Abstract
Sudden unexpected death in epilepsy (SUDEP) is a devastating event, and both DBA/1 and DBA/2 mice have been shown to be relevant animal models for studying SUDEP. DBA mice exhibit seizure-induced respiratory arrest (S-IRA), leading to cardiac arrest and subsequent sudden death after generalized audiogenic seizures (AGS). This sequence of terminal events is also observed in the majority of witnessed human SUDEP cases. Several pathophysiological mechanisms, including respiratory/cardiac dysfunction, have been proposed to contribute to human SUDEP. Several (but not all) selective serotonin (5-HT) reuptake inhibitors (SSRIs), including fluoxetine, can reversibly block S-IRA, and abnormal expression of 5-HT receptors is found in the brainstem of DBA mice. DBA mice, which do not initially show S-IRA, exhibit S-IRA after treatment with a non-selective 5-HT antagonist. These studies suggest that abnormalities of 5-HT neurotransmission are involved in the pathogenesis of S-IRA in DBA mice. 5-HT transmission plays an important role in normal respiration, and DBA mice exhibiting S-IRA can be resuscitated using a rodent ventilator. It is important and interesting to know if fluoxetine blocks S-IRA in DBA mice by enhancing respiratory ventilation. To test this, the effects of breathing stimulants, doxapram and 5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine (PK-THPP) were compared to fluoxetine on S-IRA in DBA/1 mice. Although fluoxetine reduces the incidence of S-IRA in DBA/1 mice, as reported previously, the same dose of fluoxetine fails to enhance baseline respiratory ventilation in the absence of AGS. Doxapram and PK-THPP augment the baseline ventilation in DBA/1 mice. However, these breathing stimulants are ineffective in preventing S-IRA in DBA/1 mice. These data suggest that fluoxetine blocks S-IRA in DBA/1 mice by cellular/molecular mechanisms other than enhancement of basal ventilation. Future research directions are also discussed.
Keywords: Respiratory arrest, DBA mice, Serotonin, 5-HT receptors, SSRI, Fluoxetine
DBA MICE AS RELEVANT SUDEP MODELS
Sudden unexpected death in epilepsy (SUDEP) is a fatal epileptic event, accounting for up to ~17% of deaths in epileptic patients, with an incidence of 5.1/1000 person-years [1–3]. Although the annual number of deaths for SUDEP is not as high as that for many common neurological disorders such as Alzheimer disease and stroke, the public health burden of SUDEP ranks second only after stroke when the mortality is calculated as years of potential life lost, in which more weight is given to premature deaths [4]. A number of pathophysiological mechanisms have been proposed for SUDEP [5–8], of which the respiratory/cardiac dysfunction has received considerable attention [9, 10]. Many patients with SUDEP or near-SUDEP display difficulties in breathing after generalized tonic-clonic seizures [11–15]. A recent retrospective study (MORTEMUS) observed a consistent pattern of respiratory/cardiac dysfunction in SUDEP patients by analyzing video electroencephalogram (EEG) and electrocardiogram (ECG) data; these patients developed rapid breathing after generalized tonic-clonic seizures, followed by cardiorespiratory dysfunction, which in many cases led to apnea, followed by cardiac arrest [3].
DBA mice are the oldest of all inbred strains of mice, and the genetic background of DBA/2 and DBA/1 mice is sufficiently different that they should be regarded as different strains rather than substrains of the same strain (The Jackson Laboratory website). DBA mice (both DBA/1 and DBA/2) are genetically susceptible to audiogenic seizures (AGS) evoked by acoustic stimulation (95–122 dB SPL) [16, 17]. The AGS in DBA mice are characterized by wild running, generalized clonic and/or tonic seizures, ending in tonic hind-limb extension [17, 18]. Thus, DBA/2 mice have been used as a genetic epilepsy model for many years [19]. Many DBA mice exhibit seizure-induced respiratory arrest (S-IRA) after generalized tonic seizures, which can be successfully resuscitated using a rodent ventilator if applied within ~5 sec of the onset of S-IRA [20, 21]. DBA/1 and DBA/2 mice have different patterns of S-IRA susceptibility (Table 1). Like human SUDEP, the occurrence of S-IRA is unpredictable in DBA/1 mice that have not been subjected to high intensity of acoustic stimulation, as the incidence of S-IRA (~10–30%) when tested at ~30 days of age is relatively low on initial testing [18, 22]. However, the incidence of S-IRA can reach up to ~100% after three or four consecutive days of testing (“priming”) [16]. After priming, the susceptibility to S-IRA in DBA/1 mice lasts for months [16]. DBA/2 mice have a higher incidence of S-IRA on initial testing (~75%). However, reduced seizure severity and decreased susceptibility to S-IRA are observed in DBA/2 mice after postnatal day 28 [20], which is probably due to an age-dependent hearing loss [23, 24]. It should be noted that AGS and S-IRA in DBA mice are induced by intense acoustic stimulation in the studies and, when spontaneous seizures occur, they are rarely observed. This differs from human SUDEP, which results from spontaneous seizures. However, seizure-induced sudden death evoked by acoustic stimulation in DBA mice is very useful to elucidate putative mechanisms underlying SUDEP and to evaluate potential therapeutics, because rare spontaneous seizures are difficult to study systematically.
Table 1.
Comparison of Audiogenic Seizures (AGS) and Seizure-Induced Respiratory Arrest (S-IRA) in DBA/1 and DBA/2 mice
| DBA/1 | DBA/2 | |
|---|---|---|
| AGS | Yes | Yes |
| Priming needed | Yes | No |
| Initial S-IRA | ~10–30% | 75% |
| S-IRA susceptibility | months | ~10 days |
Both DBA/1 and DBA/2 mice have been shown to be relevant animal models for studying SUDEP [25, 26]. First, both strains exhibit S-IRA after generalized convulsive seizures, leading to subsequent death if resuscitation is not rapidly instituted [16, 27]. The behaviors of these DBA mice resemble many human SUDEP and near-SUDEP cases, as these patients exhibit generalized clonic-tonic seizures and display respiratory dysfunction [3]. Second, in DBA mice, S-IRA always occurs prior to cardiac arrhythmia [16]. These sequential changes in cardiorespiratory function are also observed in sudden death evoked by maximal electroshock in phenotypically normal C57BL/6J mice [28, 29], a strain that is different from DBA mice and resistant to AGS and S-IRA. Interestingly, in line with these observations in animal models, a recent retrospective clinical study found that many SUDEP patients began with respiratory dysfunction and apnea, followed by cardiac arrest [3]. Third, several selective serotonin (5-HT) reuptake inhibitors (SSRIs) that are known to elevate 5-HT levels in synapses reduce respiratory dysfunction evoked by seizures in both DBA mice and patients [20, 30, 31] (see below). These previous studies suggest that DBA/1 and DBA/2 mice are useful animal models for studying SUDEP, and investigations using these models may shed significant light on the mechanism and treatment of SUDEP.
EVIDENCE OF 5-HT NEUROTRANSMISSION DEFECTS IN DBA MICE
The cellular/molecular mechanisms underlying SUDEP are poorly understood. Studies indicate that abnormal function of several types of ion channels and neurotransmission systems may contribute to the occurrence of SUDEP [7, 20, 28, 32–37]. Accumulating data suggest that deficits of 5-HT neurotransmission play an important role in the pathogenesis of S-IRA in animal models of SUDEP. The evidence includes:
Several SSRIs reduce the incidence of S-IRA
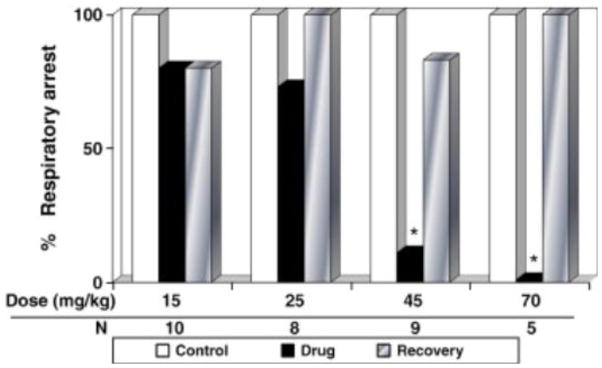
Administration of the SSRI fluoxetine at 15–25 mg/kg (i.p.) significantly and reversibly reduces the incidence of S-IRA in DBA/2 mice [20]. Spectacularly, fluoxetine at 15 and 20 mg/kg selectively prevents S-IRA without affecting the severity of AGS, although the incidence of tonic hindlimb extension is reduced when fluoxetine is administered at 25 mg/kg [20]. These data suggest that the blockade effect of fluoxetine on S-IRA may be involved in different molecular/cellular components from those for anticonvulsant effect of SSRIs [38, 39]. Although fluoxetine exerts anticonvulsant effect in vitro by blocking sodium channel currents and repetitive neuronal firing [40], it is likely that fluoxetine in moderate doses specifically suppresses S-IRA via acting at certain 5-HT receptors while high doses may produce anticonvulsant effect by blocking sodium channels. Systemic administration of fluoxetine also significantly reduces the incidence of S-IRA in DBA/1 mice [31] (Figure 1). However, the effective doses for suppressing S-IRA in DBA/1 mice are greater than those in DBA/2 mice. At 30–45 mg/kg (i.p.), fluoxetine reduces the incidence of S-IRA without altering the incidence of AGS [31, 41]. Fluoxetine at 70 mg/kg completely blocks S-IRA in DBA/1 mice. At this dose, all of the mice still exhibit wild running and clonic seizures, but the incidence of tonic seizures is decreased [31] (Figure 1). The reasons why the effective fluoxetine doses for reduction of S-IRA are different between DBA/1 and DBA/2 strains may be related, at least in part, to the differences in 5-HT receptor expression in the brainstem of the two strains of DBA mice as compared with C57BL/6J mice [42, 43]. Fluoxetine inhibits the reuptake of other monoamines, such as norepinephrine in the brain, although to a lesser degree [44]. These additional effects (see below) of fluoxetine may contribute to the differential dosing in DBA/1 and DBA/2 mice as well. The SSRI sertraline (40–75 mg/kg, i.p.) decreases the incidence of S-IRA in DBA/1 mice [21]. Sertraline at 40–50 mg/kg does not affect wild running and clonic AGS but reduces the tonic component of AGS. Sertraline at 75 mg/kg completely blocks S-IRA, with a reduction in the severity of AGS as well [21]. Another SSRI fluvoxamine (55–80 mg/kg, i.p.) reduces S-IRA in a dose-dependent manner without blocking AGS in DBA/1 mice [18]. The involvement of 5-HT neurotransmission in the pathogenesis of S-IRA is confirmed in another study that the SSRI, citalopram (20 mg/kg, i.p.), reduces the incidence of S-IRA evoked by maximal electroshock in phenotypically normal C57BL/6J mice [28, 29]. Due to species difference, the effective doses of SSRI for prevention of S-IRA in mice are larger than those used for treatment of depression in humans. However, the dose range of SSRIs that reduces the incidence of S-IRA in DBA mice appears to be appropriate for rodents, since it is similar to the doses that were originally used in rodents to establish SSRIs as potential antidepressants [45].
Figure 1. The SSRI fluoxetine reduces S-IRA in DBA/1 mice.
Systemic administration of fluoxetine, an SSRI, decreased the incidence of S-IRA in DBA/1 mice at doses that are comparable those that were previously used to establish SSRIs as potential antidepressants in rodents [45]. White bars, vehicle control; black bars, the incidence of S-IRA 30 min after fluoxetine administration; gray bars, recovery (the animals became susceptible to S-IRA again after washout of the drug) of S-IRA 24–72 hr after fluoxetine. N, number of mice tested.
* Significantly different from control at p < 0.05. From [31] with permission.
It should be noted that NOT all SSRIs produce a protective effect on S-IRA. It was reported that paroxetine administered up to 100 mg/kg is ineffective in suppressing S-IRA in DBA/1 mice. Although paroxetine at 120 mg/kg significantly reduces the incidence of S-IRA, it results in substantial toxicity to DBA/1 mice at this dose [18]. The reasons why some SSRIs, such as fluoxetine, are more effective in suppressing S-IRA than others such as paroxetine remain elusive. SSRIs are a group of drugs that selectively inhibit the reuptake of 5-HT to elevate the availability of 5-HT in the synaptic cleft and are primarily used to treat depression [46]. SSRIs also inhibit other monoamine transporters, such as norepinephrine transporter, to a lesser extent than 5-HT transporters. Moreover, these SSRIs exhibit affinity for additional receptors. For example, fluoxetine shows affinity to 5-HT2C receptors and paroxetine for muscarinic cholinergic receptors [47]. These off-target effects of SSRIs may contribute to their differential effects on S-IRA. It has been shown that fluvoxamine produces antidepressant-like effect in DBA/2 mice by activating sigma-1 receptors, but the antidepressant effect of paroxetine does NOT involve activation of sigma-1 receptors [48]. Thus, the differential actions on sigma-1 receptors may explain why fluvoxamine is effective in suppressing S-IRA but paroxetine is not [18].
Specific 5-HT receptors are involved in S-IRA
Some DBA/2 mice consistently display AGS with tonic hind-limb extension but do not exhibit S-IRA. When a non-selective 5-HT receptor antagonist, cyproheptadine, is administered (i.p.) to DBA/2 mice, a significant increase in the incidence of S-IRA occurs [20]. The occurrence of S-IRA in naïve DBA/1 mice (never exposed to acoustic stimulation) is unpredictable in the initial seizure test in response to acoustic stimulation (as low as ~10–30%) [18, 22]. Systemic administration of cyproheptadine significantly increases the incidence of S-IRA up to 60% in DBA/1 mice [18]. These data further confirm the involvement of 5-HT mechanism in the pathogenesis of S-IRA in DBA mice. Consistent with this idea, it was reported that the 5-HT2A receptors are critically involved in S-IRA evoked by electroshock [28]; S-IRA evoked by electroshock in mice is blocked by 5-HT2A receptor agonist (but not 5-HT2C agonist), and pretreatment with 5-HT2A antagonist (but not 5-HT2C antagonist) prior to citalopram administration prevents the reduction in S-IRA [28]. These data suggest that alterations of specific 5-HT receptors may contribute to S-IRA.
The expression of 5-HT receptors is abnormal in the brainstem of DBA mice
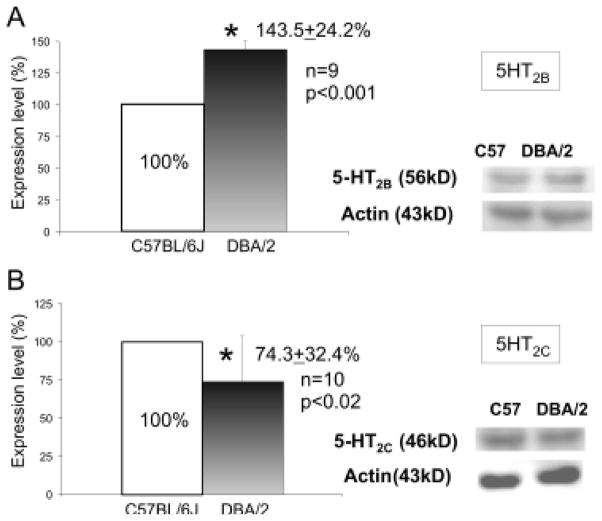
The brainstem, which is composed of midbrain, pons and medulla, controls the cardiorespiratory function in rodents [49]. SUDEP shares a significant similarity to sudden infant death syndrome [25, 50], in which deficits of 5-HT neurotransmission are observed in the brainstem medullary respiratory center [51, 52]. The brainstem is also implicated in the seizure-induced sudden death in other animal models of SUDEP [53]. Therefore, we compared the expression of several 5-HT receptors in the medial-caudal part of the brainstem in DBA/1 and DBA/2 mice with that in control C57BL/6J mice. DBA mice that displayed S-IRA and C57BL/6J mice that did not exhibit S-IRA were selected for Western blot analysis [42, 43]. As compared with C57BL/6J mice, the expression of 5-HT2C, 5-HT3B and 5-HT4 receptors is reduced, but that of 5-HT2B is increased in the brainstem of DBA/2 mice [42] (Figure 2). Abnormal expression of 5-HT receptors is also observed in the brainstem of DBA/1 mice. The expression of 5-HT2B, 5-HT2C and 5-HT3B receptors is reduced, and that of 5-HT4 receptors is not altered in the brainstem of DBA/1 mice as compared with that of C57BL/6J mice [43]. Interestingly, it was reported that mCPP, a 5-HT2B/2C agonist, reduces the incidence of S-IRA in DBA/2 mice, but this drug exerts no effect on S-IRA in DBA/1 mice [43]. This differential effect of mCPP on the incidence of S-IRA may be due to the disparate expression of 5-HT2B receptors in these two strains of DBA mice [43].
Figure 2. The abnormal expression of 5-HT2B and 5-HT2C receptors in the brainstem of DBA/2 mice.
Compared with seizure-resistant C57BL/6J mice, the expression of 5-HT2B receptors was elevated, and that of 5-HT2C receptors was reduced in the caudal brainstem of DBA/2 mice. These results may partly explain the differential effect of mCPP, a 5-HT2B/2C receptor agonist, on the incidence of S-IRA in DBA/1 and DBA/2 mice. The error bars represent SDs.
* Significantly different from C57BL/6J mice at p < 0.05. From [42] with permission.
Central enzyme for 5-HT synthesis is abnormal in DBA mice
The rate-limiting enzyme for synthesis of 5-HT in the CNS is tryptophan hydroxylase-2 (TPH2) [54]. It was reported that there exists a polymorphism (C1473G) in TPH2 among different mouse strains, and the enzymatic activity for the 1473G allele is substantially lower than that for the 1473C allele [55–57]. Several strains of mice, including DBA/1 and DBA/2, have been demonstrated to be homozygous for the 1473G allele [55, 58]. In line with this, studies showed that the activity of TPH2 in the brain of DBA/1 and DBA/2 mice is reduced as compared with C57BL/6J [55, 59].
DOES ENHANCEMENT OF VENTILATION BLOCK S-IRA IN DBA MICE?
Previous studies indicate that deficits of 5-HT neurotransmission may contribute to the pathogenesis of S-IRA [20, 28]. 5-HT is an important modulator for normal respiration [60]. It also modulates the ventilatory response to hypercapnia [61], and hypercapnia often occurs during seizures [13, 62]. Furthermore, DBA mice exhibiting S-IRA can be resuscitated using a ventilator [16, 27]. Therefore, we hypothesized that the SSRIs such as fluoxetine reduce S-IRA by enhancing respiratory ventilation, and we evaluated if breathing stimulants would suppress S-IRA in DBA mice [41].
Fluoxetine reduces S-IRA without enhancing respiratory ventilation in DBA/1 mice in the absence of seizures
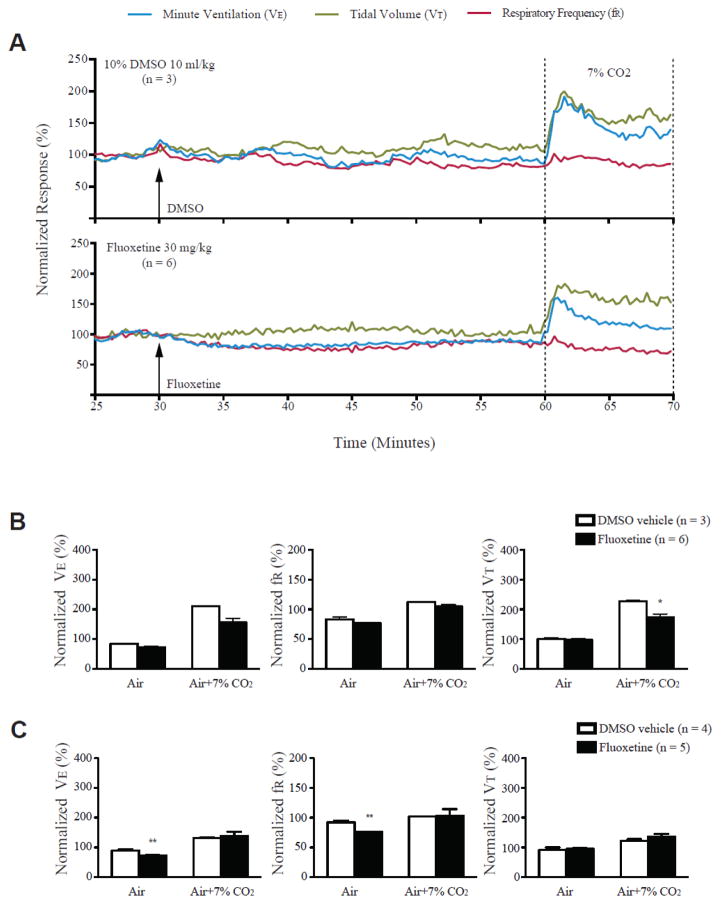
As reported previously [31], we confirmed that systemic administration of fluoxetine (30 mg/kg) significantly reduces the incidence of S-IRA in DBA/1 mice. However, fluoxetine at the same dose does not alter the respiratory ventilation in the anesthetized DBA/1 mice, although fluoxetine reduces respiratory ventilation in awake DBA/1 mice in the absence of seizures as compared with the vehicle control (Figure 3). In both anesthetized and awake DBA/1 mice, fluoxetine does not alter the ventilatory response to 7% CO2 as compared with the vehicle control in DBA/1 mice (Figure 3). These data demonstrated that fluoxetine suppression of S-IRA is NOT due to enhancement of respiratory ventilation in the absence of seizures [41]. 5-HT is an important neuromodulator for maintaining respiratory rhythmogenesis [60, 63]. If S-IRA involves disruption of respiratory rhythm, fluoxetine may increase synaptic 5-HT to restore rhythmogenesis during seizures in DBA mice. Moreover, 5-HT neurotransmission mediates arousal response [61, 64]. If S-IRA results from a deficit in the arousal system, fluoxetine may enhance arousal response to protect DBA/1 mice from S-IRA. It should be noted that the effect of fluoxetine on respiratory ventilation during AGS in DBA/1 mice was not examined due to the technical difficulty in recording reliable breathing signals in our plethysmography studies. It is possible that fluoxetine may produce a different effect on respiratory ventilation during seizures from that in the awake DBA/1 mice in the absence of seizures, as significant release of multiple neurotransmitters/neuromodulators, including 5-HT, occurs during seizures [65]. Further studies are needed to address this issue.
Figure 3. Fluoxetine did not increase basal breathing and ventilatory response to CO2 in anesthetized and conscious DBA/1 mice.
A, representative traces of minute ventilation (VE), respiratory frequency (fR) and tidal volume (VT) from anesthetized DBA/1 mice treated with fluoxetine (30 mg/kg) or vehicle, in room air or exposure to air + 7% CO2. Data were normalized to the average VE, fR or VT baseline value. Traces between the two dotted lines indicate the exposure time to 7% CO2 gas mixture. B, effects of fluoxetine on the normalized VE, fR and VT in room air and in air + 7% CO2 in anesthetized DBA/1 mice using nose-only plethysmography. C, effects of fluoxetine on the normalized VE, fR and VT in room air and in air + 7% CO2 in conscious DBA/1 mice using whole-body plethysmography. The error bars represent SEMs.
* p<0.05; ** p<0.01: significantly different from corresponding vehicle control. From [41] with permission.
Breathing stimulants enhance ventilation without affecting S-IRA in DBA/1 mice
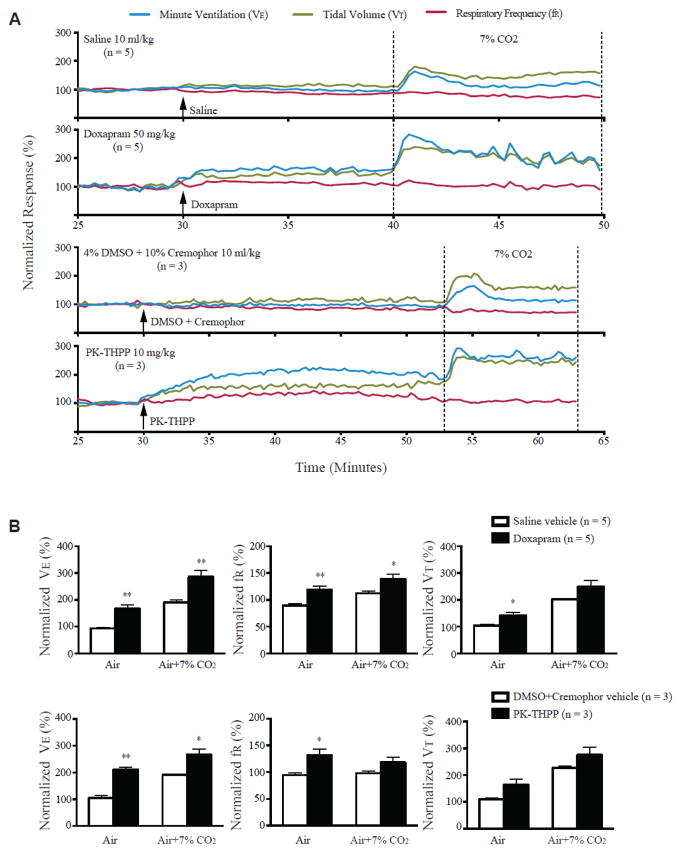
We also examined the effect of two breathing stimulants, doxapram and 5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine (PK-THPP), on respiratory function and S-IRA in DBA/1 mice [41]. Both doxapram and PK-THPP are TASK potassium channel antagonists, which have been demonstrated to stimulate breathing in anesthetized rats [66]. Systemic administration of doxapram and PK-THPP enhances the respiratory ventilation in anesthetized DBA/1 mice (Figure 4). Doxapram and PK-THPP also increase the ventilatory response to 7% CO2 in anesthetized DBA/1 mice (Figure 4). To our surprise, neither doxapram nor PK-THPP reduces the incidence of S-IRA in awake DBA/1 mice. These data indicate that drug-induced increase in basal respiratory ventilation is not sufficient to compensate S-IRA in DBA mice, while fluoxetine reduces S-IRA through mechanisms other than direct stimulation of respiratory ventilation [41].
Figure 4. Doxapram and PK-THPP stimulate basal breathing and increase ventilatory response to CO2 in anesthetized DBA/1 mice.
A, representative traces of minute ventilation (VE), respiratory frequency (fR) and tidal volume (VT) from anesthetized DBA/1 mice treated with doxapram (50 mg/kg), PK-THPP (10 mg/kg) or their corresponding vehicles, in room air or exposure to air + 7% CO2 using nose-only plethysmography. Data were normalized to the average VE, fR or VT baseline value. Traces between the two dotted lines indicate the exposure time to 7% CO2 gas mixture. B, effects of doxapram and PK-THPP on the normalized VE, fR and VT in room air and in air + 7% CO2 in anesthetized DBA/1 mice. The error bars represent SEMs.
* p<0.05; ** p<0.01: significantly different from corresponding controls. From [41] with permission.
CONCLUSION AND FURTHER RESEARCH DIRECTION
SUDEP is a devastating event for patients with epilepsy and their families. Given the unpredictable and irreversible nature of this disorder, it is impossible to study the mechanisms and treatment of SUDEP in humans. Thus, animal models play an important role in addressing these issues. Progress in this field is considerably delayed due to the paucity of relevant animal models. Recent studies demonstrate that DBA mice are relevant animal models for studying SUDEP, as S-IRA in these mice closely resembles SUDEP and near-SUDEP cases in humans. Using these models, it has been shown that deficits in 5-HT neurotransmission contribute to the pathogenesis of S-IRA in DBA mice. Emerging evidence also supports that 5-HT mechanisms may be involved in the seizure-induced respiratory depression that leads to human SUDEP, as SSRI treatment reduces the degree of respiratory dysfunction in patients with partial epilepsy [30]. However, it is currently unknown where in the brain some of the SSRIs act to reduce S-IRA, and which 5-HT receptor subtypes are involved in the suppressing effect of these SSRIs on S-IRA in DBA mice. It remains to be determined why some SSRIs are effective in reducing S-IRA, but some are not. Apparently, multiple components of 5-HT signaling may be defective in animal models of SUDEP. Which component is critically involved in the pathogenesis of S-IRA? Does a similar scenario also occur in human SUDEP? These studies demonstrate that basic research results are helpful to guide clinical investigations. Based on the differential effects of SSRIs on S-IRA, it is important to carry out epidemiological studies to examine if the SSRIs that are effective in reducing S-IRA also decrease the incidence of SUDEP in patients.
The risk factors for SUDEP are poorly understood. Alcohol has been recognized as a respiratory depressant for a long time [67]. However, its role in SUDEP is controversial. While alcohol abuse is reported to be a risk factor for SUDEP in certain populations of epileptic patients [68, 69], contradictory results are found in other case-control studies [70–72]. These discrepancies may be caused by the limitations of the epidemiological studies; due to the unpredictable nature of SUDEP and the difficulty in collecting sufficient numbers of verified SUDEP cases. Also, epileptic patients take multiple drugs to control seizures and other medical conditions. The interaction of these drugs with alcohol may mask the effect of alcohol on SUDEP. Therefore, it is crucial to investigate the effect of potential risk factors, including alcohol, on the incidence of mortality in animal models of SUDEP such as DBA mice.
Highlights.
DBA mice are relevant SUDEP animal models.
5-HT neurotransmission is defective in DBA mice.
Fluoxetine reduces S-IRA, but without enhancing the ventilation.
Breathing stimulants enhance ventilation but have no effect on S-IRA.
Potential future research directions are discussed.
Acknowledgments
This work is supported by R03NS078591, CURE (Citizens United for Research in Epilepsy) foundation grant and fund from the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital to HJF, and Excellence in Academic Medicine, Southern Illinois University, School of Medicine, CURE, and Epilepsy Foundation to CLF. We thank Gayle Stauffer for assistance with the manuscript.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hughes JR. A review of sudden unexpected death in epilepsy: prediction of patients at risk. Epilepsy Behav. 2009;14:280–7. doi: 10.1016/j.yebeh.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Nei M, Hays R. Sudden unexpected death in epilepsy. Curr Neurol Neurosci Rep. 2010;10:319–26. doi: 10.1007/s11910-010-0116-4. [DOI] [PubMed] [Google Scholar]
- 3.Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, Boon P, Crespel A, Dworetzky BA, Hogenhaven H, Lerche H, Maillard L, Malter MP, Marchal C, Murthy JM, Nitsche M, Pataraia E, Rabben T, Rheims S, Sadzot B, Schulze-Bonhage A, Seyal M, So EL, Spitz M, Szucs A, Tan M, Tao JX, Tomson T. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12:966–77. doi: 10.1016/S1474-4422(13)70214-X. [DOI] [PubMed] [Google Scholar]
- 4.Thurman DJ, Hesdorffer DC, French JA. Sudden unexpected death in epilepsy: Assessing the public health burden. Epilepsia. 2014 doi: 10.1111/epi.12666. [DOI] [PubMed] [Google Scholar]
- 5.Stollberger C, Finsterer J. Cardiorespiratory findings in sudden unexplained/unexpected death in epilepsy (SUDEP) Epilepsy Res. 2004;59:51–60. doi: 10.1016/j.eplepsyres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch LJ. Is sudden unexpected death in epilepsy due to postictal brain shutdown? Ann Neurol. 2010;68:773–5. doi: 10.1002/ana.22242. [DOI] [PubMed] [Google Scholar]
- 7.Shen HY, Li T, Boison D. A novel mouse model for sudden unexpected death in epilepsy (SUDEP): role of impaired adenosine clearance. Epilepsia. 2010;51:465–8. doi: 10.1111/j.1528-1167.2009.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klassen TL, Bomben VC, Patel A, Drabek J, Chen TT, Gu W, Zhang F, Chapman K, Lupski JR, Noebels JL, Goldman AM. High-resolution molecular genomic autopsy reveals complex sudden unexpected death in epilepsy risk profile. Epilepsia. 2014;55:e6–12. doi: 10.1111/epi.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moghimi N, Lhatoo SD. Sudden unexpected death in epilepsy or voodoo heart: analysis of heart/brain connections. Curr Cardiol Rep. 2013;15:424. doi: 10.1007/s11886-013-0424-9. [DOI] [PubMed] [Google Scholar]
- 10.Massey CA, Sowers LP, Dlouhy BJ, Richerson GB. Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nat Rev Neurol. 2014;10:271–82. doi: 10.1038/nrneurol.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langan Y, Nashef L, Sander JW. Sudden unexpected death in epilepsy: a series of witnessed deaths. J Neurol Neurosurg Psychiatry. 2000;68:211–3. doi: 10.1136/jnnp.68.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.So EL, Sam MC, Lagerlund TL. Postictal central apnea as a cause of SUDEP: evidence from near-SUDEP incident. Epilepsia. 2000;41:1494–7. doi: 10.1111/j.1528-1157.2000.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 13.Bateman LM, Li CS, Seyal M. Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain. 2008;131:3239–45. doi: 10.1093/brain/awn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blum AS. Respiratory physiology of seizures. J Clin Neurophysiol. 2009;26:309–15. doi: 10.1097/WNP.0b013e3181b7f14d. [DOI] [PubMed] [Google Scholar]
- 15.Pezzella M, Striano P, Ciampa C, Errichiello L, Penza P, Striano S. Severe pulmonary congestion in a near miss at the first seizure: further evidence for respiratory dysfunction in sudden unexpected death in epilepsy. Epilepsy Behav. 2009;14:701–2. doi: 10.1016/j.yebeh.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Faingold CL, Randall M, Tupal S. DBA/1 mice exhibit chronic susceptibility to audiogenic seizures followed by sudden death associated with respiratory arrest. Epilepsy Behav. 2010;17:436–40. doi: 10.1016/j.yebeh.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Jawahar MC, Sari CI, Wilson YM, Lawrence AJ, Brodnicki T, Murphy M. Audiogenic seizure proneness requires the contribution of two susceptibility loci in mice. Neurogenetics. 2011;12:253–7. doi: 10.1007/s10048-011-0289-2. [DOI] [PubMed] [Google Scholar]
- 18.Faingold CL, Kommajosyula SP, Long X, Plath K, Randall M. Serotonin and sudden death: differential effects of serotonergic drugs on seizure-induced respiratory arrest in DBA/1 mice. Epilepsy Behav. 2014;37:198–203. doi: 10.1016/j.yebeh.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 19.Frankel WN. Genetics of complex neurological disease: challenges and opportunities for modeling epilepsy in mice and rats. Trends Genet. 2009;25:361–7. doi: 10.1016/j.tig.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tupal S, Faingold CL. Evidence supporting a role of serotonin in modulation of sudden death induced by seizures in DBA/2 mice. Epilepsia. 2006;47:21–6. doi: 10.1111/j.1528-1167.2006.00365.x. [DOI] [PubMed] [Google Scholar]
- 21.Faingold CL, Randall M. Effects of age, sex, and sertraline administration on seizure-induced respiratory arrest in the DBA/1 mouse model of sudden unexpected death in epilepsy (SUDEP) Epilepsy Behav. 2013;28:78–82. doi: 10.1016/j.yebeh.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Partners against mortality in epilepsy conference summary. Epilepsy Curr. 2014;14:14–31. doi: 10.5698/1535-7597-14.s6.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ralls K. Auditory sensitivity in mice, Peromyscus and Mus musculus. Anim Behav. 1967;15:123–8. doi: 10.1016/s0003-3472(67)80022-8. [DOI] [PubMed] [Google Scholar]
- 24.Turner JG, Willott JF. Exposure to an augmented acoustic environment alters auditory function in hearing-impaired DBA/2J mice. Hear Res. 1998;118:101–13. doi: 10.1016/s0378-5955(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 25.Richerson GB, Buchanan GF. The serotonin axis: Shared mechanisms in seizures, depression, and SUDEP. Epilepsia. 2011;52 (Suppl 1):28–38. doi: 10.1111/j.1528-1167.2010.02908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terra VC, Cysneiros R, Cavalheiro EA, Scorza FA. Sudden unexpected death in epilepsy: from the lab to the clinic setting. Epilepsy Behav. 2013;26:415–20. doi: 10.1016/j.yebeh.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 27.Venit EL, Shepard BD, Seyfried TN. Oxygenation prevents sudden death in seizure-prone mice. Epilepsia. 2004;45:993–6. doi: 10.1111/j.0013-9580.2004.02304.x. [DOI] [PubMed] [Google Scholar]
- 28.Buchanan GF, Murray NM, Hajek MA, Richerson GB. Serotonin neurones have anticonvulsant effects and reduce seizure-induced mortality. J Physiol. 2014;592:4395–410. doi: 10.1113/jphysiol.2014.277574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau RWt, Johnson RL, Deneris ES, Chen ZF. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci. 2006;26:12781–8. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bateman LM, Li CS, Lin TC, Seyal M. Serotonin reuptake inhibitors are associated with reduced severity of ictal hypoxemia in medically refractory partial epilepsy. Epilepsia. 2010;51:2211–4. doi: 10.1111/j.1528-1167.2010.02594.x. [DOI] [PubMed] [Google Scholar]
- 31.Faingold CL, Tupal S, Randall M. Prevention of seizure-induced sudden death in a chronic SUDEP model by semichronic administration of a selective serotonin reuptake inhibitor. Epilepsy Behav. 2011;22:186–90. doi: 10.1016/j.yebeh.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Glasscock E, Yoo JW, Chen TT, Klassen TL, Noebels JL. Kv1.1 potassium channel deficiency reveals brain-driven cardiac dysfunction as a candidate mechanism for sudden unexplained death in epilepsy. J Neurosci. 2010;30:5167–75. doi: 10.1523/JNEUROSCI.5591-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalume F, Westenbroek RE, Cheah CS, Yu FH, Oakley JC, Scheuer T, Catterall WA. Sudden unexpected death in a mouse model of Dravet syndrome. J Clin Invest. 2013;123:1798–808. doi: 10.1172/JCI66220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qi Y, Wang J, Bomben VC, Li DP, Chen SR, Sun H, Xi Y, Reed JG, Cheng J, Pan HL, Noebels JL, Yeh ET. Hyper-SUMOylation of the Kv7 potassium channel diminishes the M-current leading to seizures and sudden death. Neuron. 2014;83:1159–71. doi: 10.1016/j.neuron.2014.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tevoufouet EE, Nembo EN, Dibue-Adjei M, Hescheler J, Nguemo F, Schneider T. Cardiac Functions of Voltage-Gated Ca(2+) Channels: Role of the Pharmacoresistant Type (E-/R-Type) in Cardiac Modulation and Putative Implication in Sudden Unexpected Death in Epilepsy (SUDEP) Rev Physiol Biochem Pharmacol. 2014;167:115–39. doi: 10.1007/112_2014_21. [DOI] [PubMed] [Google Scholar]
- 36.Moore BM, Jerry Jou C, Tatalovic M, Kaufman ES, Kline DD, Kunze DL. The Kv1.1 null mouse, a model of sudden unexpected death in epilepsy (SUDEP) Epilepsia. 2014;55:1808–16. doi: 10.1111/epi.12793. [DOI] [PubMed] [Google Scholar]
- 37.Wagnon JL, Korn MJ, Parent R, Tarpey TA, Jones JM, Hammer MF, Murphy GG, Parent JM, Meisler MH. Convulsive seizures and SUDEP in a mouse model of SCN8A epileptic encephalopathy. Hum Mol Genet. 2015;24:506–15. doi: 10.1093/hmg/ddu470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan QS, Jobe PC, Dailey JW. Further evidence of anticonvulsant role for 5-hydroxytryptamine in genetically epilepsy-prone rats. Br J Pharmacol. 1995;115:1314–8. doi: 10.1111/j.1476-5381.1995.tb15042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Igelstrom KM. Preclinical antiepileptic actions of selective serotonin reuptake inhibitors--implications for clinical trial design. Epilepsia. 2012;53:596–605. doi: 10.1111/j.1528-1167.2012.03427.x. [DOI] [PubMed] [Google Scholar]
- 40.Igelstrom KM, Heyward PM. The antidepressant drug fluoxetine inhibits persistent sodium currents and seizure-like events. Epilepsy Res. 2012;101:174–81. doi: 10.1016/j.eplepsyres.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 41.Zeng C, Long X, Cotten JF, Forman SA, Solt K, Faingold CL, Feng HJ. Fluoxetine prevents respiratory arrest without enhancing ventilation in DBA/1 mice. Epilepsy Behav. 2015;45:1–7. doi: 10.1016/j.yebeh.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uteshev VV, Tupal S, Mhaskar Y, Faingold CL. Abnormal serotonin receptor expression in DBA/2 mice associated with susceptibility to sudden death due to respiratory arrest. Epilepsy Res. 2010;88:183–8. doi: 10.1016/j.eplepsyres.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Faingold CL, Randall M, Mhaskar Y, Uteshev VV. Differences in serotonin receptor expression in the brainstem may explain the differential ability of a serotonin agonist to block seizure-induced sudden death in DBA/2 vs. DBA/1 mice Brain Res. 2011;1418:104–10. doi: 10.1016/j.brainres.2011.08.043. [DOI] [PubMed] [Google Scholar]
- 44.Shank RP, Vaught JL, Pelley KA, Setler PE, McComsey DF, Maryanoff BE. McN-5652: a highly potent inhibitor of serotonin uptake. J Pharmacol Exp Ther. 1988;247:1032–8. [PubMed] [Google Scholar]
- 45.Fuller RW, Perry KW, Molloy BB. Effect of an uptake inhibitor on serotonin metabolism in rat brain: studies with 3-(p-trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine (Lilly 110140) Life Sci. 1974;15:1161–71. doi: 10.1016/s0024-3205(74)80012-3. [DOI] [PubMed] [Google Scholar]
- 46.Strawn JR, Welge JA, Wehry AM, Keeshin B, Rynn MA. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2015;32:149–57. doi: 10.1002/da.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dale E, Bang-Andersen B, Sanchez C. Emerging mechanisms and treatments for depression beyond SSRIs and SNRIs. Biochem Pharmacol. 2015;95:81–97. doi: 10.1016/j.bcp.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 48.Sugimoto Y, Tagawa N, Kobayashi Y, Mitsui-Saito K, Hotta Y, Yamada J. Involvement of the sigma1 receptor in the antidepressant-like effects of fluvoxamine in the forced swimming test in comparison with the effects elicited by paroxetine. Eur J Pharmacol. 2012;696:96–100. doi: 10.1016/j.ejphar.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 49.Garcia AJ, 3rd, Koschnitzky JE, Dashevskiy T, Ramirez JM. Cardiorespiratory coupling in health and disease. Auton Neurosci. 2013;175:26–37. doi: 10.1016/j.autneu.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moon RY, Horne RS, Hauck FR. Sudden infant death syndrome. Lancet. 2007;370:1578–87. doi: 10.1016/S0140-6736(07)61662-6. [DOI] [PubMed] [Google Scholar]
- 51.Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: neuromodulatory and trophic effects. Respir Physiol Neurobiol. 2008;164:222–32. doi: 10.1016/j.resp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol. 2009;4:517–50. doi: 10.1146/annurev.pathol.4.110807.092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aiba I, Noebels JL. Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models. Sci Transl Med. 2015;7:282ra46. doi: 10.1126/scitranslmed.aaa4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 55.Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]
- 56.Kulikov AV, Osipova DV, Naumenko VS, Popova NK. Association between Tph2 gene polymorphism, brain tryptophan hydroxylase activity and aggressiveness in mouse strains. Genes Brain Behav. 2005;4:482–5. doi: 10.1111/j.1601-183X.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- 57.Osipova DV, Kulikov AV, Popova NK. C1473G polymorphism in mouse tph2 gene is linked to tryptophan hydroxylase-2 activity in the brain, intermale aggression, and depressive-like behavior in the forced swim test. J Neurosci Res. 2009;87:1168–74. doi: 10.1002/jnr.21928. [DOI] [PubMed] [Google Scholar]
- 58.Osipova DV, Kulikov AV, Mekada K, Yoshiki A, Moshkin MP, Kotenkova EV, Popova NK. Distribution of the C1473G polymorphism in tryptophan hydroxylase 2 gene in laboratory and wild mice. Genes Brain Behav. 2010;9:537–43. doi: 10.1111/j.1601-183X.2010.00586.x. [DOI] [PubMed] [Google Scholar]
- 59.Diez JA, Sze PY, Ginsburg BE. Genetic and developmental variation in mouse brain tryptophan hydroxylase activity. Brain Res. 1976;109:413–7. doi: 10.1016/0006-8993(76)90545-x. [DOI] [PubMed] [Google Scholar]
- 60.Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–66. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci U S A. 2010;107:16354–9. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moseley BD, Nickels K, Britton J, Wirrell E. How common is ictal hypoxemia and bradycardia in children with partial complex and generalized convulsive seizures? Epilepsia. 2010;51:1219–24. doi: 10.1111/j.1528-1167.2009.02490.x. [DOI] [PubMed] [Google Scholar]
- 63.Depuy SD, Kanbar R, Coates MB, Stornetta RL, Guyenet PG. Control of breathing by raphe obscurus serotonergic neurons in mice. J Neurosci. 2011;31:1981–90. doi: 10.1523/JNEUROSCI.4639-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sowers LP, Massey CA, Gehlbach BK, Granner MA, Richerson GB. Sudden unexpected death in epilepsy: fatal post-ictal respiratory and arousal mechanisms. Respir Physiol Neurobiol. 2013;189:315–23. doi: 10.1016/j.resp.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fisher RS, Schachter SC. The Postictal State: A Neglected Entity in the Management of Epilepsy. Epilepsy Behav. 2000;1:52–59. doi: 10.1006/ebeh.2000.0023. [DOI] [PubMed] [Google Scholar]
- 66.Cotten JF. TASK-1 (KCNK3) and TASK-3 (KCNK9) tandem pore potassium channel antagonists stimulate breathing in isoflurane-anesthetized rats. Anesth Analg. 2013;116:810–6. doi: 10.1213/ANE.0b013e318284469d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith AA, Engelsher C, Crofford M. Respiratory depressant effects of ethanol: mediation by serotonin. Adv Exp Med Biol. 1975;59:407–17. doi: 10.1007/978-1-4757-0632-1_29. [DOI] [PubMed] [Google Scholar]
- 68.Leestma JE, Walczak T, Hughes JR, Kalelkar MB, Teas SS. A prospective study on sudden unexpected death in epilepsy. Ann Neurol. 1989;26:195–203. doi: 10.1002/ana.410260203. [DOI] [PubMed] [Google Scholar]
- 69.Hesdorffer DC, Tomson T, Benn E, Sander JW, Nilsson L, Langan Y, Walczak TS, Beghi E, Brodie MJ, Hauser A, Epidemiology ICo Subcommission on M. Combined analysis of risk factors for SUDEP. Epilepsia. 2011;52:1150–9. doi: 10.1111/j.1528-1167.2010.02952.x. [DOI] [PubMed] [Google Scholar]
- 70.Opeskin K, Berkovic SF. Risk factors for sudden unexpected death in epilepsy: a controlled prospective study based on coroners cases. Seizure. 2003;12:456–64. doi: 10.1016/s1059-1311(02)00352-7. [DOI] [PubMed] [Google Scholar]
- 71.Nilsson L, Farahmand BY, Persson PG, Thiblin I, Tomson T. Risk factors for sudden unexpected death in epilepsy: a case-control study. Lancet. 1999;353:888–93. doi: 10.1016/s0140-6736(98)05114-9. [DOI] [PubMed] [Google Scholar]
- 72.Beran RG, Weber S, Sungaran R, Venn N, Hung A. Review of the legal obligations of the doctor to discuss Sudden Unexplained Death in Epilepsy (SUDEP)--a cohort controlled comparative cross-matched study in an outpatient epilepsy clinic. Seizure. 2004;13:523–8. doi: 10.1016/j.seizure.2003.12.008. [DOI] [PubMed] [Google Scholar]