Abstract
Background and Aims
Vaporized cannabis and concurrent cannabis and alcohol intake are commonplace. We evaluated cannabis’ subjective effects, with and without alcohol, relative to blood and oral fluid (OF, advantageous for cannabis exposure screening) cannabinoid concentrations and OF/blood and OF/plasma vaporized-cannabinoid relationships.
Methods
Healthy adult occasional-to-moderate cannabis smokers received vaporized placebo or active cannabis (2.9% and 6.7% Δ9-tetrahydrocannabinol, THC) with or without oral low-dose alcohol (~0.065g/210L peak breath alcohol concentration [BrAC]) in a within-subjects design. Blood and OF were collected up to 8.3h post-dose and subjective effects measured at matched time points with visual-analogue scales and 5-point Likert scales. Linear mixed models evaluated subjective effects by THC concentration, BrAC, and interactions. Effects by time point were evaluated by dose-wise analysis of variance (ANOVA). OF versus blood or plasma cannabinoid ratios and correlations were evaluated in paired-positive specimens.
Results
Nineteen participants (13 men) completed the study. Blood THC concentration or BrAC significantly associated with subjective effects including “high,” while OF contamination prevented significant OF concentration associations <1.4h post-dose. Subjective effects persisted through 3.3–4.3h, with alcohol potentiating cannabis effects’ duration. Effect-versus-THC concentration and effect-versus-alcohol concentration hystereses were counterclockwise and clockwise, respectively. OF/blood and OF/plasma THC significantly correlated (all Spearman r≥0.71), but variability was high.
Conclusions
Vaporized cannabis subjective effects were similar to those previously reported after smoking, with duration extended by concurrent alcohol. Cannabis intake was identified by OF testing, but OF concentration variability limited interpretation. Blood THC concentrations were more consistent across subjects and more accurate at predicting cannabis’ subjective effects.
Keywords: Cannabis, Alcohol, Subjective, Blood, Oral Fluid
Graphical abstract
Vaporized cannabis’ subjective effects are comprehensively characterized, with and without alcohol, and cannabinoid blood and oral fluid relationships evaluated. Oral fluid significantly correlated with blood, plasma, and subjective effects, with high intersubject variability. Vaporized cannabis produced similar subjective effects to smoked, but alcohol extended cannabis’ effects duration.
Introduction
Twenty-three US states and the District of Columbia legalized medical marijuana[1], with Colorado, Washington, Oregon, and Alaska decriminalizing recreational cannabis. Smoking, the most common administration route[2], is disadvantageous as pharmacotherapy, delivering hazardous pyrolytic byproducts[3]. Volatilizing cannabinoids at sub-combustion temperatures (vaporizing) should provide similar subjective effects[4–6], with decreased pyrolytic byproducts[7–8] leading to decreased reports of respiratory symptoms[9]. However, limited clinical data are available on vaporized cannabis. As cannabis vaporization prevalence increases, it is important for clinical and forensic purposes to fully characterize subjective effects, blood and oral fluid (OF) disposition, and their relationships.
Cannabis is the most common illicit drug identified in driving under the influence (DUI) cases[10]. States with legalized medical or recreational cannabis had increased DUI-cannabis (DUIC) cases[11–12], with enforcement complicated by changing cannabis laws. Blood Δ9-tetrahydrocannabinol (THC) and its non-psychoactive metabolite (11-nor-9-carboxy-THC, THCCOOH) concentrations may provide information regarding time since last intake and cannabis consumption frequency[13–14]. However, blood collection is invasive and may be delayed 90min-4h after a DUI event[15–16]. OF, a valuable alternative sampling matrix, is non-invasively collected, more difficult to adulterate than urine, and provides information about recent intake[17–19]. Some jurisdictions already adopted OF-specific legislation for DUIC[20–22]. However, OF correlation with cannabis effects or blood concentrations is not fully understood, limiting interpretation, and thus requires evaluation. Additionally, cannabis and alcohol are often identified together in DUI cases[10], making understanding their combined effects critical for forensic interpretation.
In this vaporized cannabis and oral alcohol controlled administration study, we evaluated subjective effects and OF and blood/plasma cannabinoid concentration relationships, with and without low-dose alcohol.
Methods
This protocol was approved by the University of Iowa Institutional Review Board. The study was performed at the University of Iowa Hospitals and Clinics Clinical Research Unit (UIHC-CRU) and National Advanced Driving Simulator (NADS).
Participants
Participants were recruited from the NADS subject database and provided written informed consent for the study. Inclusion criteria were ages 21–55 years; self-reported average cannabis consumption ≥1x/3months but ≤3days/week over the past 3months (Cannabis Use Disorders Identification Test [CUDIT][23]); self-reported “light” or “moderate” alcohol consumption according to a Quantity-Frequency-Variability (QFV) scale[24]; or if “heavy”, not more than 3–4 servings in a typical drinking occasion. Exclusion criteria included past or current clinically significant medical illness; history of clinically significant adverse event associated with cannabis or alcohol intoxication; ≥450mL blood donation in 2weeks preceding drug administration; pregnant or nursing; interest in drug abuse treatment within past 60days; and currently taking drugs contraindicated with cannabis or alcohol or known to impact driving.
Study Design
Participants entered the clinical research unit 10–16h before dosing to preclude intoxication. Participants drank 90% grain alcohol (to ~0.065% peak breath alcohol concentration [BrAC][25]) mixed with juice or placebo-alcohol (juice with alcohol-swabbed rim, topped with 1mL alcohol to mimic taste and odor) ad libitum over 10min; then inhaled 500mg placebo (0.008±0.002% THC), low (2.9±0.14% THC, ~14.5mg)-, or high (6.7±0.05% THC, ~33.5mg)-dose vaporized ground bulk cannabis (210°C, Volcano® Medic, Storz & Bickel, Tuttlingen, Germany) ad libitum over 10min. Cannabis was obtained from NIDA Chemistry and Physiological Systems Research Branch. Participants received all six alcohol/cannabis combinations in randomized order, one combination per session, separated by ≥1week.
OF was collected with Quantisal™ collection devices (Immunalysis, Pomona, CA) −0.8, 0.17, 1.4, 2.3, 3.3, 4.3, 5.3, 6.3, 7.3, and 8.3h after start of cannabis dosing[26]. Devices were placed under the tongue until indicators turned blue (collecting 1.0±0.1mL OF) or for 10min maximum, and placed into the stabilizing buffer. OF was stored in Nunc® cryotubes (Thomas Scientific, Swedesboro, NJ) at 4°C for analysis within a month[27]. Oral intake was prohibited 10min prior to OF collection. Blood was collected via indwelling peripheral venous catheter into grey-top potassium oxalate/sodium fluoride Vacutainer® tubes (BD, Franklin Lakes, NY) concurrently with OF (except 4.3 and 5.3h due to blood volume limits), with a second sample centrifuged at 1600×g, 15min. Blood and plasma were stored at −20°C in 3.6mL Nunc cryotubes, and analyzed within 3months[28]. BrAC was measured by Alco-Sensor® IV (Intoximeters, St. Louis, MO), a portable breath alcohol testing device, at the same times as OF and additionally at 0.42h post-dose. It reports alcohol in g/210L breath (limit of quantification [LOQ] 0.006g/210L), equivalent to approximate blood alcohol concentration (BAC) in g/dL.
Subjective effects were measured at the same times as OF collection by 100mm visual-analogue scales (VAS; “high”, “good drug effect”, “stimulated”, “stoned”, “anxious”, “sedated”, and “restless”) anchored by “Not At All”-”Most Ever”; and 5-point (“none”, “slight”, “mild”, “moderate”, “severe”) Likert scales (“difficulty concentrating”, “altered sense of time”, “slowed or slurred speech”, “body feels sluggish/heavy”, “feel hungry”, “feel thirsty”, “shakiness/tremulousness”, “nausea”, “headache”, “palpitations”, “upset stomach”, “dizzy”, and “dry mouth or throat”).
Specimen Analysis
OF was quantified for THC, THCCOOH, cannabidiol (CBD), and cannabinol (CBN) by two-dimensional gas chromatography-mass spectrometry[29], modified by adding 0.4mL hexane to solid-phase extraction columns before loading the initial elution solvent. THC, THCCOOH, CBD and CBN linear ranges were 0.5–50μg/L, 15–500ng/L, 1–50μg/L and 1–50μg/L, respectively. Inter- and intra-assay imprecision were ≤12.3%; analytical bias, ≤14.4% (n=21). For concentrations >upper limit of quantification (LOQ), OF was diluted with drug-free Quantisal buffer. Blood and plasma cannabinoids were quantified by liquid chromatography-tandem mass spectrometry (LCMSMS)[30]. Briefly, 0.5mL blood or plasma was protein-precipitated with ice-cold acetonitrile, supernatants diluted and solid-phase extracted with Bond-Elut Plexa cartridges (Agilent Technologies, Santa Clara, CA). THC, THCCOOH, CBD, and CBN linear ranges were 1–100μg/L. Inter-assay (n=30) analytical bias and imprecision were ≤9.3% and ≤10.0%.
Data Analysis
VAS and Likert results were assessed via linear mixed models in SPSS® version 19 for Windows (IBM, Armonk, NY). Initial data review and analyses indicated insufficiently different low-versus-high cannabis-dose THC concentrations; consequently, mixed-model analyses utilized blood THC and BrAC concentrations (continuous variables), producing the best-fit models. THC, BrAC, time, THC*BrAC, time*THC, time*BrAC, and time*THC*BrAC were evaluated as fixed effects; subject*THC and intercepts as random effects (heterogeneous (1) autoregressive). Two-tailed p<0.05 indicated significance. The same analyses were conducted with OF THC concentrations, including and excluding t=0.17h. For analytical purposes, concentrations <lower LOQ were set to 0, VAS responses were converted to percentages (0–100), and Likert responses to 5-point numerical scales (0≡”None”−4≡”Severe”). Likert linear mixed models for “feel hungry” and “feel thirsty” were only evaluated through 3.3h due to lunch. Friedman’s [factorial] repeated measures analysis of variance (ANOVA, factors: cannabis, alcohol; cannabis*alcohol interaction term, pairwise post-hoc comparisons) evaluated within-subject dose differences by time point. The Greenhouse-Geisser correction was utilized for sphericity violations (Mauchly’s test). For time point analyses, the conservative Bonferroni correction was utilized for multiple comparisons (p<0.005 significance level), and Bonferroni post-hoc testing for subjective effects differences from baseline by dosing condition at each time point. OF versus blood and plasma correlations and regression comparisons were performed with GraphPad Prism®6 (La Jolla, CA). OF/blood and OF/plasma cannabinoid ratios were calculated when quantifiable data were available for both. Dose and baseline differences were calculated via ANOVA.
Results
Participants
Nineteen cannabis smokers (13 men, ages 21–37 years, 74% white) reported cannabis consumption ≥2x/month (but ≤3days/week), and last use within a week prior to admission (Table 1). One participant (13) self-reported last intake 4months ago, despite reporting overall average consumption ≥1x/3months.
Table 1.
Self-reported demographic characteristics and recent cannabis and alcohol consumption history of 19 healthy adult occasional-to-moderate cannabis smokers
| Participant | Sex | Age (years) | Race and ethnicity | BMI (kg/m2) | Alcohol intake frequency | Typical drinks per occasion | Cannabis intake frequency | Hours “stoned” on typical cannabis occasiona | Time since last cannabis consumed (days) | Amount last consumedb (joint or joint equivalent) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 23.7 | W | 24.3 | 2–3x/wk | 2–4 | 2–4x/m | 1–2 | 1 | 1 |
| 2 | F | 28.4 | AA | 23.8 | ≥4x/wk | 2–4 | 2–4x/m | 3–4 | 14 | 1 |
| 3 | M | 21.9 | W | 24.7 | 2–3x/wk | 5–6 | 2–4x/m | 1–2 | 6 | 1 |
| 4 | M | 37.8 | W | 26.1 | 2–3x/wk | 2–4 | 2–3x/wk | 1–2 | 3 | 2.5 |
| 5 | M | 26.6 | W | 21.6 | ≤1x/m | 2–4 | ≤1x/m | 1–2 | 11 | 3.5 |
| 6 | F | 26.3 | W | 20.0 | 2–3x/wk | 2–4 | 2–3x/wk | 3–4 | 1 | 0.25 |
| 7 | M | 25.8 | W | 40.6 | 2–4x/m | 2–4 | 2–3x/wk | 1–2 | 0.3 | 0.5 |
| 8 | M | 26.1 | H | 31.5 | 2–4x/m | 1–2 | 2–3x/wk | 1–2 | 3 | 1 |
| 9 | M | 23.2 | W | 19.5 | 2–3x/wk | 2–4 | 2–3x/wk | 3–4 | 2 | 1 |
| 10 | M | 23.1 | W | 23.9 | 2–4x/m | 2–4 | ≤1x/m | 1–2 | 2 | 0.25 |
| 11 | M | 32.3 | O, H | 28.9 | 2–3x/wk | 2–4 | 2–3x/wk | 1–2 | 4 | 1 |
| 12 | F | 23.4 | W | 23.3 | 2–3x/wk | 2–4 | 2–4x/m | 3–4 | 4 | 1 |
| 13 | F | 30.3 | AA | 24.1 | 2–3x/wk | 2–4 | ≤1x/m | <1 | 120 | 1 |
| 14 | M | 24.6 | W | 23.3 | 2–3x/wk | 2–4 | 2–4x/m | 1–2 | 7 | 0.8 |
| 15 | M | 21.8 | W | 32.7 | ≤1x/m | 1–2 | 2–4x/m | 1–2 | 7 | 0.13 |
| 16 | F | 21.7 | AA, W | 23.0 | 2–4x/m | 1–2 | 2–3x/wk | 1–2 | 1.1 | 1.5 |
| 17 | M | 28.7 | W | 18.3 | 2–3x/wk | 2–4 | ≤1x/m | 3–4 | 45 | 0.5 |
| 18 | M | 28.1 | W | 48.3 | 2–4x/m | 2–4 | 2–4x/m | 3–4 | 5 | 1 |
| 19 | F | 22.9 | W | 21.6 | 2–4x/m | 5–6 | 2–3x/wk | 3–4 | 1 | 1 |
| Median | 25.8 | 23.9 | 4.0 | 1.0 | ||||||
| Mean | 26.1 | 26.3 | 12.5 | 1.0 | ||||||
| StDev | 4.1 | 7.5 | 27.9 | 0.8 |
’Hours “stoned” ‘ wording originates from Cannabis Use Disorders Identification Test, source of self-reported cannabis frequency data
Cannabis amount last consumed is based on empirically-normalized joint consumption, to account for various administration routes and self-reported “sharing” between multiple individuals
Abbreviations: W, White; AA, African American; H, Hispanic or Latino; As, Asian; O, Other; AI, American Indian/Native American; StDev, standard deviation
Subjective effects
Table 2 presents linear mixed models subjective effects by THC and alcohol concentrations. The overall equation tested is represented by . Non-significant effects (p>0.05) were not included in the final model. In these models, b is the coefficient estimate for each contributing factor (negative or positive b indicates parameter decreases or increases effect, respectively). It represents a scaling factor by which each tested effect (e.g., blood THC, BrAC) can be multiplied to produce the best overall model for our data, thereby describing the contribution of each effect to the final model. Non-significant effects were not included in models (b=0). Blood THC was positively associated with “high”, “good drug effect”, “stimulated”, “stoned”, “anxious”, and “restless” (Table 2, Figure 1, Supplemental Figure 1), and feelings of altered time, “slowed/slurred speech”, “dizziness”, and “dry mouth/throat” (Table 2, Supplemental Figure 2). BrAC was positively associated with “high”, “good drug effect”, and “stimulated” and “difficulty concentrating”, “slowed/slurred speech”, and “body feels sluggish/heavy”. Most models contained negative time terms, indicating effects generally were highest immediately post-dose, decreasing over time. Significant negative THC*BrAC interactions were observed for “high”, “good drug effect”, “stoned”, “stimulated”, “anxious”, and “slowed/slurred speech”, but the first three contained additional significant positive time*THC*BrAC interactions. Supplemental Table 1 provides model results where subject covariance parameters could not be calculated (thus resultant model is less certain). Models produced from OF THC were different than for blood (Supplemental Tables 2–3). For multiple subjective effects, significant main effects for blood THC were not detected in OF when the time course included <1.4h; but “high”, “good drug effect”, “anxious”, “stimulated”, “stoned”, “altered sense of time”, “feel thirsty”, and “dry mouth/throat” had significant main OF effects for models that only included times ≥1.4h, after oromucosal contamination cleared. For “anxious” and “sedated”, significant (but small) OF THC*time effects were present but blood THC*time effects were not significant. Several models (“good drug effect”, “high”, “stimulated”, “stoned”, “difficulty concentrating”, “altered sense of time”, “body feels sluggish/heavy”, “feel thirsty”, “dry mouth/throat”) had significant THC*time interactions common to blood and OF.
Table 2.
Overall effect of blood Δ9-tetrahydrocannabinol (THC) concentration (μg/L), breath alcohol concentration (BrAC, g/210L), time, and interactions (THC*BrAC, time*THC, time*BrAC, time*THC*BrAC) on Visual analogue (VAS) or Likert scales subjective effects in 19 occasional-to-moderate cannabis smokers following controlled vaporized cannabis administration with and without oral alcohol.
| Parameter | b | SEb | df | t | pa | 95% Confidence Interval of b | |
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| VAS Anxious | |||||||
| Intercept | 6.777 | 1.294 | 47.2 | 5.2 | <0.001 | 4.174 | 9.380 |
| Blood THC | 0.307 | 0.076 | 19.8 | 4.0 | 0.001 | 0.147 | 0.466 |
| BrAC | 630.5 | 0.2 | 0.826 | ||||
| Time | −0.615 | 0.156 | 634.0 | −4.0 | <0.001 | −0.921 | −0.309 |
| THC*BrAC | −4.204 | 1.090 | 639.0 | −3.9 | <0.001 | −6.344 | −2.065 |
| Time*THC | 489.0 | 1.7 | 0.085 | ||||
| Time*BrAC | 632.3 | 0.6 | 0.525 | ||||
| Time*THC*BrAC | 628.9 | 1.5 | 0.125 | ||||
| Subject Variance in Intercepts (THC) | 17.188 | 6.483 | 0.008 | 8.206 | 36.000 | ||
| Subject variance in Slopes (THC) | 0.086 | 0.035 | 0.014 | 0.039 | 0.190 | ||
| ARH1 rho (slope-intercept covariance) | 0.316 | ||||||
| VAS Good Drug Effect | |||||||
| Intercept | 20.542 | 2.942 | 27.2 | 7.0 | <0.001 | 14.507 | 26.578 |
| Blood THC | 0.488 | 0.088 | 27.2 | 5.5 | <0.001 | 0.307 | 0.668 |
| BrAC | 249.443 | 46.260 | 637.8 | 5.4 | <0.001 | 158.603 | 340.283 |
| Time | −3.150 | 0.251 | 643.0 | −12.6 | <0.001 | −3.643 | −2.658 |
| THC*BrAC | −8.023 | 1.755 | 649.1 | −4.6 | <0.001 | −11.470 | −4.577 |
| Time*THC | 0.764 | 0.105 | 624.7 | 7.3 | <0.001 | 0.558 | 0.971 |
| Time*BrAC | 640.0 | −1.8 | 0.079 | ||||
| Time*THC*BrAC | 12.821 | 5.427 | 632.8 | 2.4 | 0.018 | 2.164 | 23.478 |
| Subject Variance in Intercepts (THC) | 126.563 | 44.553 | 0.005 | 63.485 | 252.318 | ||
| Subject variance in Slopes (THC) | 0.085 | 0.038 | 0.024 | 0.036 | 0.203 | ||
| ARH1 rho (slope-intercept covariance) | 0.276 | 0.250 | 0.269 | −0.242 | 0.672 | ||
| VAS High | |||||||
| Intercept | 21.541 | 3.016 | 27.3 | 7.1 | <0.001 | 15.356 | 27.726 |
| Blood THC | 0.552 | 0.091 | 24.5 | 6.1 | <0.001 | 0.364 | 0.740 |
| BrAC | 119.404 | 48.271 | 639.8 | 2.5 | 0.014 | 24.614 | 214.193 |
| Time | −3.394 | 0.262 | 645.9 | −13.0 | <0.001 | −3.908 | −2.879 |
| THC*BrAC | −7.440 | 1.823 | 652.2 | −4.1 | <0.001 | −11.020 | −3.861 |
| Time*THC | 0.829 | 0.108 | 558.3 | 7.7 | <0.001 | 0.617 | 1.042 |
| Time*BrAC | 642.5 | −0.9 | 0.375 | ||||
| Time*THC*BrAC | 22.343 | 5.665 | 633.4 | 3.9 | <0.001 | 11.220 | 33.467 |
| Subject Variance in Intercepts (THC) | 131.518 | 46.704 | 0.005 | 65.570 | 263.792 | ||
| Subject variance in Slopes (THC) | 0.093 | 0.043 | 0.029 | 0.038 | 0.229 | ||
| ARH1 rho (slope-intercept covariance) | 0.588 | 0.190 | 0.002 | 0.105 | 0.847 | ||
| VAS Restless | |||||||
| Intercept | 11.466 | 2.266 | 36.6 | 5.1 | <0.001 | 6.873 | 16.059 |
| Blood THC | 0.156 | 0.064 | 25.4 | 2.4 | 0.022 | 0.024 | 0.288 |
| BrAC | 635.9 | 0.1 | 0.903 | ||||
| Time | 643.2 | −0.2 | 0.860 | ||||
| THC*BrAC | 648.3 | −1.5 | 0.136 | ||||
| Time*THC | 593.6 | 0.8 | 0.436 | ||||
| Time*BrAC | 638.2 | −0.1 | 0.952 | ||||
| Time*THC*BrAC | 623.8 | 0.8 | 0.439 | ||||
| Subject Variance in Intercepts (THC) | 63.440 | 22.696 | 0.005 | 31.467 | 127.903 | ||
| Subject variance in Slopes (THC) | 0.146 | ||||||
| ARH1 rho (slope-intercept covariance) | 0.549 | ||||||
| VAS Sedated | |||||||
| Intercept | 17.942 | 2.893 | 28.9 | 6.2 | <0.001 | 12.023 | 23.860 |
| Blood THC | 15.9 | 0.2 | 0.879 | ||||
| BrAC | 632.9 | 0.0 | 0.984 | ||||
| Time | −1.444 | 0.253 | 639.0 | −5.7 | <0.001 | −1.942 | −0.947 |
| THC*BrAC | 647.7 | −0.4 | 0.701 | ||||
| Time*THC | 593.0 | 1.6 | 0.119 | ||||
| Time*BrAC | 634.8 | 1.3 | 0.186 | ||||
| Time*THC*BrAC | 627.4 | 0.1 | 0.941 | ||||
| Subject Variance in Intercepts (THC) | 120.808 | 41.818 | 0.004 | 61.298 | 238.091 | ||
| Subject variance in Slopes (THC) | 0.149 | 0.074 | 0.043 | 0.057 | 0.392 | ||
| ARH1 rho (slope-intercept covariance) | −0.749 | 0.118 | <0.001 | −0.905 | −0.417 | ||
| VAS Stimulated | |||||||
| Intercept | 21.682 | 2.838 | 27.1 | 7.6 | <0.001 | 15.860 | 27.503 |
| Blood THC | 0.297 | 0.080 | 19.2 | 3.7 | 0.001 | 0.130 | 0.464 |
| BrAC | 168.759 | 43.163 | 633.6 | 3.9 | <0.001 | 84.000 | 253.518 |
| Time | −2.827 | 0.232 | 645.0 | −12.2 | <0.001 | −3.284 | −2.371 |
| THC*BrAC | −3.620 | 1.624 | 652.8 | −2.2 | 0.026 | −6.809 | −0.431 |
| Time*THC | 0.568 | 0.097 | 611.4 | 5.8 | <0.001 | 0.377 | 0.759 |
| Time*BrAC | −60.558 | 23.436 | 637.7 | −2.6 | 0.010 | −106.58 | −14.536 |
| Time*THC*BrAC | 628.8 | 1.5 | 0.133 | ||||
| Subject Variance in Intercepts (THC) | 120.357 | 41.508 | 0.004 | 61.223 | 236.608 | ||
| Subject variance in Slopes (THC) | 0.069 | 0.037 | 0.060 | 0.024 | 0.196 | ||
| ARH1 rho (slope-intercept covariance) | 0.469 | 0.214 | 0.028 | −0.028 | 0.780 | ||
| VAS Stoned | |||||||
| Intercept | 19.446 | 2.790 | 28.8 | 7.0 | <0.001 | 13.737 | 25.154 |
| Blood THC | 0.398 | 0.109 | 18.1 | 3.7 | 0.002 | 0.170 | 0.627 |
| BrAC | 630.2 | 1.2 | 0.236 | ||||
| Time | −2.875 | 0.254 | 634.7 | −11.3 | <0.001 | −3.374 | −2.375 |
| THC*BrAC | −5.502 | 1.780 | 640.5 | −3.1 | 0.002 | −8.997 | −2.007 |
| Time*THC | 0.687 | 0.107 | 634.4 | 6.4 | <0.001 | 0.477 | 0.896 |
| Time*BrAC | 632.1 | −0.7 | 0.489 | ||||
| Time*THC*BrAC | 19.712 | 5.496 | 625.9 | 3.6 | <0.001 | 8.919 | 30.505 |
| Subject Variance in Intercepts (THC) | 109.037 | 38.954 | 0.005 | 54.135 | 219.620 | ||
| Subject variance in Slopes (THC) | 0.159 | 0.073 | 0.030 | 0.065 | 0.392 | ||
| ARH1 rho (slope-intercept covariance) | 0.631 | ||||||
| Likert Difficulty Concentrating | |||||||
| Intercept | 0.554 | 0.111 | 27.3 | 5.0 | <0.001 | 0.327 | 0.781 |
| Blood THC | 21.8 | 1.9 | 0.077 | ||||
| BrAC | 5.151 | 1.672 | 645.3 | 3.1 | 0.002 | 1.868 | 8.434 |
| Time | −0.062 | 0.009 | 652.2 | −6.9 | <0.001 | −0.080 | −0.044 |
| THC*BrAC | 657.5 | 0.4 | 0.658 | ||||
| Time*THC | 0.012 | 0.004 | 441.4 | 3.2 | 0.001 | 0.005 | 0.019 |
| Time*BrAC | 648.6 | −0.9 | 0.390 | ||||
| Time*THC*BrAC | 638.0 | 0.5 | 0.611 | ||||
| Subject Variance in Intercepts (THC) | 0.183 | 0.063 | 0.004 | 0.093 | 0.358 | ||
| Subject variance in Slopes (THC) | 0.000 | 0.000 | 0.019 | 0.000 | 0.000 | ||
| ARH1 rho (slope-intercept covariance) | 0.787 | 0.130 | <0.001 | 0.373 | 0.940 | ||
| Likert Altered Sense of Time | |||||||
| Intercept | 0.406 | 0.106 | 29.6 | 3.8 | 0.001 | 0.190 | 0.623 |
| Blood THC | 0.010 | 0.004 | 18.5 | 2.6 | 0.018 | 0.002 | 0.018 |
| BrAC | 652.8 | 1.2 | 0.227 | ||||
| Time | −0.059 | 0.009 | 656.0 | −6.3 | <0.001 | −0.078 | −0.041 |
| THC*BrAC | 603.0 | −0.2 | 0.806 | ||||
| Time*THC | 0.013 | 0.003 | 83.3 | 3.7 | <0.001 | 0.006 | 0.020 |
| Time*BrAC | 654.0 | 0.9 | 0.386 | ||||
| Time*THC*BrAC | 645.5 | 0.1 | 0.917 | ||||
| Subject Variance in Intercepts (THC) | 0.159 | 0.055 | 0.004 | 0.080 | 0.314 | ||
| Subject variance in Slopes (THC) | 0.000 | 0.000 | 0.021 | 0.000 | 0.000 | ||
| ARH1 rho (slope-intercept covariance) | 0.987 | 0.047 | <0.001 | −0.807 | 1.000 | ||
| Likert Slowed/Slurred Speech | |||||||
| Intercept | 0.163 | 0.071 | 32.4 | 2.3 | 0.030 | 0.017 | 0.308 |
| Blood THC | 0.008 | 0.003 | 20.2 | 2.7 | 0.015 | 0.002 | 0.014 |
| BrAC | 3.774 | 1.280 | 647.3 | 2.9 | 0.003 | 1.261 | 6.287 |
| Time | −0.028 | 0.007 | 652.8 | −4.1 | <0.001 | −0.042 | −0.015 |
| THC*BrAC | −0.095 | 0.048 | 650.5 | −2.0 | 0.049 | −0.189 | −0.001 |
| Time*THC | 0.009 | 0.003 | 210.0 | 3.6 | <0.001 | 0.004 | 0.015 |
| Time*BrAC | 649.9 | 0.1 | 0.903 | ||||
| Time*THC*BrAC | 641.5 | 1.2 | 0.228 | ||||
| Subject Variance in Intercepts (THC) | 0.068 | 0.024 | 0.005 | 0.034 | 0.136 | ||
| Subject variance in Slopes (THC) | 0.000 | 0.000 | 0.015 | 0.000 | 0.000 | ||
| ARH1 rho (slope-intercept covariance) | 0.912 | 0.075 | <0.001 | 0.585 | 0.984 | ||
| Likert Body Feels Sluggish/Heavy | |||||||
| Intercept | 0.600 | 0.101 | 34.5 | 6.0 | <0.001 | 0.396 | 0.805 |
| Blood THC | 28.8 | 1.6 | 0.115 | ||||
| BrAC | 4.568 | 1.936 | 645.0 | 2.4 | 0.019 | 0.767 | 8.369 |
| Time | −0.066 | 0.010 | 651.1 | −6.3 | <0.001 | −0.087 | −0.046 |
| THC*BrAC | 658.3 | 0.0 | 0.965 | ||||
| Time*THC | 0.015 | 0.004 | 558.1 | 3.6 | <0.001 | 0.007 | 0.024 |
| Time*BrAC | 647.6 | 0.4 | 0.691 | ||||
| Time*THC*BrAC | 638.7 | 0.0 | 0.993 | ||||
| Subject Variance in Intercepts (THC) | 0.127 | 0.046 | 0.006 | 0.062 | 0.259 | ||
| Subject variance in Slopes (THC) | 0.000 | 0.000 | 0.038 | 0.000 | 0.000 | ||
| ARH1 rho (slope-intercept covariance) | 0.322 | ||||||
| Likert Feel Thirsty | |||||||
| Intercept | 0.728 | 0.166 | 64.2 | 4.4 | <0.001 | 0.396 | 1.059 |
| Blood THC | 45.3 | −0.1 | 0.949 | ||||
| BrAC | 419.1 | −0.1 | 0.944 | ||||
| Time | 432.8 | 0.9 | 0.377 | ||||
| THC*BrAC | 433.4 | 0.6 | 0.524 | ||||
| Time*THC | 0.083 | 0.014 | 429.9 | 6.1 | <0.001 | 0.057 | 0.110 |
| Time*BrAC | 6.077 | 1.836 | 416.1 | 3.3 | 0.001 | 2.468 | 9.687 |
| Time*THC*BrAC | 412.8 | −1.3 | 0.181 | ||||
| Subject Variance in Intercepts (THC) | 0.236 | 0.091 | 0.009 | 0.111 | 0.501 | ||
| Subject variance in Slopes (THC) | 0.000 | 0.000 | 0.135 | 0.000 | 0.000 | ||
| ARH1 rho (slope-intercept covariance) | 0.407 | ||||||
| Likert Dizzy | |||||||
| Intercept | 0.125 | 0.040 | 55.1 | 3.1 | 0.003 | 0.045 | 0.206 |
| Blood THC | 0.007 | 0.002 | 25.9 | 2.8 | 0.009 | 0.002 | 0.011 |
| BrAC | 646.1 | 1.5 | 0.141 | ||||
| Time | −0.017 | 0.005 | 651.9 | −3.2 | 0.001 | −0.027 | −0.006 |
| THC*BrAC | 656.1 | −1.8 | 0.065 | ||||
| Time*THC | 144.2 | 1.0 | 0.318 | ||||
| Time*BrAC | 649.3 | −0.4 | 0.717 | ||||
| Time*THC*BrAC | 645.6 | −0.1 | 0.899 | ||||
| Subject Variance in Intercepts (THC) | 0.014 | 0.006 | 0.012 | 0.007 | 0.032 | ||
| Subject variance in Slopes (THC) | 0.000 | 0.000 | 0.006 | 0.000 | 0.000 | ||
| ARH1 rho (slope-intercept covariance) | 0.829 | 0.147 | <0.001 | 0.257 | 0.971 | ||
| Likert Dry Mouth or Throat | |||||||
| Intercept | 0.917 | 0.131 | 34.1 | 7.0 | <0.001 | 0.651 | 1.183 |
| Blood THC | 0.008 | 0.003 | 20.5 | 2.3 | 0.034 | 0.001 | 0.015 |
| BrAC | 646.3 | −0.8 | 0.414 | ||||
| Time | −0.120 | 0.014 | 654.5 | −8.7 | <0.001 | −0.147 | −0.093 |
| THC*BrAC | 624.2 | −1.9 | 0.057 | ||||
| Time*THC | 0.033 | 0.006 | 399.8 | 5.8 | <0.001 | 0.022 | 0.044 |
| Time*BrAC | 649.1 | 1.4 | 0.157 | ||||
| Time*THC*BrAC | 1.308 | 0.301 | 626.2 | 4.3 | <0.001 | 0.716 | 1.900 |
| Subject Variance in Intercepts (THC) | 0.211 | 0.079 | 0.007 | 0.102 | 0.438 | ||
| Subject variance in Slopes (THC) | 0.212 | ||||||
| ARH1 rho (slope-intercept covariance) | 0.147 | ||||||
Data from 19 healthy, adult cannabis smokers who participated in all dosing sessions. Subjective effects were measured by 100 mm VAS or 5-point Likert scales with choices 0≡”none”, 1≡”slight”, 2≡”mild”, 3≡”moderate”, 4≡”severe” after drinking placebo or active alcohol (calculated to produce approximate peak 0.065% BrAC) and inhaling placebo, 2.9% THC, or 6.7% THC vaporized cannabis (500 mg, Volcano® Medic vaporizer).
Linear mixed model results; b is parameter (coefficient) estimate for each factor (negative b indicates the parameter decreases the subjective effect; positive b indicates the parameter increases the overall effect).
Overall equation:
Values in bold are statistically significant (p<0.05); only significant predictors are considered in the final model.
Abbreviations: SE, standard error; df, degrees of freedom; VAS, 100mm visual-analogue scale; Likert, 5-point Likert scale; THC, Δ9-tetrahydrocannabinol; BrAC, breath alcohol concentration.
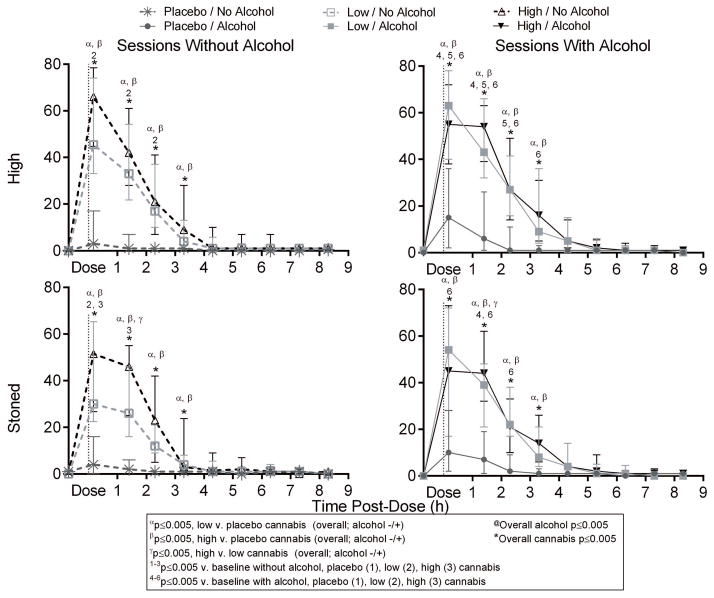
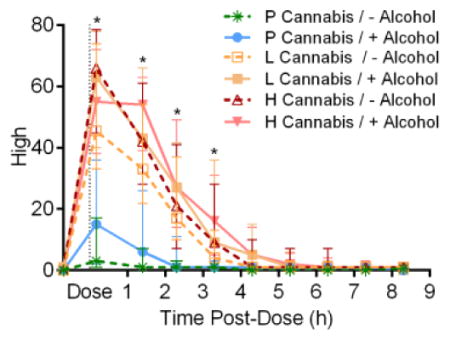
Figure 1.
Median [interquartile range] “high” and “stoned” visual-analogue scales (VAS) results versus time in 19 participants after low (2.9% THC) and high (6.7% THC) vaporized cannabis doses with and without low-dose oral alcohol. All VAS were out of 100.
All active-drug interventions were positively associated with subjective “good drug effect” 0.17 and 1.4h post-dose relative to baseline (time-point analyses, Supplemental Figure 1). Although alcohol only displayed a significant main dose effect at 0.17h, significant increases from baseline persisted 3.3 and 4.3h with combined cannabis and alcohol. Both low (2.9%-THC) and high (6.7%-THC) cannabis doses were positively associated with “high”, “good drug effect”, “stimulated”, and “stoned” over the first 3.3h (Figure 1 and Supplemental Figure 1). Significant alcohol-dose effects were detected 0.17h after cannabis dosing initiation (0.24h after drinking initiation) for “good drug effect” and “stimulated”. We observed only two significant low-versus-high cannabis differences by time point: “stoned” 1.4h post-dose and “anxious” 0.17h post-dose. Significant cannabis effects on “sedated” occurred at time points 2.3–4.3h post-dose. Cannabis also affected “altered sense of time” (1.7–2.3h), “feel thirsty” (0.17–2.3h), and “dry mouth/throat” (0.17–3.3h) (Supplemental Figure 2). Subjective effects versus blood and OF THC concentrations displayed counterclockwise hysteresis; whereas subjective effects versus BrAC showed clockwise hysteresis (Figure 2, Supplemental Figure 3).
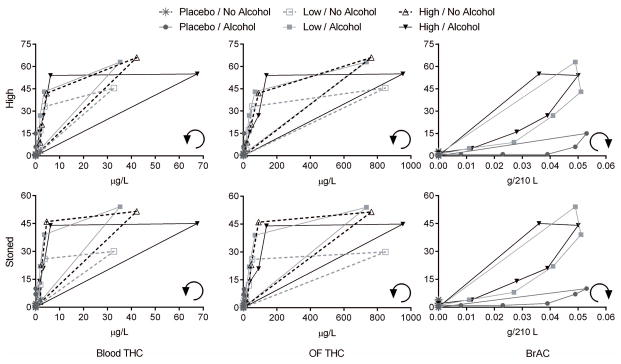
Figure 2.
Median “high” and “stoned” visual-analogue scales (VAS) results versus median blood Δ9-tetrahydrocannabinol (THC) concentrations, oral fluid (OF) THC, and breath alcohol concentration (BrAC) in 19 participants after placebo, low (2.9% THC) and high (6.7% THC) vaporized cannabis doses with and without low-dose oral alcohol. Counterclockwise and clockwise arrows represent hysteresis curve progressions over time.
OF/Blood and OF/Plasma
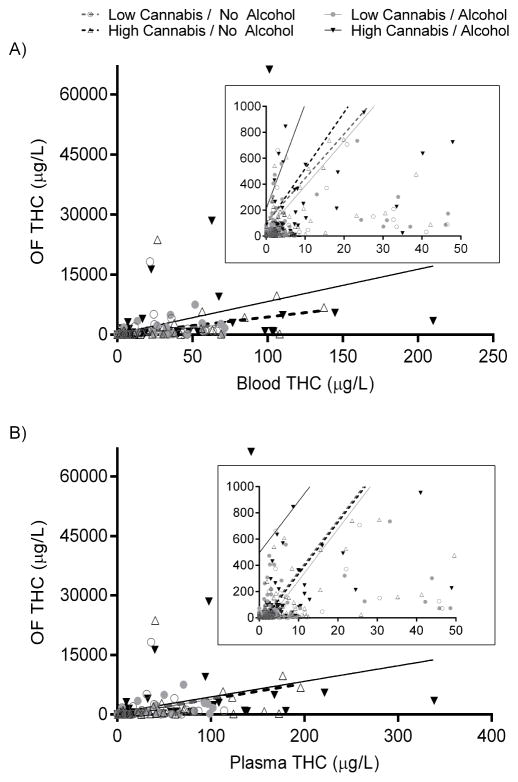
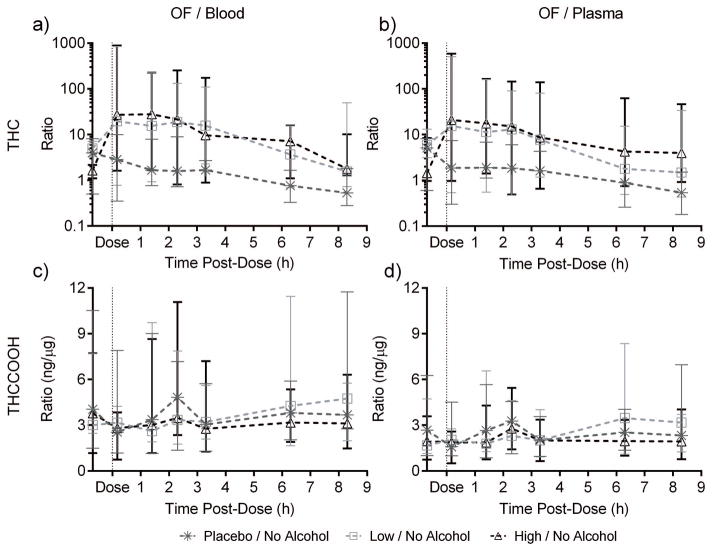
OF/blood and OF/plasma ratios showed large variability. Median [range] paired-positive OF/blood ratios were 9.4 [0.3–887, N=413] THC and 3.7 [0.6–20.9]ng/μg, N=339] THCCOOH (Supplemental Table 4). Median [range] OF/plasma ratios were 7.3 [0.2–585, N=455] THC and 2.4 [0.4–13.3]ng/μg, N=341] THCCOOH. Paired-positive CBD and CBN specimens occurred only 0.17h post-dose (9–12 pairs) and showed high variability. OF THC concentration significantly correlated (p<0.001) with blood THC concentration (Figure 3, Spearman r [95%CI]=0.7469 [0.6574–0.8156] and 0.8057 [0.7339–0.8598] for low- and high-dose cannabis without alcohol, r=0.7321 [0.6389–0.8042] and 0.8447 [0.7858–0.8884] for cannabis with alcohol) and with plasma THC (Spearman r≥0.7066 in either matrix for every dose) (Supplemental Table 5). Alcohol presence did not significantly affect ratios. Due to high variability, the only significant dose effect by time point was an overall cannabis effect on OF/plasma 8.3h post-dose (Figure 4). Ratio differences between time points could not be statistically evaluated because ratio variability was high with few paired-positives (Figure 4, Supplemental Table 4).
Figure 3.
Oral fluid (OF) Δ9-tetrahydrocannabinol (THC) concentrations versus blood (A) and plasma (B) THC, and least-squares linear regressions from 19 participants after low (2.9% THC) and high (6.7% THC) vaporized cannabis doses with and without low-dose oral alcohol. Insets illustrate (zoom) densest regions; note graph scales. OF significantly correlated (p<0.001) with blood and plasma (Spearman r≥0.7066 in either matrix for every dose). See Supplemental Table 5 for regression equations and comparisons.
Figure 4.
Median [range] oral fluid (OF)/blood and OF/plasma Δ9-tetrahydrocannabinol (THC) and 11-nor-9-carboxy-THC (THCCOOH) ratios over time in paired-positive specimens from 19 participants after low (2.9% THC) and high (6.7% THC) vaporized cannabis doses.
Discussion
Blood THC concentration after vaporization was significantly and positively associated with subjective effects (Table 2), while there generally was no significant differentiation between effects of low (2.9% THC) and high (6.7% THC) dose cannabis. This is consistent with pharmacokinetic results from these participants[26, 31], and supports previous findings that THC concentrations are a better predictor of subjective effects than cannabis dose[32]. Observed effect sizes (represented by coefficient b) for most Likert measures generally were much lower than VAS for the same factors, possibly because of the shorter Likert measurement scale. Blood THC concentration was not significantly associated with “sedated” in the overall linear mixed model, although time point dose-wise ANOVA showed significant increases over 1.4–4.4h (Supplemental Figure 1). This may result from higher variability and less-consistent results throughout the time course, or possibly other study procedures (e.g., simulated driving). “High”, “good drug effect”, and “stimulated” are likely desirable effects for recreational intake, whereas “anxious” and “restless” are likely undesirable. “Stoned” and “sedated” could be either, but would be undesirable for pharmacotherapy. Vaporized cannabis significantly increased these measures immediately post-dose, lasting 3.3 or 4.3h. “Anxious” showed significant cannabis-dose effects through 1.4h. Undesirable effects including “feel thirsty” and “dry mouth/throat” increased for the first 3.3h post-cannabis. “Difficulty concentrating” and “altered sense of time” produced mixed effects over 2.3h. Only time significantly increased “feel hungry” in the hours prior to lunch, unexpectedly with no significant THC effect. Another study found cannabis significantly increased “feel hungry” relative to baseline on a 5-point Likert scale after smoking a 6.8% THC cigarette[33]; however, as there was no placebo, possibly the observed effect was due to time since last eating.
There is growing interest in correlating cannabis’ subjective effects directly to OF THC concentrations, due to OF advantages as a sampling matrix[17–19]. However, our results indicate caution in interpreting effects from OF concentrations. Unlike blood models, OF regression models (full time course, Supplemental Table 3) had low b-values even when main effects or interactions were statistically significant, probably due to high inter-individual variability in OF THC concentrations and a time course influenced by OF oral contamination rather than systemic cannabinoid concentrations[17–18]. THC concentrations after active doses ranged from 22.7–66,200μg/L[26]. OF THC b-values represented concentration coefficients, so b in the thousandths (order of magnitude) would indicate clinically significant effects for OF THC>1000μg/L. Considering only times ≥1.4h post-dose (Supplemental Table 2) produced models with more robust significant OF main effects, as initial OF contamination decreased. However, active-dose OF THC concentrations still ranged ~1000-fold, 3.0–3940μg/L at 1.4h and 1.6–1541μg/L at 2.3h. The ≥1000-fold concentration differences impose challenges to reliably assess effects based on OF; blood THC at 0.17, 1.4, and 2.4h ranged only 11.4–210μg/L, 0–18.4μg/L, and 0–9.6μg/L, respectively. Additionally, this may account for the high variability of OF/blood and OF/plasma THC ratios (Figure 4), although the influence of OF contamination should be greatest immediately post-inhalation. In other words, OF did not closely track blood or plasma THC changes during this 8.3h time course. Overall, OF THC concentrations were not reliable indices of blood and plasma THC concentration, accounting for the former’s weak association with subjective effects. The relationship between subjective effects and blood or OF THC concentration showed counterclockwise hysteresis (Figure 2), consistent with previous findings[33]. During cannabis inhalation, maximum blood and OF THC concentrations (Cmax) occurred immediately prior to last inhalation, then decreased rapidly[34], while peak subjective effects occur over the first 2h[32, 35–37]. Subjective effects are related to brain THC concentration, with THC equilibration time in brain accounting for the lag between blood THC Cmax and maximum subjective effects[38]. Blood THC rapidly decreases during distribution to highly-perfused and adipose tissues[39], producing maximum subjective effects after blood tmax, explaining the counterclockwise hysteresis. In contrast, alcohol’s slower absorption and later Cmax[26] led to observed clockwise hysteresis. Clockwise hysteresis may be caused by tachyphylaxis (acute tolerance to an effect happening within a single dose time course, possibly due to receptor down-regulation) or feedback regulation[40].
BrAC was significantly associated, albeit not robustly, with “good drug effect”, “high”, and “stimulated”. The THC*BrAC interaction was less-than-additive (i.e., significant negative interaction term), suggesting that THC+BrAC effects were less than the sum of each individual substance effect (i.e., partial mitigation of simple main effects). However, models for several subjective effects (“high”, “good drug effect”, “stoned”) included positive time*THC*BrAC interaction terms that yielded overall approximately-additive THC+BrAC effects immediately post-dose and more-than-additive (synergistic) effects as time progressed, prolonging subjective effects. Significant increases from baseline persisting in these effects longer in cannabis-alcohol combinations (extending effects beyond those of either drug alone) corroborate this finding (Figure 1, Supplemental Figure 1).
Alcohol-alone produced hystereses shifted lower than curves for cannabis+alcohol combinations (Figure 2), indicating that participants experienced more effects after alcohol combined with active cannabis compared to alcohol-alone. Low- and high-dose cannabis combined with alcohol produced superimposed curves for “high”, “good drug effect”, “stimulated”, and “stoned”, suggesting no dose differential in cannabis effects when combined with alcohol, although individual variability was high (e.g., Supplemental Figure 4 subjective “high” (N=19)). A previous study found similar variability in individual hysteresis curves[33], albeit with just one dosing condition. Only high-THC cannabis combined with alcohol produced substantially higher blood THC Cmax. This possibly resulted from increased THC-absorption rates during inhalation (due to alcohol-induced increased cardiac output[41] and pulmonary capillary flow) or less-careful cannabis self-titration during alcohol intoxication.
Vaporized cannabis produced subjective effects and time courses similar to smoking, consistent with prior findings[33, 35]. Few studies examined combined cannabis-alcohol subjective effects[32, 42–44], and none as comprehensively as reported herein. In one study, mean subjective “high” post-cannabis intake did not significantly increase with prior alcohol relative to without[42]. Although alcohol-only increased subjective cannabis-specific “high” (corroborating our findings (Figure 1)), overall, participants correctly distinguished cannabis’ from ethanol’s “high”. Participants who drank alcohol before cannabis smoking also were aware of this distinction[32]: subjective “drunkenness” was dominant before smoking, subjective [cannabis] “high” thereafter. Alcohol pretreatment significantly decreased latency to smoked-cannabis effects and increased euphoria duration[44]. In the current study, subjective effects significantly >baseline persisted longer post-cannabis dosing with alcohol than post-cannabis dosing without alcohol (“high”, “good drug effect”, “stimulated”, “stoned”, “sedated”, “difficulty concentrating”, “dry mouth/throat”).
Prior studies directly compared THC and THCCOOH relationships between OF and blood[45–46] or OF and plasma/serum[19, 47–51]. However, few included concurrent alcohol administration[49], and none provided within-subject blood and plasma data. Plasma is more commonly used for clinical and pharmacokinetic purposes, but blood is more common in forensic settings. A forensic OF-blood THC linear regression study in suspected drugged drivers had negligible (albeit statistically significant) correlation (R2=0.030)[46], likely caused by high variability in time since last intake and unknown food or drink ingestion. Our controlled-administration fits were stronger for all doses (Supplemental Table 3), and we observed higher correlations (Spearman r=0.7321–0.8447 among all active-cannabis conditions). However, consistent with prior research, we observed high variability in OF/blood and OF/plasma ratios (Figure 4), particularly for THC. Recently reported OF/serum THC ratios showed similar ranges[49], reiterating that OF/blood or OF/serum ratios are too variable to predict one concentration from the other[51–52]. Recently, 44 [95%CI 27–90]μg/L OF THC produced the same cannabis driving prevalence as 1μg/L blood THC[53], but as we showed, there is too much variability to predict blood or oral fluid THC from the other matrix concentration. OF retains its value in identifying recent cannabis exposure[46], but is more limited in predicting cannabis effects. There were no significant alcohol effects on OF/blood or OF/plasma THC (consistent with other findings[49]).
Our study found narrower OF/blood and OF/plasma THCCOOH ratio ranges because THCCOOH enters OF from systemic circulation rather than oromucosal contamination. THCCOOH was not always detected in OF in this occasional-to-moderate-smokers cohort and, when present, was in low ng/L concentrations. OF THCCOOH distinguishes passive environmental smoke exposure from cannabis intake[54] [although chronic passive exposure was not studied][26]. Alcohol did not affect OF/blood or OF/plasma THCCOOH. THCCOOH is non-psychoactive and cannot be related to subjective effects. Its value remains as a cannabis use marker. Although OF CBD and CBN persisted for hours due to oral contamination[26], they were not present in blood and plasma after 0.42h. When present, these markers help identify recent intake, but are more likely to be detected in OF than blood in forensic settings, where blood collection lag times often exceed detection windows[15–16].
Study strengths and limitations
This is the most comprehensive evaluation of which we are aware of vaporized cannabis subjective effects time courses, with and without alcohol. We observed significant cannabis subjective effects for most measures through 3.3 or 4.3h. Our robust within-subjects design, evaluation of multiple subjective effects utilizing two different types of measurement scales, and concentration-based linear mixed models approach provided in-depth analyses of cannabis, alcohol, and interaction effects over time, also comparing blood and OF concentrations. Study limitations include lack of an explicit “bad drug effect” measure, although we did measure potential negative side effects (“anxious”, “difficulty concentrating”, “body feels sluggish/heavy”), and exclusion of frequent cannabis users (>3x/week) as participants. The latter may limit the external validity of our findings, as a prior study found different subjective effects patterns in frequent versus occasional cannabis smokers[45]. To our knowledge, only one other study compared OF/serum THC concentrations after controlled vaporized cannabis in frequent smokers[19]. Authors found similar broad variability in OF/serum THC, but did not report OF/serum THCCOOH ratios.
Conclusion
We delineated subjective psychological effects of inhaled THC, with and without oral alcohol, concomitantly comparing blood and plasma to OF cannabinoid concentrations during the treatment period. Vaporized cannabis produced a notable “high” and other subjective effects through 4.3h post-dose, similar to the effect of smoked cannabis. Alcohol prolonged the duration of cannabis’ effects. Subjective effect-versus-cannabinoid concentration curves displayed counterclockwise hysteresis, but subjective effect-versus-alcohol concentration produced clockwise hysteresis possibly due to slower alcohol absorption. We observed robust OF/blood and OF/plasma correlations, but high OF cannabinoid variability challenged reliable cannabis-effects predictions. Although OF retains strong cannabis exposure screening validity, blood THC demonstrated considerably more consistent results for predicting intoxicating effects of cannabis inhalation.
Supplementary Material
Acknowledgments
We thank the nurses and staff of the University of Iowa Clinical Research Unit and National Advanced Driving Simulator, especially Cheryl Roe, Jennifer Henderson, Rose Schmitt, and Kayla Smith, for excellent contributions to successful study completion. We also thank Drs. Dereece Smither and Richard Compton, National Highway Traffic Safety Administration, for invaluable input. We acknowledge University of Maryland, Baltimore Toxicology Program, and Graduate Partnership Program, National Institutes of Health (NIH). Quantisal™ and Volcano® devices were provided by the manufacturers to NIH through Materials Transfer Agreements, but manufacturers played no role in study design, data analysis, or manuscript writing. Research was funded by the Intramural Research Program, National Institute on Drug Abuse, NIH, the United States Office of National Drug Control Policy, and the National Highway Traffic Safety Administration.
References
- 1.ProCon.org. [Accessed 2 December 2014];23 Legal Medical Marijuana States and DC: Laws, Fees, and Possession Limits. 2014 < http://medicalmarijuana.procon.org/view.resource.php?resourceID=000881>. [Updated 30 October 2014.
- 2.Baggio S, Deline S, Studer J, Mohler-Kuo M, Daeppen JB, Gmel G. Routes of administration of cannabis used for nonmedical purposes and associations with patterns of drug use. J Adolesc Health. 2014;54:235–40. doi: 10.1016/j.jadohealth.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Joy JE, Watson SJ Jr, Benson JA Jr, editors. Marijuana and Medicine: Assessing the Science Base. National Academy Press; Washington, DC: 1999. [PubMed] [Google Scholar]
- 4.Hazekamp A, Ruhaak R, Zuurman L, van Gerven J, Verpoorte R. Evaluation of a vaporizing device (Volcano) for the pulmonary administration of tetrahydrocannabinol. J Pharm Sci. 2006;95:1308–17. doi: 10.1002/jps.20574. [DOI] [PubMed] [Google Scholar]
- 5.Abrams DI, Vizoso HP, Shade SB, Jay C, Kelly ME, Benowitz NL. Vaporization as a smokeless cannabis delivery system: A pilot study. Clin Pharmacol Ther. 2007;82:572–8. doi: 10.1038/sj.clpt.6100200. [DOI] [PubMed] [Google Scholar]
- 6.Zuurman L, Roy C, Schoemaker R, Hazekamp A, den Hartigh J, Bender J, Verpoorte R, Pinquier J, Cohen A, van Gerven J. Effect of intrapulmonary tetrahydrocannabinol administration in humans. J Psychopharmacol. 2008;22:707–16. doi: 10.1177/0269881108089581. [DOI] [PubMed] [Google Scholar]
- 7.Van Dam NT, Earleywine M. Pulmonary function in cannabis users: Support for a clinical trial of the vaporizer. Int J Drug Policy. 2010;21:511–13. doi: 10.1016/j.drugpo.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Pomahacova B, Van der Kooy F, Verpoorte R. Cannabis smoke condensate III: The cannabinoid content of vaporised Cannabis sativa. Inhal Toxicol. 2009;21:1108–12. doi: 10.3109/08958370902748559. [DOI] [PubMed] [Google Scholar]
- 9.Earleywine M, Barnwell SS. Decreased respiratory symptoms in cannabis users who vaporize. Harm Reduct J. 2007;4:11. doi: 10.1186/1477-7517-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartman RL, Huestis MA. Cannabis Effects on Driving Skills. Clin Chem. 2013;59:478–92. doi: 10.1373/clinchem.2012.194381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson MB, Kelley-Baker T, Voas RB, Lacey JH. The prevalence of cannabis-involved driving in California. Drug Alcohol Depend. 2012;123:105–9. doi: 10.1016/j.drugalcdep.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocky Mountain High Intensity Drug Trafficking Area (RMHIDTA) The Legalization of Marijuana in Colorado: The Impact. 2013;1:1–66. [Google Scholar]
- 13.Karschner EL, Schwope DM, Schwilke EW, Goodwin RS, Kelly DL, Gorelick DA, Huestis MA. Predictive model accuracy in estimating last Δ9-tetrahydrocannabinol (THC) intake from plasma and whole blood cannabinoid concentrations in chronic, daily cannabis smokers administered subchronic oral THC. Drug Alcohol Depend. 2012;125:313–319. doi: 10.1016/j.drugalcdep.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabritius M, Augsburger M, Chtioui H, Favrat B, Giroud C. Fitness to drive and cannabis: Validation of two blood THCCOOH thresholds to distinguish occasional users from heavy smokers. Forensic Sci Int. 2014;242C:1–8. doi: 10.1016/j.forsciint.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Biecheler MB, Peytavin JF, Facy F, Martineau H. SAM survey on “drugs and fatal accidents”: search of substances consumed and comparison between drivers involved under the influence of alcohol or cannabis. Traffic Inj Prev. 2008;9:11–21. doi: 10.1080/15389580701737561. [DOI] [PubMed] [Google Scholar]
- 16.Jones AW, Holmgren A, Kugelberg FC. Driving under the influence of cannabis: a 10-year study of age and gender differences in the concentrations of tetrahydrocannabinol in blood. Addiction. 2008;103:452–61. doi: 10.1111/j.1360-0443.2007.02091.x. [DOI] [PubMed] [Google Scholar]
- 17.Drummer OH. Drug testing in oral fluid. Clin Biochem Rev. 2006;27:147–59. [PMC free article] [PubMed] [Google Scholar]
- 18.Bosker WM, Huestis MA. Oral Fluid Testing for Drugs of Abuse. Clin Chem. 2009;55:1910–31. doi: 10.1373/clinchem.2008.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wille SM, Di Fazio V, Toennes SW, van Wel JH, Ramaekers JG, Samyn N. Evaluation of Delta 9-tetrahydrocannabinol detection using DrugWipe5S screening and oral fluid quantification after Quantisal collection for roadside drug detection via a controlled study with chronic cannabis users. Drug Test Anal. 2014 doi: 10.1002/dta.1660. [DOI] [PubMed] [Google Scholar]
- 20.Drummer OH, Gerostamoulos D, Chu M, Swann P, Boorman M, Cairns I. Drugs in oral fluid in randomly selected drivers. Forensic Sci Int. 2007;170:105–10. doi: 10.1016/j.forsciint.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 21.Van der Linden T, Legrand SA, Silverans P, Verstraete AG. DUID: oral fluid and blood confirmation compared in Belgium. J Anal Toxicol. 2012;36:418–21. doi: 10.1093/jat/bks038. [DOI] [PubMed] [Google Scholar]
- 22.Huestis MA, Verstraete A, Kwong TC, Morland J, Vincent MJ, De La Torre R. Oral fluid testing: Promises and pitfalls. Clin Chem. 2011;57:805–10. doi: 10.1373/clinchem.2010.152124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adamson SJ, Sellman JD. A prototype screening instrument for cannabis use disorder: the Cannabis Use Disorders Identification Test (CUDIT) in an alcohol-dependent clinical sample. Drug Alcohol Rev. 2003;22:309–15. doi: 10.1080/0959523031000154454. [DOI] [PubMed] [Google Scholar]
- 24.Sobell LC, Sobell MB. Alcohol Consumption Measures. In: Allen JP, Wilson VB, editors. Assessing Alcohol Problems: A Guide for Clinicians and Researchers. 2. US Department of Health and Human Services; Bethesda: 2003. pp. 75–99. NIH Pub. No. 03–3745. [Google Scholar]
- 25.Moskowitz H, Fiorentino D. A review of the literature on the effects of low doses of alcohol on driving-related skills. National Highway Traffic Safety Administration; 2000. DOT HS 809 208. [Google Scholar]
- 26.Hartman RL, Anizan S, Jang M, Brown TL, Yun K, Gorelick DA, Milavetz G, Spurgin A, Gaffney G, Huestis MA. Cannabinoid disposition in oral fluid after controlled vaporizer administration with and without alcohol. Forensic Toxicol. 2015 doi: 10.1007/s11419-015-0269-6. [DOI] [Google Scholar]
- 27.Lee D, Milman G, Schwope DM, Barnes AJ, Gorelick DA, Huestis MA. Cannabinoid Stability in Authentic Oral Fluid after Controlled Cannabis Smoking. Clin Chem. 2012;58:1101–09. doi: 10.1373/clinchem.2012.184929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheidweiler KB, Schwope DM, Karschner EL, Desrosiers NA, Gorelick DA, Huestis MA. In Vitro Stability of Free and Glucuronidated Cannabinoids in Blood and Plasma Following Controlled Smoked Cannabis. Clin Chem. 2013;59:1108–17. doi: 10.1373/clinchem.2012.201467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milman G, Barnes AJ, Low RH, Huestis MA. Simultaneous quantification of cannabinoids and metabolites in oral fluid by two-dimensional gas chromatography mass spectrometry. J Chrom A. 2010;1217:1513–21. doi: 10.1016/j.chroma.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwope D, Scheidweiler K, Huestis M. Direct quantification of cannabinoids and cannabinoid glucuronides in whole blood by liquid chromatography–tandem mass spectrometry. Analytical and Bioanalytical Chemistry. 2011;401:1273–83. doi: 10.1007/s00216-011-5197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartman RL, Brown TL, Milavetz G, Spurgin A, Gorelick DA, Gaffney G, Huestis MA. Controlled Cannabis Vaporizer Administration: Blood and Plasma Cannabinoids With and Without Alcohol. Clin Chem. 2015 doi: 10.1373/clinchem.2015.238287. In Press. [DOI] [PubMed] [Google Scholar]
- 32.Ramaekers J, Theunissen E, de Brouwer M, Toennes S, Moeller M, Kauert G. Tolerance and cross-tolerance to neurocognitive effects of THC and alcohol in heavy cannabis users. Psychopharmacology. 2011;214:391–401. doi: 10.1007/s00213-010-2042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwope DM, Bosker WM, Ramaekers JG, Gorelick DA, Huestis MA. Psychomotor Performance, Subjective and Physiological Effects and Whole Blood Δ9-Tetrahydrocannabinol Concentrations in Heavy, Chronic Cannabis Smokers Following Acute Smoked Cannabis. J Anal Toxicol. 2012;36:405–12. doi: 10.1093/jat/bks044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huestis MA, Sampson AH, Holicky BJ, Henningfield JE, Cone EJ. Characterization of the absorption phase of marijuana smoking. Clin Pharmacol Ther. 1992;52:31–41. doi: 10.1038/clpt.1992.100. [DOI] [PubMed] [Google Scholar]
- 35.Hunault CC, Bocker KB, Stellato RK, Kenemans JL, de Vries I, Meulenbelt J. Acute subjective effects after smoking joints containing up to 69 mg Delta9-tetrahydrocannabinol in recreational users: a randomized, crossover clinical trial. Psychopharmacology (Berl) 2014;231:4723–33. doi: 10.1007/s00213-014-3630-2. [DOI] [PubMed] [Google Scholar]
- 36.Ramaekers JG, Kauert G, van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31:2296–303. doi: 10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- 37.Ramaekers J, Kauert G, Theunissen E, Toennes S, Moeller M. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacology. 2009;23:266–77. doi: 10.1177/0269881108092393. [DOI] [PubMed] [Google Scholar]
- 38.Chiang CWN, Barnett G. Marijuana effect and delta-9-tetrahydrocannabinol plasma level. Clin Pharmacol Ther. 1984;36:234–38. doi: 10.1038/clpt.1984.168. [DOI] [PubMed] [Google Scholar]
- 39.Huestis MA. Pharmacokinetics and metabolism of the plant cannabinoids, Δ9-tetrahydrocannabinol, cannabidiol and cannabinol. Handb Exp Pharmacol. 2005;168:657–90. doi: 10.1007/3-540-26573-2_23. [DOI] [PubMed] [Google Scholar]
- 40.Louizos C, Yanez JA, Forrest ML, Davies NM. Understanding the hysteresis loop conundrum in pharmacokinetic/pharmacodynamic relationships. J Pharm Sci. 2014;17:34–91. [PMC free article] [PubMed] [Google Scholar]
- 41.Riff DP, Jain AC, Doyle JT. Acute hemodynamic effects of ethanol on normal human volunteers. Am Heart J. 1969;78:592–97. doi: 10.1016/0002-8703(69)90510-9. [DOI] [PubMed] [Google Scholar]
- 42.Perez-Reyes M, Hicks RE, Bumberry J, Jeffcoat AR, Cook CE. Interaction between marihuana and ethanol: effects on psychomotor performance. Alcohol Clin Exp Res. 1988;12:268–76. doi: 10.1111/j.1530-0277.1988.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 43.Lukas SE, Benedikt R, Mendelson JH, Kouri E, Sholar M, Amass L. Marihuana attenuates the rise in plasma ethanol levels in human subjects. Neuropsychopharmacology. 1992;7(1):77–81. [PubMed] [Google Scholar]
- 44.Lukas SE, Orozco S. Ethanol increases plasma delta-9-tetrahydrocannabinol (THC) levels and subjective effects after marihuana smoking in human volunteers. Drug Alcohol Depend. 2001;64:143–49. doi: 10.1016/s0376-8716(01)00118-1. [DOI] [PubMed] [Google Scholar]
- 45.Fabritius M, Chtioui H, Battistella G, Annoni JM, Dao K, Favrat B, Fornari E, Lauer E, Maeder P, Giroud C. Comparison of cannabinoid concentrations in oral fluid and whole blood between occasional and regular cannabis smokers prior to and after smoking a cannabis joint. Anal Bioanal Chem. 2013;405:9791–803. doi: 10.1007/s00216-013-7412-1. [DOI] [PubMed] [Google Scholar]
- 46.Langel K, Gjerde H, Favretto D, Lillsunde P, Oiestad EL, Ferrara SD, Verstraete AG. Comparison of drug concentrations between whole blood and oral fluid. Drug Test Anal. 2014;6:461–71. doi: 10.1002/dta.1532. [DOI] [PubMed] [Google Scholar]
- 47.Lee D, Vandrey R, Milman G, Bergamaschi M, Mendu DR, Murray JA, Barnes AJ, Huestis MA. Oral fluid/plasma cannabinoid ratios following controlled oral THC and smoked cannabis administration. Anal Bioanal Chem. 2013;405:7269–79. doi: 10.1007/s00216-013-7159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milman G, Schwope DM, Schwilke EW, Darwin WD, Kelly DL, Goodwin RS, Gorelick DA, Huestis MA. Oral Fluid and Plasma Cannabinoid Ratios after Around-the-Clock Controlled Oral {Delta}9-Tetrahydrocannabinol Administration. Clin Chem. 2011;57:1597–1606. doi: 10.1373/clinchem.2011.169490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toennes SW, Schneider K, Wunder C, Kauert GF, Moeller MR, Theunissen EL, Ramaekers JG. Influence of ethanol on the pharmacokinetic properties of Delta9-tetrahydrocannabinol in oral fluid. J Anal Toxicol. 2013;37:152–58. doi: 10.1093/jat/bkt002. [DOI] [PubMed] [Google Scholar]
- 50.Ramaekers JG, Moeller MR, van Ruitenbeek P, Theunissen EL, Schneider E, Kauert G. Cognition and motor control as a function of Δ9-THC concentration in serum and oral fluid: Limits of impairment. Drug Alcohol Depend. 2006;85:114–22. doi: 10.1016/j.drugalcdep.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 51.Kauert G, Ramaekers J, Schneider E, Moeller M, Toennes S. Pharmacokinetic properties of delta9-tetrahydrocannabinol in serum and oral fluid. J Anal Toxicol. 2007;31:288–93. doi: 10.1093/jat/31.5.288. [DOI] [PubMed] [Google Scholar]
- 52.Wille SMR, Raes E, Lillsunde P, Gunnar T, Laloup M, Samyn N, Christophersen AS, Moeller MR, Hammer KP, Verstraete AG. Relationship between oral Fluid and blood concentrations of drugs of abuse in drivers suspected of driving under the influence of drugs. Ther Drug Monit. 2009;31:511–19. doi: 10.1097/FTD.0b013e3181ae46ea. [DOI] [PubMed] [Google Scholar]
- 53.Gjerde H, Langel K, Favretto D, Verstraete AG. Estimation of equivalent cutoff thresholds in blood and oral fluid for drug prevalence studies. J Anal Toxicol. 2014;38:92–98. doi: 10.1093/jat/bkt122. [DOI] [PubMed] [Google Scholar]
- 54.Moore C, Coulter C, Uges D, Tuyay J, van der Linde S, van Leeuwen A, Garnier M, Orbita J., Jr Cannabinoids in oral fluid following passive exposure to marijuana smoke. Forensic Sci Int. 2011;212:227–30. doi: 10.1016/j.forsciint.2011.06.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.