Abstract
Purpose
The SOFT and TEXT randomized phase III trials investigated adjuvant endocrine therapies for premenopausal women with hormone receptor-positive (HR+) early breast cancer. We investigated the prognostic and predictive value of centrally-assessed levels of estrogen receptor (ER), progesterone receptor (PgR) and Ki-67 expression in women with HER2-negative disease.
Patients and Methods
Of 5707 women enrolled, 4115 with HER2-negative (HR+/HER2-) disease had ER, PgR and Ki-67 centrally assessed by immunohistochemistry. Breast cancer-free interval (BCFI) was defined from randomization to first invasive local, regional or distant recurrence or contralateral breast cancer. The prognostic and predictive values of ER, PgR and Ki-67 expression levels were assessed using Cox modeling and STEPP methodology.
Results
In this HR+/HER2- population, the median ER, PgR and Ki-67 expression were 95%, 90% and 18% immunostained cells. As most patients had strongly ER positive tumors, the predictive value of ER levels could not be investigated. Lower PgR and higher Ki-67 expression were associated with reduced BCFI. There was no consistent evidence of heterogeneity of the relative treatment effects according to PgR or Ki-67 expression levels though there was a greater 5-year absolute benefit of exemestane+ovarian function suppression (OFS) versus tamoxifen with or without OFS at lower levels of PgR and higher levels of Ki-67.
Conclusions
Women with poor prognostic features of low PgR and/or high Ki-67 have greater absolute benefit from exemestane+OFS versus tamoxifen+OFS or tamoxifen-alone, but individually PgR and Ki-67 are of limited predictive value for selecting adjuvant endocrine therapy for premenopausal women with HR+/HER2- early breast cancer.
Keywords: estrogen receptor, exemestane, Ki-67, ovarian function suppression, progresterone receptor, tamoxifen
Introduction
Two international randomized phase III trials, Tamoxifen and Exemestane Trial (TEXT) and Suppression of Ovarian Function Trial (SOFT) recently reported results evaluating three adjuvant endocrine therapy regimens for premenopausal women with endocrine-responsive early breast cancer[1-3]. The trials demonstrated that 5 years of adjuvant treatment with the aromatase inhibitor (AI) exemestane, in combination with ovarian function suppression (OFS), improved outcomes relative to tamoxifen plus OFS or to tamoxifen alone; and that tamoxifen plus OFS improves outcomes relative to tamoxifen alone in women who were at sufficient risk to warrant adjuvant chemotherapy and remained premenopausal thereafter[2,3]. SOFT further indicated that tamoxifen alone remains an appropriate option for some premenopausal women at low risk of recurrence[3]. In postmenopausal women randomized in the Breast International Group (BIG) 1-98 trial to receive 5 years of tamoxifen or the AI letrozole, as monotherapy or in sequence, very high levels of tumor Ki-67 expression were an adverse prognostic factor and also suggested predictive value for greater benefit of letrozole versus tamoxifen[4], but levels of estrogen receptor (ER) and progesterone receptor (PgR) expression were not predictive markers for treatment selection[5]. To help selection among three endocrine therapy options for premenopausal women with HER2-negative disease, we investigated the predictive value and absolute magnitude of treatment benefits according to levels of tumor ER, PgR and Ki-67 expression assessed by central pathology review. Although PgR level is not predictive of differential benefit of an AI versus tamoxifen in postmenopausal women, we hypothesized that the situation would be different for premenopausal women and that lower levels of PgR would predict for greater effects of endocrine therapy regimens. We postulated that very high Ki-67 would predict for the greatest benefit of exemestane plus OFS relative to tamoxifen, with or without OFS. The HER2-positive population, for whom trastuzumab is also given as adjuvant therapy, will be subject of a separate investigation.
Methods
Study Designs
The designs and conduct of the trials have been described previously[1-3]; the ethics committees and required health authorities of each participating center approved the trial protocols, and all patients gave written informed consent. In both trials, eligible premenopausal women had early invasive breast cancer assessed as ER and/or PgR-expressing in ≥10% of cells by local determination.
TEXT was designed to determine the role of adjuvant therapy with the AI exemestane relative to tamoxifen in premenopausal women treated with OFS from the start of adjuvant therapy. Between November 2003 through March 2011, 2672 eligible women were randomized 1:1 to 5 years of exemestane+OFS or 5 years of tamoxifen+OFS. OFS was by gonadotropin-releasing hormone (GnRH) agonist triptorelin, bilateral oophorectomy or ovarian irradiation. Chemotherapy was optional, and if administered, was started concurrently with triptorelin. Randomization was stratified according to intended use of adjuvant chemotherapy and lymph-node status.
SOFT was designed to determine the value of adding OFS to tamoxifen, and to determine the role of exemestane+OFS in two cohorts of premenopausal women, those who remained premenopausal after completion of (neo)adjuvant chemotherapy, and those for whom adjuvant tamoxifen alone was considered suitable treatment. Between December 2003 through January 2011, 3066 eligible women were randomized 1:1:1 to 5 years of exemestane+OFS or tamoxifen+OFS or tamoxifen alone. Randomization was stratified according to use of prior chemotherapy, lymph-node status, and intended initial method of OFS (if randomly assigned to OFS).
Central Pathology Review
Tumor tissue was prospectively collected and patients consented for protocol-mandated central review of histopathologic features and expression of ER, PgR, HER2, and Ki-67 labeling index (hereafter, Ki-67). IBCSG Central Pathology Office performed central review of whole sections obtained from formalin-fixed paraffin-embedded (FFPE) primary tumor specimens, including assessment of tumor type and grade, and immunohistochemical (IHC) evaluation of ER, PgR and Ki-67. If submitted material was limited, then testing was prioritized for ER, PgR, HER2, then Ki-67. All the immunoreactions were performed with an automated immunostainer (Austostainer, Dako, DK) using the ER/PgR PharmDX kit (Dako, Glostrup, DK) according to the manufacturer's instructions for hormone receptors, and the MIB1 monoclonal antibody (Dako) for Ki-67, as previously reported[4]. The results were recorded as the percentage of immunostained cells. HER2 expression was evaluated with the HercepTest kit (Dako) and scored as 0, 1+, 2+, or 3+, according to the FDA scoring system. Tumors scored as 2+ were re-tested with fluorescent in situ hybridization (FISH) using the PathVysion HER2 DNA probe kit (Vysis-Abbott, Chicago, IL, USA). To ensure the intraobserver and interobserver reliability of the central assessment, 5% of the centrally-evaluated tumors were blindly reassessed by the same pathologist and 10% by a different pathologist. If the recorded percentages of immunostained cells differed by more than 10%, then a collegial reevaluation at the multiheaded microscope was performed. Pathology assessment was done without knowledge of patients' treatment assignments or outcomes.
Tumors were considered as centrally-confirmed to be ER or PgR-expressing when ≥1% invasive tumor cells showed definite nuclear staining, irrespective of staining intensity[6]. Tumors were considered as HER2-positive if the IHC score was 3+ or FISH showed a HER2-to-chromosome 17 ratio of ≥2.0.
Analysis Population, Endpoint and Statistical Considerations
The analysis population included patients in the intention-to-treat trial populations for whom the IBCSG Central Pathology Office reviewed invasive tumor material. Although trial eligibility required ≥10% cells staining for ER and/or PgR on local testing, for this analysis population, only patients whose submitted tumor was not confirmed to express any ER or PgR by central testing were excluded. Patients for whom tumor was assessed centrally or locally as HER2-positive were also excluded.
The study endpoint was breast cancer-free interval (BCFI), defined as time from randomization to first appearance of invasive breast cancer recurrence (local, regional or distant) or invasive contralateral breast cancer; in the absence of an event, time was censored at date of last follow-up. The associations of pre-defined marker subgroups with BCFI were assessed using Cox proportional hazards models, stratified by cohort (as defined by trial and chemotherapy use) and treatment assignment; the predictive value of those subgroups in terms of relative treatment effects was assessed by test for treatment-by-marker interaction. Subgroups were pre-defined based on prior St. Gallen Consensus statements[7,8] as: ER<50% vs. ≥50% with ER<10% as an additional category in descriptive analyses; PgR<20% vs. 20-49% vs. ≥50% with PgR<10% as an additional category in descriptive analyses; and Ki-67<14% vs. 14-19% vs. 20-25% vs. ≥26% with the fourth category being data-driven corresponding to the upper 20th percentile of the distribution. Luminal A-like and B-like disease were defined according to the 2013 St. Gallen Consensus definition[8] in HER2-negative disease as: Luminal A-like if PgR≥20% and Ki-67<20%; B-like if either PgR<20% or Ki-67≥20%. The assessment of clinical utility of markers according to quantitative level of expression used the nonparametric sliding-window subpopulation treatment effect pattern plot (STEPP) methodology[9,10] for exploring treatment-covariate interaction with a continuous covariate. Separately for each of the four cohorts, STEPP investigated patterns in absolute treatment effects, as measured by Kaplan-Meier estimates of 5-year BCFI, across the continuum of centrally-determined levels of ER, PgR and Ki-67 expression, using permutation testing of treatment-by-marker interaction. The median follow-up was 6 years in TEXT and 5.6 years in SOFT.
Results
The IBCSG Central Pathology Office reviewed invasive tumor material of 4818 (84%) representative patients of 5707 patients in the intention-to-treat trial populations (Table S1). The analysis population was limited to 4115 patients with hormone receptor(HR)-positive, HER2-negative tumors (HR+/HER2-) after excluding 65 patients whose tumors were not confirmed to express some ER and/or PgR and 638 patients with HER2+ tumors (Figure S1). Characteristics of the 4115-patient HR+/HER2- analysis population according to cohort, as defined by trial and chemotherapy use, are summarized in Table 1. The 5-year BCFI was 90.7% overall (402 of 4115 patients had breast cancer events).
Table 1.
Patient and tumor characteristics of the HR+/HER2- analysis population, overall and according to cohort.
| Characteristic | Cohort | Overall HR+/HER2-Population | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No chemo SOFT | No chemo TEXT | Chemo TEXT | Prior chemo SOFT | |||||||
| N | % | N | % | N | % | N | % | N | % | |
| N Patients | 1135 | 100.0 | 844 | 100.0 | 1073 | 100.0 | 1063 | 100.0 | 4115 | 100.0 |
| Age at randomization | 15 | 1.3 | 35 | 4.1 | 116 | 10.8 | 190 | 17.9 | 356 | 8.7 |
| <35 | ||||||||||
| 35-39 | 86 | 7.6 | 89 | 10.5 | 188 | 17.5 | 307 | 28.9 | 670 | 16.3 |
| 40-44 | 302 | 26.6 | 296 | 35.1 | 364 | 33.9 | 345 | 32.5 | 1307 | 31.8 |
| 45-49 | 515 | 45.4 | 322 | 38.2 | 341 | 31.8 | 183 | 17.2 | 1361 | 33.1 |
| 50+ | 217 | 19.1 | 102 | 12.1 | 64 | 6.0 | 38 | 3.6 | 421 | 10.2 |
| No. nodes positive | 1039 | 91.5 | 661 | 78.3 | 353 | 32.9 | 444 | 41.8 | 2497 | 60.7 |
| N0 | ||||||||||
| N 1-3 | 95 | 8.4 | 181 | 21.4 | 451 | 42.0 | 429 | 40.4 | 1156 | 28.1 |
| N 4+ | 1 | 0.1 | 2 | 0.2 | 269 | 25.1 | 190 | 17.9 | 462 | 11.2 |
| Tumor size (path.; cm) | ||||||||||
| ≤2 cm | 975 | 85.9 | 670 | 79.4 | 487 | 45.4 | 532 | 50.0 | 2664 | 64.7 |
| >2 cm | 154 | 13.6 | 174 | 20.6 | 576 | 53.7 | 499 | 46.9 | 1403 | 34.1 |
| Unknown | 6 | 0.5 | - | - | 10 | 0.9 | 32 | 3.0 | 48 | 1.2 |
| Centrally-assessed | ||||||||||
| Tumor grade | ||||||||||
| 1 | 420 | 37.0 | 210 | 24.9 | 143 | 13.3 | 156 | 14.7 | 929 | 22.6 |
| 2 | 602 | 53.0 | 496 | 58.8 | 603 | 56.2 | 619 | 58.2 | 2320 | 56.4 |
| 3 | 108 | 9.5 | 135 | 16.0 | 320 | 29.8 | 282 | 26.5 | 845 | 20.5 |
| Not determined | 5 | 0.4 | 3 | 0.4 | 7 | 0.7 | 6 | 0.6 | 21 | 0.5 |
| Vessel invasion (lymphatics and/or blood vessels) | ||||||||||
| No | 1013 | 89.3 | 682 | 80.8 | 695 | 64.8 | 792 | 74.5 | 3182 | 77.3 |
| Yes | 120 | 10.6 | 161 | 19.1 | 374 | 34.9 | 268 | 25.2 | 923 | 22.4 |
| Not determined | 2 | 0.2 | 1 | 0.1 | 4 | 0.4 | 3 | 0.3 | 10 | 0.2 |
| Estrogen receptor (ER) | ||||||||||
| <10% | 1 | 0.1 | - | - | 6 | 0.6 | 11 | 1.0 | 18 | 0.4 |
| 10-49% | 13 | 1.1 | 12 | 1.4 | 35 | 3.3 | 22 | 2.1 | 82 | 2.0 |
| ≥50% | 1103 | 97.2 | 826 | 97.9 | 1020 | 95.1 | 1011 | 95.1 | 3960 | 96.2 |
| Not determined | 18 | 1.6 | 6 | 0.7 | 12 | 1.1 | 19 | 1.8 | 55 | 1.3 |
| Median and IQR | 95 | 90-99 | 95 | 90-99 | 90 | 85-99 | 95 | 90-99 | 95 | 90-99 |
| Progesterone receptor (PgR) | ||||||||||
| <10% | 28 | 2.5 | 36 | 4.3 | 92 | 8.6 | 131 | 12.3 | 287 | 7.0 |
| 10-19% | 13 | 1.1 | 10 | 1.2 | 36 | 3.4 | 47 | 4.4 | 106 | 2.6 |
| 20-49% | 42 | 3.7 | 50 | 5.9 | 110 | 10.3 | 105 | 9.9 | 307 | 7.5 |
| ≥50% | 1034 | 91.1 | 735 | 87.1 | 822 | 76.6 | 761 | 71.6 | 3352 | 81.5 |
| Not determined | 18 | 1.6 | 13 | 1.5 | 13 | 1.2 | 19 | 1.8 | 63 | 1.5 |
| Median and IQR | 95 | 90-99 | 90 | 80-99 | 90 | 60-95 | 90 | 40-95 | 90 | 70-99 |
| Ki-67 expression | ||||||||||
| <14% | 505 | 44.5 | 269 | 31.9 | 198 | 18.5 | 247 | 23.2 | 1219 | 29.6 |
| 14-19% | 324 | 28.5 | 261 | 30.9 | 293 | 27.3 | 302 | 28.4 | 1180 | 28.7 |
| 20-25% | 152 | 13.4 | 135 | 16.0 | 234 | 21.8 | 216 | 20.3 | 737 | 17.9 |
| >26% | 101 | 8.9 | 135 | 16.0 | 310 | 28.9 | 236 | 22.2 | 782 | 19.0 |
| Not determined | 53 | 4.7 | 44 | 5.2 | 38 | 3.5 | 62 | 5.8 | 197 | 4.8 |
| Median and IQR | 14 | 9-19 | 16 | 11-22 | 20 | 15-27 | 18 | 14-25 | 18 | 12-24 |
| Luminal A/B-likea | ||||||||||
| A-like | 796 | 70.1 | 507 | 60.1 | 448 | 41.8 | 480 | 45.2 | 2231 | 54.2 |
| B-like | 279 | 24.6 | 291 | 34.5 | 585 | 54.5 | 519 | 48.8 | 1674 | 40.7 |
| Not determined | 60 | 5.3 | 46 | 5.5 | 40 | 3.7 | 64 | 6.0 | 210 | 5.1 |
Abbreviations: IQR=interquartile range; SOFT=Suppression of Ovarian Function Trial; TEXT=Tamoxifen and Exemestane Trial.
a: Defined according to 2013 St. Gallen Consensus: in HR+/HER2-negative disease, Luminal A-like is PgR≥20% and Ki-67<20%; B-like is either PgR<20% or Ki-67≥20%.
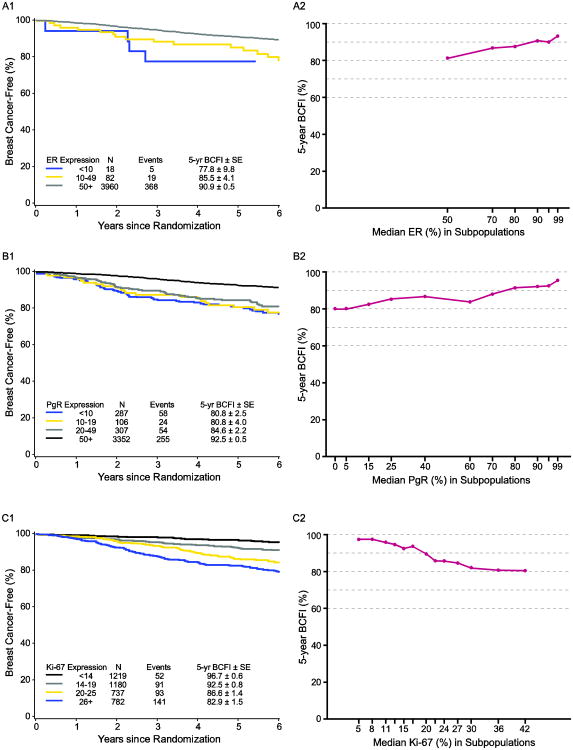
In this HR+/HER2- population, centrally-determined ER expression was ≥50% immunostained cells for 96% of tumors, of which the majority had ER expression ≥90% immunostained cells (Table 1; Figure S2). Investigation of the predictive value of ER expression level was therefore not feasible.
The median values of PgR and Ki-67 expression were 90% and 18% immunostained cells, respectively. As expected, the distribution of PgR was shifted lower, and that of Ki-67 was shifted higher, among the cohorts of patients who received chemotherapy than among the cohorts that did not (Table 1). According to the 2013 St. Gallen Consensus definitions of luminal A-like and B-like disease[8], 41% of patients overall had HER2- luminal B-like disease, including 25%, 34%, 55% and 49% of patients in the SOFT no chemotherapy, TEXT no chemotherapy, TEXT chemotherapy and SOFT prior chemotherapy cohorts, respectively.
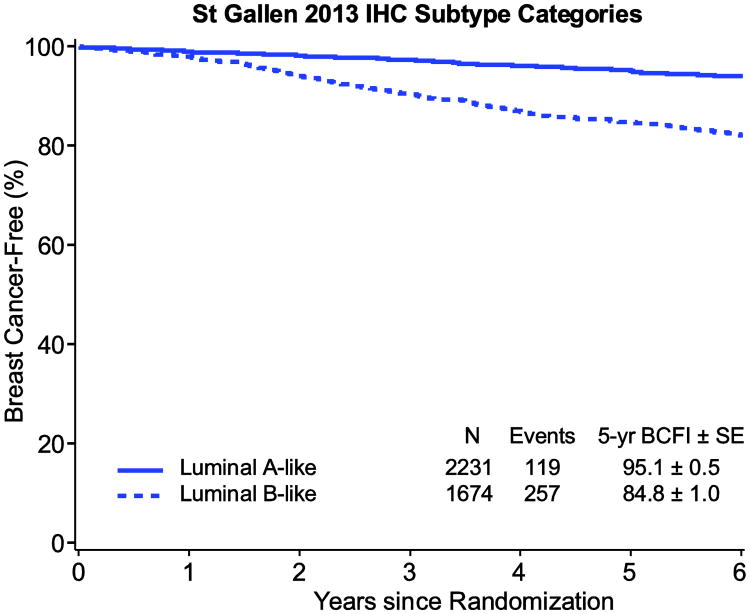
Lower tumor expression of ER and of PgR and higher expression of Ki-67 were associated with shorter BCFI (each P<0.001). Figure 1 illustrates the relation with BCFI in the overall HR+/HER2- population without regard to treatment assignment, according to marker subgroups and according to continuous levels of ER, PgR and Ki-67 expression. Consistent with the individual markers, luminal B-like status was associated with shorter BCFI (P<0.001; Figure 2).
Fig. 1.
Kaplan-Meier estimates of breast cancer-free interval (BCFI) and STEPPs of 5-year BCFI in the overall HR+/HER2- population, according to: (A) level of estrogen receptor (ER) expression; (B) level of progesterone receptor (PgR) expression; (C) level of Ki-67 expression
Abbreviations: HR+=hormone receptor positive; SE=standard error; STEPP=subpopulation treatment effect pattern plot
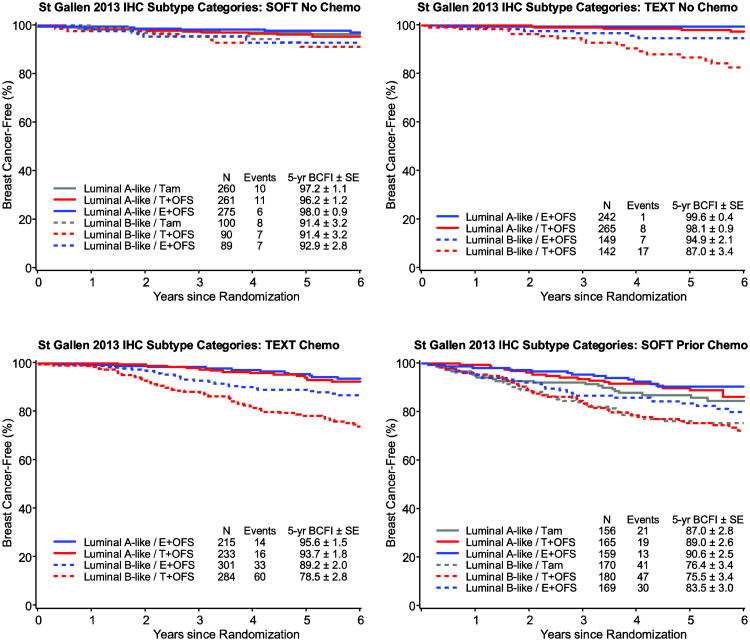
Fig. 2.
Kaplan-Meier estimates of breast cancer-free interval (BCFI) in the overall HR+/HER2- population, according to luminal A/B-like status (Status is defined by the 2013 St. Gallen Consensus: in HR+/HER2-negative disease, Luminal A-like is PgR≥20% and Ki-67<20%; B-like is either PgR<20% or Ki-67≥20%)
Abbreviations: HR+=hormone receptor positive; SE=standard error
PgR Expression
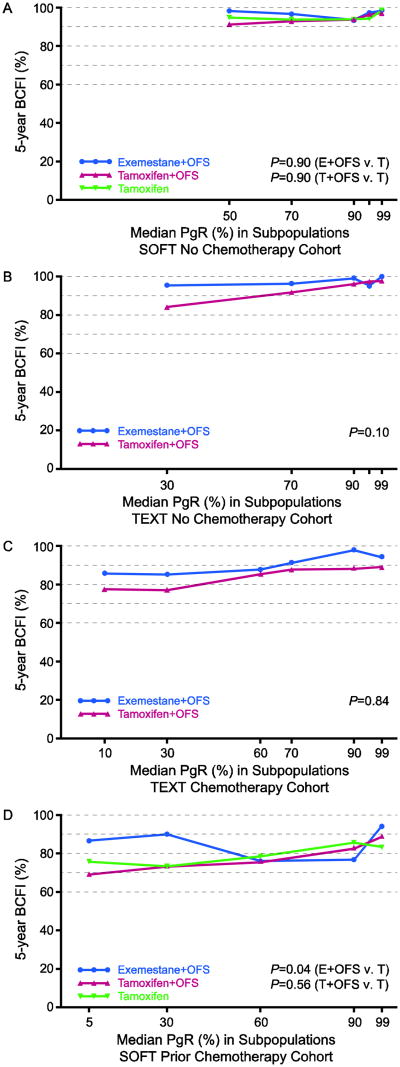
There was no evidence of heterogeneity of the relative treatment effects according to pre-defined subgroups of PgR expression (each P>0.10 for PgR-by-treatment interaction; Table 2), indicating that PgR is not a predictive marker for these treatments. In STEPP analyses of absolute treatment effects in each of the four cohorts, improvements in 5-year BCFI with exemestane+OFS versus tamoxifen+OFS or versus tamoxifen-alone appeared greater in subpopulations at lowest PgR expression levels (in the range of 7 to 17 percentage points) than at higher PgR expression levels. In the two no-chemotherapy cohorts (Figures 3A,B), the patterns suggested larger absolute improvement in 5-year BCFI with exemestane+OFS versus tamoxifen+OFS in TEXT or versus tamoxifen-alone in SOFT at low PgR that disappeared at high PgR expression levels. A similar pattern was observed in the SOFT cohort who received prior chemotherapy; there was no evident pattern for the benefit of tamoxifen+OFS versus tamoxifen across the range of PgR expression (Figure 3D). In the TEXT chemotherapy cohort, there was absolute benefit from exemestane+OFS for 5-year BCFI in the TEXT chemotherapy cohort across all PgR expression levels (Figure 3C).
Table 2.
Hazard ratios comparing relative treatment effects upon breast cancer-free interval (BCFI) in the HR+/HER2- analysis population according to marker subgroups.
| Marker and Category | Trial and Treatment Comparison | ||||||
|---|---|---|---|---|---|---|---|
| SOFT | TEXT | ||||||
| T+OFS vs Tam | E+OFS vs Tam | E+OFS vs T+OFS | |||||
| HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | ||
| All HR+/HER2- | -- | 0.90 | (0.67, 1.21) | 0.70 | (0.51, 0.96) | 0.52 | (0.37, 0.72) |
| PgR | <20% | 1.44 | (0.75, 2.78) | 0.60 | (0.29, 1.25) | 0.41 | (0.20, 0.84) |
| 20-49% | 0.90 | (0.37, 2.15) | 0.54 | (0.20, 1.50) | 0.52 | (0.24, 1.13) | |
| ≥50% | 0.88 | (0.61, 1.26) | 0.75 | (0.51, 1.11) | 0.52 | (0.34, 0.80) | |
| P(Interaction) | P= 0.42 | P= 0.76 | P= 0.85 | ||||
| Ki-67 | <14% | 0.58 | (0.28, 1.20) | 0.35 | (0.15, 0.79) | 0.73 | (0.24, 2.23) |
| 14-19% | 1.16 | (0.63, 2.12) | 0.95 | (0.50, 1.82) | 0.73 | (0.36, 1.49) | |
| 20-25% | 1.01 | (0.53, 1.93) | 0.99 | (0.51, 1.95) | 0.33 | (0.16, 0.67) | |
| ≥26% | 0.99 | (0.58, 1.70) | 0.72 | (0.39, 1.34) | 0.47 | (0.29, 0.76) | |
| P(Interaction) | P= 0.53 | P= 0.21 | P= 0.40 | ||||
| luminal A/B-like | A-like | 0.91 | (0.55, 1.50) | 0.59 | (0.33, 1.05) | 0.68 | (0.36, 1.29) |
| B-like | 1.01 | (0.69, 1.49) | 0.76 | (0.49, 1.16) | 0.45 | (0.30, 0.65) | |
| P(Interaction) | P= 0.74 | P= 0.50 | P= 0.27 | ||||
Cox models were stratified by nodal status and chemotherapy use. The Wald chi-square tests for marker-by-treatment interaction had 2, 3 or 1 degree of freedom for PgR, Ki-67 and luminal A/B-like, respectively. HRs (CI) for undetermined marker values omitted, e.g., for luminal A/B-like T+OFS vs Tam, this is why the two HRs are both greater than the overall HR.
Abbreviations: OFS=ovarian function suppression; T=tamoxifen; E=exemestane; HR=hazard ratio; LCL=lower confidence level; UCL=upper confidence level; PgR=progesterone receptor.
Fig. 3.
STEPP of 5-year breast cancer-free interval (BCFI) in the HR+/HER2- population, according to level of progesterone receptor (PgR) expression, for each of the four cohorts defined by trial and chemotherapy use: (A) SOFT no chemotherapy; (B) TEXT no chemotherapy; (C) TEXT chemotherapy; (D) SOFT prior chemotherapy
Abbreviations: HR+=hormone receptor positive; STEPP=subpopulation treatment effect pattern plot
Ki-67 Expression
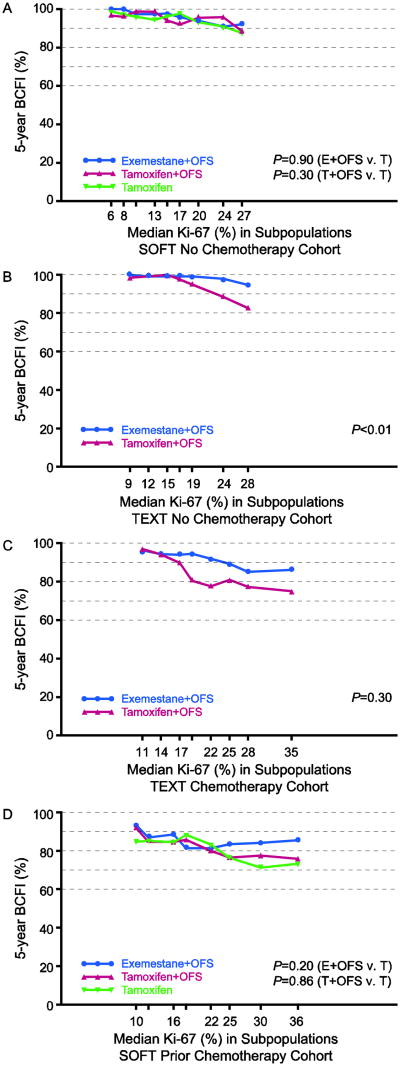
There was no evidence of heterogeneity of the relative treatment effects according to pre-defined subgroups of Ki-67 expression (each P>0.10 for Ki-67-by-treatment interaction; Table 2), indicating that Ki-67 is not a predictive marker for these treatments. In STEPP analysis of 5-year BCFI according to level of Ki-67 expression, no pattern was evident in the SOFT no chemotherapy cohort (Figure 4A). Among TEXT patients, who all received OFS from the start of adjuvant therapy, the STEPPs revealed large absolute benefits of exemestane+OFS versus tamoxifen+OFS as subpopulation median values approached and surpassed 20% Ki-67 expression (in the range of 4 to 14 percentage points), not evident at lower expression levels where 5-year BCFI was near 100% (Figures 4B,C). Among SOFT patients who received prior chemotherapy, the 5-year BCFI benefit of exemestane+OFS over tamoxifen (or tamoxifen+OFS) was also apparent at higher levels of Ki-67 expression (in the range of 7 to 12 percentage points), but without evident pattern for tamoxifen+OFS versus tamoxifen (Figure 4D).
Fig. 4.
STEPP of 5-year breast cancer-free interval (BCFI) in the HR+/HER2- population, according to level of Ki-67 expression, for each of the four cohorts defined by trial and chemotherapy use: (A) SOFT no chemotherapy; (B) TEXT no chemotherapy; (C) TEXT chemotherapy; (D) SOFT prior chemotherapy. P-values are from permutation tests of Ki-67-by- treatment interaction
Abbreviations: HR+=hormone receptor positive; OFS=ovarian function suppression; STEPP=subpopulation treatment effect pattern plot
2013 St. Gallen Luminal A/B-like (HER2-) categories
The relative treatment benefit of exemestane+OFS versus tamoxifen+OFS in TEXT and versus tamoxifen-alone in SOFT was evident in both luminal subgroups (Table 2; Figure 5). The absolute differences in 5-year BCFI among patients with luminal A-like tumors varied but was quite small in most cohorts (1.5% and 1.9% versus tamoxifen+OFS at 5 years in TEXT no-chemotherapy and chemotherapy cohorts, respectively; 0.8% and 3.6% versus tamoxifen in SOFT no-chemotherapy and prior chemotherapy cohorts, respectively). In the luminal B-like subgroup, there were larger absolute benefits of exemestane+OFS versus tamoxifen with or without OFS in each of the cohorts (7.9% and 10.7% versus tamoxifen+OFS at 5 years in TEXT no-chemotherapy and chemotherapy cohorts, respectively; 1.5% and 7.1% versus tamoxifen in SOFT no-chemotherapy and prior chemotherapy cohorts, respectively).
Fig. 5.
Kaplan-Meier estimates of breast cancer-free interval (BCFI) in the HR+/HER2- population according to luminal A/B-like status and treatment assignment, separately by cohort (Status is defined using the 2013 St. Gallen Consensus: in HR+/HER2-negative disease, Luminal A-like is PgR≥20% and Ki-67<20%; B-like is either PgR<20% or Ki-67≥20%.)
Discussion
The TEXT and SOFT trials demonstrated that, on average for premenopausal women with HR+ early breast cancer, adjuvant treatment with exemestane+OFS provides superior outcomes relative to tamoxifen with or without OFS[2,3]. SOFT also demonstrated that women who remain premenopausal after chemotherapy benefit from the addition of OFS to tamoxifen, and that tamoxifen alone remains an appropriate treatment for some premenopausal women at low risk of recurrence[2,3]. Based upon centrally-assessed expression of PgR and Ki-67, whether considering continuous expression levels or defining luminal A/B-like disease, it was apparent that patients with tumors having low PgR and/or high Ki-67 have the potential for larger absolute benefit of exemestane+OFS versus tamoxifen+OFS or tamoxifen-alone because low PgR and high Ki-67 are associated with increased risk for recurrence. However PgR and Ki-67 are not predictive markers of relative treatment efficacy and individually are not adequate for treatment selection for premenopausal women with HR+/HER2- disease.
We hypothesized that premenopausal patients whose HER2- tumors had lower expression of PgR would have greater relative benefit of exemestane+OFS versus tamoxifen with or without OFS, and of the addition of OFS to tamoxifen, than would patients with tumors having high PgR expression. This was not observed. The predictive role of PgR for adjuvant therapy selection among premenopausal and postmenopausal women may differ. Earlier IBCSG studies observed a predictive value of PgR for efficacy of chemo-endocrine versus endocrine therapy[11] and for response to perioperative chemotherapy[12] in node-negative disease for premenopausal but not postmenopausal women. A predictive value of PgR for efficacy of tamoxifen versus no endocrine therapy in ER+ disease also has been suggested in premenopausal women[13], in contrast to results of the Early Breast Cancer Trialists Collaborative Group (EBCTCG) overview which included predominantly postmenopausal patients[14]. For the efficacy of an AI versus tamoxifen in postmenopausal women with HR+ disease, low PgR expression was not predictive of the relative efficacy of AIs versus tamoxifen[5,15,16], nor of the absolute benefit in the BIG 1-98 trial[5]. Down-regulation of PgR in postmenopausal women's tumors might reflect ER activity due to low levels of circulating estrogen, in which case a differential activity of tamoxifen versus AI might not be seen. In premenopausal women, down-regulation of PgR is likely due to the (co-)activation of growth factor receptor pathways, possibly via activation of non-genomic membrane ER. It has been reported that both estrogens and tamoxifen, but not AIs, can activate or sustain growth factor receptors pathways[17], and hence the beneficial effects of tamoxifen could be reduced as compared to AIs in this scenario. However among the premenopausal patients in TEXT and SOFT, there was no evidence of heterogeneity of the relative treatment effects according to PgR expression level. In three of the four cohorts—those without chemotherapy or with prior chemotherapy—the homogeneous relative treatment effect of this prognostic marker translated into a heterogeneous absolute treatment effect. A pattern of larger, clinically-meaningful absolute improvement in 5-year BCFI from exemestane+OFS versus tamoxifen, with or without OFS, was apparent among patients with tumors having lower PgR levels than among those with high PgR expression levels.
Very high Ki-67 expression was hypothesized to be predictive of larger relative and absolute benefit of exemestane+OFS versus tamoxifen with or without OFS. This was previously observed among postmenopausal women randomized to letrozole versus tamoxifen in the BIG 1-98 trial, without regard to HER2 status[4]. A large absolute benefit of exemestane+OFS versus tamoxifen, with or without OFS, at high levels of Ki-67 that diminished at low levels of Ki-67 was apparent in the TEXT cohorts with and without chemotherapy and in the SOFT prior chemotherapy cohort.
We investigated the 2013 St. Gallen Consensus definition of luminal A/B-like disease, in which luminal B-like (HER2-) is defined by PgR<20% and/or Ki-67≥20%. Alternative definitions have been proposed for HER2- disease, including one definition in which tumors having PgR<20% and Ki-67<14% expression are defined as luminal A-like[18]. In the analysis population, only 57 of 4115 tumors had this combination of expression levels, and thus we could not contrast these two definitions in terms of prognosis or predictive value for endocrine therapy in premenopausal women with HER2- disease. Our data do also suggest that further refinements of the definitions may improve clinical utility, for example, exploring thresholds of PgR separately according to HER2-status or in a HR+, HER2-negative population.
TEXT and SOFT could not inform the use of the ER expression level to select treatment for HER2- disease, because the vast majority of patients' tumors had ER expression ≥90%. This is not unexpected considering the bimodal pattern of ER expression[19] and the trial eligibility requiring ER and/or PgR≥10% cells by local testing. With longer follow-up, more observed recurrences may provide insight. The investigation was limited to patients with HER2- disease because adjuvant trastuzumab with chemotherapy is indicated for most patients with HER2+ disease, and this subgroup was the exception for which there was evidence of treatment effect heterogeneity that requires further investigation[3,2].
The TEXT and SOFT premenopausal populations with HR+/HER2- disease do not suggest heterogeneous relative treatment efficacy according to level of PgR and Ki-67 expression as assessed in a central laboratory, nor according to luminal A/B-like status. Tumors having low PgR and/or high Ki-67 have worse prognosis and thus potential for larger absolute benefit of exemestane+OFS versus tamoxifen+OFS or tamoxifen-alone, but these markers individually are not adequate for treatment decision-making for premenospausal women with HR+/HER2-disease.
Supplementary Material
Acknowledgments
We thank the many pathologists who submitted tumor blocks and slides, and the patients, physicians, nurses and data managers who participated in the TEXT and SOFT clinical trials. We thank Stefania Andrighetto and Elvira Bianca Benini of the IBCSG Central Pathology Office and Wilbur Helfer of the IBCSG Coordinating Center.
TEXT and SOFT received financial support for trial conduct from Pfizer, the International Breast Cancer Study Group and the US National Cancer Institute. Pfizer and Ipsen provided drug supply. Support for the coordinating group, IBCSG: Frontier Science and Technology Research Foundation, Swiss Group for Clinical Cancer Research (SAKK), Cancer Research Switzerland/Oncosuisse, the Foundation for Clinical Cancer Research of Eastern Switzerland (OSKK), US National Cancer Institute (NCI) (US NIH CA75362), Susan G. Komen for the Cure Promise Grant (KG080081), Breast Cancer Research Foundation.
Grant support of cooperative groups: Australia and New Zealand Breast Cancer Trials Group (NHMRC 351161 and 510788); SWOG (US NIH CA32102); Alliance/CALGB (US NIH U10-CA180821); ECOG-ACRIN (US NIH CA21115 and CA16116); NSABP/NRG (US NIH U10-CA-12027, U10-CA-69651, U10-CA-37377, U10-CA-69974); NCIC-CTG (US NIH CA077202 and CCSRI 015469 and 021039); ICR-CTSU on behalf of the National Cancer Research Institute (NCRI) Breast Clinical Studies Group United Kingdom (NCRI-BCSG—ICR-CTSU Partnership) was supported by CRUK, CRUKE/03/022, CRUKE/03/023, A15955, NIHR RM/ICR Biomedical Research Centre and by NIHR Cambridge Biomedical Research Centre.
Footnotes
Conflicts of Interest: Dr. Francis reports uncompensated presentation of results of SOFT and TEXT for Pfizer at an international meeting.
The remaining authors declare that they have no conflict of interest.
Contributor Information
Meredith M. Regan, International Breast Cancer Study Group Statistical Center, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, USA
Olivia Pagani, Email: olivia.pagani@ibcsg.org, Institute of Oncology of Southern Switzerland, Swiss Group for Clinical Cancer Research (SAKK), and International Breast Cancer Study Group, Lugano Viganello, Switzerland.
Prudence A. Francis, Email: prue.francis@petermac.org, Peter MacCallum Cancer Center, St Vincent's Hospital, University of Melbourne, Australia; Australia & New Zealand Breast Cancer Trials Group, University of Newcastle, Australia; International Breast Cancer Study Group.
Gini F. Fleming, Email: gfleming@medicine.bsd.uchicago.edu, The University of Chicago Medical Center and Alliance for Clinical Trials in Oncology, Chicago, IL, USA.
Barbara A. Walley, Email: Barbara.Walley@albertahealthservices.ca, Tom Baker Cancer Centre and NCIC Clinical Trials Group, Calgary, AB, Canada.
Roswitha Kammler, Email: Rosita.Kammler@ibcsg.org, International Breast Cancer Study Group Coordinating Center and Central Pathology Office, Bern, Switzerland.
Patrizia Dell'Orto, Email: patrizia.dellorto@ieo.it, International Breast Cancer Study Group Central Pathology Office, Department of Pathology, European Institute of Oncology, Milan, Italy.
Leila Russo, Email: leila.russo@ieo.it, International Breast Cancer Study Group Central Pathology Office, Department of Pathology, European Institute of Oncology, Milan, Italy.
János Szőke, Email: szoke.j@oncol.hu, Pathological Department, National Institute of Oncology, Budapest, Hungary and International Breast Cancer Study Group.
Franco Doimi, Email: fdoimi@inen.sld.pe, Instituto Nacional de Enfermedades Neoplásias, Lima, Peru and International Breast Cancer Study Group.
Laura Villani, Email: laura.villani@fsm.it, Division of Pathology, Salvatore Maugeri Foundation, Pavia, Italy and Internatinal Breast Cancer Study Group.
Stefano Pizzolitto, Email: pizzolitto.stefano@aoud.sanita.fvg.it, Struttura Operativa Complessa di Anatomia Patologica, Azienda Ospedaliero-Universitaria di Udine, Udine, Italy, and International Breast Cancer Study Group.
Christian Öhlschlegel, Email: christian.oehlschlegel@kssg.ch, Kantonsspital St. Gallen, Switzerland, Swiss Group for Clinical Cancer Research (SAKK) and International Breast Cancer Study Group.
Fausto Sessa, Email: fausto.sessa@ospedale.varese.it, University of Insubria-Ospedale di Circolo and Fondazione Macchi, Varese, Italy and International Breast Cancer Study Group.
Vicente Peg Cámara, Email: vpeg@vhebron.net, Department of Pathology, Vall d'Hebron University Hospital, Barcelone, Spain.
José Luis Rodríguez Peralto, Email: jrperalto@salud.madrid.org, Pathology Department, Hospital Universitario «12 de Octubre», i+12, Universidad ComplutenseMadrid, Spain.
Gaëtan MacGrogan, Email: g.macgrogan@bordeaux.unicancer.fr, Laboratoire Anatomopathologie, Institut Bergonié, Bordeaux, France and EORTC.
Marco Colleoni, Email: marco.colleoni@ieo.it, Division of Medical Senology, European Institute of Oncology and International Breast Cancer Study Group, Milan, Italy.
Aron Goldhirsch, Email: aron.goldhirsch@ibcsg.org, European Institute of Oncology and International Breast Cancer Study Group, Milan, Italy.
Karen N. Price, International Breast Cancer Study Group Statistical Center, Frontier Science and Technology Research Foundation, Boston, MA, USA
Alan S. Coates, Email: alan.coates@ibcsg.org, International Breast Cancer Study Group and University of Sydney, Sydney, Australia.
Richard D. Gelber, Email: gelber@jimmy.harvard.edu, International Breast Cancer Study Group Statistical Center, Dana-Farber Cancer Institute, Harvard Medical School, Harvard T.H. Chan School of Public Health, Frontier Science and Technology Research Foundation, Boston, MA, USA.
Giuseppe Viale, Email: giuseppe.viale@ieo.it, International Breast Cancer Study Group Central Pathology Office, European Institute of Oncology, University of Milan, Milan, Italy.
References
- 1.Regan MM, Pagani O, Fleming GF, Walley BA, Price KN, Rabaglio M, Maibach R, Ruepp B, Coates AS, Goldhirsch A, Colleoni M, Gelber RD, Francis PA. Adjuvant treatment of premenopausal women with endocrine-responsive early breast cancer: design of the TEXT and SOFT trials. Breast. 2013;22(6):1094–1100. doi: 10.1016/j.breast.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pagani O, Regan MM, Walley BA, Fleming GF, Colleoni M, Lang I, Gomez HL, Tondini C, Burstein HJ, Perez EA, Ciruelos E, Stearns V, Bonnefoi HR, Martino S, Geyer CE, Jr, Pinotti G, Puglisi F, Crivellari D, Ruhstaller T, Winer EP, Rabaglio-Poretti M, Maibach R, Ruepp B, Giobbie-Hurder A, Price KN, Bernhard J, Luo W, Ribi K, Viale G, Coates AS, Gelber RD, Goldhirsch A, Francis PA. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371(2):107–118. doi: 10.1056/NEJMoa1404037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francis PA, Regan MM, Fleming GF, Lang I, Ciruelos E, Bellet M, Bonnefoi HR, Climent MA, Da Prada GA, Burstein HJ, Martino S, Davidson NE, Geyer CE, Jr, Walley BA, Coleman R, Kerbrat P, Buchholz S, Ingle JN, Winer EP, Rabaglio-Poretti M, Maibach R, Ruepp B, Giobbie-Hurder A, Price KN, Colleoni M, Viale G, Coates AS, Goldhirsch A, Gelber RD. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372(5):436–446. doi: 10.1056/NEJMoa1412379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viale G, Giobbie-Hurder A, Regan MM, Coates AS, Mastropasqua MG, Dell'Orto P, Maiorano E, MacGrogan G, Braye SG, Ohlschlegel C, Neven P, Orosz Z, Olszewski WP, Knox F, Thurlimann B, Price KN, Castiglione-Gertsch M, Gelber RD, Gusterson BA, Goldhirsch A. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008;26(34):5569–5575. doi: 10.1200/jco.2008.17.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viale G, Regan MM, Maiorano E, Mastropasqua MG, Dell'Orto P, Rasmussen BB, Raffoul J, Neven P, Orosz Z, Braye S, Ohlschlegel C, Thurlimann B, Gelber RD, Castiglione-Gertsch M, Price KN, Goldhirsch A, Gusterson BA, Coates AS. Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1-98. J Clin Oncol. 2007;25(25):3846–3852. doi: 10.1200/jco.2007.11.9453. [DOI] [PubMed] [Google Scholar]
- 6.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28(16):2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20(8):1319–1329. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24(9):2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazar AA, Cole BF, Bonetti M, Gelber RD. Evaluation of treatment-effect heterogeneity using biomarkers measured on a continuous scale: subpopulation treatment effect pattern plot. J Clin Oncol. 2010;28(29):4539–4544. doi: 10.1200/JCO.2009.27.9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonetti M, Gelber RD. Patterns of treatment effects in subsets of patients in clinical trials. Biostatistics. 2004;5(3):465–481. doi: 10.1093/biostatistics/5.3.465. [DOI] [PubMed] [Google Scholar]
- 11.Regan MM, Viale G, Mastropasqua MG, Maiorano E, Golouh R, Carbone A, Brown B, Suurkula M, Langman G, Mazzucchelli L, Braye S, Grigolato P, Gelber RD, Castiglione-Gertsch M, Price KN, Coates AS, Goldhirsch A, Gusterson B. Re-evaluating adjuvant breast cancer trials: assessing hormone receptor status by immunohistochemical versus extraction assays. J Natl Cancer Inst. 2006;98(21):1571–1581. doi: 10.1093/jnci/djj415. [DOI] [PubMed] [Google Scholar]
- 12.Colleoni M, Gelber S, Coates AS, Castiglione-Gertsch M, Gelber RD, Price K, Rudenstam CM, Lindtner J, Collins J, Thurlimann B, Holmberg SB, Cortes-Funes H, Simoncini E, Murray E, Fey M, Goldhirsch A International Breast Cancer Study G. Influence of endocrine-related factors on response to perioperative chemotherapy for patients with node-negative breast cancer. J Clin Oncol. 2001;19(21):4141–4149. doi: 10.1200/JCO.2001.19.21.4141. [DOI] [PubMed] [Google Scholar]
- 13.Stendahl M, Ryden L, Nordenskjold B, Jonsson PE, Landberg G, Jirstrom K. High progesterone receptor expression correlates to the effect of adjuvant tamoxifen in premenopausal breast cancer patients. Clin Cancer Res. 2006;12(15):4614–4618. doi: 10.1158/1078-0432.CCR-06-0248. [DOI] [PubMed] [Google Scholar]
- 14.Early Breast Cancer Trialists' Collaborative G. Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dowsett M, Allred C, Knox J, Quinn E, Salter J, Wale C, Cuzick J, Houghton J, Williams N, Mallon E, Bishop H, Ellis I, Larsimont D, Sasano H, Carder P, Cussac AL, Knox F, Speirs V, Forbes J, Buzdar A. Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or in Combination trial. J Clin Oncol. 2008;26(7):1059–1065. doi: 10.1200/JCO.2007.12.9437. [DOI] [PubMed] [Google Scholar]
- 16.Bartlett JM, Brookes CL, Robson T, van de Velde CJ, Billingham LJ, Campbell FM, Grant M, Hasenburg A, Hille ET, Kay C, Kieback DG, Putter H, Markopoulos C, Kranenbarg EM, Mallon EA, Dirix L, Seynaeve C, Rea D. Estrogen receptor and progesterone receptor as predictive biomarkers of response to endocrine therapy: a prospectively powered pathology study in the Tamoxifen and Exemestane Adjuvant Multinational trial. J Clin Oncol. 2011;29(12):1531–1538. doi: 10.1200/JCO.2010.30.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arpino G, Weiss H, Lee AV, Schiff R, De Placido S, Osborne CK, Elledge RM. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005;97(17):1254–1261. doi: 10.1093/jnci/dji249. [DOI] [PubMed] [Google Scholar]
- 18.Maisonneuve P, Disalvatore D, Rotmensz N, Curigliano G, Colleoni M, Dellapasqua S, Pruneri G, Mastropasqua MG, Luini A, Bassi F, Pagani G, Viale G, Goldhirsch A. Proposed new clinicopathological surrogate definitions of luminal A and luminal B (HER2-negative) intrinsic breast cancer subtypes. Breast Cancer Res. 2014;16(3):R65. doi: 10.1186/bcr3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins LC, Botero ML, Schnitt SJ. Bimodal frequency distribution of estrogen receptor immunohistochemical staining results in breast cancer: an analysis of 825 cases. American journal of clinical pathology. 2005;123(1):16–20. doi: 10.1309/hcf035n9wk40etj0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.