Abstract
Background
In response to inconsistent findings, we investigated associations between maternal serum 25-hydroxyvitamin D (25(OH)D) concentrations and infant birthweight for gestational age (BW/GA), including potential effect modification by maternal race/ethnicity and infant sex.
Methods
Data from 2,558 pregnant women were combined in a nested case-control study (preterm and term) sampled from three cohorts: the Omega study, the Pregnancy, Infection and Nutrition study, and the Pregnancy Outcomes and Community Health study. Maternal 25(OH)D concentrations were sampled at 4 to 29 weeks’ gestation (80% 14–26 weeks). BW/GA was modeled as sex-and gestational age-specific birthweight z-scores. General linear regression models (adjusting for age, education, parity, pre-pregnancy body mass index, season at blood draw, and smoking) assessed 25(OH)D concentrations in relation to BW/GA.
Results
Among non-Hispanic Black women, the positive association between 25(OH)D concentrations and BW/GA was of similar magnitude in pregnancies with female or male infants (beta (β)=0.015, standard error (SE)=0.007, P=0.025; β=0.018, SE=0.006, P=0.003, respectively). Among non-Hispanic White women, 25(OH)D-BW/GA association was observed only with male infants and the effect size was lower (β=0.008, SE=0.003, P=0.02).
Conclusions
Maternal serum concentrations of 25(OH)D in early and mid-pregnancy were positively associated with BW/GA among non-Hispanic Black male and female infants and non-Hispanic White male infants. Effect modification by race/ethnicity may be due, in part, to overall lower concentrations of 25(OH)D in non-Hispanic Blacks. Reasons for effect modification by infant sex remain unclear.
Keywords: birthweight for gestational age, race/ethnicity, pregnancy, sex difference, 25-hydroxyvitamin D
Recent research on maternal vitamin D levels in pregnancy raises questions about adequacy of maternal vitamin D levels for optimal perinatal outcomes.1 Some investigators have proposed that 27% to 91% of pregnant women in the United States have deficient or insufficient vitamin D levels due to lack of enough sunlight exposure or inadequate vitamin D intake.2 Non-Hispanic Black women are at higher risk for vitamin D deficiency because darker skin may limit vitamin D synthesis in the skin.3 A recent review reported that maternal vitamin D deficiency in pregnancy has been associated with gestational diabetes, preeclampsia, preterm delivery, and infants who are small for gestational age.4 The fetus largely depends on maternal sources of vitamin D.5 The impact of vitamin D on fetal growth may be mediated through vitamin D’s roles in calcium homeostasis, bone mineralization, and normal production and secretion of insulin.6
Epidemiologic findings on maternal vitamin D and fetal growth are inconsistent. Low birthweight and small for gestational age (SGA) are commonly used as proxy measures of fetal growth. Intervention trials of maternal vitamin D supplementation during pregnancy have reported mixed results with regard to effects on birthweight.7–10 Observational studies of vitamin D deficiency and fetal growth have likewise produced conflicting findings.11–24 The diverse results may be due to varied study methodologies or different populations studied. Two studies noted a link between maternal vitamin D levels and fetal growth in non-Hispanic White women, but not in non-Hispanic Black women.11,14 The lack of association among non-Hispanic Blacks may be explained by small sample size and a relatively narrower distribution of vitamin D levels among non-Hispanic Blacks. One recent study observed effect modification by sex, with the vitamin D-fetal growth link occurring mainly among male offspring.25
We measured maternal serum 25-hydroxyvitamin D (25(OH)D) concentrations in a nested case-control (preterm-term) study assembled from three U.S. prospective cohort studies of pregnant women. Here we test the hypotheses that low maternal 25(OH)D concentrations in pregnancy are associated with decreased infant birthweight for gestational age (BW/GA) (operationalized as infant sex-and gestational age-specific birthweight z-scores), and this association is modified by maternal race/ethnicity and infant sex.
Methods
Study design and population
This study uses data gathered in a nested case-control study of maternal 25(OH)D concentrations among preterm cases and matched term controls from three prospective cohort studies: the Omega study; the Pregnancy, Infection and Nutrition (PIN) study; and the Pregnancy Outcomes and Community Health (POUCH) study. Detailed descriptions of the cohorts are published elsewhere.26–28 In brief, the Omega study (1996–2008) examined determinants of preeclampsia, gestational diabetes, and other pregnancy outcomes of pregnant women recruited from prenatal care clinics affiliated with Swedish Medical Center in Seattle, Washington. The PIN study (1998–2005) investigated the roles of infection, stress, physical activity, and nutrition on risk of preterm delivery among pregnant women recruited from prenatal care clinics in central North Carolina. The POUCH study (1998–2004) was designed to investigate infection, maternal vascular disease, and stress pathways to preterm delivery among pregnant women recruited from selected clinics in five Michigan communities. Human subjects approval for each study was granted by the Institutional Review Boards at the respective institutions.
Cohort protocols included maternal in-person interviews and/or self-administered questionnaires, and blood draws at enrollment. Maternal blood serum samples were frozen at -70°C and stored until analysis. Gestational age at enrollment was 4 to 23 weeks in the Omega study, 11 to 29 weeks in the PIN study, and 16 to 27 weeks in the POUCH study. Medical records were reviewed after delivery to obtain information on pregnancy course, perinatal events, and pregnancy outcome. In the original nested case-control study, preterm cases and term controls were matched within cohorts on maternal race/ethnicity, gestational week at blood draw, and season within a 45 day window of blood draw. Approximately 98% of cases were matched in a 1:2 ratio to controls; 2% were matched in a 1:1 ratio. The sample was limited to singleton pregnancies among non-Hispanic White and non-Hispanic Black women with information on infant sex, birthweight, and gestational week at delivery. After excluding women with outlier values for infant birthweight (n=1) or maternal 25(OH)D concentrations (n=7), the final sample size was 2,558 women (747 from Omega study, 977 from PIN study, and 834 from POUCH study).
Outcome measurement
Infant sex- and gestational age-specific birthweight z-scores (BWz) were used to represent BW/GA. Infant birthweight (grams) and sex were abstracted from the hospital medical records. Calculation of gestational age (weeks) varied across studies: 1) In the Omega study and POUCH study, gestational age was calculated according to the first day of the self-reported last menstrual period (LMP) or early ultrasound data from medical records. If both LMP and ultrasound dates were available and were within 14 days, LMP was used to determine gestational age. Otherwise, ultrasound gestational age estimates were used. In the PIN study, gestational age was determined by first ultrasound performed prior to 22 completed weeks of gestation. If no ultrasound was performed prior to the start of week 22, then self-reported LMP was used to date the pregnancy. The BWz was calculated as (infant’s birthweight – mean birthweight of the gestational age and sex-specific stratum)/standard deviation of birthweight from the same stratum. The mean and standard deviation specific to each gestational age and sex stratum come from a referent population.29
Exposure measurement
Stored serum from the three cohorts was assayed in a single laboratory for hydroxyvitamin D2 (25(OH)D2) and hydroxyvitamin D3 (25(OH)D3) using liquid chromatography-tandem mass spectrometry with National Institute of Standards and Technology samples for quality control. The limit of detection was 1.1 ng/mL for 25(OH)D2 and 0.6 ng/mL for 25(OH)D3. The median intra-assay coefficients of variation were 7.1% (detectable range 1.4% to 23.0%) and 6.9% (range 1.5% to 12.2%) for 25(OH)D2 and 25(OH)D3, respectively. The median inter-assay coefficients of variation were 13.5% (detectable range 3.0% to 28.7%) and 7.4% (range 1.4% to 14.3%) for 25(OH)D2 and 25(OH)D3, respectively. Both 25(OH)D2 and 25(OH)D3 were combined for a measure of total serum 25-hydroxyvitamin D (25(OH)D), regarded as the best available biomarker of vitamin D status. In these analyses, total 25(OH)D is modeled as a continuous variable.
Covariates
Covariates of interest were maternal race/ethnicity (non-Hispanic Black, non-Hispanic White), maternal age, maternal education (high school or less, post high school), gestational age at blood draw, marital status (married, unmarried), parity (primiparous, multiparous), smoking status during pregnancy (yes, no), vitamins used during pregnancy (yes, no), season of blood draw, and pre-pregnancy body mass index (BMI). Less than 5% of women had missing data for any of these covariates, the largest number being for vitamin use (n=118) and smoking (n=54). Season of blood draw was classified as winter (December–February), spring (March–May), summer (June–August), and fall (September–November). Pre-pregnancy BMI, calculated according to self-reported pregnancy height and weight, was categorized as underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30 kg/m2) for descriptive statistics and was treated as a continuous measure in regression models.
Statistical analyses
The heterogeneity of cohort-specific estimates for 25(OH)D was assessed with the DerSimonian and Laird Q statistic, which indicated that the data could be combined across cohorts.30 Analysis of the distribution of 25(OH)D, using histograms and model fitting procedures, indicated it approximated a normal distribution. Analysis of variance (ANOVA) was performed to examine maternal characteristics associated with maternal 25(OH)D concentrations. Multivariable linear regression models were used to assess the association between maternal 25(OH)D concentrations and BWz. Covariates were incorporated in adjusted models if they: 1) showed associations with BW/GA and maternal 25(OH)D concentrations in the literature and in our data; 2) affected the magnitude of main effect estimates by greater than 10%; and 3) were not considered to be mediators. A model with 25(OH)D and 25(OH)D squared tested the potential for nonlinearity in the 25(OH)D-BW/GA association. Initially, separate analyses were performed for term and preterm deliveries for two reasons. First, the original study used a nested case-control design with preterm cases and term controls. Second, BWz scores for preterm infants use a mean that is potentially biased downward (i.e., at a given gestational age a baby born preterm tends to be smaller than its counterpart who remains in-utero). Our goal was to examine if the 25(OH)D-BW/GA association was similar in the two groups, term and preterm, and therefore could be combined.
Consistent with previous reports,11,14 we detected a significant difference in the distribution of 25(OH)D serum concentrations between race/ethnic groups; we tested effect modification by maternal race/ethnicity using an interaction term within a regression model. We next tested for interaction by infant sex within each race/ethnic stratum. Finally, we conducted sensitivity analyses by removing women with preeclampsia or gestational diabetes to assess if observed effects were largely explained by these pregnancy complications. Data are presented as beta (β) and standard error (SE) parameter estimates. All statistical tests were two-sided and a 5% level of significance was used throughout. Analyses were performed using SAS, version 9.3, Cary, North Carolina.
Results
The following maternal characteristics were associated with lower mean 25(OH)D concentrations: non-Hispanic Black race/ethnicity, <30 years of age, unmarried, pre-pregnancy obesity, multiparity, smoking, and fewer years of education (Table 1). Women whose blood draw occurred from December to May, and who did not use vitamins during pregnancy also had lower mean 25(OH)D concentrations. Maternal 25(OH)D concentrations did not differ by infant sex (P=0.44). Figure 1 displays the distribution of maternal 25(OH)D concentrations in pregnancy by race/ethnicity; there is a left shift in non-Hispanic Black serum 25(OH)D concentrations compared to serum 25(OH)D concentrations in non-Hispanic Whites.
Table 1.
Sample characteristics and the frequency distribution and mean 25-Hydroxyvitamin D (25(OH)D) concentrations (ng/mL), three-cohort study, 1996–2008
| N (%) | 25(OH)D concentrations (ng/mL)
|
|||
|---|---|---|---|---|
| Mean (SD) | p-value a | |||
| 25(OH)D concentrations | 2558 | 29.6 (11.0) | ||
| Race/ethnicity | <0.0001 | |||
| non-Hispanic Black | 641 (25) | 20.2 (9.4) | ||
| non-Hispanic White | 1917 (75) | 32.7 (9.6) | ||
| Educationb | <0.0001 | |||
| High school or less | 780 (31) | 26.7 (12.0) | ||
| Post high school | 1761 (69) | 30.9 (10.3) | ||
| Age (years) | <0.0001 | |||
| <20 | 231 (9) | 26.8 (11.3) | ||
| 20–29 | 1100 (43) | 28.6 (11.9) | ||
| 30–39 | 1134 (44) | 31.0 (10.0) | ||
| ≥ 40 | 93 (4) | 30.6 (8.5) | ||
| Marital statusb | <0.0001 | |||
| Married | 1654 (65) | 31.7 (10.0) | ||
| Unmarried | 902 (35) | 25.7 (11.7) | ||
| Gestation age at blood draw | 0.01 | |||
| (weeks) | <14 | 227 (9) | 28.1 (7.3) | |
| 14–26 | 2100 (82) | 29.9 (11.2) | ||
| ≥ 27 | 231 (9) | 28.3 (12.3) | ||
| Season of blood draw | <0.0001 | |||
| December-February | 592 (23) | 28.3 (11.2) | ||
| March-May | 736 (29) | 27.2 (10.8) | ||
| June-August | 607 (24) | 32.7 (10.7) | ||
| September-November | 623 (24) | 30.5 (10.5) | ||
| Prepregnancy BMIbc | <0.0001 | |||
| Underweight | 117 (5) | 29.8 (12.7) | ||
| Normal weight | 1398 (55) | 31.5 (10.3) | ||
| Overweight | 506 (20) | 29.1 (10.6) | ||
| Obese | 530 (21) | 25.2 (11.4) | ||
| Smoking status during pregnancyb | 0.004 | |||
| No | 2029 (81) | 29.9 (10.7) | ||
| Yes | 475 (19) | 28.3 (12.1) | ||
| Parityb | <0.0001 | |||
| Primiparous | 1305 (51) | 30.7 (10.2) | ||
| Multiparous | 1252 (49) | 28.4 (11.7) | ||
| Vitamins used during pregnancyb | <0.0001 | |||
| No | 327 (13) | 25.0 (12.0) | ||
| Yes | 2113 (87) | 30.5 (10.6) | ||
| Infant sex | 0.44 | |||
| Female | 1258 (49) | 29.4 (11.1) | ||
| Male | 1300 (51) | 29.8 (10.9) | ||
Abbreviations: SD, standard deviation; 25(OH)D, 25-hydroxyvitamin D; BMI, body mass index
P-value for the global comparison test within the separate variables.
There are missing values for these variables; smoking is missing 54, vitamins used during pregnancy is missing 118, and the remainders are missing less than 20.
Underweight is <18.5 kg/m2, normal is 18.5–24.9 kg/m2, overweight is 25.0–29.9 kg/m2, and obese is ≥30 kg/m2.37
Figure 1.
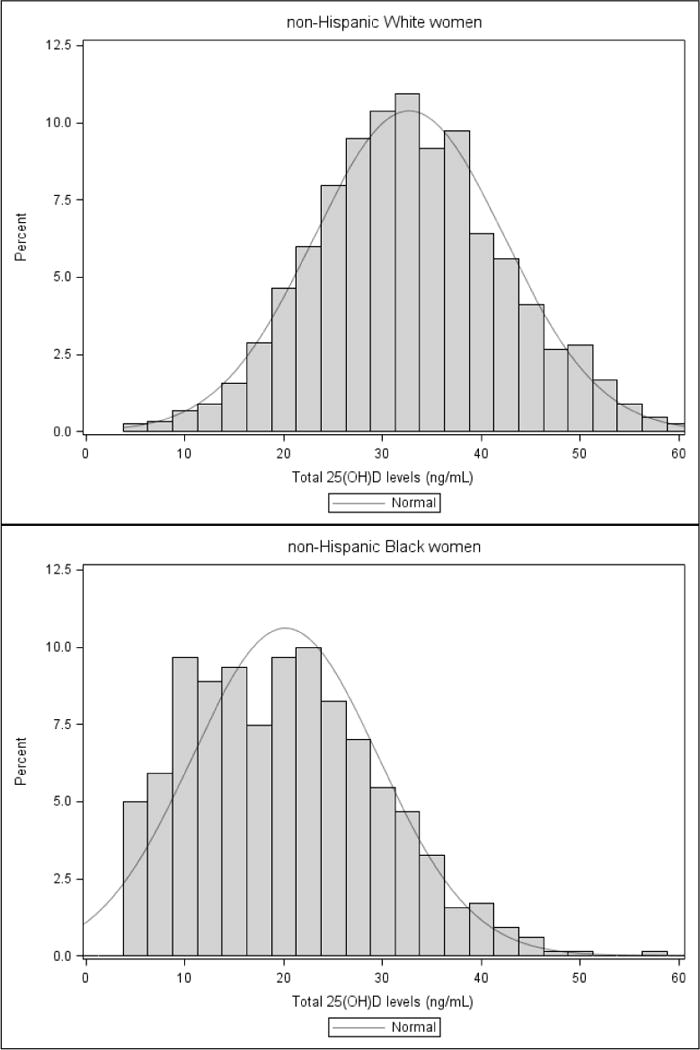
The distribution of maternal 25-Hydroxyvitamin D (25(OH)D) concentrations by maternal race/ethnicity, three-cohort study, 1996–2008
Abbreviations: 25(OH)D, 25-hydroxyvitamin D
In separate statistical models, a positive relationship between maternal 25(OH)D concentrations and BWz score was observed in both preterm and term deliveries; therefore the two were combined. We present results for the combined analyses and for the term only for comparison (Table 2). The unadjusted general linear regression analysis showed that BWz score increased by 0.012 (SE=0.002, P<0.001) per 1 ng/mL increase in maternal 25(OH)D concentrations. The addition of a quadratic term for 25(OH)D did not significantly improve the model fit (data not shown). The linear association remained after adjustment for maternal age, education, parity, pre-pregnancy BMI, season, and smoking during pregnancy. Effect modification by maternal race/ethnicity was demonstrated using an interaction term in the regression model (P=0.04, results were unchanged after removing the Omega study which consisted of 37.56% non-Hispanic White women). Tests for effect modification by infant sex within each race/ethnic stratum suggested an interaction within the non-Hispanic White women only (P=0.11). A graphical representation reinforced this suggestion (Figure 2). Among non-Hispanic Black women, the positive association between maternal 25(OH)D concentrations and BW/GA (BWz) was of similar magnitude in pregnancies with female or male infants (adjusted β=0.015, SE=0.007, P=0.025; β=0.018, SE=0.006, P=0.003, respectively) (Table 2). Among non-Hispanic White women, the 25(OH)D-BW/GA association was observed only with male infants and the magnitude was less (β=0.008, SE=0.003, P=0.02), approximately half that of non-Hispanic Black women.
Table 2.
The associations between maternal 25-Hydroxyvitamin D (25(OH)D) concentrations (ng/mL) and sex-and gestational age-specific birthweight z-scores by maternal race/ethnicity and infant sex, three-cohort study, 1996–2008
| Sex-and gestational age-specific birthweight z-scores
|
||||||
|---|---|---|---|---|---|---|
| Unadjusted model
|
Adjusted modela
|
|||||
| β | SE | P-value | β | SE | P-value | |
| All women combined (n=2,558) |
0.012 | 0.002 | <0.001 | 0.014 | 0.002 | <0.001 |
| non-Hispanic White female infants (n=934) |
−0.001 | 0.003 | 0.756 | 0.002 | 0.003 | 0.508 |
| non-Hispanic White male infants (n=983) |
0.006 | 0.003 | 0.053 | 0.008 | 0.003 | 0.022 |
| non-Hispanic Black female infants (n=324) |
0.010 | 0.006 | 0.100 | 0.015 | 0.007 | 0.025 |
| non-Hispanic Black male infants (n=317) |
0.015 | 0.006 | 0.009 | 0.018 | 0.006 | 0.003 |
| Women with term deliveries only (n=1,702) |
0.011 | 0.002 | <0.001 | 0.014 | 0.002 | <0.001 |
| non-Hispanic White female infants (n=645) |
−0.004 | 0.004 | 0.368 | 0.003 | 0.004 | 0.545 |
| non-Hispanic White male infants (n=632) |
0.004 | 0.004 | 0.386 | 0.007 | 0.004 | 0.117 |
| non-Hispanic Black female infants (n=213) |
0.007 | 0.007 | 0.311 | 0.014 | 0.007 | 0.052 |
| non-Hispanic Black male infants (n=212) |
0.014 | 0.007 | 0.050 | 0.015 | 0.008 | 0.048 |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; β, beta; SE, standard error
Adjusted for maternal age, education, parity, pre-pregnancy body mass index, season at blood draw, and smoking status during pregnancy.
Figure 2.
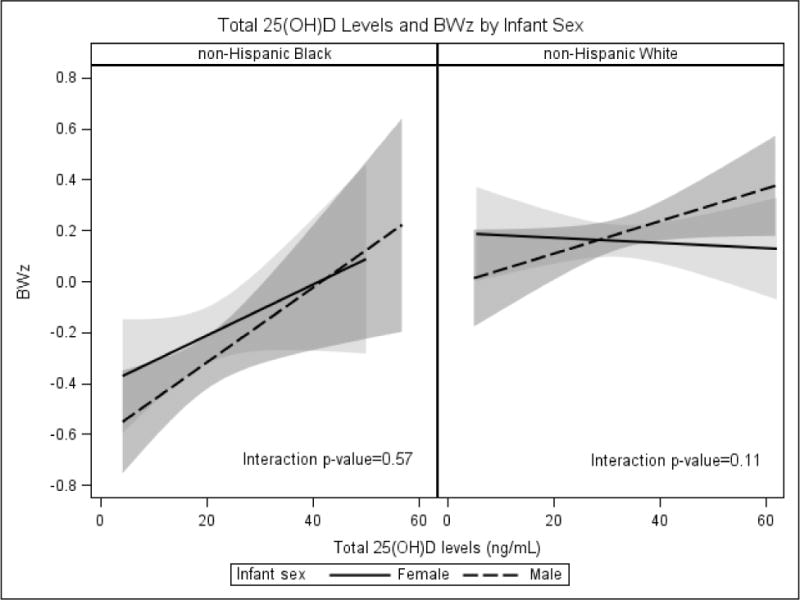
The association between maternal 25-Hydroxyvitamin D (25(OH)D) concentrations and sex-and gestational age-specific birthweight z-scores by maternal race/ethnicity and infant sex, three-cohort study, 1996–2008
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; BWz, birthweight z-scores
Analyses confined to term deliveries produced similar results, however, estimates were less stable due to limited statistical power. After removing women with preeclampsia or gestational diabetes, the positive association between maternal 25(OH)D concentrations and BWz scores persisted in all three of the four strata (data not shown).
Comment
This multi-cohort study found that maternal serum concentrations of 25(OH)D in early and mid-pregnancy were positively associated with BW/GA among non-Hispanic Black male and female infants and non-Hispanic White male infants. Comparison of our findings to those from previous studies of maternal 25(OH)D levels and BW/GA is challenging due to marked variation in study designs and analytic strategies across studies.
Results from studies examining maternal 25(OH)D concentrations and offspring birthweight, a measure affected by both fetal growth and gestational age at delivery, have been inconsistent. Observational studies following this approach have often failed to find an association.17–24 However, one multi-ethnic cohort study of 3,730 women in the Netherlands found significantly different mean birthweights for different 25(OH)D levels (<12 ng/mL: 3418g; <20 ng/mL: 3505g; ≥20 ng/mL: 3559g; P<0.001).31 A recent meta-analysis of observational studies evaluating maternal 25(OH)D levels and pregnancy outcomes by Aghajafari et al. used a binary measure of 25(OH)D with a cutoff level of <15 ng/mL.32 Their summary indicated that the low 25(OH)D group had significantly lower birthweight (mean difference −130.9 g; 95% confidence Interval (CI): −186.7, −75.1 g). Recently, data from three clinical trials were pooled by Thorne-Lyman et al.33 Results from this study suggested that 25(OH)D supplementation led to a significant 60% reduction in the risk of low birthweight. Another approach has been to study maternal 25(OH)D status and its relation to SGA, a categorical variable commonly used to denote fetal growth. A Netherlands-based cohort study showed that the odds of SGA among women with 25(OH)D <12 ng/mL was almost two-fold that of women with 25(OH)D ≥20 ng/mL (adjusted odds ratio (OR)=1.9, 95% CI: 1.4, 2.7).31 In the meta-analysis by Aghajafari et al., low 25(OH)D levels (<15 ng/mL) in pregnancy were associated with an increased risk of delivering an SGA infant, even after adjusting for potential confounders (pooled adjusted OR=2.1, 95% CI: 1.5, 2.7).32 In another meta-analysis by Wei et al., six observational studies, including 6,013 women, were pooled.4 They found an increased risk of delivering an SGA infant among the low 25(OH)D group when the 25(OH)D cutoff level was <20 ng/mL (pooled OR=1.52, 95% CI: 1.1, 2.2), whereas there was no association detected with a 25(OH)D cutoff level of <30 ng/mL. Taken together, these studies support a positive association between maternal 25(OH)D levels and fetal growth, though the threshold of 25(OH)D for effect on fetal growth is uncertain.
In our study, the stronger association between 25(OH)D concentrations and BW/GA observed in non-Hispanic Black versus non-Hispanic White women may be due to a greater effect in the lowest end of the 25(OH)D distribution where there are limited data for non-Hispanic Whites. Among other studies examining effect modification by race/ethnicity, Bodnar et al. reported evidence of a U-shaped association between maternal 25(OH)D levels and SGA in white women but not in non-Hispanic Black women.11 In Bodnar et al. nested case-control study there was a relatively small sample of non-Hispanic Black women delivering SGA infants. Ertl et al. found that at 11–13 weeks’ gestation, 25(OH)D concentrations below 10th percentile were more common among pregnancies with SGA infants.14 This association was statistically significant in white women. In non-Hispanic Black women, the effect size was slightly less and sample size was limited for adequate statistical power. Our observed effect modification by race/ethnicity might also be explained by other factors associated with race/ethnicity that are also synergistic with vitamin D in their effects on fetal growth. Examples of these synergistic factors include maternal diet quality, physical activity, and pre-pregnancy vascular health. Among non-Hispanic White women in our study, the positive association between maternal 25(OH)D concentrations and BW/GA was confined to male infants, perhaps suggesting vulnerability for males, even within the moderately low 25(OH)D range. One previous study examined effect modification by infant sex and likewise reported a stronger association between maternal 25(OH)D status and fetal growth in male offspring.25 The authors hypothesized that male and female infants respond differently to maternal nutritional stimuli, including maternal 25(OH)D status.25
The biological plausibility of an association between maternal vitamin D levels and fetal growth rests on direct and indirect mechanisms. The fetus relies on maternal sources of vitamin D for bone remodeling and muscle function. Vitamin D is also involved in pathways that affect trophoblast invasion and angiogenesis, which are essential for adequate placenta implantation and fetal growth.4
From a clinical perspective, it may be helpful to translate our birthweight z-score results to birthweight differences between infants born to mothers with deficient vs sufficient levels of 25(OH)D in pregnancy, defined here by Institute of Medicine (IOM) criteria.34 For example, among non-Hispanic Black male infants delivered at 40 weeks’ gestation (term), those born to mothers with a 25(OH)D level of 11ng/ml (the cutpoint for lowest quintile in this population of non-Hispanic Black mothers and considered deficient by IOM criteria) were, on average, 56g lighter than infants born to mothers with a 25(OH)D level of 20ng/ml (IOM cutpoint for sufficient). At 32 weeks’ gestation, this birthweight decrement is estimated to be, on average, 49g.
Our study had multiple strengths. By combining cohorts, the data included pregnant women from three different geographic areas in the United States, the sample size was relatively large, and the women represented diverse demographic characteristics. The large sample size and the diversity permitted an assessment of effect modification by maternal race/ethnicity and infant sex. In addition, the 25(OH)D concentrations were measured by liquid chromatography-tandem mass spectrometry, which is currently the most accurate technique.35 A measure of serum 25(OH)D, with a half-life of about 2–3 weeks, reflects dietary and skin sources of the vitamin. Moreover, our study collected detailed data on several possible confounding variables. We used sex- and gestational age-specific birthweight z-scores as a proxy measure for fetal growth, instead of birthweight, which reflects a mixture of fetal growth and timing of delivery.
There were also study limitations worth noting. Women’s 25(OH)D concentrations were measured only once and almost all measures were obtained before the third trimester. We do not know which trimester(s) in pregnancy are most relevant, but repeated measures of maternal 25(OH)D concentrations during pregnancy would be useful in future studies to examine this question. Due to the varying study protocols, the algorithm for determining gestational age across the three studies varied. Regardless of the small variation in approaches to calculating gestational age, results were rigorous enough to be consistent across the three population samples. The serum was assayed in 2011, thus some maternal blood samples were stored up to 15 years before being assayed for 25-Hydroxyvitamin D levels. However, if prolonged storage resulted in concentration decline it would be non-differential and therefore our results may underestimate effect sizes. Because samples were obtained 7–19 years ago, they may not reflect the current distribution of maternal vitamin D levels. We lacked information on lifestyle factors, such as diet and physical activity, which could act as confounders. While maternal early and mid-pregnancy 25(OH)D concentrations may affect risk of pregnancy complications, pregnancy conditions associated with poor fetal growth might also influence maternal 25(OH)D status (reverse causation). We attempted to partially address this concern through our sensitivity analysis that removed women with preeclampsia and gestational diabetes and showed that results were relatively unchanged. We also lacked measure of vitamin D binding protein which can impact bioavailability of 25(OH)D and may vary by race.36 Regrettably, we did not have adequate sample size to consider the categorical variable, SGA, in the context of the observed effect modifications by race/ethnicity and infant sex.
Our results suggest that low maternal 25(OH)D concentrations may affect BW/GA. However, further research is indicated to better understand the role of vitamin D in BW/GA and the adequate levels of maternal serum vitamin D needed for optimal fetal growth. The challenges of unmeasured confounding by multiple lifestyle factors and the recent controversy over the biological meaning of vitamin D status measured as total 25(OH)D suggest that observational studies alone cannot guide clinical practice.35 Large, well-designed randomized controlled trials of vitamin D supplementation in pregnancy are needed to assess effects on fetal growth and health.
Acknowledgments
CDC 200-2008-27956-12; Omega: NICHD R01 HD32562; PIN: NICHD RO1 HD28684, HD28684A, HD37584, HD39373, NIH RR0046, R24 HD050924; POUCH: NIH R01 HD034543, R01 HD34543, March of Dimes Foundation 20-FY98-0697 through 20-FY04-37, Thrasher Research Foundation grant 02816-7, CDC U01 DP000143-01.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Christesen HT, Falkenberg T, Lamont RF, Jørgensen JS. The impact of vitamin D on pregnancy: A systematic review. Acta Obstetricia Et Gynecologica Scandinavica. 2012;91:1357–1367. doi: 10.1111/aogs.12000. [DOI] [PubMed] [Google Scholar]
- 2.Hossein-Nezhad A, Holick MF. Optimize dietary intake of vitamin D: An epigenetic perspective. Current Opinion in Clinical Nutrition and Metabolic Care. 2012;15:567–579. doi: 10.1097/MCO.0b013e3283594978. [DOI] [PubMed] [Google Scholar]
- 3.Bodnar LM, Simhan HN. Vitamin D may be a link to black-white disparities in adverse birth outcomes. Obstetrical & Gynecological Survey. 2010;65:273–284. doi: 10.1097/OGX.0b013e3181dbc55b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei SQ, Qi HP, Luo ZC, Fraser WD. Maternal vitamin D status and adverse pregnancy outcomes: A systematic review and meta-analysis. The Journal of Maternal-fetal and Neonatal Medicine. 2013;26:889–899. doi: 10.3109/14767058.2013.765849. [DOI] [PubMed] [Google Scholar]
- 5.Dror DK, Allen LH. Vitamin D inadequacy in pregnancy: Biology, outcomes, and interventions. Nutrition Reviews. 2010;68:465–477. doi: 10.1111/j.1753-4887.2010.00306.x. [DOI] [PubMed] [Google Scholar]
- 6.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes: a systematic review and meta-analysis. Journal of Clinical Endocrinology and Metabolism. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooke OG, Brown IR, Bone CD, Carter ND, Cleeve HJ, Maxwell JD, et al. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. British Medical Journal. 1980;280:751–754. doi: 10.1136/bmj.280.6216.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marya RK, Rathee S, Dua V, Sangwan K. Effect of vitamin D supplementation during pregnancy on foetal growth. The Indian Journal of Medical Research. 1988;88:488–492. [PubMed] [Google Scholar]
- 9.Mallet E, Gugi B, Brunelle P, Hénocq A, Basuyau JP, Lemeur H. Vitamin D supplementation in pregnancy: a controlled trial of two methods. Obstetrics and Gynecology. 1986;68:300–304. doi: 10.1097/00006250-198609000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: Double-blind, randomized clinical trial of safety and effectiveness. Journal of Bone and Mineral Research. 2011;26:2341–2357. doi: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodnar LM, Catov JM, Zmuda JM, Cooper ME, Parrott MS, Roberts JM, et al. Maternal serum 25-Hydroxyvitamin D concentrations are associated with small-for-gestational age births in White women. The Journal of Nutrition. 2010;140:999–1006. doi: 10.3945/jn.109.119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowyer L, Catling-Paull C, Diamond T, Homer C, Davis G, Craig ME. Vitamin D, PTH and calcium levels in pregnant women and their neonates. Clinical Endocrinology. 2009;70:372–377. doi: 10.1111/j.1365-2265.2008.03316.x. [DOI] [PubMed] [Google Scholar]
- 13.Burris HH, Rifas-Shiman SL, Camargo CA, Litonjua AA, Huh SY, Rich-Edwards JW, et al. Plasma 25-hydroxyvitamin D during pregnancy and small-for-gestational age in black and white infants. Annals of Epidemiology. 2012;22:581–586. doi: 10.1016/j.annepidem.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ertl R, Yu CK, Samaha R, Akolekar R, Nicolaides KH. Maternal serum vitamin D at 11–13 weeks in pregnancies delivering small for gestational age neonates. Fetal Diagnosis and Therapy. 2012;31:103–108. doi: 10.1159/000333810. [DOI] [PubMed] [Google Scholar]
- 15.Robinson CJ, Wagner CL, Hollis BW, Baatz JE, Johnson DD. Maternal vitamin D and fetal growth in early-onset severe preeclampsia. American Journal of Obstetrics and Gynecology. 2011;204:556:e1–4. doi: 10.1016/j.ajog.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viljakainen HT, Saarnio E, Hytinantti T, Miettinen M, Surcel H, Mäkitie O, et al. Maternal Vitamin D Status Determines Bone Variables in the Newborn. The Journal of Clinical Endocrinology and Metabolism. 2010;95:1749–1757. doi: 10.1210/jc.2009-1391. [DOI] [PubMed] [Google Scholar]
- 17.Clifton-Bligh RJ, McElduff P, McElduff A. Maternal vitamin D deficiency, ethnicity and gestational diabetes. Diabetic Medicine. 2008;25:678–684. doi: 10.1111/j.1464-5491.2008.02422.x. [DOI] [PubMed] [Google Scholar]
- 18.Farrant HJW, Krishnaveni GV, Hill JC, Boucher BJ, Fisher DJ, Noonan K, et al. Vitamin D insufficiency is common in Indian mothers but is not associated with gestational diabetes or variation in newborn size. European Journal of Clinical Nutrition. 2009;63:646–652. doi: 10.1038/ejcn.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Alonso AM, Dionis-Sanchez EC, Chedraui P, González-Salmerón MD, Pérez-López FR, et al. First-trimester maternal serum 25-hydroxy vitamin D3 status and pregnancy outcome. International Journal of Gynaecology and Obstetrics. 2012;116:6–9. doi: 10.1016/j.ijgo.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 20.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, et al. Maternal vitamin D status during pregnancy and child outcomes. European Journal of Clinical Nutrition. 2008;62:68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta S, Hunter DJ, Mugusi FM, Spiegelman D, Manji KP, Giovannucci EL, et al. Perinatal outcomes, including mother-to-child transmission of HIV, and child mortality and their association with maternal vitamin D status in Tanzania. The Journal of Infectious Diseases. 2009;200:1022–1030. doi: 10.1086/605699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morley R, Carlin JB, Pasco JA, Wark JD. Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. The Journal of Clinical Endocrinology and Metabolism. 2006;91:906–912. doi: 10.1210/jc.2005-1479. [DOI] [PubMed] [Google Scholar]
- 23.Prentice A, Jarjou LMA, Goldberg GR, Bennett J, Cole TJ, Schoenmakers I. Maternal plasma 25-hydroxyvitamin D concentration and birthweight, growth and bone mineral accretion of Gambian infants. Acta Paediatrica. 2009;98:1360–1362. doi: 10.1111/j.1651-2227.2009.01352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shand AW, Nassar N, Dadelszen PV, Innis SM, Green TJ. Maternal vitamin D status in pregnancy and adverse pregnancy outcomes in a group at high risk for pre-eclampsia. British Journal of Obstetrics and Gynaecology. 2010;117:1593–1598. doi: 10.1111/j.1471-0528.2010.02742.x. [DOI] [PubMed] [Google Scholar]
- 25.Gernand AD1, Bodnar LM, Klebanoff MA, Parks WT, Simhan HN. Maternal serum 25-hydroxyvitamin D and placental vascular pathology in a multicenter US cohort. The American Journal of Clinical Nutrition. 2013;98:383–388. doi: 10.3945/ajcn.112.055426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams MA, Frederick IO, Qiu C, Meryman LJ, King IB, Walsh SW, et al. Maternal erythrocyte omega-3 and omega-6 fatty acids, and plasma lipid concentrations, are associated with habitual dietary fish consumption in early pregnancy. Clinical Biochemistry. 2006;39:1063–1070. doi: 10.1016/j.clinbiochem.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savitz DA, Dole N, Williams J, McDonald T, Carter AC, Eucker B. Determinants of participation in an epidemiological study of preterm delivery. Paediatric and perinatal epidemiology. 1999;13:114–125. doi: 10.1046/j.1365-3016.1999.00156.x. [DOI] [PubMed] [Google Scholar]
- 28.Holzman C, Bullen B, Fisher R, Paneth N, Reuss L, Prematurity Study Group Pregnancy outcomes and community health: The POUCH study of preterm delivery. Paediatric and perinatal epidemiology. 2001;15(Suppl. 2):136–158. doi: 10.1046/j.1365-3016.2001.00014.x. [DOI] [PubMed] [Google Scholar]
- 29.Kramer MS, Platt RW, Wen SW, Joseph KS, Allen A, Abrahamowicz M, et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108(2):e35. doi: 10.1542/peds.108.2.e35. [DOI] [PubMed] [Google Scholar]
- 30.Luque-Fernandez MA, Gelaye B, VanderWeele T, Ferre C, Siega-Riz AM, Holzman C, et al. Seasonal variation of 25-hydroxyvitamin D among non-Hispanic black and white pregnant women from three US pregnancy cohorts. Paediatric and Perinatal Epidemiology. 2014;28:166–176. doi: 10.1111/ppe.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leffelaar ER, Vrijkotte TGM, van Eijsden M. Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: Results of the multi-ethnic Amsterdam Born Children and their Development cohort. British Journal of Nutrition. 2010;104:108–117. doi: 10.1017/S000711451000022X. [DOI] [PubMed] [Google Scholar]
- 32.Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O’Beirne M, Rabi DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ (Clinical Research Ed) 2013;346:f1169. doi: 10.1136/bmj.f1169. [DOI] [PubMed] [Google Scholar]
- 33.Thorne-Lyman A, Fawzi WW. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis Paediatric and Perinatal Epidemiology. 2012;26:75–90. doi: 10.1111/j.1365-3016.2012.01283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. The Journal of Clinical Endocrinology and Metabolism. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen H, McCoy LF, Schleicher RL, Pfeiffer CM. Measurement of 25-hydroxyvitamin D3 (25OHD3) and 25-hydroxyvitamin D2 (25OHD2) in human serum using liquid chromatography-tandem mass spectrometry and its comparison to a radioimmunoassay method. Clinica chimica acta. 2008;391:6–12. doi: 10.1016/j.cca.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. The New England Journal of Medicine. 2013;369(21):1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.US Centers for Disease Control and Prevention (CDC) Body Mass Index: Considerations for Practitioners. CDC.gov. (accessed October 9, 2013) [Google Scholar]


