doi: 10.1038/clpt.1988.107. [DOI] [PubMed] [Google Scholar]
- 36.Benowitz NL, Jacob P, Herrera B. Nicotine intake and dose response when smoking reduced–nicotine content cigarettes. Clin Pharmacol Ther. 2006;80(6):703–14. doi: 10.1016/j.clpt.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Etter J-F, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015;147:68–75. doi: 10.1016/j.drugalcdep.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foulds J, Veldheer S, Yingst J, Hrabovsky S, Wilson SJ, Nichols TT, et al. Development of a Questionnaire for Assessing Dependence on Electronic Cigarettes Among a Large Sample of Ex-Smoking E-cigarette Users. Nicotine Tob Res. 2015;17(2):186–92. doi: 10.1093/ntr/ntu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob Res. 2013;15(1):267–70. doi: 10.1093/ntr/ntr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Etter J-F. Explaining the effects of electronic cigarettes on craving for tobacco in recent quitters. Drug Alcohol Depend. 2015;148:102–8. doi: 10.1016/j.drugalcdep.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 41.Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. e-Cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102(9):1758–66. doi: 10.2105/AJPH.2011.300526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pepper JK, Brewer NT. Electronic nicotine delivery system (electronic cigarette) awareness, use, reactions and beliefs: a systematic review. Tob Control. 2013;23(5):375–84. doi: 10.1136/tobaccocontrol-2013-051122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chapman SLC, Wu L-T. E-cigarette prevalence and correlates of use among adolescents versus adults: a review and comparison. J Psychiatr Res. 2014;54:43–54. doi: 10.1016/j.jpsychires.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore G, Hewitt G, Evans J, Littlecott HJ, Holliday J, Ahmed N, et al. Electronic-cigarette use among young people in Wales: evidence from two cross-sectional surveys. BMJ open. 2015;5(4):e007072. doi: 10.1136/bmjopen-2014-007072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brody AL, Mandelkern MA, Costello MR, Abrams AL, Scheibal D, Farahi J, et al. Brain nicotinic acetylcholine receptor occupancy: effect of smoking a denicotinized cigarette. Int J Neuropsychopharmacol. 2009;12(03):305–16. doi: 10.1017/S146114570800922X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362(24):2295–303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol Ther. 2009;122(2):125–39. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Armitage A, Dixon M, Frost B, Mariner D, Sinclair N. The effect of inhalation volume and breath-hold duration on the retention of nicotine and solanesol in the human respiratory tract and on subsequent plasma nicotine concentrations during cigarette smoking. Contributions Tob Research. 2004;21(4):240–9. doi: 10.1021/tx0340753. [DOI] [PubMed] [Google Scholar]