Abstract
Detection of 15N in multidimensional NMR experiments of proteins has sparsely been utilized because of the low gyromagnetic ratio (γ) of nitrogen and the presumed low sensitivity of such experiments. Here we show that selecting the TROSY components of proton-attached 15N nuclei (TROSY 15NH) yields high quality spectra in high field magnets (>600 MHz) by taking advantage of the slow 15N transverse relaxation and compensating for the inherently low 15N sensitivity. The 15N TROSY transverse relaxation rates increase modestly with molecular weight but the TROSY gain in peak heights depends strongly on the magnetic field strength. Theoretical simulations predict that the narrowest line width for the TROSY 15NH component can be obtained at 900 MHz, but sensitivity reaches its maximum around 1.2 GHz. Based on these considerations, a 15N-detected 2D 1H-15N TROSY-HSQC (15N-detected TROSY-HSQC) experiment was developed and high-quality 2D spectra were recorded at 800 MHz in 2 hr for 1 mM maltose-binding protein at 278K (τc ~40 ns). Unlike for 1H detected TROSY, deuteration is not mandatory to benefit 15N detected TROSY due to reduced dipolar broadening, which facilitates studies of proteins that cannot be deuterated, especially in cases where production requires eukaryotic expression systems. The option of recording 15N TROSY of proteins expressed in H2O media also alleviates the problem of incomplete amide proton back exchange, which often hampers the detection of amide groups in the core of large molecular weight proteins that are expressed in D2O culture media and cannot be refolded for amide back exchange. These results illustrate the potential of 15NH-detected TROSY experiments as a means to exploit the high resolution offered by high field magnets near and above 1 GHz.
Keywords: Nitrogen detection, TROSY, High field magnet, protein NMR, amide back exchange, deuteration
Introduction
Heteronuclear NMR experiments that use detection of nuclei with low gyromagnetic ratio (γ) and benefit from the slower relaxation properties of 13C and 15N have recently been proposed to expand the utility of NMR in structural and functional studies of macromolecules (Takeuchi et al., 2012). A variety of experiments have been developed for structure analyses of proteins using 13C-direct detection (Arnesano et al., 2005; Bermel et al., 2003; Bermel et al., 2006a; Bermel et al., 2006b; Felli and Brutscher, 2009; Hsu et al., 2009; Lee et al., 2005; Serber et al., 2001; Takeuchi et al., 2010a; Takeuchi et al., 2008) and 15N-direct detection (Gal et al., 2011; Levy and Richter, 1979; Takeuchi et al., 2012; Takeuchi et al., 2010b; Vasos et al., 2006). Since 15N has the lowest γ among NMR active nuclei found in proteins, 15N-direct detection is expected to yield the narrowest NMR resonances (Figure S1), which should help resolving signal degeneracy in high molecular-weight or unstructured systems.
Previously, 2H-attached amide 15N (15ND) has been used in 15N-direct detection experiments of proteins based on the perception of the general line-narrowing effect of deuteration (Takeuchi et al., 2012; Takeuchi et al., 2010b; Vasos et al., 2006); here we present that the TROSY (Pervushin, 2000; Pervushin et al., 1997) component of 1H-attached amide 15N (TROSY 15NH) should be observed to maximize the benefit of the low γ-nuclei detection experiments, both in terms of resolution and sensitivity in high field magnets >600 MHz. This approach was experimentally supported by the development of a 15N-detected 2D 1H-15N TROSY-HSQC (15N-detected TROSY-HSQC) experiment. We found that the 15N-detected TROSY-HSQC spectrum of a 1 mM protein with a 40 ns rotational correlation time (τc), which corresponds to 67 kDa protein at 298 K, can be recorded in 2 hr with additional benefits in resolution. The 15N TROSY effect shows strong magnetic field dependence, and the narrowest line width for the TROSY 15NH component can be obtained at 900 MHz, whereas the sensitivity reaches its maximum around 1.2 GHz. The TROSY 15N detection will also benefit the study of large systems and intrinsically disordered proteins (IDPs), which often suffer from severe spectral overlap. In addition, unlike conventional TROSY 1H detection, deuteration is not mandatory for the TROSY 15N detection. Thus, the TROSY 15NH detection reported here provides a novel opportunity for macromolecular NMR of proteins that can only be expressed in mammalian or insect cells, or systems that cannot be refolded for amide back exchange.
Results
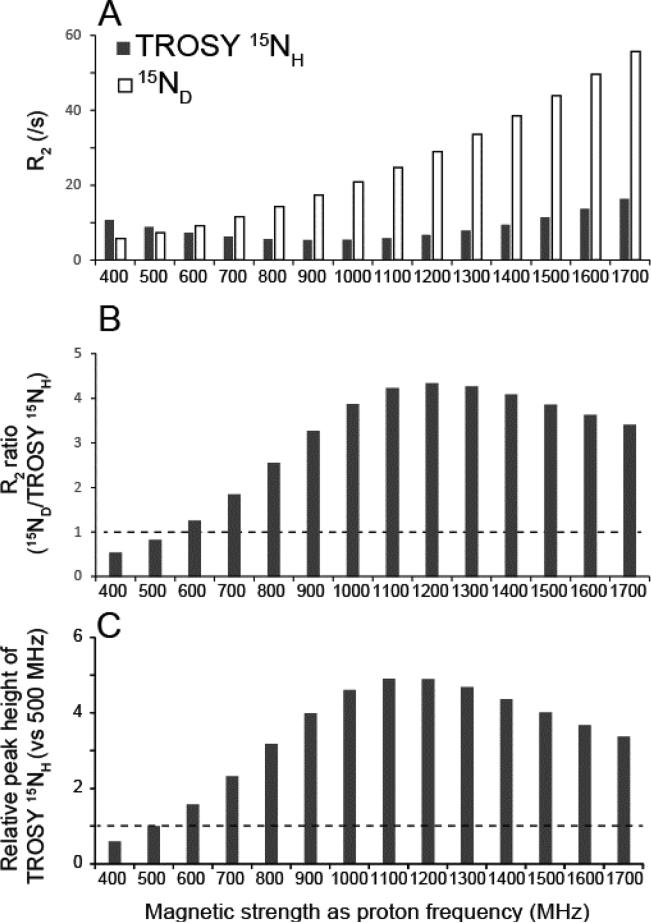
Figure 1A shows the calculated transverse relaxation rate (R2) for TROSY 15NH (gray) and deuterium-bound nitrogen, 15ND, (white) of a protein at indicated magnetic field strengths represented by the corresponding proton frequency. At lower magnetic field, the transverse relaxation is slower for 15ND; however, this is reversed between 11.7 T and 14.1 T (500 MHz and 600 MHz in proton frequency; Figure 1A). Henceforth we will use the frequency of proton resonance for the description of field strength. For the system with rotational correlation time of 20 ns (corresponding to a 33 kDa protein at 298 K), the ratio of R2 of TROSY 15NH relative to 15ND is smallest at a field strength of 1.2 GHz and the relaxation rate of TROSY 15NH is 4.3 times smaller than that of 15ND (R2; TROSY 15NH, 6.7 s−1; 15ND 29 s−1) (Figure 1B).
Figure 1. Estimated relaxation rates and relative peak heights of TROSY15NH at various magnetic field strengths.
(A) Calculated R2 for TROSY 15NH (gray) and 15ND (white) at magnetic field strengths indicated with proton frequency. (B) R2 ratio of 15ND over TROSY 15NH (C) Relative signal heights of TROSY 15HN resonances relative to 500 MHz. The instrumental efficiencies for detecting the resonance are assumed to be the same across spectrometers. The transverse relaxation rates were calculated based on equations (1) and (5) in the supplemental materials and methods.
The prime reason for this difference stems from the fact that the relaxation rate of 2H-attached 15N lacks the benefit of 15N-1H dipole-15N CSA interference, the TROSY effect that dramatically reduces relaxation for 1H-attached 15N, and this scales with the field strength reaching a maximum at 900 MHz for the 15N-1H TROSY resonances (Figure 1A). The 15N TROSY effect and associated relaxation gains compared to 15ND are rather insensitive to the molecular weight of the systems investigated but depend largely on the magnetic field strength (Figure S2A and S2B). The R2 of TROSY 15NH reaches its minimum near 900 MHz where it is 62 % slower than at 500 MHz. Importantly, the TROSY reduction in transverse relaxation rates is more effective in 15NH compared to 1HN (Figure S2C vs S2A), since the dipole-dipole interactions from surrounding protons are negligible for 15N due to its low γ. This provides the additional advantage that, even at non-deuterated conditions, the closest Hα proton introduces only about 5% of additional 15N-1H dipole-dipole contribution to R2 of TROSY 15NH for a 20 ns system at 800 MHz (Figure S3).
Since TROSY 15N-detection is favorable at higher magnetic fields, it would be interesting to estimate how much could be gained in signal height at higher field strengths. The intensity (I) of an NMR experiment is proportional to the amount of induced current in the first point of an FID and is reflected in the peak area (A) of a resonance (or volume in multidimensional experiments) after Fourier transform (FT). If the relaxation during the pulse scheme is ignored, this can be written as
| (1) |
where, γe and γe are gyromagnetic ratios for excited and detected nuclei, B0 is the magnetic field strength. In contrast, peak height is the integral over the envelop of the FID and is expected to be proportional to the intensity of signal and inversely proportional to R2.
| (2) |
The peak height of the TROSY 15NH resonance will be at maximum around 1.2 GHz and will be 4.8 times higher at 1.2 GHz compared to 500 MHz, if the efficiency in signal detection hardware is assumed to be the same (Figure 1C). The relaxation rate R2 of TROSY 15NH for a 20 ns system is expected to be 6.7 s−1 which is more than two times slower than for proton-decoupled 15NH (48 s−1) and 15ND(29 s−1). Therefore, even though half of the coherence would be lost by the TROSY-selection schemes, the resultant gain in signal height is substantial. Since the noise level after FT is proportional to the square root of the acquisition length, the signal to noise ratio (S/N) of a spectrum with fixed acquisition length, which is long enough to allow complete relaxation, would directly reflect the relaxation benefits. When the acquisition length is matched to the relaxation rates of detected components (Rovnyak et al., 2004), the benefit in S/N would be proportional to the square root of the gain in signal height.
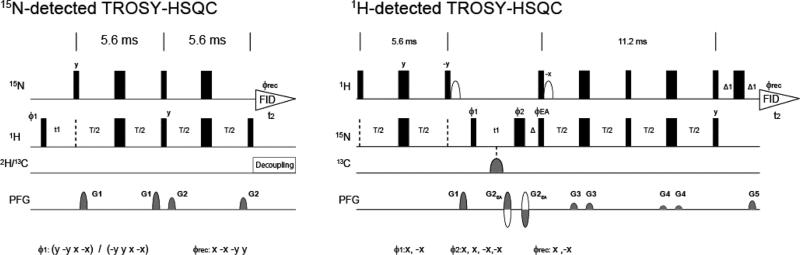
In order to experimentally observe TROSY 15NH resonances with optimal resolution and sensitivity, a 15N-detected 2D 1H-15N TROSY-HSQC (15N-detected TROSY-HSQC) pulse sequence was developed, in which the TROSY components in both 1H and 15N dimensions were recorded for optimal resolution, and proton excitation was utilized for optimal sensitivity (Figure 2). The experiment consists of a simple single transition-to-single transition polarization transfer (ST2PT) (Pervushin et al., 1998) from 1H to 15N for selecting the TROSY component in both dimensions. Importantly, a short recycling delay can be used since we excite 1H, and we only have to wait for recovery of the rapidly relaxing 1H spins, which is an advantage over 15N or 13C excitation schemes. The 15N-detected TROSY is significantly shorter than the 1H detected equivalent, which provides additional benefits (Figure 2).
Figure 2. Pulse sequence of 15N-detected (left) and 1H-detected (right) 2D TROSY-HSQC experiments.
Narrow and wide rectangular black bars indicate non-selective π/2 and π pulses, respectively. All pulses are applied along the x-axis unless indicated otherwise. The delays T were set to 2.7 ms. The phase cycle employed was ϕ1 = (y -y x –x), and ϕ rec = (x -x -y y) for 15N-detected 2D TROSY-HSQC and ϕ1 = (x –x), ϕ2 = (x x –x –x), and ϕ rec = (x -x) for 1H-detected 2D TROSY-HSQC. Phase sensitive detection in the indirect 1H dimension (t1) was obtained by incrementing the phase ϕ1 for15N-detected 2D TROSY-HSQC and the phase ϕ3 and G2 in an echo-antiecho manner (Kay et al., 1992). The recycling delay was set to 1 s. For 15N-detected 2D TROSY-HSQC, the two sine-shaped pulsed field gradients were applied along the z-axis for 1.0 ms with maximum intensities of G1 = 22.5 G/cm, and G2 = 25 G/cm. For 1H-detected 2D TROSY-HSQC, the sine-shaped pulsed field gradients were applied along the z-axis for 1.0 ms with maximum intensities of G1 = −15 G/cm, and G2 = ± 40 G/cm, G3 = 4.5 G/cm, G4 = 0.5 G/cm, G5 = 8.1 G/cm. Deuterium decoupling was achieved by using WALTZ16 (Shaka et al., 1983) (1 kHz) and carbon decoupling was achieved by using a p5m4 supercycle (Fujiwara and Nagayama, 1988) with an adiabatic CHIRP pulse of 2.5 ms length and 25% smoothing (Bohlen and Bodenhausen, 1993).
The 15N-detected TROSY-HSQC experiment was first tested at 800 MHz and 500 MHz instruments on 2H15N13C-labeled GB1 in a D2O buffer at 286K (τc = 7ns), in order to experimentally verify the enhancement of the TROSY effect at higher fields (see supplementary discussion for detail). The line widths of the TROSY 15N signals were substantially narrower than the 1H-decoupled 15NH, anti-TROSY 15NH, and 2H-decoupled 15ND resonances at 800 MHz (Figure S4A-C). The narrow line widths are directly reflected in the gain of signal height. In contrast, while benefit from TROSY-selection was also observed on a 500 MHz magnet, the 2H-decoupled 15ND resonances have almost the same linewidth as the TROSY 15NH resonances at the low field (Figure S4D). These observations clearly demonstrated the benefit of selecting the TROSY 15NH components at higher magnetic field.
Figure 3A left shows 15N-detected TROSY-HSQC spectra of 1 mM 2H13C15N-labeled maltose binding protein (MBP) in complex with β-cyclodextrin (βCD) in an 800 MHz instrument at 278 K. The τc of the system, at the above conditions was 40 ns as deduced by TRACT experiments (Lee et al., 2006). The experiments were performed with a cryogenic TXO probe with a cold 15N preamplifier. The expected line width of the TROSY 15N components is 3.5 Hz at 800 MHz. The maximum acquisition time for 15N dimension was set to 160 ms in order to make sure the resultant spectra have adequate resolution reflecting their relaxation rates. The actual line widths for dispersed signals in the MBP spectrum were broader than expected (9.5Hz ± 2.3 Hz; Average ± S.D.) with no significant dependence on the secondary structures. The broader line widths are presumably due to incomplete heterounclear decoupling from the CA and CO, exchange with water, and/or other unaccounted effects in the calculation of relaxation rates such as cross relaxation between amide protons and other remote protons by the oscillation between in-phase and anti-phase 15N coherences in the detecting period (Peng et al., 1991).
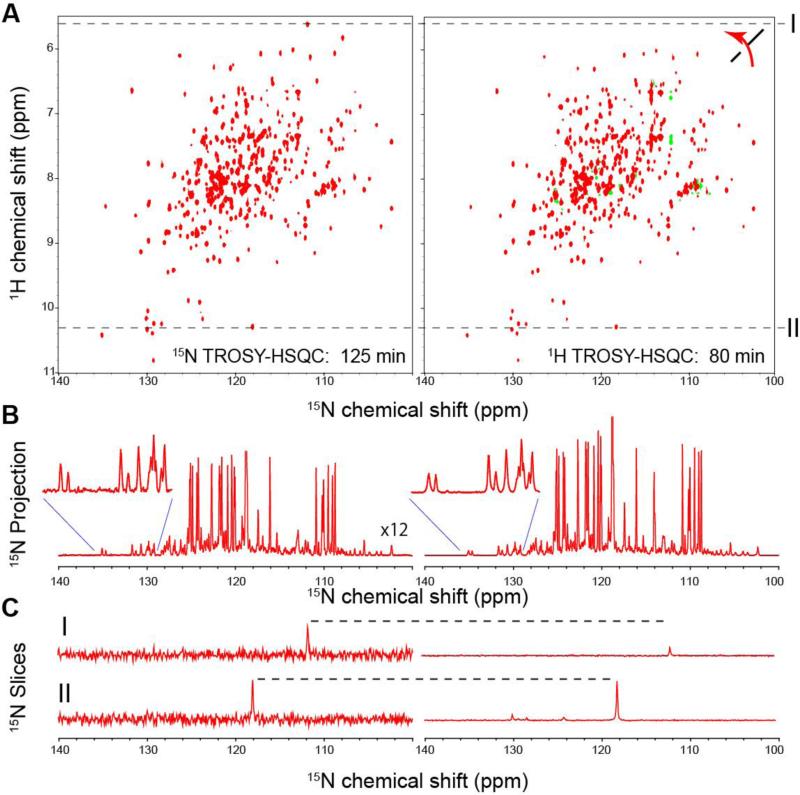
Figure 3. Comparison of 15N-detected TROSY-HSQC (left) and 1H-detected TROSY-HSQC (right) of 1 mM 2H15N-labeled MBP in complex with 2 mM βCD in 800 MHz at 278 K.
(A) The 15N-detected TROSY-HSQC was recorded in 125 min, ns=16, F1=348 pts (34ms), F2 = 1024 pts (160 ms). The 1H-detected TROSY-HSQC was recorded in 80 min, ns = 4, F1= 1024 pts (160 ms), F2=1024 pts (40 ms). (B) 15N projection of (A). The projection of the 15N-detected experiment is scaled 12-fold. (C) Two representative cross sections indicated as I and II in (A) are shown.
As shown in Figure 3, the 15N-detected TROSY-HSQC spectra (left) can be recorded in 2 hr with a reasonable quality for the 40 ns system as compared to 1H-detected TROSY-HSQC spectra (right). Both spectra were recorded with a triple-resonance cryogenic probe (TXO) designed for heteronuclear-detection experiments. The probe has cryogenic preamps for 15N and 13C, and carbon/nitrogen detection is on the inner coil. All the resonances that were observed in 1H-detected TROSY-HSQC spectrum were also observed in the 15N-detected TROSY-HSQC spectrum, with the exception of the arginine side chains, which are folded in the indirect dimension of the 1H-detected spectrum but outside the spectrum width of the direct dimension in the 15N-detected spectrum. The additional advantage of the 15N-detected experiment is that it is not affected by water and water suppression schemes as in the 1H-detected experiments. For example, the low-frequency signal in the proton dimension (see slice I in Figure 3A and C) is significantly attenuated in the 1H-detected experiment compared to the 15N-detected experiment (see slice II in Figure 3A and C) due to the Watergate solvent suppression schemes (Piotto et al., 1992). There is no loss of signals in 15N-detected spectrum, as the latter does not use any solvent suppression schemes.
Although direct comparison of 15N and 1H sensitivity is difficult since their electric/thermal noise levels, efficiency of detecting the signals, and effect of BO field inhomogeneity would be different for the individual electronic circuits, it is still interesting to compare the relative intensity of these two detection schemes. If one assumes identical noise, efficiency in signal detection, and no BO field inhomogeneity, the relative intensity (or peak area after FT) of a straightforward 15N-detected experiment that starts from 15N magnetization compared to 1H-detected experiments is 0.0032. However, if 15N-detected experiments start with the 1H magnetization, the relative intensity becomes 0.032. In a 15N-detected TROSY-HSQC experiment, proton magnetization is transverse for 5.6 ms and nitrogen magnetization is transverse for 5.6 ms, compared to a 1H-detected experiment where the faster relaxing proton magnetization is transverse for 16.8 ms (Figure 2). Thus, the pulse scheme is shorter in the 15N-detected system and coherence is transverse in 15N during half of the time. If we factor in the relaxation losses during this shorter time period for a 40 ns (~70 kDa) protein, then the relative intensity between 15N-detected TROSY-HSQC and 1H-detected TROSY-HSQC becomes 0.059. This is consistent with the experimentally observed relative intensities, and in the peak heights, as the line width for the transposed 1H-detected TROSY-HSQC spectrum is the same as in the 15N-detected TROSY-HSQC with the same maximal acquired points, and the peak heights would directly reflect the intensity of 15N-detected TROSY-HSQC and 1H-detected TROSY-HSQC (Figure 3). It should be noted that the sensitivity for the 1H-detected experiments would be slightly higher in a TXI probe. However for samples with physiological salt concentrations, there is no significant difference in 1H sensitivity between our TXO and TXI probes in our instruments.
As discussed above, deuteration is not a strict requirement to benefit from the TROSY 15NH detection. In contrast, deuteration is mandatory for the TROSY 1HN detection. For example, the Hα proton in an α-helical conformation alone, would accelerate the R2 of TROSY 1HN by 2.5-fold, and additional contributions from other remote protons would further enhance the relaxation. Since TROSY 15N detection is still beneficial for proteins expressed in 1H2O, there is no problem associated with the incomplete 1H back exchange of amides, which often happens for large proteins and hampers the detection of signals from internal amide groups. For example, malate synthetase G that is recombinantly produced in E.coli with a D2O culture media has to be unfolded in H2O to ensure complete exchange of amide protons. However, this procedure usually reduces the final protein yield due to incomplete refolding efficiencies after the proton back exchange (Tugarinov et al., 2006). Moreover, numerous other proteins cannot be refolded for amide proton back exchange.
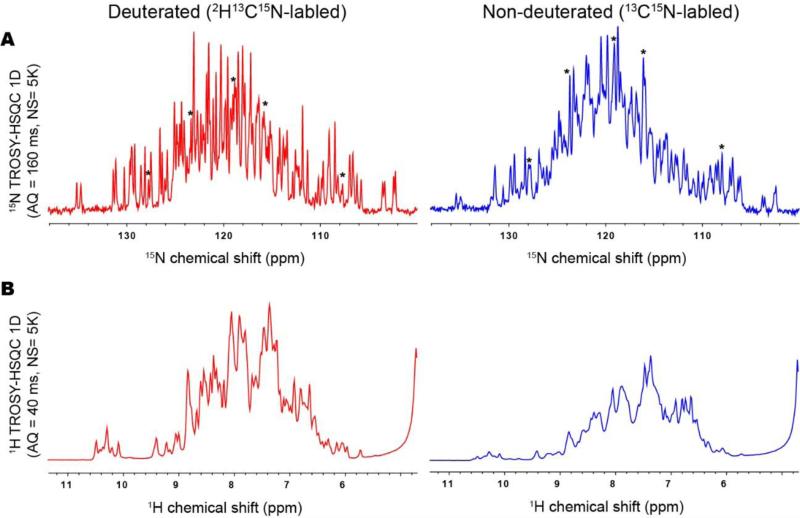
This rational was experimentally confirmed by the comparison of the first increment of 15N-detected TROSY-HSQC and 1H-detected TROSY-HSQC of 0.5 mM MBP in complex with βCD in deuterated (left) and non-deuterated (right) conditions (Figure 4). There was only a modest loss in peak heights in the 15N-detected TROSY-HSQC of the protonated compared to the deuterated sample. In contrast, the peak height of 1H-detected TROSY-HSQC was reduced to about one third of that of the deuterated sample. The intensity reduction of 15N-detected TROSY-HSQC is mainly due to relaxation loss during the coherence transfer from proton to nitrogen in the pulse program where the proton magnetization is transverse for 5.6 ms (30% signal reduction is expected) and 2J and 3J scalar coupling to Hα and Hβ, respectively (see detail in supplemental discussion). As discussed above, the cross relaxation between amide protons and other protons may cause an additional decay (Peng et al., 1991). Nevertheless, some of the resonances in the 15N-detected TROSY-HSQC spectrum showed increased signal heights at the non-deuterated compared to deuterated conditions (indicated with asterisks), which is presumably due to incomplete 1H back exchange in the deuterated protein.
Figure 4. Comparison of (A)15N-detected TROSY-HSQC and (B) 1H-detected TROSY-HSQC of 0.5 mM MBP in complex with 2 mM βCD on a deuterated (left) and non-deuterated (right) protein.
(A) The first increment of 15N-detected TROSY-HSQC was recorded in 106 min, ns=5K, F2 = 1024 pts (160 ms) in 800 MHz at 278 K. (B) The first increment of 1H-detected TROSY-HSQC was recorded in 92 min, ns=5K, F2 = 1024 pts (40 ms) in 800 MHz at 278 K. In (A) the resonances that clearly showed higher signal height in the non-deuterated compared to the deuterated sample are indicated by asterisks.
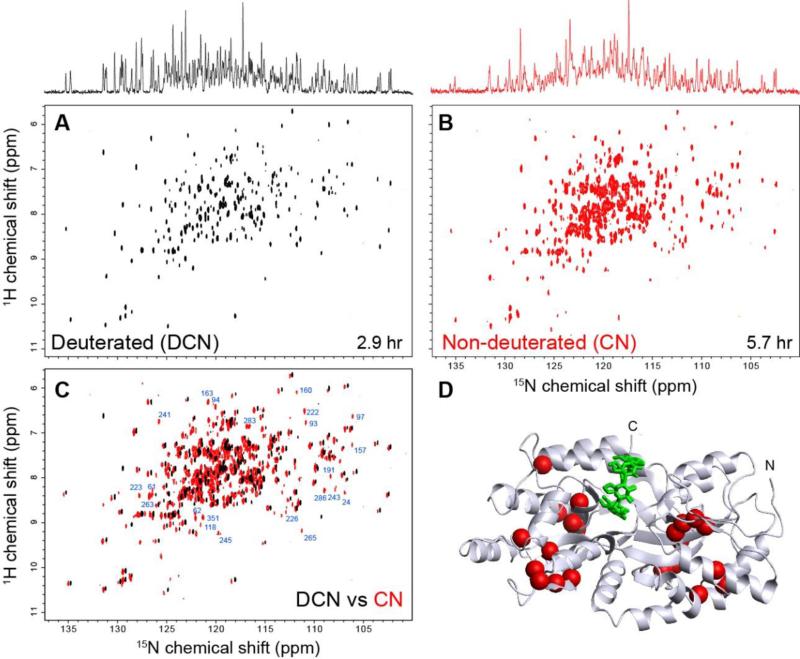
The benefit of recording the 15N-detected TROSY-HSQC spectrum without deuteration was subsequently verified by a 2D experiment. For this purpose, we have recorded the 15N-detected 2D TROSY-HSQC spectra of deuterated (2H15N13C-labled) and non-deuterated (15N13C-labled) MBP at 0.5 mM (Figure 5 A and B). The spectra for deuterated and non-deuterated MBP were recorded for 2.9 hr and 5.7 hr, respectively. Although the average signal height of the non-deuterated MBP is less compared to the deuterated MBP, we see that there are numerous peaks that are observed only in the spectrum of non-deuterated MBP but not in that of deuterated MBP. The spectra of deuterated and non-deuterated MBP were directly compared in Figure 5C. Mapping of the residues that are observed only in the non-deuterated sample clearly indicates that those resonances missing in deuterated sample are from the core of the protein that are missing due to incomplete backbone amide back-exchange (Figure 5D). Note that MBP can readily be refolded for easy amide proton back-exchange of perdeuterated protein. Thus, the assignments for the whole protein are available (Gardner et al., 1998) to identify the positions of the recovered signals in the structure.
Figure 5. Comparison of 2D 15N-detected TROSY-HSQC of (A) 2H15N13C-labled (deuterated) and (B) 15N13C-labled (non-deuterated) 0.5 mM MBP in complex with 2 mM βCD.
Both 15N-detected TROSY-HSQC spectra were recorded with F1=128 pts (12 ms), F2 = 1024 pts (160 ms) in 800 MHz at 278 K. The number of scans was set to 64 and 128 in the spectrum of 2H15N13C-labled and 15N13C-labled MBP, respectively. 15N projections of both spectra are shown on top. (C) Overlay of (A) and (B). The assignments of well-dispersed resonances that were observed only at non-deuterated conditions are indicated. (D) Mapping of residues that are observed only at non-deuterated conditions. The residues annotated in (C) are indicated by spheres in the crystal structure of the MBP-βCD complex.
Discussion
Here we report that direct 15NH detection in TROSY experiments of protonated amide groups has multiple benefits for studies of large proteins when studied at high field instruments (> 600 MHz). Due to the extremely slow transverse relaxation of the 15NH TROSY component the quality of 15N-1H correlated spectra comes close to the 1H-detected TROSYs. Although less sensitive, the 15NH detected TROSY exhibits narrower lines, does not necessarily require perdeuterated samples thus circumventing the amide back-exchange problem, and promises to work for much larger proteins. This approach was experimentally supported by the development of a 15N-detected TROSY-HSQC experiment. The TROSY-based 15N-detected schemes can be incorporated in other 15N-detected experiments and will have general benefits by enhancing the resolution in crowded spectra. The 15N detected experiments can readily be combined with NUS methods to speed up acquisition time (Figure S5).
Since 15NH TROSY relaxes slower than the 1HN equivalent (Figures S2, S6), one can benefit from longer acquisition in the direct 15N dimension to increase signal height. This benefit is compounded by the use of a smaller spectral width (~6 ppm) in the indirect 1H dimension centered at 8.5 ppm. Although the dispersion in the 15N dimension is almost invariant, 1H dispersion can vary significantly depending on the structural characteristic of the proteins. The sampling efficiency in the indirect dimension becomes even more advantageous for IDPs with a small dispersion in the 1H dimension, and the TROSY-HSQC benefits even more in the 15N detection mode relative to 1H detection.
The narrower lines of TROSY 15NH detection experiments will benefit the study of large systems as well as IDPs, which suffer from severe spectral overlap. It should be noted that the relaxation of TROSY 15NH deteriorates least with increasing molecular weight among all nuclei found in proteins (Figure S7). This promises access to much larger systems. Furthermore, the narrower line widths may enable measurement of chemical shifts more accurately, which is important for obtaining structure information from pseudo-contact shifts caused by paramagnetic centers or spin labels (Allegrozzi et al., 2000; Allen and Imperiali, 2010; Banci et al., 2004; Inagaki and Miyazawa, 1980; Keizers et al., 2007). Accurate chemical shift measurements are also important for measuring residual dipolar couplings (Tjandra and Bax, 1997; Tolman et al., 1995; Tycko et al., 2000). Consistent with this notion, 13C-detection has been shown to increase the detectability and precision of RDC measurement for broad 1H resonances (Balayssac et al., 2006). In further developments, 2D and 3D TROSY versions of additional 15N detection experiments can be developed for backbone and side-chain assignment of large proteins.
Our simulations indicate that higher magnetic fields enhance the signal heights of 15N-detected TROSY-HSQC signals compared to lower fields, and signal heights are almost independent of the molecular weight (Figure S7). The benefit of TROSY selection in 15N-detection becomes most substantial around 900 MHz with respect to resolution, and signal heights become optimal around 1.1-1.2 GHz.
Although several strategies were developed to achieve deuteration in eukaryotic expression systems (Kofuku et al., 2014; Miyazawa-Onami et al., 2013; Morgan et al., 2000; Sastry et al., 2011), it is still hard to obtain high levels of (>90%) deuteration with insect and mammalian cells. Thus, 15N-detected experiments, which are not dependent on deuteration as the 1H-detected equivalents (Figure 4), will be beneficial for large systems that can be obtained only by using those eukaryotic expression systems. Moreover, since deuteration is not strictly required for TROSY 15N detection, there is less need for amide protein back exchange, which will alleviate a most serious problem for NMR studies of large proteins that are expressed in deuterated media (Figure 5). However, the impact of protonation on relaxation rates and the associated line widths in the indirect 1H dimension has to be explored and addressed. Avoiding the traditional time-domain approach and acquiring frequency-based correlations to obtain chemical shift correlation for indirect dimension, such as the Hadamard approach (Kupce et al., 2003) would be one possibility. Although multiplet structure due to 2J and 3J scalar coupling of 15N to Hα and Hβ, respectively, might reduce the effective resolution for non-deuterated proteins, especially for the system with smaller effective molecular weight such as IDPs, selective excitation of amide resonances can be used to further reduce the T1 recovery delays (Schanda and Brutscher, 2005). It should be noted that selective decoupling of Hα and Hβ resonances would at least partially resolve the resolution losses caused by splitting of signal by the 2J and 3J scalar couplings. Thus, the combination of TROSY, Hadamard (Kupce et al., 2003), SOFAST (Schanda and Brutscher, 2005) and combination of them (Schanda and Brutscher, 2006) can further reduce the measurement time and/or be used for sensitivity gains for 15N-detected TROSY. The problem of enhanced 1H relaxation in 15NH TROSY experiments of protonated samples can also be addressed by expressing protein in glucose-deficient H2O based M9, supplemented with 2H13C15N-labeled amino acids and nutrients, which result in a sample where the amides are fully protonated and Cα is partially deuterated (Lohr et al., 2003). When considering the effect of ionic strength, the sensitivity factor, L, of a cyrogenic probes can be expressed as
| (3) |
| (4) |
where Rs, Rc, a, b, l, n, μ, ω, and σ are the resistance of the sample, the resistance of the coil, coil radius, sample radius, length, coil turns, the permeability of free space, the Larmor frequency, and the sample conductivity, respectively (Kelly et al., 2002). Since the 15N frequency is about one tenth of the 1H frequency, loss of sensitivity due to high ionic conductivity is expected to be much less in 15N- compared to 1H-detected experiments.
Direct detection of the 15N TROSY component holds great promise to study high molecular weight protein, systems with spectral overlap, such as IDPs and this advantage scales with magnetic field strength, especially near and above 1 GHz.
Supplementary Material
Acknowledgement
This work was supported by NIH grants GM047467 and AI 37581 to GW and by METI (Grant name: development of core technologies for innovative drug development based upon IT) to IS. This work was also partly supported by JST, PRESTO to KT. Maintenance of NMR instruments was in part supported by NIH grant EB002026. We would like thanks Dominique Frueh, Wolfgang Bermel and Arthur Palmer for useful discussions.
References
- Allegrozzi M, Bertini I, Janik MBL, Lee Y-M, Liu G, Luchinat C. Lanthanide-Induced Pseudocontact Shifts for Solution Structure Refinements of Macromolecules in Shells up to 40 Å from the Metal Ion. J. Am Chem Soc. 2000;122:4154–4161. [Google Scholar]
- Allen KN, Imperiali B. Lanthanide-tagged proteins--an illuminating partnership. Curr Opin Chem Biol. 2010;14:247–254. doi: 10.1016/j.cbpa.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Arnesano F, Banci L, Piccioli M. NMR structures of paramagnetic metalloproteins. Q Rev Biophys. 2005;38:167–219. doi: 10.1017/S0033583506004161. [DOI] [PubMed] [Google Scholar]
- Balayssac S, Bertini I, Luchinat C, Parigi G, Piccioli M. 13C direct detected NMR increases the detectability of residual dipolar couplings. J Am Chem Soc. 2006;128:15042–15043. doi: 10.1021/ja0645436. [DOI] [PubMed] [Google Scholar]
- Banci L, Bertini I, Cavallaro G, Giachetti A, Luchinat C, Parigi G. Paramagnetism-Based Restraints for Xplor-NIH. J Biomol NMR. 2004;28:249–261. doi: 10.1023/B:JNMR.0000013703.30623.f7. [DOI] [PubMed] [Google Scholar]
- Bermel W, Bertini I, Felli IC, Kummerle R, Pierattelli R. 13C Direct detection experiments on the paramagnetic oxidized monomeric copper, zinc superoxide dismutase. J Am Chem Soc. 2003;125:16423–16429. doi: 10.1021/ja037676p. [DOI] [PubMed] [Google Scholar]
- Bermel W, Bertini I, Felli IC, Lee YM, Luchinat C, Pierattelli R. Protonless NMR experiments for sequence-specific assignment of backbone nuclei in unfolded proteins. J Am Chem Soc. 2006a;128:3918–3919. doi: 10.1021/ja0582206. [DOI] [PubMed] [Google Scholar]
- Bermel W, Bertini I, Felli IC, Piccioli M, Pierattelli R. 13C-detected protonless NMR spectroscopy of proteins in solution. Prog Nucl Magn Res Spec. 2006b;48:25–45. [Google Scholar]
- Bohlen JM, Bodenhausen G. Experimental Aspects of Chirp NMR Spectroscopy. J Mag Res A. 1993;102:293–301. [Google Scholar]
- Felli I, Brutscher B. Recent advances in solution NMR: fast methods and heteronuclear direct detection. ChemPhysChem. 2009;10:1356–1368. doi: 10.1002/cphc.200900133. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Nagayama K. Composite inversion pulses with frequency switching and their application to broadband decoupling. J Mag Res (1969) 1988;77:53–63. [Google Scholar]
- Gal M, Edmonds KA, Milbradt AG, Takeuchi K, Wagner G. Speeding up direct (15)N detection: hCaN 2D NMR experiment. J Biomol NMR. 2011;51:497–504. doi: 10.1007/s10858-011-9580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner KH, Zhang X, Gehring K, Kay LE. Solution NMR Studies of a 42 KDa Escherichia Coli Maltose Binding Protein/β-Cyclodextrin Complex: Chemical Shift Assignments and Analysis. J Am Chem Soc. 1998;120:11738–11748. [Google Scholar]
- Hsu S-TD, Bertoncini CW, Dobson CM. Use of protonless NMR spectroscopy to alleviate the loss of information resulting from exchange-broadening. J Am Chem Soc. 2009;131:7222–7223. doi: 10.1021/ja902307q. [DOI] [PubMed] [Google Scholar]
- Inagaki F, Miyazawa T. NMR analyses of molecular conformations and conformational equilibria with the lanthanide probe method. Prog Nuc Mag Res Spec. 1980;14:67–111. [Google Scholar]
- Kay L, Keifer P, Saarinen T. Pure absorption gradient enhanced heteronuclear single quantum correlation spectroscopy with improved sensitivity. J Am Chem Soc. 1992;114:10663–10665. [Google Scholar]
- Keizers PH, Desreux JF, Overhand M, Ubbink M. Increased paramagnetic effect of a lanthanide protein probe by two-point attachment. J Am Chem Soc. 2007;129:9292–9293. doi: 10.1021/ja0725201. [DOI] [PubMed] [Google Scholar]
- Kelly AE, Ou HD, Withers R, Dötsch V. Low-Conductivity Buffers for High-Sensitivity NMR Measurements. J Am Chem Soc. 2002;124:12013–12019. doi: 10.1021/ja026121b. [DOI] [PubMed] [Google Scholar]
- Kofuku Y, Ueda T, Okude J, Shiraishi Y, Kondo K, Mizumura T, Suzuki S, Shimada I. Functional dynamics of deuterated β2-adrenergic receptor in lipid bilayers revealed by NMR spectroscopy. Angew Chem Int Ed. 2014;53:13376–13379. doi: 10.1002/anie.201406603. [DOI] [PubMed] [Google Scholar]
- Kupce E, Nishida T, Freeman R. Hadamard NMR spectroscopy. Magnetic resonance in chemistry : MRC. 2003;42:95–122. [Google Scholar]
- Lee D, Hilty C, Wider G, Wüthrich K. Effective rotational correlation times of proteins from NMR relaxation interference. J Magn Reson. 2006;178:72–76. doi: 10.1016/j.jmr.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Lee D, Vögeli B, Pervushin K. Detection of C′,Cα correlations in proteins using a new time- and sensitivity-optimal experiment. J Biomol NMR. 2005;31:273–278. doi: 10.1007/s10858-005-2361-4. [DOI] [PubMed] [Google Scholar]
- Levy G, Richter R. Nitrogen 15 Nuclear Magnetic Resonance Spectroscopy. John Wiley & Sons.; 1979. [Google Scholar]
- Lohr F, Katsemi V, Hartleib J, Gunther U, Ruterjans H. A strategy to obtain backbone resonance assignments of deuterated proteins in the presence of incomplete amide 2H/1H back-exchange. J Biomol NMR. 2003;25:291–311. doi: 10.1023/a:1023084605308. [DOI] [PubMed] [Google Scholar]
- Miyazawa-Onami M, Takeuchi K, Takano T, Sugiki T, Shimada I, Takahashi H. Perdeuteration and methyl-selective 1H, 13C-labeling by using a Kluyveromyces lactis expression system. J Biomol NMR. 2013;57:297–304. doi: 10.1007/s10858-013-9789-8. [DOI] [PubMed] [Google Scholar]
- Morgan WD, Kragt A, Feeney J. Expression of deuterium-isotope-labelled protein in the yeast pichia pastoris for NMR studies. J Biomol NMR. 2000;17:337–347. doi: 10.1023/a:1008313530207. [DOI] [PubMed] [Google Scholar]
- Peng J, Thanabal V, Wagner G. Improved Accuracy of Heteronuclear Transverse Relaxation Time Measurements in Macromolecules. Elimination of Antiphase Contributions J Mag Res. 1991;95:421–427. [Google Scholar]
- Pervushin K. Impact of transverse relaxation optimized spectroscopy (TROSY) on NMR as a technique in structural biology. Q Rev Biophys. 2000;33:161–197. doi: 10.1017/s0033583500003619. [DOI] [PubMed] [Google Scholar]
- Pervushin K, Riek R, Wider G, Wuthrich K. Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc Natl Acad Sci. 1997;94:12366–12371. doi: 10.1073/pnas.94.23.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervushin KV, Wider G, Wüthrich K. Single Transition-to-single Transition Polarization Transfer (ST2-PT) in [15N,1H]-TROSY. J Biomol NMR. 1998;12:345–348. doi: 10.1023/A:1008268930690. [DOI] [PubMed] [Google Scholar]
- Piotto M, Saudek V, Sklenar V. Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous solutions. J Biomol NMR. 1992;2:661–665. doi: 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]
- Rovnyak D, Hoch JC, Stern AS, Wagner G. Resolution and sensitivity of high field nuclear magnetic resonance spectroscopy. J Biomol NMR. 2004;30:1–10. doi: 10.1023/B:JNMR.0000042946.04002.19. [DOI] [PubMed] [Google Scholar]
- Sastry M, Xu L, Georgiev IS, Bewley CA, Nabel GJ, Kwong PD. Mammalian production of an isotopically enriched outer domain of the HIV-1 gp120 glycoprotein for NMR spectroscopy. J Biomol NMR. 2011;50:197–207. doi: 10.1007/s10858-011-9506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanda P, Brutscher B. Very fast two-dimensional NMR spectroscopy for real-time investigation of dynamic events in proteins on the time scale of seconds. J Am Chem Soc. 2005;127:8014–8015. doi: 10.1021/ja051306e. [DOI] [PubMed] [Google Scholar]
- Schanda P, Brutscher B. Hadamard frequency-encoded SOFAST-HMQC for ultrafast two-dimensional protein NMR. J Magn Reson. 2006;178:334–339. doi: 10.1016/j.jmr.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Serber Z, Richter C, Dotsch V. Carbon-detected NMR experiments to investigate structure and dynamics of biological macromolecules. Chembiochem. 2001;2:247–251. doi: 10.1002/1439-7633(20010401)2:4<247::AID-CBIC247>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Frueh DP, Hyberts SG, Sun ZJ, Wagner G. High-resolution 3D CANCA NMR experiments for complete mainchain assignments using Ca direct-detection. J Am Chem Soc. 2010a;132:2945–2951. doi: 10.1021/ja907717b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Gal M, Shimada I, Wagner G. Low γ-nuclei detection experiments for bimolecular NMR. Rec Dev Biomol NMR. 2012:25–52. [Google Scholar]
- Takeuchi K, Heffron G, Sun ZY, Frueh DP, Wagner G. Nitrogen-detected CAN and CON experiments as alternative experiments for main chain NMR resonance assignments. J Biomol NMR. 2010b;47:271–282. doi: 10.1007/s10858-010-9430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Sun ZY, Wagner G. Alternate 13C-12C labeling for complete mainchain resonance assignments using Ca direct-detection with applicability toward fast relaxing protein systems. J Am Chem Soc. 2008;130:17210–17211. doi: 10.1021/ja806956p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjandra N, Bax A. Direct Measurement of Distances and Angles in Biomolecules by NMR in a Dilute Liquid Crystalline Medium. Science. 1997;278:1111–1114. doi: 10.1126/science.278.5340.1111. [DOI] [PubMed] [Google Scholar]
- Tolman JR, Flanagan JM, Kennedy MA, Prestegard JH. Nuclear magnetic dipole interactions in field-oriented proteins: information for structure determination in solution. Proc Natl Acad Sci. 1995;92:9279–9283. doi: 10.1073/pnas.92.20.9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugarinov V, Kanelis V, Kay LE. Isotope labeling strategies for the study of high-molecular-weight proteins by solution NMR spectroscopy. Nat Protocols. 2006;1:749–754. doi: 10.1038/nprot.2006.101. [DOI] [PubMed] [Google Scholar]
- Tycko R, Blanco FJ, Ishii Y. Alignment of Biopolymers in Strained Gels: A New Way To Create Detectable Dipole−Dipole Couplings in High-Resolution Biomolecular NMR. J Am Chem Soc. 2000;122:9340–9341. [Google Scholar]
- Vasos PR, Hall JB, Kummerle R, Fushman D. Measurement of 15N relaxation in deuterated amide groups in proteins using direct nitrogen detection. J Biomol NMR. 2006;36:27–36. doi: 10.1007/s10858-006-9063-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.