Abstract
Type 1 diabetes in mice is characterized by autoimmune destruction of insulin-producing pancreatic β-cells. Disease pathogenesis involves invasion of pancreatic islets by immune cells, including macrophages and T cells, and production of antibodies to self-antigens, including insulin. Activation of the inflammatory reflex, the neural circuit that inhibits inflammation, culminates on cholinergic receptor signals on immune cells to attenuate cytokine release and inhibit B-cell antibody production. Here, we show that galantamine, a centrally acting acetylcholinesterase inhibitor and an activator of the inflammatory reflex, attenuates murine experimental type 1 diabetes. Administration of galantamine to animals immunized with keyhole limpet hemocyanin (KLH) significantly suppressed splenocyte release of immunoglobulin G (IgG) and interleukin (IL)-4 and IL-6 during KLH challenge ex vivo. Administration of galantamine beginning at 1 month of age in nonobese diabetic (NOD) mice significantly delayed the onset of hyperglycemia, attenuated immune cell infiltration in pancreatic islets and decreased anti-insulin antibodies in serum. These observations indicate that galantamine attenuates experimental type 1 diabetes in mice and suggest that activation of the inflammatory reflex should be further studied as a potential therapeutic approach.
INTRODUCTION
Type 1 diabetes is associated with substantially increased rates of morbidity and mortality, accounting for costs exceeding $14.9 billion in health care costs in the U.S. each year (1–11). It is characterized by decreased insulin secretion and severe hyperglycemia, which can lead to ketoacidosis, coma and death. The disease pathogenesis is attributed to immune-mediated destruction of the insulin-producing β-cells of pancreatic islets. Histopathological findings in type 1 diabetes includes inflammation and destruction of pancreatic islets with infiltration of macrophages, T cells and other immune cells (12–19). Titers of autoreactive antibodies are significantly increased in patients suffering from type 1 diabetes, including antibodies specific for insulin (IAA), glutamic acid decarboxylase (GAD), protein tyrosine phosphatase (ICA512 or IA2A) and zinc transporter protein (ZnT8) (20–23). There are currently no effective treatments for type 1 diabetes. Anti–B-cell antibodies (rituximab), anti-CD3 antibodies (otelixizumab and teplizumab) targeting T cells and the Diamyd vaccine (GAD immunotherapy) have all failed to meet endpoints in recent clinical trials (24–30).
Recent advances in understanding neural control of innate immunity reveal that neural reflexes, including the inflammatory reflex and the cholinergic antiinflammatory pathway, control cytokine release and inflammation (31–39). The cholinergic antiinflammatory pathway is defined as a vagus nerve signal that culminates on T-cell acetylcholine release and activation of α7 nicotinic acetylcholine receptor (α7nAChR) on splenic macrophages, which inhibits proinflammatory cytokine release (40–44). Nerve stimulation and administration of α7 receptor agonists are in clinical development for treatment of inflammatory diseases (39,45–47). Recent evidence also links activation of the cholinergic antiinflammatory pathway to a reduction in antibody production in spleen, specifically lower antibody titers and B-cell activity during Streptococcus pneumoniae infection (48). Here we reasoned that the cholinergic antiinflammatory pathway would attenuate inflammation and serum antibody titers in murine type 1 diabetes.
Galantamine, a centrally acting acetylcholinesterase (AChE) inhibitor clinically approved to treat Alzheimer’s disease (49,50), is an activator of the cholinergic antiinflammatory pathway (35,51). We recently reported that galantamine treatment of mice with high fat diet–induced obesity significantly alleviates weigh gain, obesity-associated inflammation, hyperglycemia and insulin resistance (52). The nonobese diabetic (NOD) mouse is a model of type 1 diabetes, spontaneously developing antibodies against self-antigens, with islet infiltration starting at 3–4 wks, leading to later islet destruction and hyperglycemia around 16–18 wks (53). In addition, similar antibodies, as observed in humans, have been described in the nonobese diabetic (NOD) mouse model of type 1 diabetes (54–56). Accordingly, here we administered galantamine to NOD mice beginning at a preclinical stage and measured blood glucose and serum antibodies. We found that galantamine administration attenuates type 1 diabetes–associated hyperglycemia, confers protection against islet inflammation and decreases serum titers of diabetes-related autoimmune antibodies.
MATERIALS AND METHODS
Animals
NOD (4–5 wks old) and Balb/c (6–8 wks old) mice were obtained from The Jackson Laboratory. Food and water were available ad libitum. Mice were used in subsequent experiments after at least a 14-d adaptation period. All procedures were performed in accordance with the National Institutes of Health (NIH) guidelines (57) under protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the Feinstein Institute for Medical Research.
Cytokine and Antibody Determination
Balb/c mice were injected intraperitoneally with 100 μg keyhole limpet hemocyanin (KLH) (Calbiochem) + 50% Imject Alum (Thermo Scientific) in 200 μL saline two times, 2 wks apart. Two weeks after the second injection, 4 mg/kg galantamine was injected intraperitoneally. Splenocytes were harvested 4 h later, and erythrocytes were lysed with red blood cell lysis buffer (Sigma-Aldrich) and cultured in RPMI, HEPES, penicillin/streptomycin, l-glutamine, 1% nonessential amino acids and β-mercaptoethanol. Cells were exposed to increasing concentrations of KLH for 48 h, and media were analyzed for cytokine and antibody content. Cytokines were measured by multiplex enzyme-linked immunosorbent assay (ELISA) (Quansys), and IgG antibodies were measured by ELISA. Briefly, plates were coated with 20 μg/mL KLH antigen in phosphate-buffered saline (PBS) (Life Technologies) overnight, followed by blocking with 1% bovine serum albumin (BSA) in PBS for 2 h. Plates were washed with PBS + 0.01% Tween-20 (PBST) three times. Blood collected by capillary tube from nicked mouse tail was spun at 5,000g, and then serum was extracted and diluted 1:1,000 to 1:10,000 in PBS + BSA. A total of 100 μL diluted serum was incubated on coated plates for 1 h at room temperature and was then washed three times with PBST. A 1:2,000 dilution of horseradish peroxidase–conjugated sheep anti-mouse IgG (GE Healthcare) was added to the plates for 1 h at room temperature and was then washed again. Plates were then developed using OptEIA TMB substrate (BD Biosciences), and optical density at 450 nm was measured. Total IgE levels were determined by ELISA with rat anti-mouse IgE (BD Biosciences) coated plates, probed with biotinylated rat anti-mouse IgE antibody, followed by horseradish peroxidase–conjugated streptavidin.
Drug Administration
Five-week-old female NOD/ShiLtJ mice were injected intraperitoneally with 1 mg/kg galantamine (Calbiochem) or vehicle control in 200 μL normal saline, daily, until the end of the experiment. Blood glucose was measured once weekly by using a Freestyle Freedom Lite meter (Abbott). Mice were deemed diabetic after 2 consecutive blood glucose readings >200 mg/dL. For therapeutic experiments, NOD/ShiLtJ mice with overt diabetes (blood glucose >200 mg/dL for 2 wks in a row, 16–22 wks of age) were injected intraperitoneally with 1 mg/kg galantamine or saline, daily, with monitored blood glucose. At the end of the experiment, mice were euthanized with CO2, and pancreas, spleen and serum were collected and processed for further analyses.
Tissue Processing and Insulitis Scores
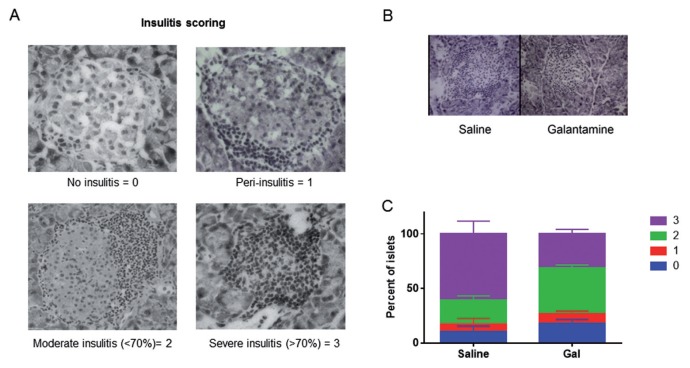
Mice were euthanized at 17 wks, and pancreas was fresh-frozen in optimal cutting temperature (O.C.T.) medium. Diabetic and nondiabetic mice were both included in the analysis of insulitis. The 5-to 7-μm slices were obtained by cryostat sectioning, mounted and fixed in acetone, allowed to dry at room temperature and stained with Mayer’s hematoxylin for 5 min. Insulitis was scored by using a bright-field microscope in double-blinded manner (blinded to both treatment groups and diabetic state). At least 50 islets from at least three disparate sections of each mouse pancreas were then scored as follows: 0, no insulitis; 1, peri-insulitis; 2, moderate (<70%) insulitis; 3, complete (>70%) insulitis (Supplemental Figure S1).
Antibody Determination
Plates were coated with antigens including insulin (human insulin; Sigma), histone II-A (from calf thymus; Sigma), myelin basic protein (MBP) (from mouse; Sigma), myelin oligodendrocyte glycoprotein (MOG) (immunodominant epitope of mouse MOG; Sigma) and deoxyribonucleic acid (DNA) (from calf thymus; Sigma). IgG levels were determined by ELISA as previously described using plates coated with each respective antigen.
Statistics
Blood glucose, KLH antibody responses and cytokine responses were analyzed by two-way analysis of variance (ANOVA) followed by Bonferroni posttest. Diabetes-onset survival curves were analyzed by log-rank (Mantel-Cox). Islet scores were analyzed by the χ2 test. All tests with a p value of <0.05 were considered statistically significant. Statistical analyses were performed by using Graphpad Prism 6 software. Unless otherwise stated, all numbers are given as average ± standard error of the mean.
All supplementary materials are available online at www.molmed.org.
RESULTS
Galantamine Attenuates Antibody Release by Splenocytes
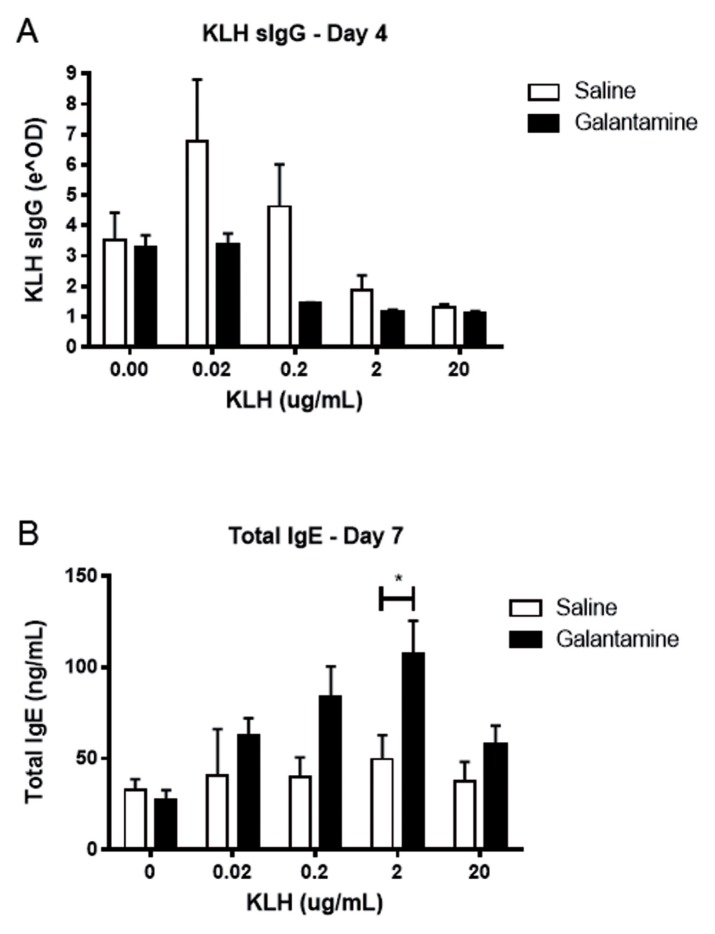
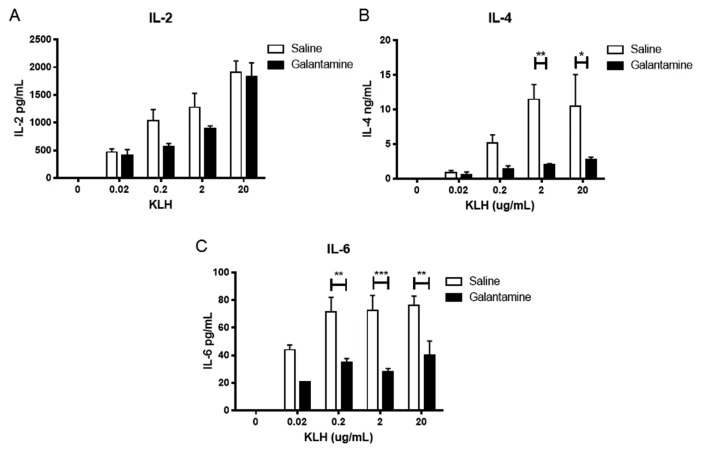
We first examined the effect of galantamine administration on splenocyte antibody release. Mice were immunized intraperitoneally with keyhole limpet hemocyanin in alum twice, 2 wks apart. Fourteen days after the second immunization, mice were injected with galantamine (4 mg/kg) or saline intraperitoneally 4 h before euthanasia, and isolated splenocytes were cultured in the presence of KLH in vitro. Splenocytes from mice administered galantamine produced less KLH-specific IgG, while producing more total IgE (Figure 1). In addition, galantamine administration resulted in reduced interleukin (IL)-4 and IL-6, but not IL-2, release by splenocytes (Figure 2). No changes in cell numbers were observed.
Figure 1.
Galantamine alters antibody responses in immunized mice. KLH immunized mice were administered galantamine (4 mg/kg) or saline 4 h before death. Splenocytes were extracted, plated at 2 × 105 in 96-well plates and incubated with indicated μg/well KLH. KLH-specific IgG (A) and total IgE (B) was determined by ELISA. Galantamine affected antibody levels (IgG, p < 0.05; IgE, p < 0.01 by two-way ANOVA; *p < 0.05 by Bonferroni posttest).
Figure 2.
Galantamine alters cytokine responses in immunized mice. KLH immunized mice were administered galantamine (4 mg/kg) or saline 4 h before death. Splenocytes were extracted, plated at 2 × 105 in 96-well plates and incubated with indicated μg/well KLH. Cytokines were determined on d 7 by Quansys multiplex ELISA. (A) Galantamine did not significantly affect IL-2. (B) IL-4 (p < 0.001) and IL-6 (p < 0.0001) were significantly different in galantamine-treated animals (two-way ANOVA; *p < 0.05, **p < 0.01, ***p < 0.001, by Bonferroni posttest).
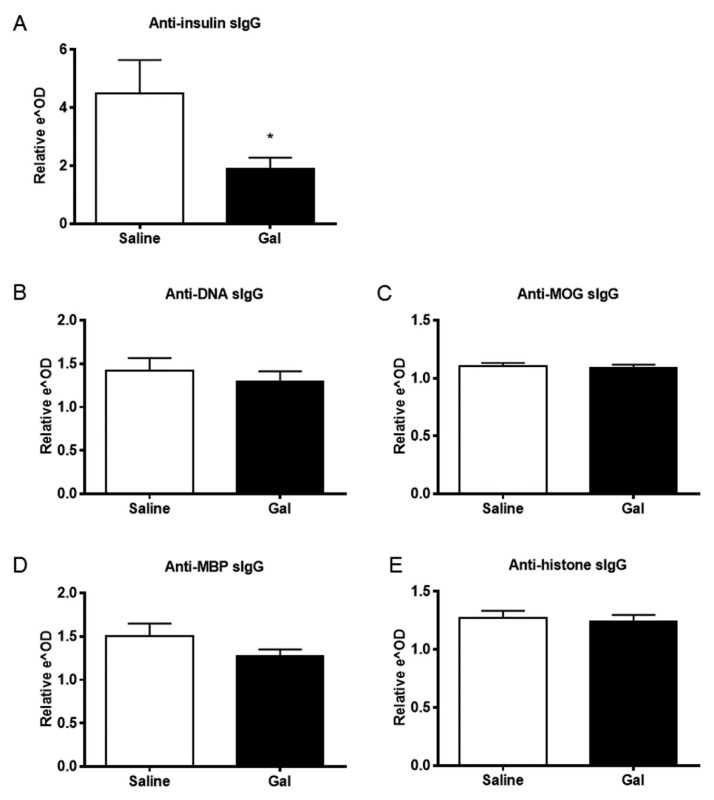
Galantamine Reduces Serum Levels of Anti-Insulin Antibodies in NOD Mice
NOD mice have elevated levels of autoantibodies (55). To investigate the effect of galantamine on serum antibody levels, titers of anti-insulin, anti-histone, anti-DNA, anti-MOG and anti-MBP antibodies were measured in galantamine-administered and control NOD mice. IgG antibodies specific to insulin were significantly reduced in galantamine-administered animals (Figure 3A), whereas antibodies directed against histone, DNA, MOG and MBP were unaffected (Figures 3B–E). These results indicate a selective suppressive effect of galantamine administration on insulin-specific antibody production in this model.
Figure 3.
Galantamine administration reduces levels of circulating pathogenic anti-insulin antibodies. Serum from galantamine- or saline-administered was applied to ELISA plates coated with antigens and then probed with anti-mouse antibodies. Serum anti-insulin antibodies were reduced in animals administered galantamine (A) (p > 0.05), but other common NOD autoantibodies were not affected (B–E).
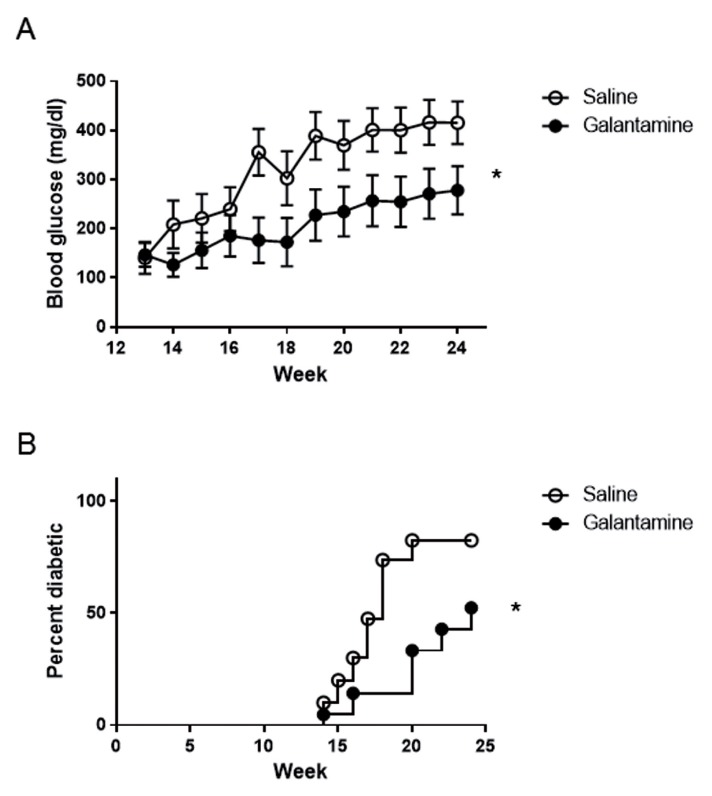
Galantamine Prevents Hyperglycemia and Diabetes Onset
To study the preventative effects of galantamine in type 1 diabetes, 4- to 5-wk-old NOD mice were administered 1 mg/kg galantamine (n = 11) or saline (n = 10) intraperitoneally, 200 μL daily for 20 wks. Blood glucose was monitored weekly to determine the onset of hyperglycemia, defined as glucose >200 mg/dL. Mice administered galantamine did not develop hyperglycemia until wk 19, whereas almost all mice administered saline rapidly developed hyperglycemia by wk 14 (Figure 4A). The average glucose levels in saline-administered animals was 226.6 ± 60.3 mg/dL by wk 14; similar levels were not reached in galantamine-administered animals until wk 19 (227.8 ± 52.3 mg/dL) (Figure 4A). Additionally, the prevalence of diabetes (two consecutive blood glucose levels >200 mg/dL) in mice administered galantamine was lower (Figure 4B, p < 0.05). Galantamine-administered mice that did develop diabetes rapidly progressed to high levels of blood glucose (data not shown).
Figure 4.
Galantamine delays the onset of hyperglycemia and diabetes. The 1 mg/kg galantamine (n = 11) or saline (n = 10) was administered intraperitoneally daily to NOD mice beginning at 5 wks of age. Blood glucose levels were measured weekly, with two successive weeks of blood glucose >199 mg/dL, indicating onset of diabetes. Administration of galantamine (A) significantly reduced the average blood glucose over time (p < 0.05, two-way ANOVA), and (B) significantly delayed onset of diabetes (p < 0.05, Mantel-Cox, results of two combined experiments).
To determine the efficacy of galantamine in treating established diabetes, NOD mice with 2 consecutive weeks of blood glucose >200 mg/dL were administered 1 mg/kg galantamine daily. Galantamine treatment did not significantly alter blood glucose levels, compared with vehicle treatment (Supplementary Figure S1). Together, these results indicate that galantamine in the context of preclinical type 1 diabetes at relatively early stages of disease progression is efficacious in delaying onset of hyperglycemia.
Galantamine Attenuates Pancreatic Islet Inflammation
To determine to effect of galantamine on immune cell infiltration in pancreatic islets, pancreata from 17-wk-old female NOD mice administered 1 mg/kg galantamine or saline intraperitoneally daily were investigated by using histochemical methods. The 7-μm sections were obtained from throughout the pancreas, at least 100 μm apart. Insulitis was scored from 0 to 3, as indicated in Figure 5A. Administration of galantamine reduced the severity of insulitis (Figure 5B) and improved the overall insulitis score in galantamine-administered animals (Figure 5C, χ2 p < 0.0005).
Figure 5.
Daily administration of galantamine decreases islet infiltration by immune cells. (A) Insulitis scoring scheme examplars. Islets with no infiltrating mononuclear cells were scored as 0, peri-islet inflammation only = 1, moderate intra-islet inflammation occupying <70% of the islet = 2, severe-complete intra-islet inflammation occupying >70% of the islet = 3. (B, C) Pancreata from 16-wk-old NOD mice injected intraperitoneally with saline or galantamine from 5 wks of age were isolated and frozen in OCT media and then sliced at 10 μm. At least 50 total islets from three disparate areas of each pancreas were scored blindly. Administration of galantamine (B) reduced the severity of insulitis in NOD mice (representative images), as well as (C) improved the overall level of insulitis in the pancreas (n = 5, χ2 p < 0.0005).
DISCUSSION
Here, we show that administration of the acetylcholinesterase inhibitor galantamine (35,51,58) delays elevation of blood glucose levels and onset of diabetes in the NOD model. In this disease model, galantamine reduces pancreatic islet inflammation and lowers disease-specific antibody levels without reducing levels of other tested IgG antibodies. Furthermore, splenocytes derived from galantamine-administered mice released less IL-4, IL-6 and IgG in response to antigen re-exposure in vitro.
The observation that splenocytes derived from galantamine-administered mice released less IgG and higher IgE levels in response to immunized antigen in vitro suggests that galantamine stimulates class-switching of B cells from IgG to IgE. In addition, the lower IL-4 and IL-6 secretion by splenocytes from galantamine-administered animals after antigen exposure suggests an environment less supportive of B-cell antibody release, including suppression of Th2 helper T cells. These findings highlight a previously unrecognized antiinflammatory effect of galantamine in preclinical type 1 diabetes.
Autoimmunity in NOD mice is initiated in the pancreatic lymph nodes, but not in the spleen, at 2–3 wks of age (59). Treatment starting from 5 wks of age could influence a mechanism that slows the progression of insulitis and ultimately the onset of clinical diabetes separate from the initial priming for autoimmunity to islet autoantigens. We examined cytokine content by ELISA from pancreatic samples at 12 wks of age after 8 wks of galantamine administration. The lysate of these samples included embedded pancreatic lymph nodes. We found no difference in levels of interferon (IFN)-γ, IL-17, monocyte chemotactic protein 1 (MCP-1) or IL-1β (data not shown).
Changes in cellular populations could be an important component of galantamine’s antiinflammatory effects. To examine changes in populations, we obtained single cell isolates from both spleen and total pancreas, including lymph nodes, taken from mice administered galantamine for 8 wks. We examined CD3+CD4+, CD3+CD4+FoxP3+ and CD3+CD8+ populations by flow cytometry. We observed no difference in percentages of these populations in treated animals compared with vehicle control in both spleen and pancreas (data not shown). Fewer cells were observed in the pancreata of galantamine-treated animals (data not shown). It is plausible that galantamine may suppress the inflammatory activity of the immune cells, but does not alter the immune cell composition. The specific effect of galantamine on cellular function remains to be elucidated, and additional analyses would provide useful data.
Galantamine has previously been shown to exert antiinflammatory effects through brain-mediated and vagus nerve–dependent signaling (35,51). The vagus nerve innervates the pancreas, and vagus nerve cholinergic output controls pancreatic endocrine and exocrine secretion (60–62). A role for the vagus nerve has also been shown in suppressing pancreatic inflammation; surgical transection of the vagus nerve (vagotomy) results in exacerbated murine pancreatitis, thus indicating a tonic antiinflammatory role of these innervations (63). The vagus nerve also innervates the liver, and it is known that vagus nerve–mediated signaling suppresses hepatic glucose production (64–66), one of the main determinants of fasting blood glucose levels. We suggest that galantamine-mediated activation of the vagus nerve reduces pancreatic inflammation and delays onset of diabetes. In this context, it is possible that metabolic effects of vagus nerve activation on hepatic glucose release may also play a role (67). Future studies should address the role of these and other mechanisms in mediating galantamine efficacy in type 1 diabetes.
CONCLUSION
Galantamine is in clinical use for treatment of Alzheimer’s disease patients in the United States and has been used for decades in Europe in treating myasthenia gravis and Alzheimer’s disease and in children with autism spectrum disorders (49,50,68). This abundant clinical experience in adult and pediatric populations should facilitate potential experimental use of galantamine in the treatment of type 1 diabetes. Other therapeutic approaches, including β-cell transplantation or β-cell regeneration have been explored (69–76). In light of our current findings, it would be interesting to further study whether galantamine treatment in combination with islet restoration would improve clinical outcomes in established type 1 diabetes.
Supplemental Data
ACKNOWLEDGMENTS
This work was supported by a grant from the Juvenile Diabetes Research Fund and the following grants from the National Institute of General Medical Sciences, National Institutes of Health: R01GM057226 (to KJ Tracey) and R01GM089807 (to KJ Tracey and VA Pavlov).
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Cite this article as: Hanes WM, et al. (2015) Galantamine attenuates type 1 diabetes and inhibits anti-insulin antibodies in nonobese diabetic mice. Mol. Med. 21:702–8.
REFERENCES
- 1.Orchard TJ, et al. Type 1 diabetes and coronary artery disease. Diabetes Care. 2006;29:2528–38. doi: 10.2337/dc06-1161. [DOI] [PubMed] [Google Scholar]
- 2.Secrest AM, et al. All-cause mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes: the Allegheny County type 1 diabetes registry. Diabetes Care. 2010;33:2573–9. doi: 10.2337/dc10-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soedamah-Muthu SS, et al. All-cause mortality rates in patients with type 1 diabetes mellitus compared with a non-diabetic population from the UK general practice research database, 1992–1999. Diabetologia. 2006;49:660–6. doi: 10.1007/s00125-005-0120-4. [DOI] [PubMed] [Google Scholar]
- 4.Laing SP, et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia. 2003;46:760–5. doi: 10.1007/s00125-003-1116-6. [DOI] [PubMed] [Google Scholar]
- 5.Laing SP, et al. The British Diabetic Association Cohort Study, I: all-cause mortality in patients with insulin-treated diabetes mellitus. Diabet Med. 1999;16:459–65. doi: 10.1046/j.1464-5491.1999.00075.x. [DOI] [PubMed] [Google Scholar]
- 6.Skrivarhaug T, et al. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia. 2006;49:298–305. doi: 10.1007/s00125-005-0082-6. [DOI] [PubMed] [Google Scholar]
- 7.Secrest AM, et al. Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes. 2010;59:3216–22. doi: 10.2337/db10-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Livingstone SJ, et al. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med. 2012;9:e1001321. doi: 10.1371/journal.pmed.1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lind M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371:1972–82. doi: 10.1056/NEJMoa1408214. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. National diabetes statistics report, 2014: estimates of diabetes and its burden in the United States. Atlanta (GA): US Department of Health and Human Services; 2014. p. 12. Available from: http://www.cdc.gov/diabetes/data/statistics/2014statisticsreport.html. [Google Scholar]
- 11.Dall TM, et al. Distinguishing the economic costs associated with type 1 and type 2 diabetes. Popul Health Manag. 2009;12:103–10. doi: 10.1089/pop.2009.12203. [DOI] [PubMed] [Google Scholar]
- 12.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–9. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 13.Nelson P, et al. Modeling dynamic changes in type 1 diabetes progression: quantifying beta-cell variation after the appearance of islet-specific autoimmune responses. Math Biosci Eng. 2009;6:753–78. doi: 10.3934/mbe.2009.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morran MP, Omenn GS, Pietropaolo M. Immunology and genetics of type 1 diabetes. Mt Sinai J Med. 2008;75:314–27. doi: 10.1002/msj.20052. [DOI] [PubMed] [Google Scholar]
- 15.Kay TW, Campbell IL, Harrison LC. Characterization of pancreatic T lymphocytes associated with beta cell destruction in the non-obese diabetic (NOD) mouse. J Autoimmun. 1991;4:263–76. doi: 10.1016/0896-8411(91)90023-6. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins TA, Gala RR, Dunbar JC. The lymphocyte and macrophage profile in the pancreas and spleen of NOD mice: percentage of interleukin-2 and prolactin receptors on immunocompetent cell subsets. J Reprod Immunol. 1996;32:55–71. doi: 10.1016/s0165-0378(96)00986-2. [DOI] [PubMed] [Google Scholar]
- 17.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–85. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 18.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91:79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- 19.Pugliese A, et al. Self-antigen–presenting cells expressing diabetes-associated autoantigens exist in both thymus and peripheral lymphoid organs. J Clin Invest. 2001;107:555–64. doi: 10.1172/JCI10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miao D, Yu L, Eisenbarth GS. Role of autoantibodies in type 1 diabetes. Front Biosci. 2007;12:1889–98. doi: 10.2741/2195. [DOI] [PubMed] [Google Scholar]
- 21.Knip M, et al. Environmental triggers and determinants of type 1 diabetes. Diabetes. 2005;54(Suppl 2):S125–36. doi: 10.2337/diabetes.54.suppl_2.s125. [DOI] [PubMed] [Google Scholar]
- 22.Taplin CE, Barker JM. Autoantibodies in type 1 diabetes. Autoimmunity. 2008;41:11–8. doi: 10.1080/08916930701619169. [DOI] [PubMed] [Google Scholar]
- 23.Wenzlau JM, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A. 2007;104:17040–5. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pescovitz MD, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361:2143–52. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wherrett DK, et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet. 2011;378:319–27. doi: 10.1016/S0140-6736(11)60895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludvigsson J, et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med. 2012;366:433–42. doi: 10.1056/NEJMoa1107096. [DOI] [PubMed] [Google Scholar]
- 28.Bach JF. Anti-CD3 antibodies for type 1 diabetes: beyond expectations. Lancet. 2011;378:459–60. doi: 10.1016/S0140-6736(11)60980-X. [DOI] [PubMed] [Google Scholar]
- 29.Orban T, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–9. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherry N, et al. Teplizumab for treatment of type 1 diabetes (Protege study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378:487–97. doi: 10.1016/S0140-6736(11)60931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 32.Pavlov VA, et al. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med. 2003;9:125–34. [PMC free article] [PubMed] [Google Scholar]
- 33.Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun. 2005;19:493–9. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Pavlov VA, et al. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Natl Acad Sci U S A. 2006;103:5219–23. doi: 10.1073/pnas.0600506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pavlov VA, et al. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun. 2009;23:41–5. doi: 10.1016/j.bbi.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–96. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parrish WR, et al. Modulation of TNF release by choline requires α7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol Med. 2008;14:567. doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–28. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity. J Exp Med. 2012;209:1057–68. doi: 10.1084/jem.20120571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–8. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 41.Rosas-Ballina M, et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A. 2008;105:11008–13. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosas-Ballina M, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bencherif M, et al. Alpha7 nicotinic receptors as novel therapeutic targets for inflammation-based diseases. Cell Mol Life Sci. 2011;68:931–49. doi: 10.1007/s00018-010-0525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olofsson PS, et al. a7 Nicotinic acetylcholine receptor (a7nAChR) expression in bone marrow–derived non–T cells is required for the inflammatory reflex. Mol Med. 2012;18:539–43. doi: 10.2119/molmed.2011.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mabley JG, et al. Nicotine reduces the incidence of type I diabetes in mice. J Pharmacol Exp Ther. 2002;300:876–81. doi: 10.1124/jpet.300.3.876. [DOI] [PubMed] [Google Scholar]
- 46.Olofsson PS, et al. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev. 2012;248:188–204. doi: 10.1111/j.1600-065X.2012.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zitnik RJ. Treatment of chronic inflammatory diseases with implantable medical devices. Cleve Clin J Med. 2011;78(Suppl 1):S30–4. doi: 10.3949/ccjm.78.s1.05. [DOI] [PubMed] [Google Scholar]
- 48.Mina-Osorio P, et al. Neural signaling in the spleen controls B-cell responses to blood-borne antigen. Mol Med. 2012;18:618–27. doi: 10.2119/molmed.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reichman WE. Current pharmacologic options for patients with Alzheimer’s disease. Ann Gen Hosp Psychiatry. 2003;2:1. doi: 10.1186/1475-2832-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ellis JM. Cholinesterase inhibitors in the treatment of dementia. J Am Osteopath Assoc. 2005;105:145–58. [PubMed] [Google Scholar]
- 51.Waldburger JM, et al. Spinal p38 MAP kinase regulates peripheral cholinergic outflow. Arthritis Rheum. 2008;58:2919–21. doi: 10.1002/art.23807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Satapathy SK, et al. Galantamine alleviates inflammation and other obesity-associated complications in high-fat diet-fed mice. Mol Med. 2011;17:599–606. doi: 10.2119/molmed.2011.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–85. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 54.Abiru N, et al. Transient insulin autoanti-body expression independent of development of diabetes: comparison of NOD and NOR strains. J Autoimmun. 2001;17:1–6. doi: 10.1006/jaut.2001.0530. [DOI] [PubMed] [Google Scholar]
- 55.Quintana FJ, Cohen IR. Autoantibody patterns in diabetes-prone NOD mice and in standard C57BL/6 mice. J Autoimmun. 2001;17:191–7. doi: 10.1006/jaut.2001.0544. [DOI] [PubMed] [Google Scholar]
- 56.Tisch R, et al. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993;366:72–5. doi: 10.1038/366072a0. [DOI] [PubMed] [Google Scholar]
- 57.Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and Life Studies, National Research Council of the National Academies. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: National Academies Press; 2011. [Google Scholar]
- 58.Ji H, et al. Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunol. 2014;7:335–47. doi: 10.1038/mi.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jaakkola I, Jalkanen S, Hänninen A. Diabetogenic T cells are primed both in pancreatic and gut-associated lymph nodes in NOD mice. Eur J Immunol. 2003;33:3255–64. doi: 10.1002/eji.200324405. [DOI] [PubMed] [Google Scholar]
- 60.Holst JJ, et al. Autonomic nervous control of the endocrine secretion from the isolated, perfused pig pancreas. J Auton Nerv Syst. 1986;17:71–84. doi: 10.1016/0165-1838(86)90045-7. [DOI] [PubMed] [Google Scholar]
- 61.Miller RE. Pancreatic neuroendocrinology: peripheral neural mechanisms in the regulation of the Islets of Langerhans. Endocr Rev. 1981;2:471–94. doi: 10.1210/edrv-2-4-471. [DOI] [PubMed] [Google Scholar]
- 62.Woods SC, Porte D., Jr Neural control of the endocrine pancreas. Physiol Rev. 1974;54:596–619. doi: 10.1152/physrev.1974.54.3.596. [DOI] [PubMed] [Google Scholar]
- 63.van Westerloo DJ, et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–30. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 64.Yi CX, et al. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim Biophys Acta. 2010;1802:416–31. doi: 10.1016/j.bbadis.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Owyang C, Heldsinger A. Vagal control of satiety and hormonal regulation of appetite. J Neurogastroenterol Motil. 2011;17:338–48. doi: 10.5056/jnm.2011.17.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang PY, et al. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature. 2008;452:1012–6. doi: 10.1038/nature06852. [DOI] [PubMed] [Google Scholar]
- 67.Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex: linking immunity and metabolism. Nat Rev Endocrinol. 2012;8:743–54. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nicolson R, Craven-Thuss B, Smith J. A prospective, open-label trial of galantamine in autistic disorder. J Child Adolesc Psychopharmacol. 2006;16:621–9. doi: 10.1089/cap.2006.16.621. [DOI] [PubMed] [Google Scholar]
- 69.Chakravarthy BK, Gupta S, Gode KD. Functional beta cell regeneration in the islets of pancreas in alloxan induced diabetic rats by (−)-epicatechin. Life Sci. 1982;31:2693–7. doi: 10.1016/0024-3205(82)90713-5. [DOI] [PubMed] [Google Scholar]
- 70.Montana E, Bonner-Weir S, Weir GC. Beta cell mass and growth after syngeneic islet cell transplantation in normal and streptozocin diabetic C57BL/6 mice. J Clin Invest. 1993;91:780–7. doi: 10.1172/JCI116297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoon K-H, et al. Differentiation and expansion of beta cell mass in porcine neonatal pancreatic cell clusters transplanted into nude mice. Cell Transplant. 1998;8:673–89. doi: 10.1177/096368979900800613. [DOI] [PubMed] [Google Scholar]
- 72.Xu G, et al. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–6. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 73.Shapiro AM, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 74.Ramiya VK, et al. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med. 2000;6:278–82. doi: 10.1038/73128. [DOI] [PubMed] [Google Scholar]
- 75.Ryan EA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–9. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 76.Gibly RF, et al. Advancing islet transplantation: from engraftment to the immune response. Diabetologia. 2011;54:2494–505. doi: 10.1007/s00125-011-2243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.