Abstract
The classical role of hemoglobin in the erythrocytes is to carry oxygen from the lungs to the tissues via the circulation. However, hemoglobin also acts as a redox regulator and as a scavenger of the gaseous mediators nitric oxide (NO) and hydrogen sulfide (H2S). Here we show that upregulation of hemoglobin (α, β and δ variants of globin proteins) occurs in human peripheral blood mononuclear cells (PBMCs) in critical illness (patients with severe third-degree burn injury and patients with sepsis). The increase in intracellular hemoglobin concentration is a result of a combination of enhanced protein expression and uptake from the extra-cellular space via a CD163-dependent mechanism. Intracellular hemoglobin preferentially localizes to the mitochondria, where it interacts with complex I and, on the one hand, increases mitochondrial respiratory rate and mitochondrial membrane potential, and on the other hand, protects from H2O2-induced cytotoxicity and mitochondrial DNA damage. Both burn injury and sepsis were associated with increased plasma levels of H2S. Incubation of mononuclear cells with H2S induced hemoglobin mRNA upregulation in PBMCs in vitro. Intracellular hemoglobin upregulation conferred a protective effect against cell dysfunction elicited by H2S. Hemoglobin uptake also was associated with a protection from, and induced the upregulation of, HIF-1α and Nrf2 mRNA. In conclusion, PBMCs in critical illness upregulate their intracellular hemoglobin levels by a combination of active synthesis and uptake from the extracellular medium. We propose that this process serves as a defense mechanism protecting the cell against cytotoxic concentrations of H2S and other gaseous transmitters, oxidants and free radicals produced in critically ill patients.
INTRODUCTION
Although hemoglobin is best known for its role as an oxygen carrier in erythrocytes, many biological functions of hemoglobin predate the evolution of the circulatory system and are used to modulate various redox functions and interactions with gaseous transmitters such as nitric oxide (NO) and hydrogen sulfide (H2S) (1–5). For instance, clams living in H2S-rich waters upregulate intracellular hemoglobin levels as a protective mechanism against H2S toxicity (5).
The current report describes a significant upregulation of hemoglobin in human peripheral blood mononuclear cells (PBMCs) in two distinct forms of critical illness (sepsis and severe burn injury) and highlights the consequences of this phenomenon in the context of oxidant-, free radical- and gasotransmitter-associated cytotoxicity.
MATERIALS AND METHODS
Materials
All chemicals were obtained from Sigma-Aldrich, unless stated otherwise.
Cell Culture
Human monocyte histiocytic lymphoma cells (U937) and human embryonic kidney epithelial cells (HEK239T17) were obtained from ATCC and maintained in RPMI1640 with 10% fetal bovine serum (Life Technologies).
Isolation of Human PBMCs
Blood samples from critically ill patients and from healthy volunteers were collected with the permission of the local institutional review boards (IRBs). For the component of the clinical study involving burn patients, adult patients with burns covering ≥30% of the total body surface area (TBSA) were enrolled. Patients received standard burn care (6) and blood samples were obtained at the time of admission (0 wk) and 3 wks later (3 wks). For the component of the clinical study involving septic patients, adult patients with freshly diagnosed sepsis according to the established sepsis criteria (7) were enrolled at the time of diagnosis (0 wk) and 1 wk later (1 wk). Age-matched healthy human subjects served as healthy controls. Leukocytes and plasma were isolated using Histopaque (Sigma-Aldrich) (8).
Protein Identification by Mass Spectrometry
Protein extract of isolated PBMCs were separated on SDS-PAGE, stained with Coomassie Brilliant Blue (Bio-Rad), and ~15-kDa bands were excised from gel and subjected for identification by ESI-LC/MS/MS (4000 QTRAP with LC Packings capillary LC system, Applied Biosystems) (9).
Western Blotting
Western blotting was performed as before (9–11) using: anti–hemoglobin α (Santa Cruz Biotechnology), anti–hemoglobin-β (Santa Cruz Biotechnology) and anti–hemoglobin β/γ/δ (Santa Cruz Biotechnology). Loading was normalized with anti–β-actin-HRP (Santa Cruz Biotechnology). Blots were developed with Supersignal West Pico Chemiluminescent (Thermo Scientific).
RT-qPCR
Total RNA was extracted with TriReagent (MRC), cDNA was synthesized by high-capacity cDNA reverse transcriptase kit (Life Technologies) and expression of the following genes was determined: hemoglobin α, β, γ, δ, CD10, CD19, CD34, CD38, CD40, CD45, CD117, CD127, Integrin 1β, Integrin 4α, HIF1-α, Nrf2. Actin, 36B4, 18S was used as an internal control. Sequences of the primers are listed in Table 1.
Table 1.
List of primers used in the present study.
| Name | Forward | Reverse |
|---|---|---|
| Hemoglobin α | 5-CAG AGA GAA CCC ACC ATG GTG-3 | 5-GGT GGT GGT TCT GGA TGA AGG-3 |
| Hemoglobin β | 5-CAG ACA CCA TGG TGC ATC TG-3 | 5-CTC CAA GAA ACT CAG GAA ACC C-3 |
| Hemoglobin γ | 5-GGA AGG CTC CTG GTT GTC TAC C-3 | 5-CGT TCT TCC ACG ACT GAA GGG-3 |
| Hemoglobin δ | 5-CAA ACA GAC ACC ATG GTG CAT C-3 | 5-GTC CAA GGG TAG ACC ACC AG-3 |
| CD10 | 5-CTG GAA TCT ATA AAG AGG CTT GTA CAG C-3 | 5-GGT TTA GCC GTA GCA TTG GC-3 |
| CD19 | 5-CCT CAG CCA GGA CCT CAC-3 | 5-CGT CTC CAT TAC CCA CAT ATC TC-3 |
| CD34 | 5-CCA ACA GAA CAG AAA TTT CCA GC-3 | 5-GGT GAC CAG TGC AAT CAG G-3 |
| CD38 | 5-CCT GAG ATG AGA CAT GTA GAC TGC-3 | 5-GAG AAC TGA TGG GCC AGA TC-3 |
| CD40 | 5-GGT CAA GCA GAT TGC TAC AGG-3 | 5-GTC TCA CAG CTT GTC CAA GG-3 |
| CD45 | 5-CCA CAG GTG TTT CAT CAG TAC AGA C-3 | 5-CCT CTC TCC TGG GAC ATC TGA G-3 |
| CD117 | 5-CCA GAC AGG CTC TTC TCA ACC-3 | 5-GAT CAG TGC ATA ACA GCC TAA TCT C-3 |
| CD127 | 5-GAC GCA TGT GAA TTT ATC CAG C-3 | 5-GGA CTC CAT TCA CTC CAG AAG C-3 |
| Integrin 1b | 5-CCC ATT GAC CTC TAC TAC CTT ATG G-3 | 5-GCC AAA TCC AAT TCT GAA GTC C-3 |
| Integrin 4a | 5-GGA TCC ATC GTG ACT TGT GG-3 | 5-GGA TAT TCC AGC TTG ACA TGA TGC-3 |
Measurement of Hemoglobin Concentration
Concentration of hemoglobin in plasma and RPMI was determined using the Hemoglobin Assay Kit (Sigma-Aldrich).
Flow Cytometric Analysis
PMBCs were fixed in 3.7% paraformaldehyde for 30 min at 4°C followed by three washes with PBS and permeabilized with 0.1% Triton-X containing PBS for 30 min, followed by three washes with PBS. CD45 (Santa Cruz Biotechnology Biotechnology) and primary antibody against hemoglobin-β was applied at 500× dilution in blocking buffer (1% BSA in PBS) for 1 h, followed by washing with PBS and subsequent incubation with Alexa Fluor–conjugated secondary antibodies (Life Technologies) for 30 min. Samples were analyzed using a FACSArray Bioanalyzer (BD Biosciences).
Immunocytochemistry
U937 cells were treated for 24 h with 2, 20 or 200 mg/dL of purified hemoglobin, isolated from the blood of healthy volunteers, then washed two times with RPMI and fixed with 4% paraformaldehyde in slide chambers (Lab-Tek). Cells were triple stained with: DAPI (Life Technologies), Alexa Fluor 594 conjugated (Mix-n-Stain, Sigma-Aldrich) to anti-CD163 antibody and Alexa Fluor 488 conjugated (Mix-n-Stain, Sigma-Aldrich) to anti–hemoglobin α. Images were visualized using Nikon Eclipse 80i fluorescent microscope with CoolSNAP HQ camera and analyzed with NIS Elements BR3.10 software.
Proximity Ligation Assay (PLA)
In situ protein:protein proximity/interaction studies were performed with Duolink in situ (Sigma-Aldrich) as before (10) using the following antibodies: anti-CD163 (Santa Cruz Biotechnology), anti–hemoglobin α (Abcam), anti-cadherin (Santa Cruz Biotechnology), anti-Na+K+ATPase (Santa Cruz Biotechnology), anti-lamin (Cell Signaling Technology), anti-PDI (Cell Signaling Technology), anti-histone (Cell Signaling Technology), anti-LDH (Cell Signaling Technology), anti-NdufS3 (mitochondrial complex I subunit, Abcam), anti–mitochondrial complex II 70 kDa subunit (Life Technologies), anti–mitochondrial complex IV subunit (cytochrome c oxidase subunit II, Life Technologies), anti–mitochondrial complex V 56 kDa subunit (Life Technologies) and anti-TFAM (mitochondrial transcription factor A, Genetex). Images were visualized using Nikon Eclipse 80i fluorescent microscope with CoolSNAP HQ camera and analyzed with NIS Elements BR3.10 software.
Quantification of Cell Death using LDH Release
U937 cells (5 × 105 cells) were incubated with hemoglobin (2–20–200 mg/dL) for 24 h and washed two times with RPMI media and challenged with 5 mmol/L NaSH, 500 μmol/L H2O2, 100 μmol/L SIN or 500 μmol/L SNAP for 24 h. LDH activity in the culture medium, an indicator of cell necrosis, was measured as described (10).
Measurement of Mitochondrial and Total Cellular Oxidant Production
Detection of mitochondrial superoxide was achieved with MitoSOX (Life Technologies) and total ROS levels were quantified with H2DCF (Life Technologies) as described (11).
Citrate Synthase Activity Measurement
Specific activity of citrate synthase was detected in total cell lysate of 5 × 105 U937 cells previously incubated with hemoglobin (2–20–200 mg/dl) for 24 h using Citrate Synthase Assay Kit (Sigma-Aldrich) as described (11).
Measurement of Mitochondrial Membrane Potential
Mitochondrial membrane potential was measured using TMRE (Life Technologies) in 5 × 105 of U937 cells as described (9).
Measurement of Intracellular ATP Content
ATP content was measured using CellTiter-Glo Luminescent Cell Viability Assay (Promega) as described (11).
Analysis of Bioenergetic Parameters by Extracellular Flux Analysis
U937 cells (2 × 105) were plated in 24-well Seahorse culture plates (BD Biosciences) followed by 24 h incubation with hemoglobin (2–20–200 mg/dl). Bioenergetic parameters were measured with XF24 Extracellular Flux Analyzer (Seahorse Bioscience) as described (12).
Measurement of Plasma H2S Content
Concentration of H2S in human plasma samples or in samples of animals subjected to burn injury according to a model previously described (13) were detected with 7-azido-4-methylcoumarine (AzMC) H2S-specific fluorescent probe as described (14).
Transient Transfection of HEK293T17 Cells with Hemoglobin
HEK293T17 cells were transfected on 24-well plates with full-length human hemoglobin cDNA inserted into pCMV6-XL4 vector purchased from Origene Technologies. β-galactosidase-inserted plasmid was used as a control (Origene). Transfection of HEK293T17 cells was performed using Lipofectamine 2000 (Life Technologies) with 0.5 μg of plas-mid DNA per well. After 48 h HEK293T17 cells were challenged with 7.5 mmol/L NaSH for 1 h. LDH release into the media was quantified and hemoglobin expression was confirmed by Western blotting.
Measurement of Mitochondrial DNA Damage
Damage to mitochondrial DNA was determined as described (10).
Statistical Analysis
All data are presented as means ± SEM and were analyzed using GraphPad Prism software. Statistical analysis was performed by ANOVA followed by Bonferroni’s multiple comparisons.
RESULTS
Hemoglobin Protein and mRNA Levels Are Increased in PBMCs during Critical Illness
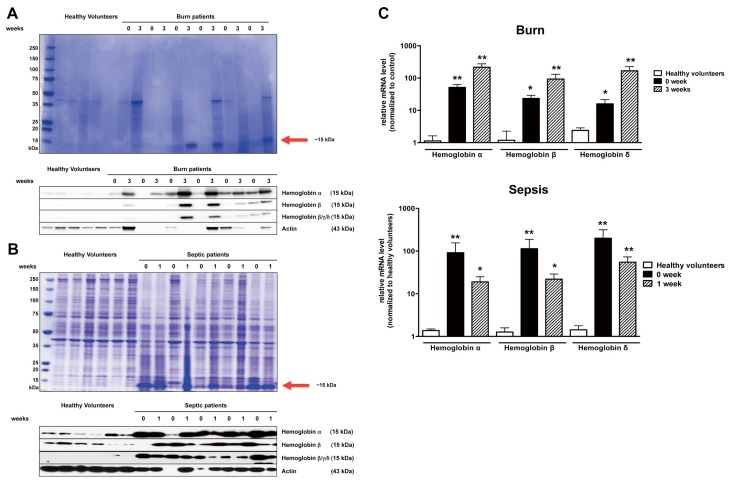
SDS-PAGE electrophoresis gels prepared from homogenates of PBMCs from patients with severe third-degree burns (Figure 1A) and patients with sepsis (Figure 1B) showed a marked increase in the amount of a protein in the 15–20 kDa range. Mass spectroscopic analysis identified this protein as hemoglobin. Western blotting confirmed abundant levels of hemoglobin, primarily the α isoform, in the same set of samples (Figures 1A, B). Subsequent mRNA analysis of the PMBCs isolated from the blood of patients demonstrated an upregulation of the Hb-α subcluster genes, and, to a lesser extent, Hb-β and Hb-δ mRNA levels (Figure 1C).
Figure 1.
PBMCs of burn and septic patients accumulate hemoglobin. Coomassie-stained and Western analysis of protein extract isolated from PBMCs of patients with burn injury (A) and septic shock (B) showed accumulation of a 15 kDa protein (in comparison to healthy volunteers), identified as hemoglobin. Western blot analysis of PBMCs extract confirmed accumulation of hemoglobin α, β and γ/δ. (C) Expression of hemoglobin α, β and γ/δ was markedly elevated in patients with burn or sepsis at early and later times post admission, in comparison to healthy volunteers. Week 0 represents patient admission day to the hospital (in burn patients) and diagnosis of sepsis (in patients with sepsis). Blood was collected and analyzed 3 wks after admission (burn patients) or 1 wk after diagnosis of sepsis (septic patients). Even though equal protein loading was performed, actin bands were markedly different because in critical illness many proteins (including proteins normally used for loading control, such as actin) can be up-or downregulated. For lanes where no detectable actin lanes were observed, we have utilized an average of the densitometry values of all lanes evaluated for the particular patient population for the densitometry analysis. Data represent mean with ± S.E.M.; n = 6; *p < 0.05; **p < 0.01.
Potential Mechanisms of the Upregulation of Hemoglobin in Critical Illness
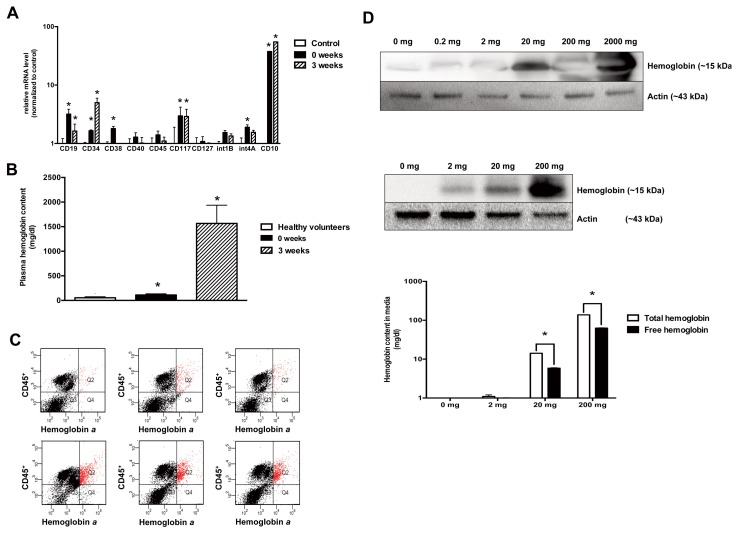
One possibility for the expression of hemoglobin is related to a mobilization of stem cells into the circulation during critical illness, as early hematopoietic progenitor cells (as opposed to mature leukocytes) express significant levels of hemoglobin, including the fetal variants (15). qPCR analysis of the PBMCs from burn patients showed an increase in various hematopoietic cell markers for common myeloid progenitors (D34, FLT3) and lymphoid progenitors (CD34, CD38, CD45, CD117, CD127, CD10) (Figure 2A), indicating that there is an increase in hematopoietic stem cell/immature leukocyte content in PBMCs during critical illness.
Figure 2.
Immature leukocyte populations are enriched after burn injury, and hemoglobin is associated with CD45+ positive PBMCs. (A) The expression levels of various bone marrow derived progenitor cell CD markers are significantly elevated in PBMCs of burn patients. Data represent mean ± S.E.M.; n = 6; *p < 0.05. (B) An increased amount of free hemoglobin detected in serum of burn patients. (C) Flow cytometric analysis shows increased CD45+/hemoglobin α+ double positive cells in total PBMC population (from 2% ± 1% in healthy volunteer samples to 16% ± 4% in burn patient samples; n = 3; **p < 0.01.) (D) PBMCs (top subpanel) and U937 cells (middle and bottom subpanel) bind hemoglobin onto the cell surface in a dose-dependent manner. This causes a decrease free hemoglobin content of media in proportion to hemoglobin uptake by U937 cells (bottom subpanel, showing the decrease of total and free hemoglobin from the cell culture media after incubation of the hemoglobin-containing culture medium with U937 cells). Data represent mean with ± S.D.; n = 3; *p < 0.05
Another possibility that may contribute to the observed increase in the hemoglobin content of PBMCs may be the uptake of hemoglobin from the circulation, since burn and sepsis is known to induce significant hemolysis, which can markedly increase plasma-free hemoglobin concentrations (16). A significant hemolysis was also confirmed in the present samples (Figure 2B). Flow cytometric analysis showed double staining of PBMCs isolated from burn patients for the presence of hemoglobin and CD45+ (leukocyte common antigen) in the PBMCs of burn patients (Figure 2C), thereby localizing the hemoglobin to the leukocytes. In subsequent studies, we have confirmed the ability of PBMCs and U937 cells to take up hemoglobin from the culture medium in vitro (Figure 2D).
CD163-Mediated Uptake and Mitochondrial Sequestration of Hemoglobin
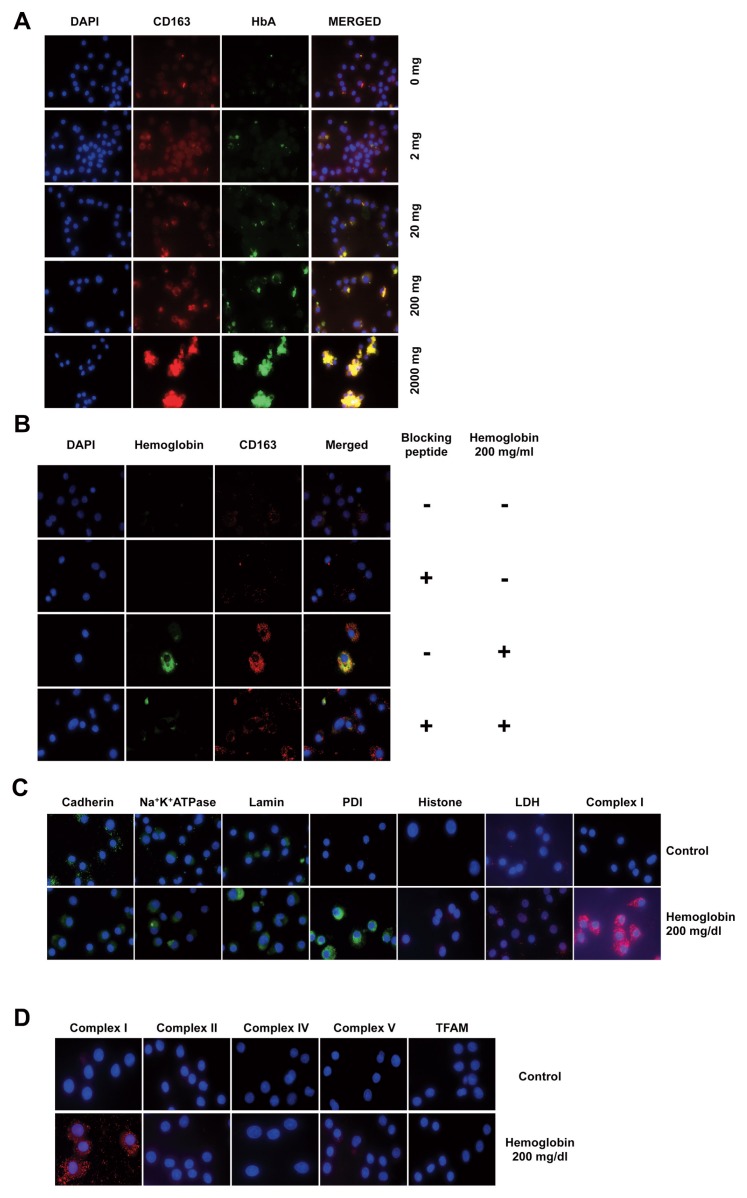
Cellular hemoglobin uptake is mediated by CD163 (scavenger receptor cysteine-rich type 1 protein M130), a membrane transporter protein (17). CD163 exhibits a significant upregulation on the surface of PBMCs in sepsis and other forms of critical illness (18). Upon incubation of U937 cells with hemoglobin, there was a significant colocalization of hemoglobin with CD163 (Figure 3A); inhibition of the interaction of CD163 with hemoglobin by preincubating the cells with a CD163-blocking peptide attenuated hemoglobin uptake (Figure 3B). PLA studies localized the intracellular hemoglobin to close proximity with the mitochondrial complex I, but not with LDH (an abundant cytosolic protein), nor with various nuclear or cell membrane proteins (Figure 3C). Further investigation of the potential interaction of hemoglobin with several additional mitochondrial proteins revealed that hemoglobin does not show any significant interaction with complexes II, IV or V, nor with TFAM (a mitochondrial DNA-associated protein) (Figure 3D), suggesting the specificity of the hemoglobin–complex I interaction.
Figure 3.
Hemoglobin is taken up by U937 via the CD163 receptor and localizes to mitochondria where it interacts with complex I. (A) Immunocytochemistry analysis of U937 cells incubated with hemoglobin indicates the colocalization of hemoglobin with the CD163 receptor in a dose-dependent manner. (B) Proximity ligation assay confirms the interaction between CD163 receptor and hemoglobin; this interaction is inhibited by preincubation of U937 cells with a blocking peptide of the CD163 receptor. (C) Hemoglobin interacts with mitochondrial complex I, (D) but not with other mitochondrial oxidative phosphorylation complexes or the mitochondrial transcription factor A (TFAM), as assessed by in situ PLA analysis. Figures show representative images of at least three independent determinations conducted on different experimental days (n = 3).
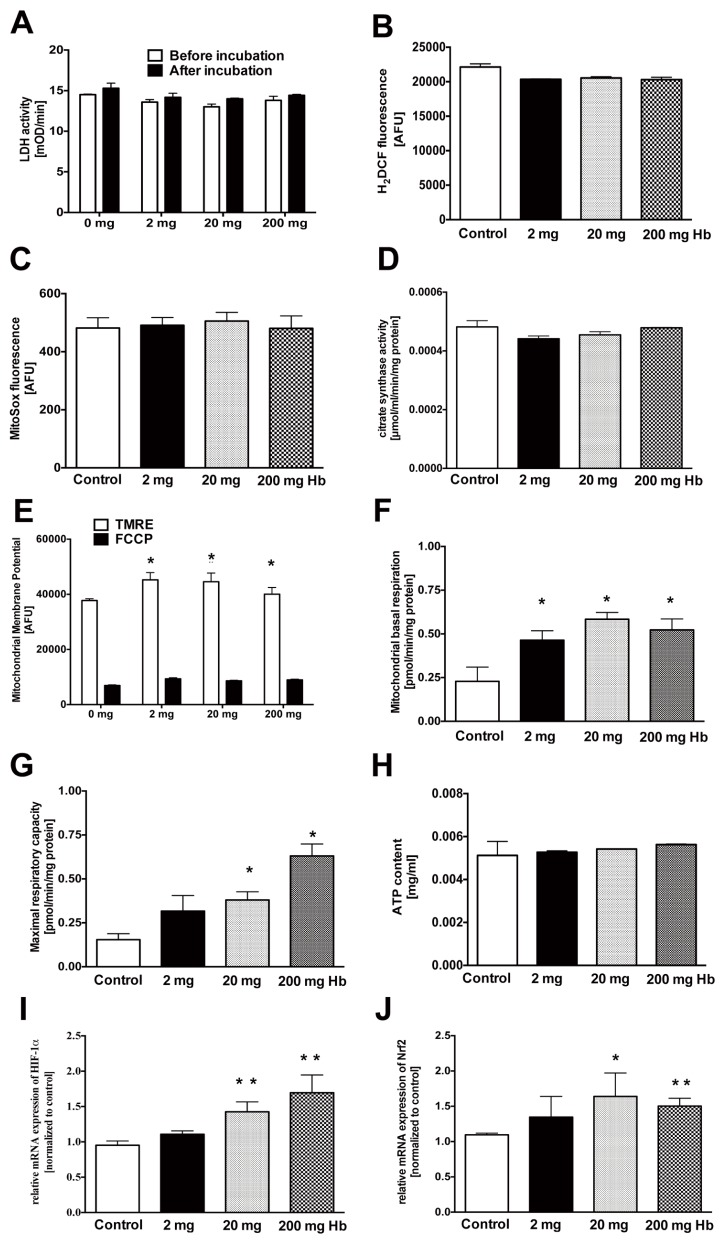
Uptake of hemoglobin into U937 cells did not adversely affect cell viability (Figure 4A), failed to induce reactive oxygen species (ROS) production (Figures 4B, C) and did not affect mitochondrial volume (Figure 4D), but did cause a slight increase in mitochondrial membrane potential and a slight increase of mitochondrial basal and maximal respiratory rates (Figures 4E–G), without significantly affecting total cellular ATP content (Figure 4H). Hemoglobin also induced an upregulation of HIF-1α and Nrf-2 mRNA, two significant cellular regulators of redox homeostasis (Figures 4I, J).
Figure 4.
Increased intracellular level of hemoglobin affects mitochondrial parameters. The effect of various concentrations of hemoglobin on U937 cells on (A) cytotoxicity measured by LDH release, (B) levels of intracellular reactive oxygen species, (C) levels of mitochondrial-specific reactive oxygen species, (D) mitochondrial content, (E) mitochondrial membrane potential, (F) basal mitochondrial respiratory capacity, (G) maximal mitochondrial respiratory capacity, (H) cellular ATP content and expression of (I) HIF1-α and (J) Nrf2 mRNA. Incubation of U937 cells with hemoglobin stimulates mitochondrial membrane potential, basal and maximal respiratory capacity. Data represent mean ± S.E.M.; n = 3; *p < 0.05.
H2S Induces Hemoglobin Upregulation; Intracellular Hemoglobin Protects from H2S Cytotoxicity
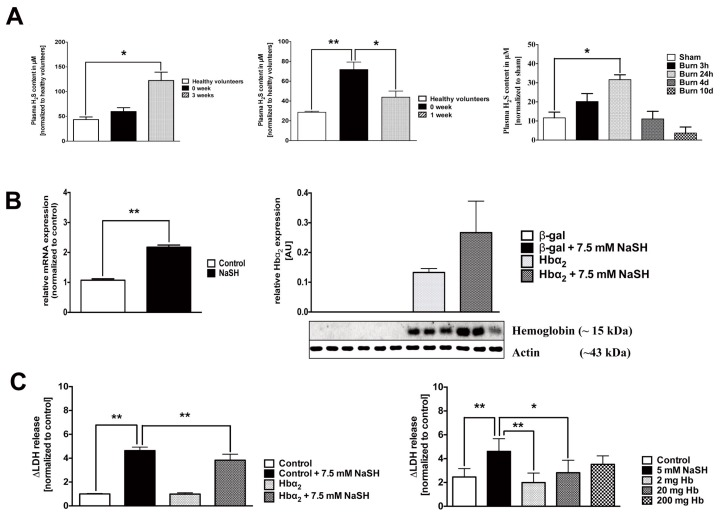
We hypothesized that the increased Hb mRNA expression of PBMCs constitutes an active response to various oxidants, free radicals and gaseous mediators that PMBCs may be exposed to in critical illness. H2S is a novel gaseous biological mediator with multiple pathogenetic roles in critical illness (19), which has been shown to cause an upregulation of hemoglobin in lower organisms (5). We have detected marked elevations in the plasma H2S in burn and septic patients, as well as in a murine model of burn (Figure 5A). Furthermore, incubation of PBMCs or U937 cells with H2S resulted in the upregulation of the Hb-α subunit mRNA (Figure 5B). Increased intracellular hemoglobin content (either as a result of active upregulation by the cells, or after uptake from the extracellular space) attenuated the cellular damage elicited by incubation of the leukocytes with high concentrations of H2S (Figure 5C).
Figure 5.
Cellular upregulation of hemoglobin protects against H2S-induced cytotoxicity. (A) H2S levels are elevated in the plasma of severe burn and septic patients and in the plasma of mice subjected to burn injury. Data represent mean ± S.E.M.; n = 6–8; *p < 0.05; **p < 0.01. (B) Left panel: Treatment of PBMCs isolated from healthy volunteers with 1 mmol/L NaSH for 1 h induced the expression of hemoglobin mRNA. HEK293T17 cells transfected with a hemoglobin α overexpression construct showed a further increase in the expression of intra-cellular hemoglobin when the transfection was combined with H2S treatment (7.5 mmol/L). Hb-α expression was quantified by Western blotting at 48 h. Data represent mean ± S.E.M; n = 3; **p < 0.01 (C) Expression of hemoglobin in HEK293T17 cells and uptake of hemoglobin into U937 cells protected against the cytotoxic effect of a high concentration (7.5 mmol/L) of NaSH, as measured by LDH release into the medium. Data represent mean ± S.E.M.; n = 3; *p < 0.05; **p < 0.01.
The protection afforded by hemoglobin extended to a protection against the loss of cellular viability by SIN-1 (a simultaneous donor of superoxide and NO, that is, a generator of peroxynitrite) (Figure 6A); hemoglobin also attenuated the cell injury induced by SNAP (a NO donor) (Figure 6B) or by hydrogen peroxide (H2O2) (Figure 6C). H2O2-induced damage to the mitochondrial DNA was also attenuated by hemoglobin (Figure 6D).
Figure 6.
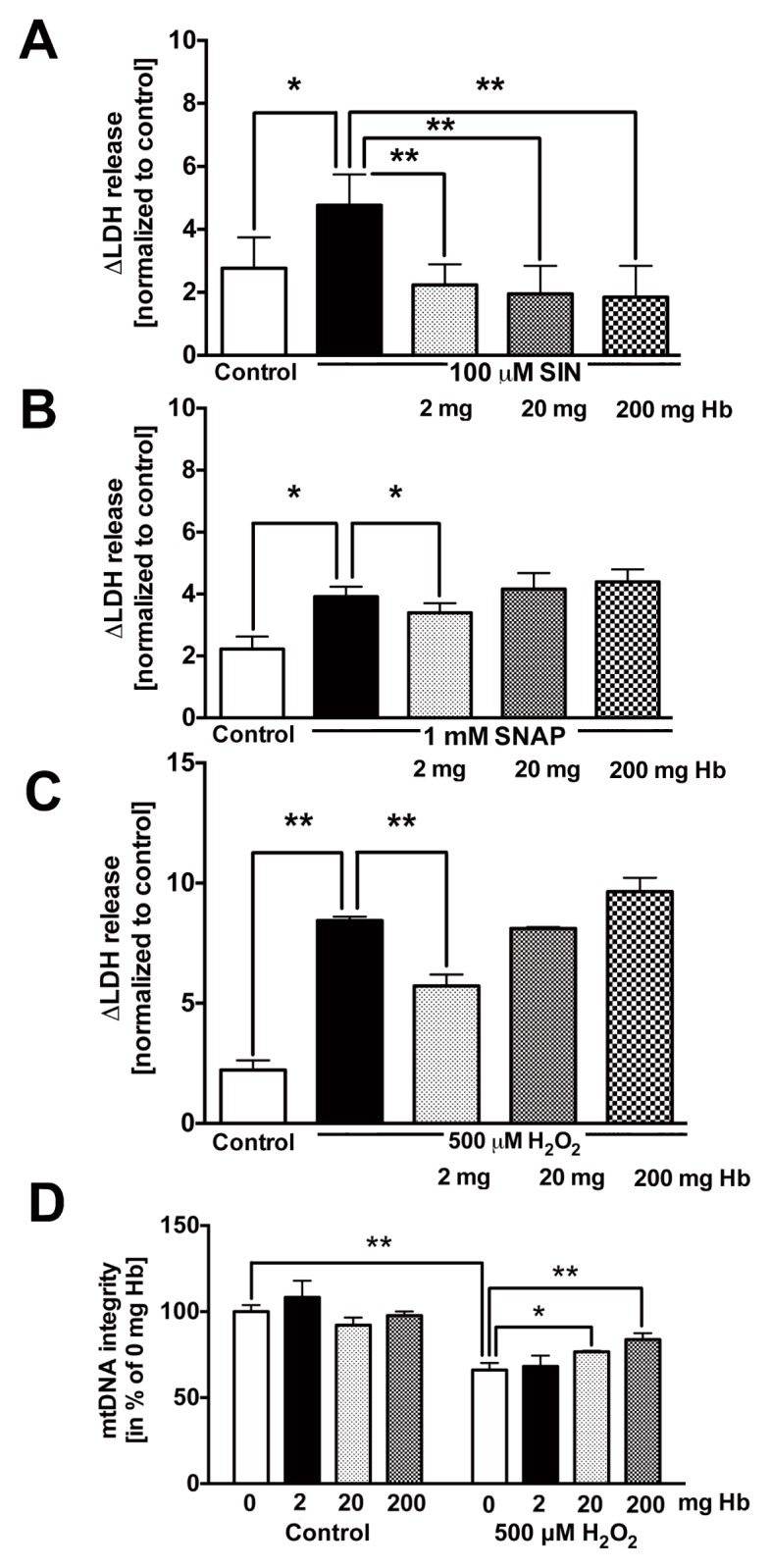
Hemoglobin protects U937 cells against ROS/RNS induced cytotoxicity. Incubation of U937 cells with hemoglobin protects against cell death initiated by (A) SIN-1 (100 μmol/L), (B) SNAP (500 μmol/L) and (C) H2O2 (500μmol/L). (D) Hemoglobin protects against H2O2-induced mitochondrial and nuclear DNA damage. Data represent mean ± S.E.M; n = 4–6. *p < 0.05; **p < 0.01.
DISCUSSION
Circulating hemoglobin, or cell-free hemoglobin, as a result of excessive intravascular hemolysis (for instance, as a consequence of infusion of stored blood products, ABO incompatibility or various forms of critical illness) (15,16,20) is generally considered a deleterious molecule, which exerts its toxic effects via several distinct mechanisms including pro-oxidant redox cycles catalyzed by heme (4), scavenging physiologically essential NO from the vascular space, leading to endothelial dysfunction and vasoconstriction (15), activation of proinflammatory pathways via TLR4 receptors (21) and other mechanisms. In this context, uptake of hemoglobin into mononuclear cells from the circulation via CD163 is viewed as a protective pathway that serves to reduce circulating free hemoglobin levels (17).
Several lines of independent studies, however, implicate hemoglobin as a cytoprotective protein. For instance, hypoxia upregulates hemoglobin expression in alveolar epithelial cells in vitro (22) and bacterial lipopolysaccharide upregulates the β hemoglobin subunit in murine macrophages (23). H2O2 increases Hb-α1 and Hb-β expression in HepG2 and HEK293 cells, which, in turn, protects the cells from oxidative stress (24). Hemoglobin has been localized in kidney mesangial cells, where it confers oxidative stress resistance (25). The expression of Hb-α1 and Hb-β also has been demonstrated in mesencephalic dopaminergic neurons and glial cells, and has been linked to the modulation of oxygen homeostasis, oxidative phosphorylation, oxidative stress and NO biosynthesis (26). Finally, red blood cell hemolysate and hemoglobin both reduce the effect of oxidative stress on peripheral blood mononuclear cell DNA damage induced by H2O2 (27). These studies, when taken together with the results of the current study, indicate that upregulation of intracellular hemoglobin (either via uptake from the extracellular space, and/or through de novo biosynthesis) can be a reactive response of the cell to adverse conditions such as oxidative stress, hypoxia or high concentrations of gaseous mediators, which, in turn, serves to protect the cell from the damage.
We conclude that in the current study, the upregulation of hemoglobin in the PBMCs of critically ill patients probably results from a combination of uptake from the extracellular space and an active biosynthesis of hemoglobin, at least in part by immature leukocytes mobilized from the bone marrow. The in vitro data (upregulation of Hb mRNA in U937 cells after incubation with H2S) coupled with the findings showing that (a) circulating H2S levels are increased in critical illness and (b) cells with elevated intra-cellular hemoglobin levels are protected from H2S-mediated cytotoxicity are consistent with the existence of a reactive process whereby upregulation of hemoglobin serves as a protective mechanism in PBMCs against H2S toxicity in critical illness. This model is not in disagreement with prior work showing that extracellular circulating hemoglobin can have multiple deleterious effects (20); in fact, uptake and sequestration of hemoglobin from the plasma into the PBMCs via CD163 may contribute to a reduction of circulating free hemoglobin levels.
We do not propose that the sole purpose of intracellular hemoglobin in PBMCs is the modulation of H2S homeostasis or protection from H2S-mediated injury. It is more likely that hemoglobin exerts pleiotropic effects in these cells; it modulates the cellular responses to various oxidants and free radicals (NO, superoxide, H2O2, peroxynitrite, and so on); hemoglobin, either through acting on membrane receptors or via modulation of intracellular pathways and signal transduction processes, may also regulate the expression of various oxidant-responsive elements (as evidenced, in our study, by the hemoglobin-induced upregulation of HIF-1α and NRF2). Upregulation of both of these factors is known to confer cytoprotection and ischemic/hypoxic preconditioning via a variety of interacting downstream pathways (28,29).
Intracellular hemoglobin shows remarkable compartmentalization; it concentrates to the mitochondria, where it shows a particularly close association with mitochondrial complex I. It is interesting to note that a recent proteomic analysis of control brains and brains from multiple sclerosis patients has identified the mitochondrial localization of the hemoglobin β chain (30). The functional consequence of mitochondrial hemoglobin remains to be further elucidated; based on the current results, we speculate that it may either contribute to a specific mitochondrial protection (for example, against mitochondrial oxidative stress), or it may play a more specific role in the regulation of mitochondrial electron transport; the latter hypothesis is supported by our extracellular flux analysis findings indicating an increased basal and FCCP-uncoupled maximal rate of mitochondrial respiration in cells with increased mitochondrial hemoglobin content. Our results also indicate that mitochondrial hemoglobin may serve to protect the integrity of mitochondrial DNA from oxidative damage.
The conditions and factors determining the deleterious versus protective roles of hemoglobin in various pathophysiological conditions are likely to depend on the localization of hemoglobin (intracellular versus extracellular), the degree of oxidative stress, the rate of the gaseous transmitters produced, as well as many additional factors. Based on several sets of independent investigations, it appears that when oxidative stress is relatively low, hemoglobin (and its degradation products, for example, heme) increases oxidative stress, and scavenges physiologically necessary amounts of NO, but when the degree of oxidative stress is high, hemoglobin tends to protect from cellular damage (22–27,31). One also has to keep in mind that hemoglobin decomposes into multiple products, including heme (which increases oxidative stress, but also acts as a substrate of the gasotransmitter carbon monoxide, produced by heme oxygenase), while other degradation products (for example, bilirubin) have significant antioxidant effects (31). These factors remain to be carefully evaluated to dissect the multiple roles of hemoglobin in the regulation of various pathophysiological conditions.
There are a number of limitations of the current study, as well as a number of follow-up issues that remain to be elucidated in future experiments. (a) In addition to the upregulation of Hbs, there are a number of additional protein bands that show enrichment in the PBMCs of critically ill patients (Figure 1); changes in the entire PBMC proteome, as well as additional individual proteins that are up- or downregulated remain to be studied in future experiments. (b) What is the relative contribution of Hb mRNA/protein upregulation and uptake of Hbs from the extracellular space to the observed enrichment of PBMC Hbs in burn and in sepsis? Furthermore, with respect to the upregulation of Hb mRNA, what is the contribution of upregulation in mature PBMCs versus in immature/stem forms that are mobilized into the circulation during the critical illness insult? These issues may be more directly addressed by future preclinical studies (for example, rodent models of sepsis or burns). (c) We have only studied the reactive upregulation of Hbs in response to H2S in the current project. It remains to be elucidated if similar reactive responses can also be elicited by other stimuli (for example, hypoxia, oxidative stress, NO, CO and others) in PBMCs. In this context, it is worth mentioning that, in other cell types, Hb up-regulation has been demonstrated in response to bacterial lipopolysaccharide stimulation in murine monocyte-macrophages (23), in response to hypoxia in epithelial cells (22) or in response to oxidative stress in hepatocytes (24). (d) What is the exact functional role of Hbs in the mitochondria, and how does Hbs translocation occurs into the mitochondria? Since Hbs are large molecules, the process is likely to involve active transport mechanisms. It also remains to be determined whether Hbs incorporate into respiratory complex I and, if so, do they directly participate in mitochondrial electron transport? One limitation of the results is that PLA studies show close vicinity to the two proteins studied (in this case complex I and Hb) but direct binding requires confirmation by additional methods (for example, confocal microscopy, immunoprecipitation and/or pull-down techniques). In this context it is important to keep in mind that mitochondrial complex I consists of a large number of subunits, including the NADH dehydrogenase module (N module), the electron transfer module (Q module) and the proton translocation module (P module), which are made up of 14 core subunits (32). Specific interaction(s) of Hbs with these subunits remain to be studied in further experiments. (e) The exact kinetics and reactions of Hbs with NO, H2O2 and H2S remain to be further studied in the cytoprotective context. With respect to the reaction of NO with Hbs, the literature is substantial and goes back to a century; it has been put into the biological (vascular) context by the original work of Murad, Ignarro and Furchgott (33–36), who have identified Hbs as scavengers of NO (as well as of endothelium-derived relaxing factor [EDRF], which later has been demonstrated to be identical with NO). Similarly, with respect to the interactions of Hbs with oxyradicals, substantial work has been conducted already, which can be reviewed in the literature (37–39). With respect to the interactions of Hbs with H2S, several lines of studies have demonstrated the formation of sulfhemoglobins (40,41), and Banerjee and colleagues have recently outlined several novel aspects of the interaction, especially with respect to the interactions of H2S with methemoglobin (42).
CONCLUSION
Although much additional work remains to be conducted to explore the above issues, in summary, at least under the conditions studied in the current report, we can conclude that cellular uptake of hemoglobin does not adversely affect the viability of leukocytes, and protects them from various gaseous and oxidative insults. Therefore, we hypothesize that the upregulation of hemoglobin in PBMCs during critical illness constitutes a cytoprotective mechanism.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (P50GM060338 to DN Herndon and R01GM107846 to C Szabo) and by a grant from the Shriners of North America (#85800) to C Szabo.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Cite this article as: Brunyanszki A, et al. (2015) Up-regulation and mitochondrial sequestration of hemoglobin occur in circulating leukocytes during critical illness, conferring a cytoprotective phenotype. Mol. Med. 21:666–75.
REFERENCES
- 1.Doctor A, Stamler JS. Nitric oxide transport in blood: a third gas in the respiratory cycle. Compr Physiol. 2011;1:541–68. doi: 10.1002/cphy.c090009. [DOI] [PubMed] [Google Scholar]
- 2.Rifkind JM, Nagababu E. Hemoglobin redox reactions and red blood cell aging. Antioxid Redox Signal. 2013;18:2274–83. doi: 10.1089/ars.2012.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quaye IK. Extracellular hemoglobin: the case of a friend turned foe. Front Physiol. 2015;6:96. doi: 10.3389/fphys.2015.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardison R. Hemoglobins from bacteria to man: evolution of different patterns of gene expression. J Exp Biol. 1998;201:1099–117. doi: 10.1242/jeb.201.8.1099. [DOI] [PubMed] [Google Scholar]
- 5.Bailly X, Vinogradov S. The sulfide binding function of annelid hemoglobins: relic of an old biosystem? J Inorg Biochem. 2005;99:142–50. doi: 10.1016/j.jinorgbio.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Ali A, et al. Propranolol attenuates hemorrhage and accelerates wound healing in severely burned adults. Crit Care. 2015;4:217. doi: 10.1186/s13054-015-0913-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva SC, et al. Evaluation of Toll-like, chemokine, and integrin receptors on monocytes and neutrophils from peripheral blood of septic patients and their correlation with clinical outcomes. Braz J Med Biol Res. 2014;47:384–93. doi: 10.1590/1414-431X20143190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tóth-Zsámboki E, et al. Activation of poly(ADP-ribose) polymerase by myocardial ischemia and coronary reperfusion in human circulating leukocytes. Mol Med. 2006;12:221–8. doi: 10.2119/2006-00055.Toth-Zsamboki. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunyanszki A, Olah G, Coletta C, Szczesny B, Szabo C. Regulation of mitochondrial poly(ADP-ribose) polymerase activation by the β-adrenoceptor/cAMP/protein kinase A axis during oxidative stress. Mol Pharmacol. 2014;86:450–62. doi: 10.1124/mol.114.094318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szczesny B, Brunyanszki A, Olah G, Mitra S, Szabo C. Opposing roles of mitochondrial and nuclear PARP1 in the regulation of mitochondrial and nuclear DNA integrity: implications for the regulation of mitochondrial function. Nucleic Acids Res. 2014;42:13161–73. doi: 10.1093/nar/gku1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oláh G, et al. Differentiation-associated downregulation of poly(ADP-ribose) polymerase-1 expression in myoblasts serves to increase their resistance to oxidative stress. PLoS One. 2015;10:e0134227. doi: 10.1371/journal.pone.0134227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szabo C, et al. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc Natl Acad Sci U S A. 2013;110:12474–9. doi: 10.1073/pnas.1306241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porter C, et al. Severe burn injury induces thermogenically functional mitochondria in murine white adipose tissue. Shock. 2015;44:258–64. doi: 10.1097/SHK.0000000000000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coletta C, et al. Regulation of vascular tone, angiogenesis and cellular bioenergetics by the 3-mercaptopyruvate sulfurtransferase/H2S pathway: functional impairment by hyperglycemia and restoration by -α-lipoic acid. Mol Med. 2015;21:1–14. doi: 10.2119/molmed.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantú I, Philipsen S. Flicking the switch: adult hemoglobin expression in erythroid cells derived from cord blood and human induced pluripotent stem cells. Haematologica. 2014;99:1647–9. doi: 10.3324/haematol.2014.116483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence C, Atac B. Hematologic changes in massive burn injury. Crit Care Med. 1992;20:1284–8. doi: 10.1097/00003246-199209000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Schaer CA, Vallelian F, Imhof A, Schoedon G, Schaer DJ. CD163-expressing monocytes constitute an endotoxin-sensitive Hb clearance compartment within the vascular system. J Leukoc Biol. 2007;82:106–10. doi: 10.1189/jlb.0706453. [DOI] [PubMed] [Google Scholar]
- 18.Brunialti MK, et al. Increased percentages of T helper cells producing IL-17 and monocytes expressing markers of alternative activation in patients with sepsis. PLoS One. 2012;7:e37393. doi: 10.1371/journal.pone.0037393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coletta C, Szabo C. Potential role of hydrogen sulfide in the pathogenesis of vascular dysfunction in septic shock. Curr Vasc Pharmacol. 2013;11:208–21. [PubMed] [Google Scholar]
- 20.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–62. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 21.Lin T, et al. Identification of hemopexin as an anti-inflammatory factor that inhibits synergy of hemoglobin with HMGB1 in sterile and infectious inflammation. J Immunol. 2012;189:2017–22. doi: 10.4049/jimmunol.1103623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grek CL, Newton DA, Spyropoulos DD, Baatz JE. Hypoxia up-regulates expression of hemoglobin in alveolar epithelial cells. Am J Respir Cell Mol Biol. 2011;44:439–47. doi: 10.1165/rcmb.2009-0307OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Zeng M, Stamler JS. Hemoglobin induction in mouse macrophages. Proc Natl Acad Sci U S A. 1999;96:6643–7. doi: 10.1073/pnas.96.12.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W, Baker SS, Baker RD, Nowak NJ, Zhu L. Upregulation of hemoglobin expression by oxidative stress in hepatocytes and its implication in nonalcoholic steatohepatitis. PLoS One. 2011;6:e24363. doi: 10.1371/journal.pone.0024363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishi H, et al. Hemoglobin is expressed by mesangial cells and reduces oxidant stress. J Am Soc Nephrol. 2008;19:1500–8. doi: 10.1681/ASN.2007101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biagioli M, et al. Unexpected expression of alpha- and beta-globin in mesencephalic dopaminergic neurons and glial cells. Proc Natl Acad Sci U S A. 2009;106:15454–9. doi: 10.1073/pnas.0813216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gafter-Gvili A, et al. Oxidative stress-induced DNA damage and repair in human peripheral blood mononuclear cells: protective role of hemoglobin. PLoS One. 2013;8:e68341. doi: 10.1371/journal.pone.0068341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semenza GL. Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Bio-phys Acta. 2011;1813:1263–8. doi: 10.1016/j.bbamcr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang KW, Lee SJ, Kim SG. Molecular mechanism of Nrf2 activation by oxidative stress. Antioxid Redox Signal. 2005;7:1664–73. doi: 10.1089/ars.2005.7.1664. [DOI] [PubMed] [Google Scholar]
- 30.Broadwater L, et al. Analysis of the mitochondrial proteome in multiple sclerosis cortex. Biochim Biophys Acta. 2011;1812:630–41. doi: 10.1016/j.bbadis.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morimatsu H, et al. Heme Proteins, Heme Oxygenase-1 and Oxidative Stress. In: Lushchak V, Semchyshyn HM, editors. Oxidative Stress - Molecular Mechanisms and Biological Effects. Intech; Rijeka (Croatia): 2012. pp. 109–124. [Google Scholar]
- 32.Mimakia M, Wanga X, McKenzieb M, Thorburn DR, Ryan MC. Understanding mitochondrial complex I assembly in health and disease. Biochim Biophys Acta - Bioenergetics. 2012;1817:851–62. doi: 10.1016/j.bbabio.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Anson ML, Mirsky AE. On the combination of nitric oxide with hemoglobin. J Physiol. 1925;60:100–2. doi: 10.1113/jphysiol.1925.sp002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murad F, Mittal CK, Arnold WP, Katsuki S, Kimura H. Guanylate cyclase: activation by azide, nitro compounds, nitric oxide, and hydroxyl radical and inhibition by hemoglobin and myoglobin. Adv Cyclic Nucleotide Res. 1978;9:145–58. [PubMed] [Google Scholar]
- 35.Martin W, Villani GM, Jothianandan D, Furchgott RF. Selective blockade of endothelium- dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985;232:708–16. [PubMed] [Google Scholar]
- 36.Ignarro LJ, Buga GM, Byrns RE, Wood KS, Chaudhuri G. Endothelium-derived relaxing factor and nitric oxide possess identical pharmacologic properties as relaxants of bovine arterial and venous smooth muscle. J Pharmacol Exp Ther. 1988;246:218–26. [PubMed] [Google Scholar]
- 37.Minetti M, et al. Role of oxygen and carbon radicals in hemoglobin oxidation. Arch Biochem Biophys. 1993;302:233–44. doi: 10.1006/abbi.1993.1205. [DOI] [PubMed] [Google Scholar]
- 38.Deliconstantinos G, Villiotou V, Stavrides JC. Scavenging effects of hemoglobin and related heme containing compounds on nitric oxide, reactive oxidants and carcinogenic volatile nitrosocompounds of cigarette smoke. A new method for protection against the dangerous cigarette constituents. Anticancer Res. 1994;14:2717–26. [PubMed] [Google Scholar]
- 39.Ascenzi P, De Marinis E, Coletta M, Visca P. H2O2 and · NO scavenging by Mycobacterium leprae truncated hemoglobin O. Biochem Biophys Res Commun. 2008;373:197–201. doi: 10.1016/j.bbrc.2008.05.168. [DOI] [PubMed] [Google Scholar]
- 40.Nichol AW, Hendry I, Morell DB. Mechanism of formation of sulphhaemoglobin. Biochim Biophys Acta. 1968;156:97–108. doi: 10.1016/0304-4165(68)90108-6. [DOI] [PubMed] [Google Scholar]
- 41.Ríos-González BB, Román-Morales EM, Pietri R, López-Garriga J. Hydrogen sulfide activation in hemeproteins: the sulfheme scenario. J Inorg Biochem. 2014;133:78–86. doi: 10.1016/j.jinorgbio.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vitvitsky V, Yadav PK, Kurthen A, Banerjee R. Sulfide oxidation by a noncanonical pathway in red blood cells generates thiosulfate and polysulfides. J Biol Chem. 2015;290:8310–20. doi: 10.1074/jbc.M115.639831. [DOI] [PMC free article] [PubMed] [Google Scholar]