Abstract
Sensory threshold (ST) was measured using an electric von Frey anesthesiometer (VFA) in all limbs of 20 normal dogs and 29 dogs with acute thoracolumbar spinal cord injury (SCI) caused by spontaneous intervertebral disc extrusion. ST values were measured at three separate time points in normal dogs and on days 3, 10 and 30 following decompressive surgery in dogs with SCI. ST values were compared between groups and correlated with locomotor recovery in SCI-affected dogs.
ST values were significantly higher (consistent with hypoalgesia) in the pelvic limbs of SCI-affected dogs at day 3, day 10 and day 30 when compared to normal dogs (P < 0.05) while no significant difference in thoracic limb ST values was observed between groups. A progressive decrease in pelvic limb ST values occurred in SCI-affected dogs over time, consistent with improvement toward normal sensation or development of allodynia. This finding correlated inversely with locomotor score at 3 and 10 days after surgery. A significant decline in ST values across testing sessions was observed for all limbs of normal and SCI-affected dogs and may be related to patient acclimation, operator training effect, or effect of analgesic medications. This study supports the feasibility of VFA to assess differences in ST between normal and SCI-affected dogs. However, future studies must focus on techniques to minimize or compensate for clinical, environmental and behavioral factors which may impact ST values in the clinical setting.
Keywords: Spinal cord injury, Quantitative sensory testing, von Frey anesthesiometer, Sensory threshold, Canine
Introduction
Acute spinal cord injury (SCI) is a common neurological problem in dogs (Olby et al., 2003). Despite the prognostic significance of diminished conscious pain perception in canine SCI, routine clinical evaluation of dogs with SCI has historically focused on locomotor scoring and only a crude assessment of the ‘presence’ or ‘absence’ of a behavioral response to a painful stimulus (Olby et al., 2001; Levine et al., 2009; Lascelles, 2013). Abnormalities in sensory processing such as allodynia and hyperesthesia are reported in up to 90% of human patients after SCI (Boldt et al., 2014). Sensory abnormalities have yet to be thoroughly explored in dogs with SCI, despite being repeatedly documented in rodent models of SCI and in the human clinical setting (Carlton et al., 2009; Felix et al., 2009; Densmore et al., 2010; Lindsey et al., 2010; Hoschouer et al., 2010; Hayes et al., 2012).
Recent studies suggest that an electronic von Frey anesthesiometer (VFA) may prove useful in dogs as an objective mechanical quantitative sensory test (QST) (Moore et al., 2013; Briley et al., 2014). This technique has been used previously to assess hyperalgesia in dogs with orthopedic disease (Brydges et al., 2012), anti-nociceptive effects of analgesics (KuKanich et al., 2005a,b; KuKanich and Papich, 2011), and to evaluate sensory threshold (ST) in a small number of dogs with acute SCI (Moore et al., 2013). ST in these studies has been defined as the strength of mechanical stimulus required to produce a conscious behavioral response to that stimulus. When assessing patients with SCI, increases in ST above baseline are generally interpreted to represent hypoalgesia while decreases in ST below baseline are representative of allodynia or hyperesthesia (Detloff et al., 2010; Moore et al., 2013).
The goal of our study was to explore the feasibility of VFA to measure differences in ST values between normal dogs and dogs with acute thoracolumbar SCI caused by intervertebral disc extrusion (IVDE) in the clinical setting. We also aimed to document how ST values changed in SCI-affected dogs over a 30-day period of neurological recovery. We hypothesized that pelvic limb ST values would differ between normal dogs and those with thoracolumbar SCI, while thoracic limb ST values would not. Based on our previous work, we also hypothesized that pelvic limb ST values in SCI-affected dogs would have an inverse correlation with improving locomotor scores, consistent with recovery of sensory function in the weeks following SCI.
Materials and methods
The study was approved by the Ohio State University (OSU) Clinical Research Advisory Committee and the Institutional Animal Care and Use Committee (2012A00000149). Written owner consent was obtained prior to study enrolment.
Normal dogs
Twenty apparently healthy adult dogs were recruited from the OSU Veterinary Medical Center. Dogs had no prior history of neurological or orthopedic disease and were of a small breed (≤ 20 kg). All dogs were assessed to be neurologically and orthopedically normal based on examination by two of the investigators (RBS and SAM), with the exception that valgus and varus conformational limb abnormalities typical for chondrodystrophic breeds were considered acceptable for enrollment to facilitate generalization of our results across a realistic clinical population.
An electronic VFA device (IITC) was used for ST measurement in all four limbs. This device is comprised of a load cell, a recording device, a handle, and a rigid 0.8 mm diameter plastic disposable tip. The one used for this study measured, stored and digitally displayed the maximum force applied to the limb between 0.1 and 1000 g during a test.
ST testing was performed in a quiet room with minimal traffic, as previously described (Moore et al., 2013). Testing order of the limbs was decided by a coin toss and recorded. Dogs were positioned in lateral recumbency and maintained in this position using the minimum amount of restraint. They were placed in left lateral recumbency for testing of the right-sided limbs and vice versa. The limb being tested was allowed to rest on the floor in a neutral position. For the pelvic limbs, the electronic VFA probe was applied perpendicular to the dorsal surface of the metatarsus, halfway between the tarsometatarsal and metatarsophalangeal joints between digits IV and V; this region lies within the cutaneous autonomous zone of the fibular branch of the sciatic nerve. For thoracic limbs, the electronic VFA probe was applied perpendicular to the dorsal surface of metacarpus, halfway between the carpometacarpal and metacarpophalangeal joints between digits IV and V; this region lies within the cutaneous autonomous zone of the radial nerve.
Dogs were prevented f rom visualizing the device during application to ensure behavioral responses were due to tactile stimulation (Detloff et al., 2010). Steady, progressively increasing pressure was applied until the dog displayed a behavioral response to the stimulus, regarded as a conscious response such as vocalization, or lip licking. This response generally occurred in conjunction with withdrawal of the limb, but not in all cases. Immediate withdrawal of the limb upon application of the probe before application of pressure was considered a reflexive movement or a product of proprioceptive input rather than a conscious response to tactile stimulus and was discarded and the stimulus repeated after 1 min (Kloos et al., 2005; KuKanich et al., 2005a; Detloff et al., 2010).
The evaluator (RBS) was blinded to the pressure readings obtained during testing. The minimum pressure required to elicit a behavioral response was recorded. The test was repeated five times in each limb, with each test separated by 1 min to avoid windup, ST decay, and hypersensitization (KuKanich et al., 2005b; Detloff et al., 2010, 2012). The highest and lowest ST values were excluded and the three middle values averaged to assign a single ST value to each limb (Moore et al., 2013). ST testing was repeated three times at least 48 h apart in all normal dogs.
Affected dogs
Twenty-nine dogs adult dogs with acute T3-L3 myelopathy caused by IVDE were consecutively and prospectively enrolled from the general patient population at OSU Veterinary Medical Center. Dogs were eligible for enrollment if diagnostic testing (CT, CT and myelogram, or MRI) confirmed IVDE, and they weighed ≤ 20 kg. A subjective assessment of intact conscious response to pain stimulus, as assessed by both the attending clinician and the investigators, was required for enrollment. All dogs underwent surgical decompression for their IVDE. ST testing of all four limbs using the technique described above was performed at three time points: 3, 10 and 30 days after surgery. Each affected dog was also assigned a locomotor score by the investigators using the Olby Spinal Cord Injury Scale (OSCIS) (Olby et al., 2001) at each time point. Analgesic and/or anti-inflammatory medications were prescribed for all patients during the perioperative period with dosing at the discretion of the attending clinician. All medications that the subjects were receiving at the time of testing were recorded.
Statistics
Summary statistics including mean and standard error of the mean (SEM), or median and range where appropriate, are reported for clinical data on all dogs, and for ST values for all testing sessions. Normality of data was verified by the Anderson-Darling method. Data for ST values was compared across three testing sessions in normal dogs and in SCI-affected dogs using a mixed effect model, incorporating repeated measures for each subject (Verbeke and Molenberghs, 2000). Spearman correlations were calculated to assess the relationship between ST values and locomotor scores in SCI-affected dogs. A P-value of < 0.05 was considered significant for all analyses. Analyses were conducted using SAS software.
Results
Normal dogs
Normal dogs ranged in age from 8 months to 6.5 years (median 3 years) and weighed between 3.7 kg to 17.2 kg (median 9.4 kg). There were eight spayed females and 12 castrated males. Breeds were as follows: mixed breed dogs (6), Dachshunds (4), Miniature Schnauzers (2), Sealyham terriers (2), Beagle (1), Bichon frise (1), Cocker spaniel (1), Pembroke Welsh corgi (1), Miniature Pinscher (1), and Shih Tzu (1). Time period between testing sessions for each dog ranged from 2 to 27 days (median 6 days).
Affected dogs
A total of 29 dogs with acute SCI caused by IVDE were enrolled. Dogs ranged in age from 2 to 11 years (median 5 years) and weighed between 3.9 kg to 17.0 kg (median 8.0 kg). There were 14 spayed females, 13 castrated males, and two intact males. Breeds were as follows: Dachshunds (12), mixed breed dogs (6), French bulldogs (4), Beagles (2), Pembroke Welsh corgis (2), Shih Tzus (2), and Cocker spaniel (1).
All dogs underwent decompressive hemilaminectomy or pediculectomy at one or multiple sites between T10–11 and L3–4 intervertebral disc spaces, with or without one or more lateral disc fenestrations dependent on imaging results and discretion of the surgeon. Postoperative analgesic dosage and type was dependent upon the surgeon’s preference but included a fentanyl constant rate infusion for 12–24 h post-operatively, a fentanyl patch placed immediately post-operatively, and combinations of tramadol, gabapentin, methocarbamol, or diazepam. Post-operative anti-inflammatory therapy generally included tapering anti-inflammatory doses of prednisone, or a non-steroidal anti-inflammatory drug (NSAID). The medication doses, frequency of administration, and number of total medications were recorded for each dog at each session.
von Frey anesthesiometry sensory threshold (ST) values of normal dogs
Mean ST values for normal dogs across three testing sessions are summarized in Table 1. Mean ± SEM sensory threshold values in grams for normal dogs across three testing sessions were as follows: 161.9 ± 14.8, 128.4 ± 11.7, 102.7 ± 10.0 (left thoracic limb- LTL); 145.7 ± 9.8, 116.5 ± 11.9, 94.8 ± 8.0 (left pelvic limb- LPL); 147.1 ± 11.6, 127.1 ± 9.0, 116.7 ± 9.0 (right thoracic limb- RTL); 142.8 ± 12.6, 121.4 ± 10.5, 100.6 ± 9.3 (right pelvic limb- RPL). A significant difference was not identified between ST values obtained from the LTL, RTL, LPL, RPL of normal dogs between sessions 1 and 2 (P = 0.18, 0.43, 0.25, 0.39, respectively) or between sessions 2 and 3 (P = 0.31, 0.68, 0.39, 0.41). When comparing sessions 1 and 3, a significant decrease in session 3 was noted in ST values for the LTL (P = 0.02) and LPL (P = 0.04).
Table 1.
Individual and mean sensory threshold (ST) values of thoracic and pelvic limbs across three testing session in normal dogs (n=20). LTL, left thoracic limb; LPL, left pelvic limb; RTL, right thoracic limb; RPL, right pelvic limb.
| von Frey (g) LTL | von Frey (g) LPL | von Frey (g) RTL | von Frey (g) RPL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal dogs | Session 1 |
Session 2 |
Session 3 |
Session 1 |
Session 2 |
Session 3 |
Session 1 |
Session 2 |
Session 3 |
Session 1 |
Session 2 |
Session 3 |
| Beagle | 177.3 | 134.4 | 86.3 | 205.7 | 184.9 | 111.8 | 100.8 | 157.1 | 90.9 | 264.6 | 206.2 | 147.4 |
| Bichon Frise | 111.4 | 95.4 | 60.4 | 161.6 | 59.7 | 61.4 | 80.6 | 71.6 | 84.8 | 92.4 | 58.5 | 41.9 |
| Cocker Spaniel | 134.1 | 169.0 | 114.4 | 117.6 | 137.4 | 133.4 | 154.2 | 138.6 | 112.0 | 139.9 | 146.0 | 132.4 |
| Corgi | 188.9 | 131.7 | 94.5 | 178.5 | 68.8 | 77.8 | 227.4 | 145.9 | 99.4 | 158.2 | 129.8 | 94.6 |
| Dachshund | 174.8 | 120.7 | 136.4 | 159.7 | 117.2 | 139.5 | 162.6 | 161.5 | 138.3 | 108.6 | 150.2 | 117.4 |
| Dachshund | 133.0 | 85.6 | 66.0 | 143.4 | 90.1 | 89.0 | 108.1 | 132.6 | 39.1 | 74.8 | 101.1 | 65.2 |
| Dachshund | 95.0 | 81.4 | 57.7 | 77.8 | 90.8 | 56.9 | 229.6 | 83.6 | 107.9 | 202.0 | 128.6 | 72.2 |
| Dachshund | 170.7 | 128.7 | 160.1 | 181.2 | 111.5 | 82.2 | 169.9 | 165.6 | 169.2 | 75.9 | 126.5 | 155.5 |
| Miniature pinscher | 143.8 | 141.2 | 234.4 | 126.2 | 169.0 | 166.8 | 132.2 | 161.1 | 164.5 | 156.8 | 180.7 | 203.0 |
| Mixed breed | 269.4 | 133.3 | 118.4 | 204.3 | 102.7 | 65.1 | 123.3 | 120.2 | 112.2 | 197.3 | 127.1 | 80.7 |
| Mixed breed (Puggle/Corgi) | 186.5 | 68.8 | 55.8 | 158.1 | 100.2 | 55.6 | 180.6 | 69.5 | 104.2 | 178.1 | 123.8 | 89.5 |
| Mixed breed (Dachshund) | 244.8 | 225.9 | 138.8 | 192.5 | 233.4 | 178.2 | 151.1 | 197.3 | 201.8 | 237.6 | 218.3 | 159.3 |
| Mixed breed (beagle/hound) | 126.5 | 123.3 | 93.7 | 135.1 | 149.1 | 113.3 | 144.8 | 144.3 | 93.9 | 185.9 | 128.2 | 89.0 |
| Mixed (Dachshund/Scottie) | 311.6 | 274.4 | 121.0 | 229.7 | 225.0 | 101.1 | 204.5 | 168.9 | 161.0 | 147.0 | 96.3 | 87.5 |
| Mixed breed (Pomeranian) | 135.1 | 106.9 | 96.7 | 109.1 | 71.3 | 79.2 | 156.8 | 116.0 | 142.3 | 106.4 | 90.5 | 115.1 |
| Schnauzer | 93.6 | 75.6 | 70.1 | 92.3 | 41.3 | 80.7 | 86.0 | 101.4 | 122.7 | 61.5 | 59.7 | 72.8 |
| Schnauzer | 105.5 | 62.3 | 66.8 | 106.9 | 80.9 | 70.4 | 92.5 | 90.6 | 84.8 | 109.7 | 66.9 | 43.0 |
| Sealyham terrier | 198.4 | 146.6 | 117.5 | 113.6 | 113.7 | 95.4 | 236.6 | 131.7 | 123.6 | 154.2 | 92.3 | 76.8 |
| Sealyham terrier | 209.1 | 165.1 | 123.3 | 144.7 | 119.5 | 76.0 | 147.2 | 144.0 | 133.9 | 131.9 | 150.3 | 102.7 |
| Shih Tzu | 27.7 | 97.5 | 42.1 | 75.3 | 62.7 | 61.2 | 52.9 | 39.7 | 46.6 | 73.3 | 46.9 | 66.9 |
| MEAN | 161.9 | 128.4 | 102.7 | 145.7 | 116.5 | 94.8 | 147.1 | 127.1 | 116.7 | 142.8 | 121.4 | 100.6 |
| STD ERROR OF THE MEAN | 14.8 | 11.7 | 10.0 | 9.8 | 11.9 | 8.0 | 11.6 | 9.0 | 9.0 | 12.6 | 10.5 | 9.3 |
ST values differ in the Pelvic limbs between normal and SCI-affected dogs
ST values obtained from SCI-affected dogs at three time points after injury are summarized in Table 2. Mean ± SEM sensory threshold values in grams for SCI-affected dogs at days 3,10, and 30 after surgery were as follows; 199.0 ±15.6, 156.3 ± 12.0, 148.3 ± 10.1 (LTL); 349.6 ± 27.1, 254.7 ± 23.9, 205.6 ± 18.1 (LPL); 196.9 ± 20.2, 151.9 ± 11.7, 163.4 ± 11.0 (RTL); 356.9 ± 31.6, 235.6 ± 22.9, 214.5 ± 16.2 (RPL). ST values from the limbs of normal dogs at session 1 were compared to ST values from SCI-affected dogs at days 3, 10, and 30 (Fig. 1). ST values derived from session 1 were used for comparison as they had greater variability and minimized the effect of acclimation to better reflect values that would be obtained in a clinical setting.
Table 2.
Individual and mean sensory threshold (ST) values of thoracic and pelvic limbs in dogs with acute thoracolumbar spinal cord injury (SCI) caused by intervertebral disc extrusion (n=29). LTL, left thoracic limb; LPL, left pelvic limb; RTL, right thoracic limb; RPL, right pelvic limb.
| von Frey (g) LTL | von Frey (g) LPL | von Frey (g) RTL | von Frey (g) RPL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCI-affected dogs | Day 3 | Day 10 | Day 30 | Day 3 | Day 10 | Day 30 | Day 3 | Day 10 | Day 30 | Day 3 | Day 10 | Day 30 |
| Beagle | 160.8 | 97.9 | 105.7 | 246.9 | 199.8 | 286.4 | 104.4 | 148.5 | 168.0 | 229.0 | 167.5 | 243.5 |
| Beagle | 258.7 | 137.3 | 178.5 | 487.4 | 380.7 | 235.6 | 170.5 | 155.4 | 170.5 | 383.6 | 251.1 | 240.1 |
| Beagle | 180.1 | 199.8 | 110.9 | 284.8 | 258.5 | 165.2 | 218.9 | 224.7 | 162.3 | 269.0 | 300.3 | 138.9 |
| Cocker Spaniel | 176.8 | 249.1 | 172.3 | 327.3 | 266.4 | 231.2 | 219.9 | 256.5 | 172.3 | 248.5 | 319.3 | 281.3 |
| Corgi | 251.5 | 152.5 | 107.1 | 407.4 | 256.9 | 276.4 | 279.5 | 157.1 | 260.6 | 381.6 | 282.2 | 325.8 |
| Corgi | 155.2 | 154.6 | 126.4 | 343.1 | 269.6 | 293.7 | 219.2 | 100.9 | 195.7 | 589.8 | 218.5 | 231.6 |
| Dachshund | 98.1 | 82.4 | 77.6 | 168.3 | 131.0 | 85.6 | 164.6 | 123.2 | 81.7 | 174.0 | 116.8 | 114.6 |
| Dachshund | 74.7 | 77.9 | 90.5 | 239.0 | 71.7 | 44.0 | 128.3 | 98.8 | 105.5 | 302.9 | 142.8 | 109.2 |
| Dachshund | 293.9 | 194.0 | 232.4 | 573.8 | 405.3 | 187.8 | 190.8 | 168.9 | 197.8 | 713.6 | 99.7 | 288.5 |
| Dachshund | 139.2 | 57.4 | 103.9 | 550.6 | 356.0 | 126.9 | 84.2 | 34.4 | 110.9 | 481.5 | 352.4 | 149.9 |
| Dachshund | 150.3 | 174.5 | 200.3 | 295.8 | 280.6 | 224.1 | 160.7 | 180.8 | 197.2 | 237.2 | 220.1 | 154.9 |
| Dachshund | 178.3 | 132.7 | 174.5 | 503.4 | 444.2 | 326.7 | 211.4 | 145.2 | 92.2 | 394.1 | 358.4 | 275.6 |
| Dachshund | 94.7 | 173.6 | 228.1 | 440.5 | 237.4 | 305.0 | 84.0 | 167.9 | 253.2 | 222.1 | 141.2 | 248.0 |
| Dachshund | 271.3 | 124.4 | 97.2 | 511.7 | 275.3 | 132.4 | 209.0 | 117.0 | 80.1 | 636.1 | 333.6 | 171.3 |
| Dachshund | 109.3 | 83.7 | 94.6 | 168.5 | 109.6 | 167.3 | 160.6 | 109.9 | 98.1 | 251.5 | 135.4 | 103.8 |
| Dachshund | 184.4 | 118.9 | 120.8 | 319.1 | 213.3 | 173.8 | 142.2 | 98.7 | 120.6 | 340.0 | 210.7 | 245.7 |
| Dachshund | 166.8 | 113.3 | 237.0 | 194.8 | 166.0 | 226.1 | 236.4 | 85.8 | 184.0 | 227.4 | 146.2 | 228.2 |
| Dachshund | 114.7 | 113.7 | 115.0 | 187.6 | 217.1 | 158.4 | 153.7 | 165.6 | 142.6 | 220.1 | 189.6 | 176.9 |
| French Bulldog | 171.4 | 92.8 | 125.6 | 253.3 | 134.2 | 120.4 | 238.0 | 69.6 | 128.6 | 188.8 | 95.9 | 103.0 |
| French Bulldog | 212.0 | 350.8 | 202.8 | 318.7 | 277.9 | 284.8 | 285.0 | 331.7 | 227.4 | 313.6 | 368.6 | 308.2 |
| French Bulldog | 368.1 | 268.9 | 177.8 | 640.7 | 482.5 | 339.8 | 645.7 | 248.6 | 197.9 | 783.8 | 580.7 | 323.9 |
| French Bulldog | 407.4 | 164.4 | 173.0 | 291.3 | 139.4 | 145.2 | 396.7 | 175.2 | 248.1 | 615.2 | 187.3 | 158.3 |
| Mixed breed (Pitbull/Basset) | 235.9 | 146.4 | 145.8 | 334.5 | 226.7 | 289.0 | 163.5 | 128.3 | 238.9 | 361.2 | 186.4 | 220.4 |
| Mixed breed (Yorkie) | 147.6 | 122.3 | 54.2 | 161.0 | 99.5 | 41.8 | 200.1 | 63.5 | 80.9 | 173.2 | 60.4 | 84.4 |
| Mixed breed (Dachshund) | 82.1 | 116.4 | 127.3 | 171.4 | 175.8 | 119.0 | 106.1 | 103.2 | 109.0 | 145.9 | 149.3 | 192.1 |
| Mixed breed (Cocker spaniel/Poodle) | 252.1 | 235.4 | 204.1 | 299.1 | 344.8 | 203.6 | 187.8 | 226.7 | 220.3 | 348.0 | 170.6 | 182.0 |
| Mixed breed (Dachshund/Yorkie) | 263.5 | 219.6 | 124.3 | 701.2 | 621.8 | 374.2 | 164.8 | 177.7 | 87.7 | 555.7 | 568.9 | 390.3 |
| Shih Tzu | 222.7 | 221.6 | 281.6 | 300.4 | 318.9 | 379.1 | 104.2 | 173.0 | 265.6 | 289.0 | 247.1 | 424.2 |
| Shih Tzu | 349.2 | 157.7 | 109.9 | 416.2 | 25.0 | 19.7 | 77.0 | 167.6 | 140.4 | 273.2 | 231.1 | 106.6 |
| MEAN | 199.0 | 156.3 | 148.3 | 349.6 | 254.7 | 205.6 | 196.9 | 151.9 | 163.4 | 356.9 | 235.6 | 214.5 |
| STD ERROR OF THE MEAN | 15.6 | 12.0 | 10.1 | 27.1 | 23.9 | 18.1 | 20.2 | 11.7 | 11.0 | 31.6 | 22.9 | 16.2 |
Figure 1.
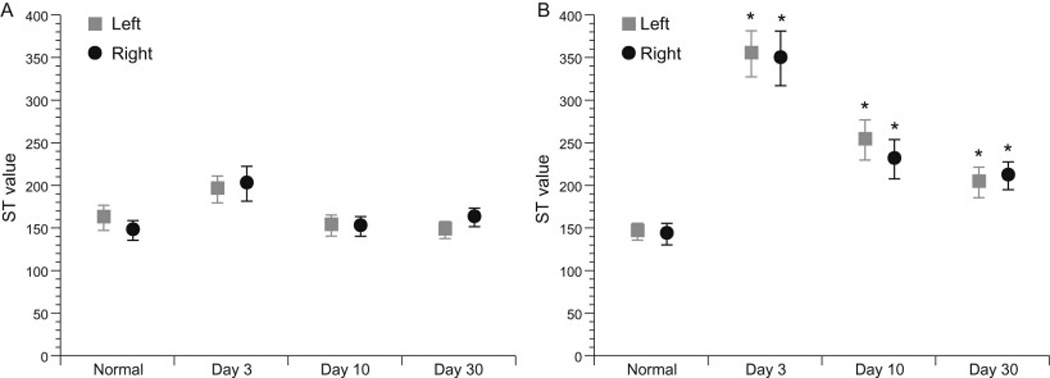
Comparison of left and right thoracic limb (A) and pelvic limb (B) sensory threshold (ST) values between normal dogs at session one and spinal cord injury (SCI)-affected dogs at days 3, 10, and 30 following decompressive surgery. ST values were significant higher in the pelvic limbs of SCI-affected dogs at all three time points evaluated, while no differences were noted in thoracic limb ST values between groups. Mean ± SEM are presented and asterisk denotes P<0.05.
A significant difference was not identified between the mean ST values in the thoracic limbs of normal dogs when compared to the mean ST values in the thoracic limbs of SCI-affected dogs at any time point after injury (Fig. 1A). Significant differences were observed in mean ST values in the pelvic limbs between normal dogs (142.8 ± 12.6- RPL and 145.7 ± 9.8-LPL) and SCI-affected dogs on day 3 (356.9 ± 31.6, P < 0.0001 RPL; 349.6 ± 27.1, P < 0.0001 LPL), day 10 (235.6 ± 22.9, P = 0.006 RPL; 254.7 ± 23.9, P = 0.04 LPL) and day 30 (214.5 ± 16.2, P = 0.006 RPL; 205.6 ± 18.1, P = 0.01 LPL) (Fig. 1B).
Pelvic limb ST values decrease with time and correlate inversely with locomotor recovery in SCI-affected dogs
Pelvic limb ST values in SCI-affected dogs were compared across testing sessions (Fig. 2). A significant decline in ST was noted between days 3 and 10 (349.6 ± 27.1 vs. 254.7 ± 23.9, P< 0.0001 LPL; 356.9 ± 31.6 vs. 235.6 ± 22.9, P < 0.0001 RPL) and between days 3 and 30 (349.6 + 27.1 vs. 205.6 ±18.1, P < 0.0001 LPL; 356.9 ± 31.6 vs. 214.5 ± 16.2, P < 0.0001 RPL).
Figure 2.
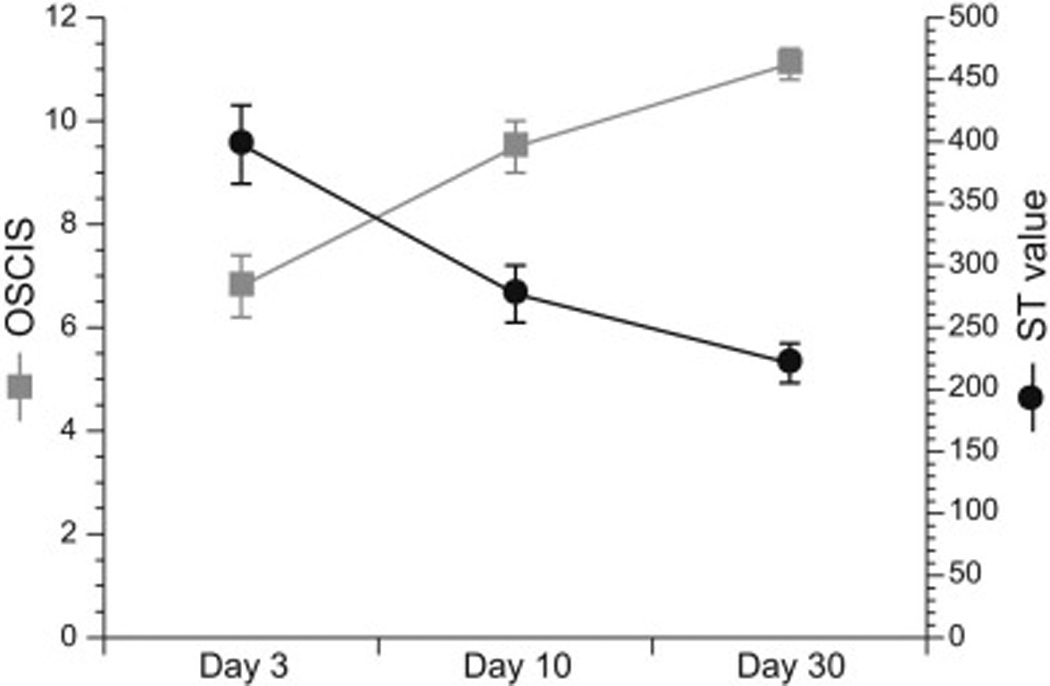
Relationship between Olby Spinal Cord Injury Scale (OSCIS) locomotor score and pelvic limb sensory threshold (ST) value in spinal cord injury affected dogs over time following decompressive surgery. A significant inverse correlation between locomotor score and pelvic limb ST value is observed at days 3 and 10 after injury. ST value displayed is the mean ± SEM value for the pelvic limb with the highest ST value.
At 3 days postoperatively, the median locomotor score for SCI-affected dogs was 6 (range 1–11). This increased to a median score of 10 (range 4–14) by day 10, and a median score of 11 (7–14) by day 30. A significant inverse correlation was observed between locomotor score and pelvic limb ST values at days 3 and 10 after surgery (Fig. 2): day 3 ρ = −0.71, P <0.0001 (LPL) and ρ = −0.5, P = 0.005 (RPL); day 10 ρ = −0.46, P = 0.01 (LPL) and ρ = −0.39, P = 0.037 (RPL).
Thoracic limb ST values change with repeated measures in SCI-affected dogs
A significant difference was not identified between ST values at days 3 and 10 for the thoracic limbs of SCI-affected dogs (P= 0.07 LTL, P= 0.053 RTL); however, a significant difference was observed when comparing values for the LTL between days 3 (199.0 ± 15.6) and 30 (148.3 ± 10.1) (P = 0.03). A significant correlation between thoracic limb ST values and locomotor score in SCI-affected dogs was not identified at any time point.
Discussion
Our study provides the first objective evaluation of ST in a large cohort of dogs with acute SCI. ST values obtained from the pelvic limbs of dogs with thoracolumbar SCI were significantly higher than ST values obtained from the pelvic limbs of normal dogs in our study, while no differences were observed between ST values from the thoracic limbs of the same two groups.
Pelvic limb ST values significantly decreased in the 30-day postoperative period in dogs with acute thoracolumbar SCI. This change correlated inversely with locomotor scores, indicating that as motor function improves, sensory thresholds decrease in neurologically affected limbs. Given the significant difference in pelvic limb ST values observed between normal dogs and SCI-affected d ogs during neurological recovery, coupled with the lack of statistically significant difference when comparing thoracic limb ST values between the same groups, it is likely that this change represents a true decline in ST. This finding may be explained by improvement of sensory function towards preinjury status, or may represent trends towards development of central sensitization or mechanical allodynia (Kloos et al., 2005; Walk et al., 2009).
Interpretation of the observed changes in pelvic limb ST values in SCI-affected dogs during neurological recovery are complicated by a concurrent smaller but statistically significant decrease in ST values measured from the thoracic limbs of the same patients over the same time period. Because patients with thoracolumbar SCI are expected to have neurologically normal thoracic limbs, improvement of sensory function to pre-injury status cannot explain this observation. It is possible that central sensitization or the development of allodynia could explain this finding (Carlton et al., 2009; Densmore et al., 2010). It is equally possible that the changes in thoracic ST values represent a decline in analgesic administration, acclimation of subjects to the testing environment, investigator training effect, or a combination of all of these factors. These factors also likely contribute in part to changes noted in pelvic limb ST values.
All of the SCI-affected dogs were administered analgesics including fentanyl, gabapentin, NSAIDs and tramadol. Such medications have been shown to influence the results of QST in various species (Lascelles et al., 1998; Matthews and Dickenson, 2002; Wegner et al., 2008; KuKanich and Papich, 2011; Kögel et al., 2014). Although the specific effect of some of these medications on ST values in dogs is unknown, the expected effect is an increase in ST values for the duration of administration. This effect could be expected to manifest in thoracic and pelvic limbs equally, and would also be expected to cease with discontinuation of medication administration.
A small but significant decline in ST values was also observed in normal dogs between sessions 1 and 3 in thoracic and pelvic limbs. This finding may be explained by ST decay, acclimation, or investigator training effect. Tactile sensory threshold decays (lowered sensory thresholds) can occur within a testing session if too many stimuli are given, or repeated stimuli are given too closely together (Detloff et al., 2010). We adhered to a 1-min delay between stimuli in order to minimize this concern (KuKanich et al., 2005b; Detloff et al., 2010, 2012; Moore et al., 2013). Feeding during the testing session is suggested to minimize the effect of sensory threshold decay in rodent studies (Detloff et al., 2010, 2012), but proved too distracting in dogs during pilot studies (S.A. Moore, unpublished data). With repeated testing sessions dogs may acclimate to the testing environment, which can also decrease ST values (Detloff et al., 2010). Testing sessions in all dogs were separated by no less than 48 h, but a longer period may be needed to minimize this phenomenon.
Our data highlight several hurdles to the use of VFA which must be addressed prior to its routine use in the clinical setting. These include patient, observer, environmental and analgesic medication factors which must be controlled to ensure reliable results. For obvious reasons, it would be unethical to withhold or restrict analgesics for veterinary patients in a clinical t rial. Prolonged acclimation of subjects to the testing environment is also not feasible for studies using client-owned animals with spontaneous SCI. Given our results, the utility of this measurement for assessment of ST in dogs with SCI is promising, but requires further investigation.
Conclusions
Our results support the feasibility of VFA to objectively measure differences in ST between normal and SCI-affected dogs and to document changes in sensory function during recovery after SCI. A significant decline in pelvic limb ST values correlated inversely with locomotor recovery in SCI-affected dogs, indicating improvement toward normal sensation, development of hyperesthesia, or a combination of both phenomena. We also observed small but significant declines in ST values in normal dogs with repeated testing and in thoracic limbs of SCI-affected dogs over time. These changes may be explained by analgesic medications in SCI-affected dogs, or by environmental and behavioral confounders in both groups. Future studies must focus on techniques to minimize or compensate for clinical, environmental, and behavioral factors that may impact ST values in the clinical setting.
Highlights.
We compared sensory threshold (ST) values obtained from normal dogs (n=20) with those from dogs with acute spinal cord injury (SCI) caused by intervertebral disc extrusion (n=29).
Pelvic limb ST values were significantly higher in SCI-affected dogs when compared to normal dogs at 3, 10, and 30 days after decompressive surgery.
Thoracic limb ST values did not differ between SCI-affected dogs and normal dogs at any time-point.
Pelvic limb ST values were inversely correlated with locomotor scores at all three time-points in SCI-affected dogs.
Small but significant differences in ST values occurred between testing sessions in normal dogs, and in the thoracic limbs of SCI-affected dogs which indicates that certain clinical factors beyond sensorimotor impairment may affect ST values in the clinical setting.
Acknowledgments
This study was funded by the Morris Animal Foundation D13CA-024, NIH CCTS UL1TR001070, and NCRR UL1RR025755. The authors also gratefully acknowledge Mrs. Amanda Disher and Ms. Heather Myers for their assistance with data collection and Mr. Tim Vojt for his assistance with preparation of the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None of the authors of this paper has a financial or personal 329 relationship with other people or organisations that could inappropriately influence 330 or bias the content of the paper.
References
- Boldt I, Eriks-Hoogland I, Brinkhof MW, de Bie R, Joggi D, von Elm E. Non-pharmacological intervention for chronic pain in people with spinal cord injury. The Cochrane Database of Systematic Reviews. 2014;11:CD009177. doi: 10.1002/14651858.CD009177.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briley JD, Williams MD, Freire M, Griffith EH, Lascelles BD. Feasability and repeatability of cold and mechanical quantitative sensory testing in normal dog. The Veterinary Journal. 2014;199:245–250. doi: 10.1016/j.tvjl.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydges NM, Argyle DJ, Mosley JR, Duncan JC, Fleetwood-Walker S, Clements DN. Clinical assessments of increased sensory sensitivity in dogs with cranial cruciate ligament rupture. The Veterinary Journal. 2012;193:545–550. doi: 10.1016/j.tvjl.2012.01.019. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Junhui D, Tan HY, Nesic O, Hargett GL, Bopp AC, Yamani A, Lin Q, Willis WD, Hulsebosch CE. Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain. 2009;147:265–276. doi: 10.1016/j.pain.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Densmore VS, Kalous A, Keast JR, Osborne PB. Above-level mechanical hyperalgesia in rats develops after incomplete spinal cord injury but not after cord transection, and is reversed by amitriptyline, morphine and gabapentin. Pain. 2010;151:184–193. doi: 10.1016/j.pain.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Detloff MR, Clark LM, Hutchinson KJ, Kllos AD, Fisher LC, Basso DM. Validity of acute and chronic tactile sensory testing after spinal cord injury in rats. Experimental Neurology. 2010;225:366–376. doi: 10.1016/j.expneurol.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detloff MR, Fisher LC, Deibert RJ, Basso DM. Acute and chronic tactile sensory testing after spinal cord injury in rats. Journal of Visualized Experiments. 2012;62:e3247. doi: 10.3791/3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix ER, Widerstrom-Noga EG. Reliability and validity of quantitative sensory testing in persons with spinal cord injury and neuropathic pain. Journal of Rehabilitation Research and Development. 2009;46:69–84. [PubMed] [Google Scholar]
- Hayes KC, Wolfe DL, Hsieh JT, Potter PJ, Drassioukov A, Durham CE. Clinical and electrophysiologic correlates of quantitative sensory testing in patients with incomplete spinal cord injury. Archives of Physical Medicine and Rehabilitation. 2002;83:1612–1619. doi: 10.1053/apmr.2002.35101. [DOI] [PubMed] [Google Scholar]
- Hoschouer EL, Basso DM, Jakeman LB. Aberrant sensory responses are dependent on lesion severity after spinal cord contusion injury in mice. Pain. 2010;148:328–342. doi: 10.1016/j.pain.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloos AD, Fisher LC, Detloff MR, Hassenzahi DL, Basso DM. Stepwise motor and all-or-none sensory recovery is associated with nonlinear sparing after incremental spinal cord injury in rats. Experimental Neurology. 2005;191:251–265. doi: 10.1016/j.expneurol.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kögel B, Terlinden R, Schneider J. Characterisation of tramadol, morphine and tapentadol in an acute pain model in Beagle dogs. Veterinary Anaesthesia and Analgesia. 2014;41:297–304. doi: 10.1111/vaa.12140. [DOI] [PubMed] [Google Scholar]
- KuKanich B, Lascelles BD, Papich MG. Assessment of a von Frey device for evaluation of the antinociceptive effects of morphine and its application in pharmacodynamics modeling of morphine in dogs. American Journal of Veterinary Research. 2005a;66:1616–1622. doi: 10.2460/ajvr.2005.66.1616. [DOI] [PubMed] [Google Scholar]
- KuKanich B, Lascelles BD, Papich MG. Use of a von Frey device for evaluation of pharmacokinetics and pharmacodynamics of morphine after intravenous administration as an infusion or multiple doses in dogs. American Journal of Veterinary Research. 2005b;66:1968–1974. doi: 10.2460/ajvr.2005.66.1968. [DOI] [PubMed] [Google Scholar]
- KuKanich B, Papich MG. Pharmacokinetics and antinociceptive effects of oral tramadol hydrochloride administration in Greyhounds. American Journal of Veterinary Research. 2011;72:256–262. doi: 10.2460/ajvr.72.2.256. [DOI] [PubMed] [Google Scholar]
- Lascelles BD, Cripps PJ, Jones A, Waterman-Pearson AE. Efficacy and kinetics of carprofen, administered preoperatively or postoperatively, for the prevention of pain in dogs undergoing ovariohysterectomy. Veterinary Surgery. 1998;27:568–582. doi: 10.1111/j.1532-950x.1998.tb00533.x. [DOI] [PubMed] [Google Scholar]
- Lascelles BD. Getting a sense of sensations. The Veterinary Journal. 2013;197:115–117. doi: 10.1016/j.tvjl.2013.02.025. [DOI] [PubMed] [Google Scholar]
- Levine GJ, Levine JM, Budke CM, Kerwin SC, Au J, Vinayak A, Hettlich BF, Slater MR. Description and repeatability of a newly developed spinal cord injury scale for dog. Preventive Veterinary Medicine. 2009;89:121–127. doi: 10.1016/j.prevetmed.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Lindsey AE, LoVerso RL, Tovar A, Hill CE, Beattie MS, Bresnahan JC. An analysis of changes in STs to mild tactile and cold stimuli after experimental spinal cord injury in the rat. Neurorehabilitation and Neural Repair. 2000;14:287–300. doi: 10.1177/154596830001400405. [DOI] [PubMed] [Google Scholar]
- Matthews EA, Dickenson AH. A combination of gabapentin and morphine mediates enhanced inhibitory effects on dorsal horn neuronal responses in a rat model of neuropathy. Anesthesiology. 2002;96:633–640. doi: 10.1097/00000542-200203000-00020. [DOI] [PubMed] [Google Scholar]
- Moore SA, Hettlich BF, Waln A. The use of an electronic von Frey device for evaluation of ST in neurologically normal dogs and those with acute spinal cord injury. The Veterinary Journal. 2013;197:216–219. doi: 10.1016/j.tvjl.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Olby NJ, De Risio L, Muñana K, Wosar MA, Skeen TM, Sharp NJ, Keene BW. Development of a functional scoring system in dogs with acute spinal cord injuries. American Journal of Veterinary Research. 2001;62:1624–1628. doi: 10.2460/ajvr.2001.62.1624. [DOI] [PubMed] [Google Scholar]
- Olby N, Levine J, Harris T, Muñana K, Skeen T, Sharp N. Long-term functional outcome of dogs with severe injuries of the thoracolumbar spinal cord: 87 cases (1996–2001) Journal of the American Veterinary Medical Association. 2003;222:762–769. doi: 10.2460/javma.2003.222.762. [DOI] [PubMed] [Google Scholar]
- Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. First. Verbag, NY, USA: Springer; 2000. [Google Scholar]
- Walk D, Sehgal N, Moeller-Bertram T, Edwards RR, Wasan A, Wallace M, Irving G, Argoff C, Backonja MM. Quantitative sensory testing and mapping – A review of nonautomated quantitative methods for examination of the patient with neuropathic pain. The Clinical Journal of Pain. 2009;25:632–640. doi: 10.1097/AJP.0b013e3181a68c64. [DOI] [PubMed] [Google Scholar]
- Wegner K, Horais KA, Tozier NA, Rathbun ML, Shtaerman Y, Yaksh TL. Development of a canine nociceptive thermal escape model. Journal of Neuroscience Methods. 2008;168:88–97. doi: 10.1016/j.jneumeth.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


