Abstract
Combining effective treatments with diverse mechanisms of action for smoking cessation may provide better therapy by targeting multiple points of control in the neural circuits underlying addiction. Previous research in a rat model has shown that dextromethorphan, which has α3β4 nicotinic and NMDA glutamatergic antagonist actions, significantly decreases nicotine self-administration. We have found in the rat model that the H1 histamine antagonist pyrilamine and the serotonin 5HT2c agonist lorcaserin also significantly reduce nicotine self-administration. The current studies were conducted to determine the interactive effects of dextromethorphan with pyrilamine and lorcaserin on nicotine self-administration in rats. Young adult female rats were fitted with jugular IV catheters and trained to self-administer a nicotine infusion dose of 0.03-mg/kg/infusion. In an initial dose-effect function study of dextromethorphan, we found a monotonic decrease in nicotine self-administration over a dose range of 1 to 30-mg/kg with a lowest effective dose of 3-mg/kg. Then, with two separate cohorts of rats, dextromethorphan (0, 3.3, and 10-mg/kg) interactions with pyrilamine (0, 4.43, and 13.3-mg/kg) were investigated as well as interactions with lorcaserin (0, 0.3125 and 0.625-mg/kg). In the pyrilamine-dextromethorphan interaction study, the acute dose of pyrilamine (13.3-mg/kg) as well as an acute dose of dextromethorphan caused a significant decrease in nicotine self-administration. There were mutually augmenting effects of these two drugs. The combination of dextromethorphan (10-mg/kg) and pyrilamine (13.3-mg/kg) significantly lowered nicotine self-administration relative to either 10-mg/kg of dextromethorphan alone (p<0.05) or 13.3-mg/kg of pyrilamine alone (p<0.0005). In the lorcaserin-dextromethorphan study, an acute dose of lorcaserin (0.312-mg/kg) as well as an acute dose of dextromethorphan (10-mg/kg) caused a significant decrease in nicotine self-administration replicating previous findings. Augmenting interactions were observed with dextromethorphan and pyrilamine as well as lorcaserin. These findings suggest that combination therapy may be more effective smoking cessation treatments than monotherapy.
Keywords: Dextromethorphan, Histamine H1 Receptors, Pyrilamine, Serotonin 5HT2c Receptors, Lorcaserin, Nicotine Self-administration
Introduction
Tobacco addiction is estimated to cause over 540,000 premature deaths per year in the United States and millions more worldwide (Carter et al., 2015). It is estimated that tobacco use is responsible for almost 20% of all deaths in developed countries, making it the single largest cause of premature death worldwide (Dani and Heinemann, 1996). Although American tobacco consumption has declined significantly since the 1950s, a half century later still one in five Americans classified themselves as smokers (Giovino, 2007). Only three to five percent of cigarette smokers who attempt to quit without assistance are able to remain abstinent for six to twelve months (Hughes et al., 2004). Despite a wide variety of current treatments available for smoking cessation including nicotine replacement, bupropion and varenicline, relapse rates remain high, often >80%. Smokers who quit successfully usually only do so after numerous attempts. Clearly, more effective treatments are needed to help tobacco cessation. Recently, there has been an increased interest in the use of combination therapy with pharmacological agents as a potential option for smoking cessation treatment. The results of both clinical and preclinical studies investigating this potential treatment mechanism have been promising. Studies in humans have shown that combining FDA-approved smoking cessation aids results in significantly improved outcomes, with combinations of bupropion with either the nicotine patch or nicotine replacement therapy (NRT) (Jorenby et al., 1999; Rose and Behm, 2013), and combinations of varenicline with NRT or bupropion (Ebbert et al., 2014) (Ebbert et al., 2009; Koegelenberg et al., 2014; Rose and Behm, 2014) having improved efficacy to monotherapy with these treatments. We have previously shown that combining varenicline and bupropion reduces nicotine self-administration in rats more effectively than either treatment alone (Hall et al., 2015). This study was designed to explore the effects of combination therapy with other currently available pharmacological agents that have been shown to reduce nicotine self-administration in the rat model when given alone.
Dextromethorphan is an over-the-counter antitussive agent with multiple mechanisms of action on neurotransmitter systems in the brain. It acts as an NMDA glutamate receptor antagonist, a nonselective serotonin reuptake inhibitor, and sigma-1 receptor agonist (Henderson and Fuller, 1992; Maurice et al., 2001; Netzer et al., 1993). Dextromethorphan also been shown to act as a noncompetitive antagonist at α3β4, α4β2, and α7 nicotinic receptors (Damaj et al., 2005; Hernandez et al., 2000). The compound has received attention as a potential smoking cessation agent due to its ability to reduce nicotine self-administration in rats (Glick et al., 2001) as well as block nicotine's anti-nociceptive effects in thermal pain assays (Damaj et al., 2005).
Pyrilamine is an H1 histamine antagonist that is often used as an antihistaminergic agent in over-the-counter cold medications. We have previously shown that pyrilamine decreases nicotine self-administration in rats (Cousins et al., 2014; Levin et al., 2011b). Although pyrilamine significantly reduces nicotine self-administration independently, combining this histamine antagonist with other drugs may result in neurotransmitter system interactions that reduce nicotine self-administration more significantly. A close relationship is known to exist between the histaminergic and cholinergic systems, and pyrilamine has been shown to interact with nicotinic receptors whereby the compound appears to inhibit catecholamine secretion (Chen et al., 2001; Kim et al., 2014). These qualities make pyrliamine a promising potential agent for combination therapy.
Lorcaserin is a serotonin 5HT2C agonist. The compound has been shown to be effective in the treatment of weight gain and is currently FDA-approved for the treatment of obesity (Higgins et al., 2015; Johnson and Oliver, 2014). Lorcaserin has also been shown to interact with nicotinic systems. We and others have shown that lorcaserin significantly reduces nicotine self-administration as well as alcohol intake in rats (Higgins et al., 2012; Levin et al., 2011a; Rezvani and Levin, 2014). A recent Phase 2 trial shoed efficacy of lorcaserin in smoking cessation treatment (Shanahan et al., 2015). Lorcaserin also blocks intracranial self-stimulation and nicotine-enhanced responding for a conditioned reinforcer (Guy et al., 2014; Zeeb et al., 2015). Taken together, these findings suggest a role for 5HT2C receptors in the reinforcement and reward-related learning processes in the brain.
The current studies investigated combinations of dextromethorphan and pyrilamine as well as dextromethorphan and lorcaserin in a rat model of nicotine self-administration to determine if the combined effects of these drugs actions on different receptor types exceed that of each alone. It was hypothesized that treatment with these combinations would result in greater reductions in nicotine self-administration than would be observed with mono-treatment with any of these drugs, that therapy acting of more than one part of the addiction circuitry would have better effects.
Methods
Subjects
Young adult female Sprague-Dawley rats (Charles River, Raleigh, NC, USA) used in these studies were housed singly in colony rooms close to the self-administration facility so that they could be moved with minimal stress. Females were used to be congruent with our previous studies. The housing room was kept on a reverse 12:12 h (lights on 7:00 AM) day/night cycle to ensure that rats were in an active phase during the self-administration sessions. Rats were fed daily after behavioral testing with an amount of chow to keep them at a lean health weight approximately 85% of ad lib weight, and were given constant access to water through standard cage bottles or through automatic delivery lines in housing room racks. The procedures of this study were approved by the Duke University Institutional animal care and use committee in accordance with state and federal regulations.
Experimental Design
All of the studies tested acute drug treatment effects on nicotine self-administration using a repeated measures counterbalanced design. Each subject received each of the doses in an order of dose combinations that was counterbalanced across subjects, avoiding possible confounding of dose effect with the order of dose administration. The full range of dose conditions and vehicle control were administered to each subject twice. All doses of drugs and drug combinations used in each study were initially assessed for adverse effects on locomotor activity and food-motivated responding.
Study 1
Doses of 1, 3, 10 and 30-mg/kg of dextromethorphan and saline vehicle were injected (sc) 10-min prior to the onset of the test sessions. The acute dose-effect function of dextromethorphan on locomotor activity, food motivated responding and nicotine self-administration was evaluated. The doses were given in a repeated measures counterbalanced design, twice for the nicotine self-administration study and once each for the food motivated responding and locomotor activity studies.
Study 2
Interactions of dextromethorphan (0, 3.3, and 10-mg/kg) and pyrilamine (0, 4.43 and 13.3-mg/kg) was studied via s.c. injection 10 min prior to sessions using a repeated measures counterbalanced design. Following the results of the locomotor activity and food motivated responding sessions, the dose ranges of dextromethorphan and pyrilamine for the nicotine self-administration trials were tested.
Study 3
Combinations of dextromethorphan (0, 3.3, 10-mg/kg) and lorcaserin (0, 0.625 and 1.25-mg/kg) were administered via s.c. injection 10 minutes prior to sessions using a repeated measures counterbalanced design. Following the results of the locomotor activity and pellet sessions, the dose ranges of dextromethorphan and lorcaserin for the nicotine self-administration trials were conducted.
Preparation of Drug Solutions
Solutions of 0.03-mg/kg nicotine ditartrate were prepared according to the nicotine base weight. Using sterile glassware, the nicotine salt was dissolved in sterile saline and adjusted to a standard pH between 7.0 and 7.2. After being adjusted to a pH appropriate for intravenous infusion, the nicotine solution was filtered through a 0.22-μ Nalgene filter for sterilization. These solutions were stored in conical tubes wrapped with aluminum foil to prevent exposure to light. Between sessions nicotine solutions were refrigerated for no longer than two weeks before replacement. Pyrilamine, lorcaserin and dextromethorphan were dissolved in saline solution and were injected s.c. in a volume of 1 ml/kg.
Nicotine Self-Administration Procedure
Rats were trained to self-administer nicotine in a manner described previously (Hall et al., 2015; Hall et al., 2014). Briefly, animals were trained to press a lever to receive a 45 mg food pellet reward (FR1), and subsequently underwent jugular catheterization surgery. The rats then began nicotine self-administration sessions. Responses on the active lever (signaled via illuminated cue-light) delivered a 0.03-mg/kg/infusion dose of nicotine via the delivery line and catheter (FR1). Responses on the opposite (inactive) lever had no effect. Following each lever press and nicotine delivery, the cue light extinguished for a one-minute time out, the house light was illuminated, and the responses on the active lever were recorded but were without consequence. Prior to the start of the experiment, the rats were given five baseline nicotine self-administration sessions with no drug pretreatment. Before sessions, catheters were flushed with 0.3-ml of a 100 units/ml heparinized saline solution. Sessions lasted for 45 min and responses were operated using MED-PC software. Following sessions, nicotine was drawn out of the delivery port and replaced with 0.3-ml of a saline solution containing 8-mg/ml of the antibiotic gentamicin and 500 units/ml of heparin.
Food Self-Administration
Dose combinations used in the study for each drug were examined for effects on food-motivated lever responding. Over nine days, rats were injected with each combination of dextromethorphan (0, 10, 30-mg/kg), pyrilamine (0, 13.3, 40-mg/kg), and lorcaserin (0, 0.625 and 1.25-mg/kg) 10-min prior to pellet sessions in a counterbalanced order. Each session lasted 30 min. with an FR1 schedule.
Locomotor Activity
Locomotor activity was indexed using a Figure-8 apparatus. This maze is a continuous enclosed alley (10-cm by 10-cm) in the shape of a Figure-8 (70-cm long and 42-cm wide with a 21-cm by 16-cm central arena) with two blind alleys extending 20 cm from either side. Eight infrared photobeams crossing the maze alleys are used to measure locomotor activity, one located on each of the two blind alleys and three on each of two loops of the Figure 8. Activity was determined based on the number of breaks in the photobeams over one hour, and trends in locomotor activity were analyzed to test for potential locomotor effects of dextromethorphan and pyrilamine. Following four days of acclimation, rats were tested once for baseline locomotor activity without any drug injections prior to entering the maze. The rats were tested with each dose of dextromethorphan (0, 3, 10, 30-mg/kg) in Study 1, each combination of dextromethorphan (0, 10, 30-mg/kg) and pyrilamine (0, 13.3, 40-mg/kg) in Study 2 and dextromethorphan (0, 10, 30-mg/kg) and lorcaserin (0, 0.625 and 1.25-mg/kg) in Study 3 injected 10 min prior to testing in counterbalanced order.
Jugular Catheterization Surgery
Following successful completion of operant training sessions, jugular catheterization surgery was performed in a servile, aseptic environment as described previously (Hall et al., 2014). Briefly, animals were anesthetized with ketamine (60 mg/kg, i.p.) and dexmedetomidine (0.15 mg/kg, i.p.) and a sterile catheter was inserted into the jugular vein just anterior to the heart and secured using sterile silk. The catheter was sutured to the deep muscle and exited the body on the dorsal side of the rat just posterior to the scapulae. The end exiting the body was shortened and attached to a stainless steel extension of the plastic delivery port on a self-administration harness (SAI Infusion Technologies, Libertyville, IL, USA). Then, it was flushed with sterile saline heparinized 100 Units/ml. to prevent coagulation following surgery.
Data Analysis
The data were evaluated using analysis of variance. The within-subjects factors were dextromethorphan, pyrilamine and lorcaserin doses. Interactions p<0.10 were followed-up with simple main effects comparisons as recommended by Snedecor and Cochran (1967). An alpha level of p<0.05 (two-tailed) was considered significant in all final analyses.
Results
Study 1: Dextromethorphan Dose-effect Function
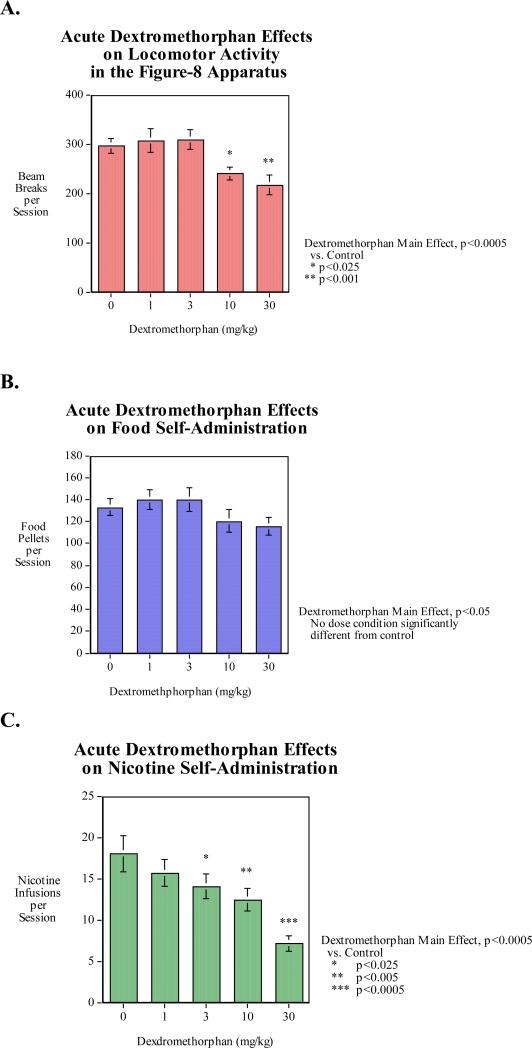
With locomotor activity there was a significant main effect of dextromethorphan (p<0.0005). Individual comparisons showed that the 10-mg/kg (p<0.025) and the higher 30-mg/kg (p<0.001) doses of dextromethorphan significantly decreased locomotor activity relative to control (Fig. 1A). With food-motivated responding there was a significant main effect of dextromethorphan (p<0.05); however, none of the individual doses caused effects that were significantly different from control (Fig. 1B).
Figure 1. Dextromethorphan dose effect function (N=15, mean±sem).
A) Locomotor activity: The dextromethorphan effect was significant (p<0.0005) with the 30 mg/kg dose significantly (p<0.005) decreasing activity relative to control.
B) Food motivated responding: The dextromethorphan effect was significant.
C) Nicotine self-administration: The dextromethorphan effect was significant.
As shown in figure 1C, acute dextromethorphan administration caused a dose-related decrease in nicotine self-administration. The main effect of dextromethorphan was significant (p<0.0005). Means comparisons of each dose condition to control showed a threshold between 3-mg/kg and 10-mg/kg for significantly (p<0.025) decreasing nicotine self-administration. The higher doses of 10-mg/kg (p<0.005) and 30-mg/kg (p<0.0005) of dextromethorphan caused progressively greater effects of reducing nicotine self-administration.
To directly compare dextromethorphan effects on food and nicotine self-administration the two sets of data were normalized to percent of control mean for each type of reinforcer. In an overall analysis with reinforcer as a factor there were main effects of reinforcer (F(1,14)=7.69, p<0.025), dextromethorphan (F(4,56)=12.45, p<0.0005). Importantly there was a significant interaction of reinforcer x dextromethorphan (F(4,56)=4.60, p<0.005) characterized by nicotine self-administration having a significantly greater effect of dextromethorphan than food self-administration. Food self-administration was only depressed to 92.4% and 89.0% of the control mean with 10 and 30 mg/kg of dextromethorphan, whereas nicotine was reduced to 87.1% of control mean by the lowerest 1 mg/kg dextromethorphan dose and showed greater effects down to 78.2%, 68.9% and 39.6% by the 3, 10 and 30 mg/kg dextromethorphan doses respectively, all three of which significantly lowered nicotine self-administration as shown in the analysis of the infusion data above.
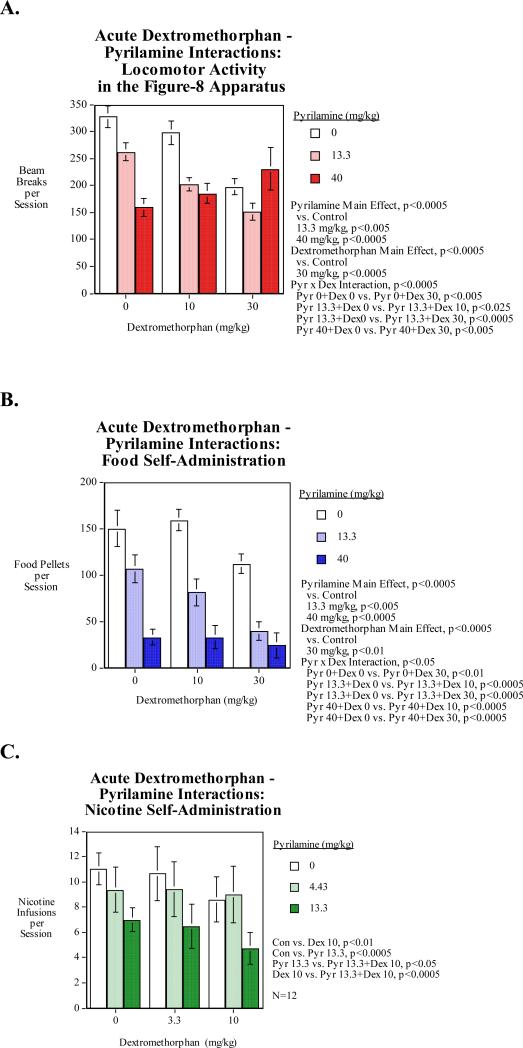
Study 2: Dextromethorphan – Pyrilamine Interactions for Reducing Nicotine SA
Locomotor activity was significantly reduced by the 13.3-mg/kg (p<0.005) and the 40-mg/kg (p<0.0005) doses of pyrilamine. The 30-mg/kg dose of dextromethorphan also significantly (p<0.0005) reduced locomotor activity relative to control. Pyrilamine significantly augmented both the lower 10-mg/kg dose and the higher 30-mg/kg dose of dextromethorphan in reducing locomotor activity by a greater margin than either dose alone (Fig. 2A). Treatment with the higher 40-mg/kg dose of pyrilamine also significantly interacted with the lower 10-mg/kg dose of dextromethorphan in decreasing locomotor activity. Both the higher 40-mg/kg dose and the lower 13.3 mg/kg dose of pyrilamine resulted in a significant decrease in locomotor activity relative to saline injection (Fig. 2A).
Figure 2. Dextromethorphan-Pyrilamine Interactions (N=15, mean±sem).
A) Locomotor activity: The dextromethorphan main effect was significant The pyrilamine main effect was significant (p<0.0005), with both the 13.3 (p<0.005) and 40 mg/kg (p<0.005) pyrilamine doses significantly decreasing locomotor activity relative to control.
B) Food motivated responding: The dextromethorphan main effect was significant (p<0.0005), with the 30 mg/kg dose significantly decreasing (p<0.01) food self-administration relative to control. The main effect of pyrilamine was significant (p<0.0005), decrease food self-administration in a dose-related manner (n=11). Both the low and high doses (p<0.005) of pyrilamine resulted in a significant decrease in food self-administration relative to saline injection. The 13.3 mg/kg dose of pyrilamine significant reductive interactions with both the low (p<0.0005) and high dose of dextromethorphan, (p<0.0005) compared to saline injection (n=11). Treatment with the high dose of pyrilamine also had significant reductive interactions with both the low dose of dextromethorphan (p<0.0005) and the high dose of dextromethorphan, (p<0.0005) compared to saline injection.
C. Nicotine self-administration: The dextromethorphan main effect was significant. Pyrilamine had a significant (p<0.001), main effect (n=11). The 13.3 mg/kg pyrilamine dose significantly (p<0.0005) decreased nicotine self-administration relative to control.
Food motivated responding was significantly reduced by both the 13.3-mg/kg (p<0.05) and the 40-mg/kg (p<0.0005) doses relative to control (Fig. 2B). The 30-mg/kg dextromethorphan dose significantly (p<0.01) reduced food motivated responding. The lower 13.3-mg/kg dose of pyrilamine significantly (p<0.0005) interacted with both the lower 10-mg/kg and the higher 30-mg/kg doses of dextromethorphan in reducing food self-administration by a greater margin than either dose alone (Fig. 5a). Treatment with the higher 40-mg/kg dose of pyrilamine also significantly (p<0.0005) interacted with both the lower 10-mg/kg and the higher 30-mg/kg doses of dextromethorphan in reducing food self-administration.
Nicotine self-administration was significantly (p<0.01) reduced by treatment with 10-mg/kg dextromethorphan relative to control. Pyrilamine at 13.3-mg/kg significantly (p<0.0005) decreased nicotine self-administration relative to control (Fig. 2C). There were mutually augmenting effects of these two drugs. The combination of dextromethorphan (10-mg/kg) and pyrilamine (13.3-mg/kg) significantly lowered nicotine self-administration relative to either 10-mg/kg of dextromethorphan alone (p<0.05) or 13.3-mg/kg of pyrilamine alone (p<0.0005).
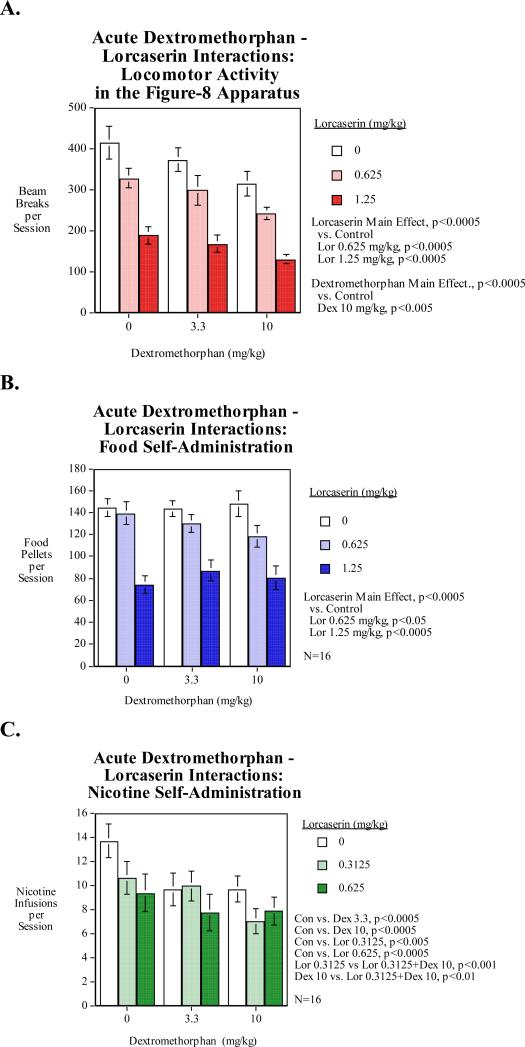
Study 3: Dextromethorphan – Lorcaserin Interactions for Reducing Nicotine SA
Significant interactions in nicotine self-administration were observed between dextromethorphan and lorcaserin. Treatment with the lower 0.3125-mg/kg dose of lorcaserin significantly (p<0.01) interacted with the higher 10-mg/kg dose of dextromethorphan in reducing nicotine self-administration with the combination more effective than either drug alone.
Both the higher 1.25-mg/kg dose and the lower 0.625-mg/kg dose of lorcaserin resulted in a significant decrease in food self-administration relative to saline injection (Fig. 3B). No significant interactions were observed between dextromethorphan and lorcaserin in food self-administration (Fig. 3B). Treatment with the lower 0.625-mg/kg dose of lorcaserin appeared to interact with both doses of dextromethorphan, but these results were not significant.
Figure 3. Dextromethorphan-Lorcaserin Interactions (N=16, mean±sem).
A) Locomotor activity: The dextromethorphan main effect was significant (p<0.0005) with the 10-mg/kg dose causing a significant (p<0.005) activitydecrease. The main effect of lorcaserin was also significant (p<0.0005) with both the 0.625-mg/kg and the 1.25-mg/kg doses causing significant decrease in locomotor activity.
B) Food motivated responding: The lorcaserin main effect was significant (p<0.0005) with the 0.625-mg/kg (p<0.05) and 1.25-mg/kg (p<0.0005) significantly decreased food motivated responding.
C) Nicotine self-administration: Both dextromethorphan doses significantly (p<0.0005) decreased nicotine self-administration. Both lorcaserin doses (0.3125-mg/kg p<0.005, 0.625 p<0.0005) also significantly reduced nicotine self-administration. The combination of 10-mg/kg of dextromethorphan and 0.3125-mg/kg of lorcaserin significantly reduced nicotine self-administration than 10-mg/kg dextromethorphan (p<0.01) or 0.3125-mg/kg lorcaserin (p<0.001) alone.
Both dextromethorphan and lorcaserin significantly decreased nicotine self-administration independently in a dose-dependent manner. Both the lower 3.3-mg/kg and the higher 10-mg/kg dose of dextromethorphan significantly decreased nicotine self-administration relative to saline injection (Fig. 3C). Both the lower 0.3125-mg/kg and the higher 0.625-mg/kg dose of lorcaserin caused significant decreases in nicotine self-administration relative to saline injection. There were significant mutually augmenting effects of dextromethorphan and lorcaserin. The combination of the higher dose of dextromethorphan (10-mg/kg) and the lower dose of lorcaserin (0.3125-mg/kg) reduced nicotine self-administration significantly more than 10-mg/kg of dextromethorphan (p<0.01) or 0.3125-mg/kg of lorcaserin (p<0.001) alone.
Discussion
Dextromethorphan was shown to cause a dose-related decrease in nicotine self-administration. This replicates the previous finding of Glick et al (Glick et al., 2001). Replicating our own previous work, we also showed that pyrilamine significantly reduced nicotine self-administration (Cousins et al., 2014; Levin et al., 2011b). Finally, replicating work from our lab and others (Higgins et al., 2012; Levin et al., 2011a), lorcaserin was shown to significantly reduce nicotine self-administration. The combinations of dextromethorphan with both pyrilamine and lorcaserin were shown to have mutually augmenting effects in significantly reducing nicotine self-administration compared with either drug alone.
The effect of dextromethorphan on nicotine self-administration was seen at a lower dose than the threshold for the effect on food self self-administration. There may be rate dependent effects when comparing dextromethorphan effects on food self-administration and nicotine self-administration. That is, dextromethorphan may have more potent effects on lower response rates for reinforcement. This could affect the interpretation of the generality of the effect of dextromethorphan but not the fact that it does effectively decrease nicotine-self-administration.
In the dextromethorphan-pyrilamine study, as has been demonstrated by our laboratory in previous studies, pyrilamine significantly reduced nicotine self-administration in a dose-dependent manner when compared to saline injection (Levin et al., 2011b) and the combination of pyrilamine and dextromethorphan was more effective in reducing nicotine self-administration than either drug alone. In the dextromethorphan-lorcaserin study, dextromethorphan was observed to significantly reduce nicotine self-administration in a dose-dependent manner in accordance with the results seen by Glick et al. (Glick et al., 2001) and the again the combination of dextromethorphan and lorcaserin was more effective than either drug alone.
Further inquiry into the use of dextromethorphan in other drug addiction treatments is warranted, as previous studies have shown that dextromethorphan may be useful in the treatment of opioid and methamphetamine addiction (Glick et al., 2001) as well as cocaine addiction (Pulvirenti et al., 1997). It should be recognized, however, that dextromethorphan has the potential for recreational abuse as a dissociative hallucinogen. Any drug addiction treatment containing dextromethorphan should not simply substitute one addictive drug for another. Future studies should investigate if rats will self-administer dextromethorphan alone and in combination with other agents to evaluate its potential for addiction, and new applications for this drug should be carefully considered. There is evidence that dextromethorphan is self-administered by rats when offered in combination with an antihistamine, whereas neither drug alone was found to support self-administration (Jun et al., 2004). However, simply because a drug is self-administration does not discount its therapeutic utility. Nicotine itself is therapeutically useful for promoting smoking cessation.
The dextromethorphan-lorcaserin study also corroborates the results of Higgins et al. (Higgins et al., 2012) in showing that lorcaserin reduces nicotine self-administration in a dose-dependent manner. Furthermore, significant interactions were observed between the 0.625-mg/kg lorcaserin dose and the 3.3-mg/kg dextromethorphan dose and between the 0.3125-mg/kg lorcaserin dose and the 10-mg/kg dextromethorphan dose. While these results are exciting and warrant further investigation into a combination therapy of dextromethorphan and lorcaserin as a novel treatment for smoking cessation and nicotine addiction, more work must first be done to further determine the relationship between these two drugs and the optimal combination of the two drug treatments. Future research should also focus on the neurological foundations of interactions between the NMDA glutamate and α3β4 nicotinic antagonism do dextromethorphan with he 5HT2C serotonin agonism of lorcaserin in the reward pathway as suggested by the observed dextromethorphan-lorcaserin interactions.
Independently, all three drugs significantly decreased locomotor activity in a dose-dependent manner when administered ten minutes prior to locomotor activity session. The results of the locomotor activity trials suggest that the 30-mg/kg dose of dextromethorphan, the 13.3-mg/kg and 40-mg/kg doses of pyrilamine, and the 0.625 and 1.25-mg/kg doses of lorcaserin cause significant hypoactivity. Since potential sedating effects are considered problematic for a potential nicotine addiction treatment and should be avoided, the investigation of the effects of lower doses of these drugs on locomotor activity may be in order. Interestingly, the 30 mg/kg dose of dextromethorphan significantly (p<0005) reduced the hypoactivity caused by 40 mg/kg of pyrilamine. Possible mechanisms for this are not readily apparent but may be related to the NMDA glutamate blocking effects of dextromethorphan. Previously, we found that the NMDA antagonist dizocilpine significantly quickened locomotor response (Levin et al., 1998).
Following the general trend observed with locomotor activity, all three drugs significantly decreased food pellet self-administration independently in a dose-dependent manner. Increased appetite and weight gain are common side effects associated with most traditional smoking cessation therapies, and novel treatment options should look to mitigate these side effects. With this in mind, the significant decreases in food self-administration observed with dextromethorphan, pyrilamine, and lorcaserin and the interactions between dextromethorphan and pyrilamine are promising for a potential smoking cessation drug. More work should be done to determine if these effects on food self-administration attenuate with longer exposure.
There may be drug-drug interactions between lorcaserin and dextromethorphan both of which are metabolized by CYP2D6 (Rodrigues and Roberts, 1997). In addition, pyrilamine has also been found to be metabolized by CYP2D6.(Hiroi et al., 1995). It not as clear what the acute interactions would be with these drugs.
Looking forward, the results of these nicotine self-administration studies suggest that dextromethorphan, pyrilamine, and lorcaserin all have great promise for potential use in treating tobacco addiction. In addition, there should be research with these drug combinations with other techniques to assess reinforcement and abuse liability of nicotine including tests of relapse prevention (drug self-administration reinstatement), conditioned place preference and drug discrimination. These tests combined with drug self-administration build a more complete understanding of the potential for new treatments for treating drug addiction. Future work should explore the interactions of pyrilamine and lorcaserin. Additional interaction studies should also be conducted on dextromethorphan with a number of other agonists and antagonists to neurotransmitter molecules in the reward pathway and in related neurological systems. The nicotinic acetylcholine receptor antagonist mecamylamine (Zevin et al., 2000) and α4β2 partial agonist sazetidine-A have (Levin et al., 2010) also been previously shown to significantly decrease nicotine self-administration independently, and are thus ideal candidates for future interaction studies. The interaction of these drug treatments with currently approved smoking cession treatments nicotine replacement, buopropion and varenicline should be assessed in the effort to improve smoking cessation efficacy.
Highlights.
Dextromethorphan decreased nicotine self-administration with a lowest effective dose of 3-mg/kg.
Dextromethorphan combined with pyrilamine lowered nicotine self-administration relative to either alone.
Dextromethorphan also has mutually augmenting effects with lorcaserin.
Combination therapy may be more effective smoking cessation treatments than monotherapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Carter BD, Abnet CC, Feskanich D, Freedman ND, Hartge P, Lewis CE, Ockene JK, Prentice RL, Speizer FE, Thun MJ, Jacobs EJ. Smoking and mortality — Beyond established causes. New England Journal of Medicine. 2015;372:631–640. doi: 10.1056/NEJMsa1407211. [DOI] [PubMed] [Google Scholar]
- Chen Z, Chen JQ, Kamei C. Effect of H1-antagonists on spatial memory deficit evaluated by 8-arm radial maze in rats. Acta Pharmacologica Sinica. 2001;22:609–613. [PubMed] [Google Scholar]
- Cousins V, Rose JE, Levin ED. Nicotine IV self-administration in rats using the consummatory operant licking response: Pharmacologic effects. Progress in Neuropsychopharmacology & Biological Psychiatry. 2014;54:200–205. doi: 10.1016/j.pnpbp.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Flood P, Ho KK, May EL, Martin BR. Effect of dextrometorphan and dextrorphan on nicotine and neuronal nicotinic receptors: in vitro and in vivo selectivity. J Pharmacol Exp Ther. 2005;312:780–785. doi: 10.1124/jpet.104.075093. [DOI] [PubMed] [Google Scholar]
- Dani JA, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16:905–908. doi: 10.1016/s0896-6273(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Burke MV, Hays JT, Hurt RD. Combination treatment with varenicline and nicotine replacement therapy. Nicotine & Tobacco Research. 2009;11:572–576. doi: 10.1093/ntr/ntp042. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Hatsukami DK, Croghan IT, Schroeder DR, Allen SS, Hays JT, Hurt RD. Combination Varenicline and Bupropion SR for Tobacco-Dependence Treatment in Cigarette Smokers: A Randomized Trial. Journal of the American Medical Association. 2014;311:155–163. doi: 10.1001/jama.2013.283185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovino GA. The tobacco epidemic in the United States. American Journal of Preventative Medicine. 2007;33:S318–S326. doi: 10.1016/j.amepre.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Dickinson HA, Kitchen BA. Comparative effects of dextromethorphan and dextrorphan on morphine, methamphetamine, and nicotine self-administration in rats. Eur J Pharmacol. 2001;422:87–90. doi: 10.1016/s0014-2999(01)01066-4. [DOI] [PubMed] [Google Scholar]
- Guy EG, Fisher DC, Higgins GA, Fletcher PJ. Examination of the effects of varenicline, bupropion, lorcaserin, or naltrexone on responding for conditioned reinforcement in nicotine-exposed rats. Behavioural Pharmacology. 2014;25:775–783. doi: 10.1097/FBP.0000000000000092. [DOI] [PubMed] [Google Scholar]
- Hall BJ, Slade S, Wells C, Rose JE, Levin ED. Bupropion-varenicline interactions and nicotine self-administration behavior in rats. Pharmacology, Biochemistry, and Behavior. 2015;130:84–89. doi: 10.1016/j.pbb.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BJ, Wells C, Allenby C, Lin M, Hao I, Marshall L, Rose JE, Levin ED. Differential effects of non-nicotine tobacco constituent compounds on nicotine self-administration in rats. Pharmacology, Biochemistry and Behavior. 2014;120:103–108. doi: 10.1016/j.pbb.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson MG, Fuller RW. Dextromethorphan antagonizes the acute depletion of brain serotonin by p-chloroamphetamine and H75/12 in rats. Brain Res. 1992;594:323–326. doi: 10.1016/0006-8993(92)91144-4. [DOI] [PubMed] [Google Scholar]
- Hernandez SC, Bertolino M, Xiao Y, Pringle KE, Caruso FS, Kellar KJ. Dextromethorphan and its metabolite dextrorphan block alpha3beta4 neuronal nicotinic receptors. The Journal of Pharmacology and Experimental Therapeutics. 2000;293:962–967. [PubMed] [Google Scholar]
- Higgins GA, Desnoyer J, Van Niekerk A, Silenieks LB, Lau W, Thevarkunnel S, Izhakova J, DeLannoy IA, Fletcher PJ, DeLay J, Dobson H. Characterization of the 5-HT2C receptor agonist lorcaserin on efficacy and safety measures in a rat model of diet-induced obesity. Pharmacology Research & Perspectives. 2015;3:e00084. doi: 10.1002/prp2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Rossmann A, Rizos Z, Noble K, Soko AD, Fletcher PJ. The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology. 2012;37:1177–1191. doi: 10.1038/npp.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi T, Ohishi N, Imaoka S, Yabusaki Y, Fukui H, Funae Y. Mepyramine, a histamine H1 receptor antagonist, inhibits the metabolic activity of rat and human P450 2D forms. Journal of Pharmacology and Experimental Therapeutics. 1995;272:939–944. [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Society for the Study of Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Johnson G, Oliver MN. Lorcaserin (Belviq) for weight loss. American Family Physician. 2014;90:863–866. [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, Fiore MC, Baker TB. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. Neuroscience. 1999;90:303–317. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Jun JH, Thorndike EB, Schindler CW. Abuse liability and stimulant properties of dextromethorphan and diphenhydramine combinations in rats. Psychopharmacology. 2004;172:277–282. doi: 10.1007/s00213-003-1650-4. [DOI] [PubMed] [Google Scholar]
- Kim DC, Yun SJ, Park YS, Jun DJ, Kim D, Jiten Singh N, Kim S, Kim KT. Pyrilamine inhibits nicotine-induced catecholamine secretion. Neurochemistry International. 2014;74:42–45. doi: 10.1016/j.neuint.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Koegelenberg CF, Noor F, Bateman ED, van Zyl-Smit RN, Bruning A, O'Brien JA, Smith C, Abdool-Gaffar MS, Emanuel S, Esterhuizen TM, Irusen EM. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: a randomized clinical trial. Journal of the American Medical Association. 2014;312:155–161. doi: 10.1001/jama.2014.7195. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bettegowda C, Weaver T, Christopher NC. Nicotine-dizocilpine interactions and working and reference memory performance of rats in the radial-arm maze. Pharmacology, Biochemistry and Behavior. 1998;61:335–340. doi: 10.1016/s0091-3057(98)00109-9. [DOI] [PubMed] [Google Scholar]
- Levin ED, Johnson J, Slade S, Wells C, Cauley M, Petro A, Rose JE. Lorcaserin decreases nicotine self-administration in female rats. Journal of Pharmacology and Experimental Therapeutics. 2011a;338:890–896. doi: 10.1124/jpet.111.183525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Xiao Y, Slade S, Cauley M, Wells D, Hampton D, Petro A, Rose JE, Brown ML, Paige MA, McDowell BE, Kellar K. Sazetidine-A, a selective α4β2 nicotinic receptor desensitizing agent and partial agonist reduces nicotine self-administration in rats. Journal of Pharmacology and Experimental Therapeutics. 2010;332:933–939. doi: 10.1124/jpet.109.162073. [DOI] [PubMed] [Google Scholar]
- Levin ED, Slade S, Wells C, Pruitt M, Cousins V, Cauley M, Petro A, Hampton D, Rose JE. Histamine H1 antagonist treatment with pyrilamine reduces nicotine self-administration in rats. Eur J Pharmacol. 2011b;650:256–260. doi: 10.1016/j.ejphar.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Maurice T, Urani A, Phan VL, Romieu P. The interaction between neuroactive steroids and the sigma1 receptor function: behavioral consequences and therapeutic opportunities. Brain Research Reviews. 2001;37:116–132. doi: 10.1016/s0165-0173(01)00112-6. [DOI] [PubMed] [Google Scholar]
- Netzer R, Pflimlin P, Trube G. Dextromethorphan blocks N-methyl-D-aspartate-induced currents and voltage-operated inward currents in cultured cortical neurons. Eur J Pharmacol. 1993;238:209–216. doi: 10.1016/0014-2999(93)90849-d. [DOI] [PubMed] [Google Scholar]
- Pulvirenti L, Balducci C, Koob GF. Dextromethorphan reduces intravenous cocaine self-administration in the rat. Eur. J. Pharmacol. 1997;321:279–283. doi: 10.1016/s0014-2999(96)00970-3. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Lorcaserin, a selective 5-HT2c receptor agonist, decreases alcohol intake in female alcohol preferring rats. Pharmacology, Biochemical and Behavior. 2014 doi: 10.1016/j.pbb.2014.07.017. in press. [DOI] [PubMed] [Google Scholar]
- Rodrigues AD, Roberts EM. The in vitro interaction of dexmedetomidine with human liver microsomal cytochrome P4502D6 (CYP2D6). Drug Metab Dispos. 1997;25:651–655. [PubMed] [Google Scholar]
- Rose JE, Behm FM. Adapting smoking cessation treatment according to initial response to precessation nicotine patch. The American Journal of Psychiatry. 2013;170:860–867. doi: 10.1176/appi.ajp.2013.12070919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Combination treatment with varenicline and bupropion in an adaptive smoking cessation paradigm. The American Journal of Psychiatry. 2014;171:1199–1205. doi: 10.1176/appi.ajp.2014.13050595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan W, Rose JE, Glicklich A, Stubbe S, Halliday D, Zhang J, Krolikowski J, Sanchez-Kam M. Lorcaserin For Smoking Cessation Treatment In Cigarette Smokers: A Randomized Phase 2 Trial.. American Thoracic Society 2015 International Conference; Denver, CO. 2015. [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. Iowa State University Press; Ames, Iowa: 1967. [Google Scholar]
- Zeeb FD, Higgins GA, Fletcher PJ. The serotonin 2C receptor agonist lorcaserin attenuates Intracranial self-stimulation and blocks the reward-enhancing effects of nicotine. ACS Chemical Neuroscience. 2015;6:1231–1240. doi: 10.1021/acschemneuro.5b00017. [DOI] [PubMed] [Google Scholar]
- Zevin S, Jacob P, Benowitz NL. Nicotine-mecamylamine interactions. Clinical Pharmacology and Therapeutics. 2000;68:58–66. doi: 10.1067/mcp.2000.108066. [DOI] [PubMed] [Google Scholar]