Abstract
Extracellular high-mobility group box 1 (HMGB1) (disulfide form), via activation of toll-like receptor 4 (TLR4)-dependent signaling, is a strong driver of pathologic inflammation in both acute and chronic conditions. Identification of selective inhibitors of HMGB1-TLR4 signaling could offer novel therapies that selectively target proximal endogenous activators of inflammation. A cell-based screening strategy led us to identify first generation HIV-protease inhibitors (PI) as potential inhibitors of HMGB1-TLR4 driven cytokine production. Here we report that the first-generation HIV-PI saquinavir (SQV), as well as a newly identified mammalian protease inhibitor STO33438 (334), potently block disulfide HMGB1-induced TLR4 activation, as assayed by the production of TNF-α by human monocyte-derived macrophages (THP-1). We further report on the identification of mammalian cathepsin V, a protease, as a novel target of these inhibitors. Cellular as well as recombinant protein studies show that the mechanism of action involves a direct interaction between cathepsin V with TLR4 and its adaptor protein MyD88. Treatment with SQV, 334 or the known cathepsin inhibitor SID26681509 (SID) significantly improved survival in murine models of sepsis and reduced liver damage following warm liver ischemia/reperfusion (I/R) models, both characterized by strong HMGB1-TLR4 driven pathology. The current study demonstrates a novel role for cathepsin V in TLR4 signaling and implicates cathepsin V as a novel target for first-generation HIV-PI compounds. The identification of cathepsin V as a target to block HMGB1-TLR4-driven inflammation could allow for a rapid transition of the discovery from the bench to the bedside. Disulfide HMGB1 drives pathologic inflammation in many models by activating signaling through TLR4. Cell-based screening identified the mammalian protease cathepsin V as a novel therapeutic target to inhibit TLR4-mediated inflammation induced by extracellular HMGB1 (disulfide form). We identified two protease inhibitors (PIs) that block cathepsin V and thereby inhibit disulfide HMGB1-induced TLR4 activation: saquinavir (SQV), a first-generation PI targeting viral HIV protease and STO33438 (334), targeting mammalian proteases. We discovered that cathepsin V binds TLR4 under basal and HMGB1-stimulated conditions, but dissociates in the presence of SQV over time. Thus cathepsin V is a novel target for first-generation HIV PIs and represents a potential therapeutic target of pathologic inflammation.
INTRODUCTION
High-mobility group box 1 (HMGB1) regulates cell functions in a diverse and compartment-specific manner (1,2). These include the regulation of gene transcription and DNA repair responses in the nucleus, nucleic acid sensing and cell metabolism in the cytoplasm and mitochondria and complex messenger functions in the extracellular space (1,2). HMGB1 released from cells either passively (that is, during necrosis) or actively (that is, via secretion) triggers signaling through numerous receptors to regulate cytokine production, cell chemotaxis and tissue regeneration (1,2). The pleotropic nature of HMGB1-receptor interactions involves at least two mechanisms. First, HMGB1 interacts with a wide range of other ligands, such as CXCL12 (3) and IL-1 (4) to enhance the interaction of the complex with the cognate receptor of the ligand-binding partner (3). Second, the redox status of the three cysteines (C23, C45 and C106) within HMGB1 determines both the efficiency of binding to other ligands as well as the specificity of HMGB1 for certain receptors (5). For example, when in the all-thiol configuration, HMGB1 binds to CXCL12 (SDF1) to amplify the activation of CXCR4 on immune and progenitor cells promoting chemotaxis. In contrast, under mild oxidizing conditions, C23 and C45 can form a disulfide bond and, if C106 remains in the thiol configuration, HMGB1 binds avidly to MD2 to activate the MD2/toll-like receptor 4 (TLR4) receptor complex leading to inflammatory signaling (6). This isoform is called the disulfide form of HMGB1 (7).
Experimental studies suggest that excessive or sustained HMGB1-TLR4 activation can lead to immunopathology in the acute setting. This has been observed in ischemia/reperfusion (I/R) (8), hemorrhagic shock (9), trauma (10), NSAID toxicity (11), acute lung injury (12) and islet survival after transplantation (13); and in the more chronic setting of inflammation associated with epileptic seizures (14), pulmonary arterial hypertension, (15) as well as responses to chemotherapy (16). Thus, inhibitors that limit HMGB1-mediated TLR4 activation would be beneficial in a number of clinically relevant settings. The discovery that disulfide HMGB1 is the isoform that triggers TLR4/MD2 signaling allowed us to screen for agents that suppress disulfide HMGB1-induced TNF-α production by macrophages (17). A screen of 5,546 FDA-approved drugs yielded first generation HIV-protease inhibitors (PI) as effective inhibitors (18).
As shown in the current report, our search for mammalian targets of first generation HIV-PI involved in HMGB1-induced TLR4 activation identified cathepsin V (also called cathepsin L2) as both susceptible to inhibition by HIV-PI and required for TLR4 signaling. These findings open the way for the therapeutic repurposing of saquinavir and other HIV-PI inhibitors for the experimental treatment of inflammation.
MATERIALS AND METHODS
Reagents
OPTI-MEM (Invitrogen) was used as treatment buffer. Recombinant rat HMGB1, expressed in E. coli, was provided by KJ Tracey (24). LPS was an InvivoGen product. STO33438 (5-nitro-2-[(5-[phenoxymethyl]-4-phenyl-1,2,4-triazol-3-yl)sulfanyl]pyridine), (Timtec) and SID26681509 (SID; Tocris) were dissolved to 10 mmol/L in 100% DMSO. All the HIV protease inhibitors were provided by Y Al-Abed.
Animals
Male C57BL/J6 or BALB/c mice were from The Jackson Laboratory. All animal procedures were carried out in accordance with the guidelines set by the University of Pittsburgh or Feinstein Institute’s Institutional Animal Care and Use Committee (IACUC) and with IACUC approval.
Antibodies
Anti-human cathepsin V (MAB10801, R&D Systems), anti-HA (ab-hatag, In-vivoGen), anti-human TLR4 (IMG-578A, IMGENEX), anti-human MyD88 (4283, Cell Signaling), anti-human IRAK4 (4363, Cell Signaling), anti-human IRAK1 (4504, Cell Signaling).
Cell Culture and Plasmids
Peritoneal macrophages were isolated and cultured as described previously (17). Human monocytic THP-1 cells were differentiated for 12 h with 200 ng/mL PMA. HEK293 (ATCC) and HEK293 stably transfected with CD14/MD2 and TLR4-HA (InvivoGen) were grown at 37°C and 5% CO2, in DMEM (10% FBS, 2 mmol/L L-GLN, 100 U/mL PS, 1 mmol/L NaP, 100 μg/mL Normocin) (InvivoGen).
Plasmids
pUNO-hTLR4-HA plasmid expressing the human TLR4 gene fused at the 3’ end to the influenza hemaglutinine (HA) and pDUO-MD2/CD14 plasmid coexpressing MD2 and CD14 genes (InvivoGen) were transfected into HEK293 cells using Genejammer transfection agent (Agilent) and Blasticidin (InvivoGen).
Histology
Formalin-fixed, paraffin-embedded livers were sectioned, hematoxylineosin stained and assessed for tissue damage.
Coimmunoprecipitation and Immunoblot
Cells were lysed in 1% NP-40, 50 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 10% glycerol and protease inhibitors (Sigma-Aldrich, Roche). After clearing by centrifugation, total protein content was quantified using a BCA kit and 1000 μg were immunoprecipitated (IP) overnight using anti-mouse/rabbit beads (Rockland) and corresponding antibodies. IP and input fractions were subjected to SDS-PAGE and Western blot analysis.
LDH Cytotoxicity
LDH cytotoxicity was performed according to the manufacturer’s instructions (LDH kit, Takara Bio).
Cytokine and Chemokine Release
Cell supernatants were assayed by enzyme-linked immunosorbent assay (ELISA) for TNF-α, IL-6 (R&D Systems) according to the manufacturer’s instructions. IL-12p40, IP-10, MCP-1, MIP-1α and KC were determined by Luminex (Millipore).
Cecal Ligation and Puncture (CLP)
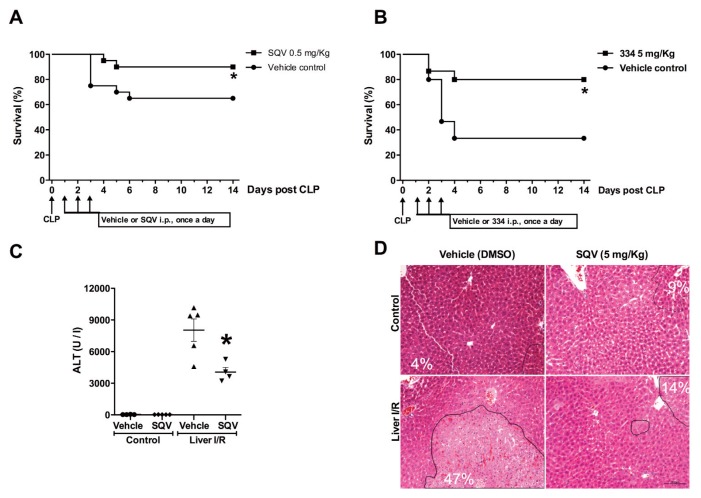
To establish live intraabdominal infection and sepsis, mice were subjected to the CLP procedure (19). After intramuscular anesthesia (ketamine: 75 mg/kg, Fort Dodge Lab and xlyazine: 20 mg/kg, Boehringer Ingelheim), a 15-mm midline incision was made to expose the cecum. After ligation 5.0 mm from the tip, the cecal stump was punctured once with a 22G needle and a small amount of stool extruded. The cecum was placed back into its normal position and the wound closed with two layers of running suture. All animals received saline solution (0.9% NaCl, 20 mL/kg of body weight.) resuscitation and a single dose of antibiotic (NaCl 0.5 mg imipenem/mouse in saline; Primaxin, Merck) 30 min after the surgery. Saquinavir (SQV), 334 was given intraperitoneal (IP) each day for 3 d starting at 24 h after CLP surgery. Controls mice received injection of vehicles. Survival was monitored for 2 wks.
Warm Liver Ischemia/Reperfusion
A midline laparotomy was performed in anesthetized mice (ketamine 100 mg/kg and xylazine 10 mg/kg) and the hepatic lobes freed by dividing the surrounding ligaments. By using a microatraumatic vascular clamp (Fine Science Tools), 70% segmental ischemia was achieved, whereas the blood supply to the remaining liver lobes remained patent to avoid intestinal venous congestion. The incision was covered with a piece of saline-soaked gauze and plastic film to reduce dehydration, and mice were placed on a heating pad to maintain a rectal temperature of 37°C. The clamp was removed after 60 min to initiate hepatic reperfusion and the abdominal incision was closed with running 4-0 Vicryl-plus sutures (Ethicon). Sham-treated mice underwent the same manipulation, except that the vascular clamp was not applied (20).
Surface-Plasmon Resonance Analysis (BIAcore)
Analysis of binding of recombinant human cathepsin V (1080-CY-010, R&D Systems) to recombinant human TLR4 (1478-TR-050, R&D Systems) was conducted using BIAcore 3000 instrument (BIAcore Inc.). Binding reactions were done in HBS-EP buffer (BIAcore Inc.). The CM5 dextran chip (flow cell 2) was activated with an injection of 35 μL of 0.1 mol/L N-ethyl-N′-[3-diethylaminopropyl]-carbodiimide and 0.1 mol/L N-hydroxysuccinimide. TLR4 protein was coated on the surface of a CM5 dextran sensor chips by direct immobilization. An aliquot of 100 μL of 10 μg/mL dilution of human TLR4 protein in 10 mmol/L NaOAc, pH 4.5 was injected into flow cell-2 of CM5 chip to get the levels of 650 resonance units for the immobilization, followed by 35 μL injection of 1 mol/L ETA, pH 8.2 to block the remaining active sites. The flow cell-1, without immobilized TLR4-coated, was activated, blocked and evaluated for nonspecific binding. Binding analyses were performed at a flow rate of 30 μL/min at 25°C. To evaluate binding, the analytes (60 μL of cathepsin V, 0–10 μmol/L) were injected into flow cell-1 and -2 and the association of analyte and ligand was recorded respectively by surface-plasmon resonance. The signal from the blank channel (flow cell-1) was subtracted from the channel containing purified TLR4 proteins (cell 2). Results were analyzed using the software BIAeval 3.2 (BIAcore Inc.). For all samples, a blank injection with buffer alone was subtracted from the resulting reaction surface data. Data were globally fitted to the Lagmuir model for a 1:1 binding (21).
siRNA Transfection of THP-1 Cells
siCathepsin V (4392420) and siControl (Ambion) were reconstituted to 100 μmol/L. Cells were transfected using Lipofectamine2000 (Invitrogen) according to the manufacturer’s instructions (20 nmol/L siRNA) and differentiated after 12 h with PMA (100 nmol/L for 2 h), rinsed and incubated O/N before treatment.
Assessment of Cathepsin V Activity
Recombinant cathepsin V, reconstituted in 25 mmol/L MES, 5 mmol/L DTT, pH 5.5 was kept on ice for 20 min. The reconstituted enzyme was mixed with vehicle (DMSO 0.1%) or increasing concentrations of inhibitor (0.1; 0.3; 1; 3; 10; 30 μmol/L) followed by 20 min incubation on ice. The fluorogenic peptide substrate Z-LR-AMC was added and activity assessed for 10 min using a spectrophotometer. Final amount of cathep-sin V was 8 ng/well and 10 μmol/L for substrate. The IC50 values were calculated using GraphPad Prism 6.
Statistical Analysis
Data are expressed as means ± SEM from at least two or three independent experiments. One-way analysis of variance (ANOVA) with Tukey post hoc test was used for comparison among all different groups. Survival curves were analyzed by Fisher exact test in Figure 4. A P value of < 0.05 was considered statistically significant: *P < 0.05; **P < 0.01; #P < 0.001, unless otherwise indicated.
Figure 4.
Inhibition of cathepsin is protective in in vivo models known to be driven via the HMGB1-TLR4 axis (A,B) Survival curves of male BALB/c mice that underwent cecal ligation and puncture (CLP). SQV or 334 was administered at 0.5 mg (SQV) or 5 mg (334) per mouse (IP in 200 μL PBS-DMSO) per day for 3 d starting at 24 h after CLP surgery. Control mice in received vehicle (PBS-DMSO) only. Animal survival was monitored for up to 2 wks. N = 20 mice per group. *P < 0.05 versus control group. (C-D) C57BL6/J mice underwent 70% warm liver ischemia for 1 h followed by reperfusion for 6 h. Mice were treated with SQV or vehicle 1 h prior to ischemia and again at the time of reperfusion. SQV attenuated liver I/R injury as measured by (C) plasma ALT levels and (D) histological examination of H&E-stained liver sections with indicated percent necrosis quantitated in each image.
All supplementary materials are available online at www.molmed.org.
RESULTS
First-Generation HIV-Protease Inhibitors Block HMGB1-Induced TNF-α Production
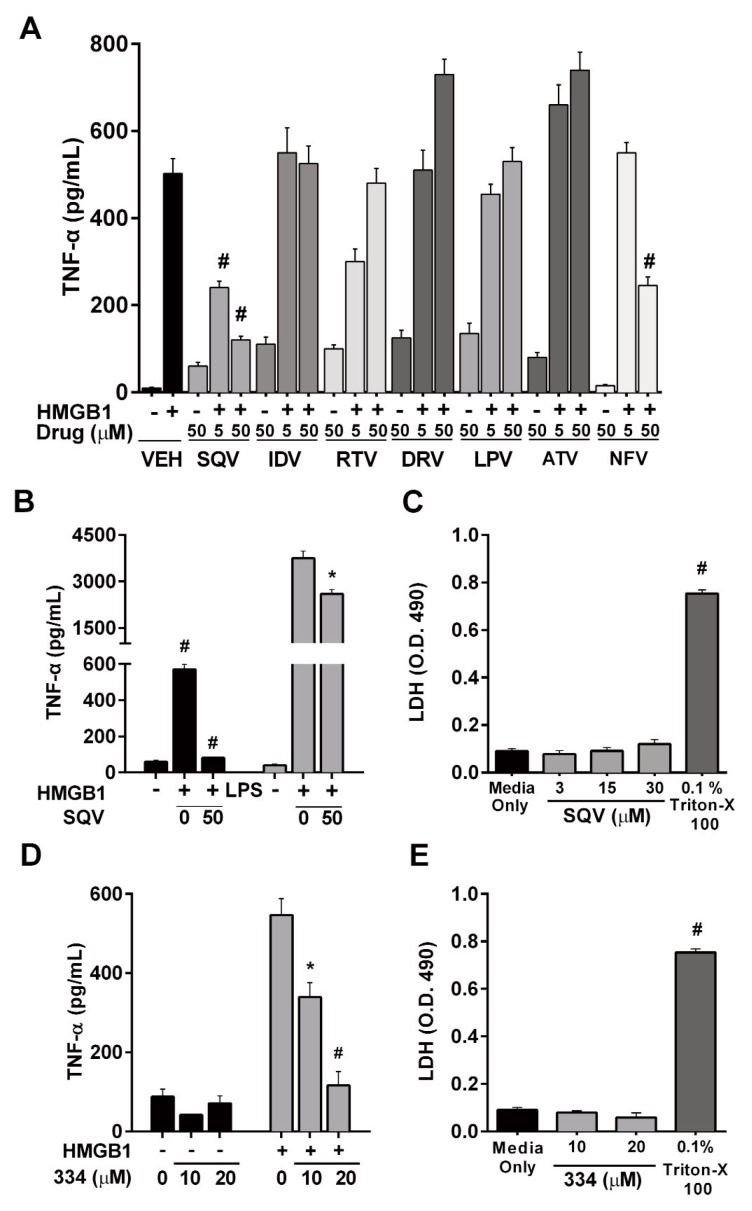
Our recent screening of 5,546 pharmacologically active compounds and clinically used drugs identified several classes of compounds, including HIV-protease inhibitors as potent inhibitors of disulfide HMGB1-induced TNF-α production in RAW 246.7 macrophages (18). Therefore, we tested the capacity of seven HIV-protease inhibitors currently in clinical use, to inhibit TNF-α production in response to disulfide HMGB1 in cultured human monocyte-derived-macrophages. These cells are human THP-1 cells stimulated with PMA to allow for differentiation into macrophages. SQV and nelfinavir (NFV), two first generation HIV-protease inhibitors, were most effective in suppressing TNF-α production (Figure 1A). A similar profile was observed in parallel experiments using freshly isolated mouse peritoneal macrophages. These cells also responded to SQV and nelfinavir as well as indinavir (Supplementary Figure S1A).
Figure 1.
Protease inhibitor screen in human monocyte-derived macrophages identifies Saquinavir (SQV) and ST033438 (334) as potent inhibitors of HMGB1-induced TNF-α production. (A) The effect of various first generation HIV protease inhibitors on HMGB1-induced TNF-α production as assessed by ELISA (VEH, vehicle; SQV, saquinavir; IDV, indinavir; RTV, ritonavir; DRV, darunavir; LPV, lopinavir; ATV, atazanavir; NFV, nelfinavir) after 1 h of drug pre-treatment followed by 18 h HMGB1 stimulation (B) Effect of SQV on HMGB1-induced versus LPS-induced TNF-α production in human monocyte-derived macrophages. (C,E) Cytotoxi-city of SQV and 334 was determined by measuring LDH release into cell media after 18 h of treatment. (D) Effect of STO33438 (334) on HMGB1-induced TNF-α in human monocyte-derived macrophages.
Because SQV exhibited the greatest potency, we further characterized its inhibitory activity and mechanism of action. SQV also suppressed, but to a lesser extent, TNF-α production in response to LPS, another TLR4 agonist in human monocyte-derived macrophages (Figure 1B) and did not induce toxicity (Figure 1C). In parallel experiments using freshly isolated mouse peritoneal macrophages, SQV not only inhibited the HMGB1-stimulated production of TNF-α but also IL-6, IL-12p40, IP10, MCP-1 and MIP-1α, without inducing toxicity (Supplementary Figures S1B–E).
Screening of Known Protease Inhibitors Identifies STO33438
HIV-protease inhibitors were designed to block the viral HIV protease. We hypothesized that the observed effects of HMGB1-induced inflammation relate to their off-target action on a mammalian protease involved in HMGB1-TLR4 signaling. To aid in the identification of this putative mammalian protease target we screened the TimTec ACTITARG-P library composed of known inhibitors of mammalian proteases. Of the 1,520 inhibitors tested in Raw 264.7 cells, we identified three hit compounds: ST033438 (334), ST057867 (578) and ST056797 (567) that were able to inhibit HMGB1-induced TNF-α production by 45.1%, 41.3% and 36.2% respectively (Supplementary Table 1). We further confirmed that 334 blocked HMGB1-induced TNF-α production in human monocyte-derived macrophages and does not induce toxicity (Figures 1D, E). We obtained parallel results in freshly isolated mouse peritoneal macrophages (Supplementary Figures S1F, G). Like SQV, the inhibitory activity of 334 was not limited to TNF-α but included several other cytokines and chemokines (Supplementary Figure 2F).
Identification of Cathepsin V as a Target for SQV and 334
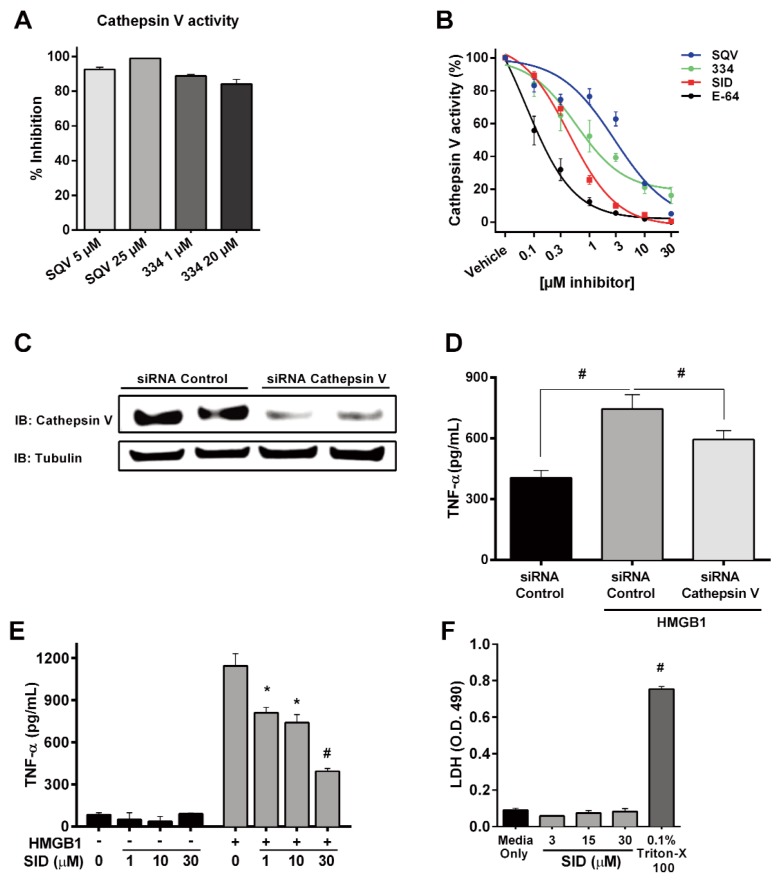
To identify the mammalian targets of SQV and 334, the inhibitory activity of these compounds was assessed against 58 known human proteases (Supplementary Table 2). Only human cathepsin V was substantially inhibited by both compounds tested (Supplementary Table 2). Protease activity assays demonstrated strong inhibition of recombinant human cathepsin V by SQV and 334 (Figure 2A) and IC50 values for SQV and 334 were calculated and compared with a known inhibitor of cathepsin, SID, as well as a broad acting protease inhibitor E64 (Figure 2B). SID, originally synthesized to inhibit cathepsin L (22) (now a Tocris product), also has been shown to inhibit cathepsin V (23). In our experiments, SQV, 334, SID and E-64 inhibited cathepsin V activity with IC50 values of 3.30 μmol/L, 1.13 μmol/L, 0.50 μmol/L and 0.12 μmol/L respectively as shown in Figure 2B. To further explore the role of cathepsin V in HMGB1-induced TNF-α production, we suppressed cathepsin V expression in human monocyte-derived macrophages using siRNA prior to HMGB1 treatment. Inhibition of cathepsin V protein expression attenuated TNF-α production in response to HMGB1 (Figures 2C, D; p < 0.001). As a secondary method to confirm the role of cathepsin V in TNF-α production, human monocyte-derived macrophages were also treated with the known cathepsin inhibitor, SID. As expected, SID blocked HMGB1- induced TNF-α production dose dependently (Figure 2E) without altering cell viability (Figure 2F). We obtained parallel results in freshly isolated mouse peritoneal macrophages (Supplementary Figures S2A–C).
Figure 2.
Identification of cathepsin V as a target for SQV and STO33438. (A) Effect of SQV and 334 on cathepsin V activity. (B) Dose-dependent inhibition of recombinant cathepsin V by SQV, 334, the cathepsin inhibitor, SID and the broad protease inhibitor E64. (C) Protein expression of cathepsin V and tubulin in human monocyte-derived macrophages transfected with control or cathepsin V siRNA. (D) Human monocyte-derived macrophages were transfected with control or cathepsin V siRNA and then assayed for HMGB1-dependent TNF-α production by ELISA. (E) Dose-dependent effect of the cathepsin inhibitor SID on HMGB1-induced TNF-α release from human monocyte derived macrophages. (F) Cytotoxicity was measured by LDH release into the cell media after 18 h of treatment.
These combined findings identify human cathepsin V as a target for the protease inhibitor SQV and confirm a role for cathepsin V in HMGB1-induced macrophage activation through TLR4.
SQV Inhibits the Interaction between Cathepsin V and the TLR4-MyD88 Receptor Complex
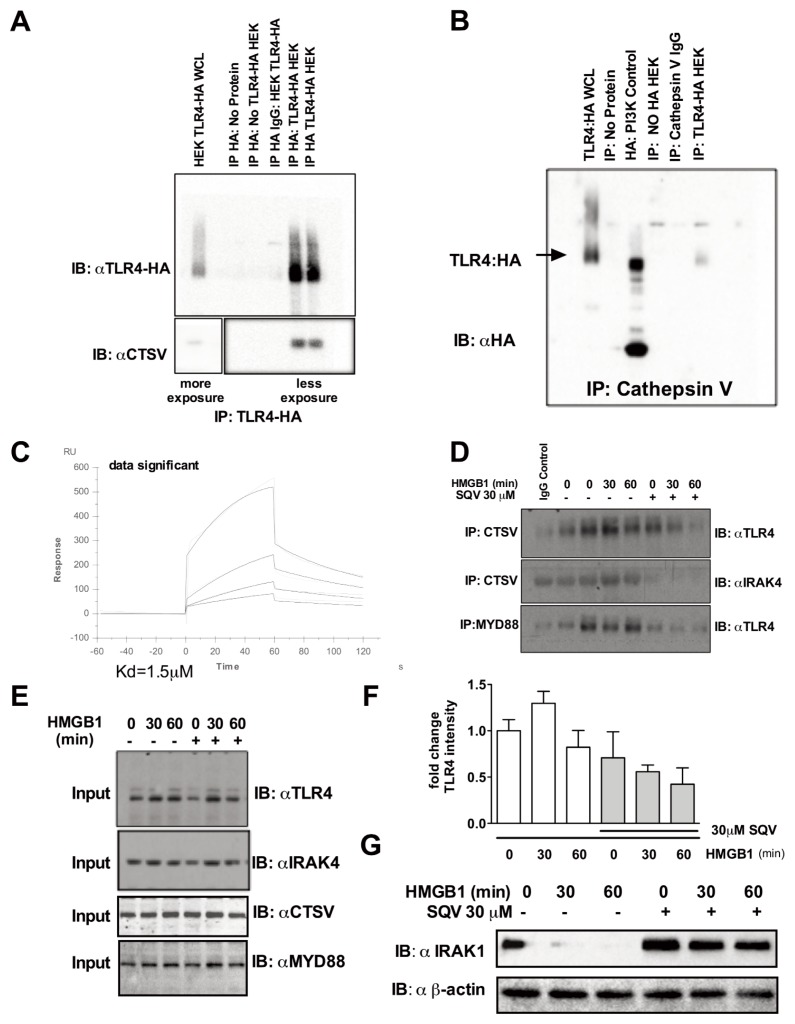
To determine if cathepsin V interacts directly with TLR4, HA-tagged human TLR4 was transfected into HEK 293 cells and reciprocal immunoprecipitation experiments were performed (Figures 3A, B). Cathepsin V coimmunoprecipitated with HA-TLR4 after HA pull-down and HA-TLR4 coimmunoprecipitated with cathepsin V after cathepsin V pull-down (Figures 3A, B). A direct interaction of cathepsin V with TLR4 was also established using biacore analysis. Recombinant cathepsin V was found to bind to human TLR4 with a Kd of 1.5 μmol/L (Figure 3C). This interaction was dose dependently inhibited by SQV (0.625, 1.25, 2.5 and 5 μmol/L). Experiments using human monocyte-derived macrophages revealed basal interaction between cathepsin V and TLR4 that increased due to HMGB1 stimulation and was disrupted by SQV (Figure 3D). Further exploration of the TLR4/Myd88/IRAK4 complex demonstrated additional interactions of cathepsin V with IRAK4 as well as TLR4 with Myd88 in response to HMGB1 and was followed by its dissociation after SQV treatment (Figure 3D). A quantitation of multiple immunoprecipitation experiments shows an increased formation in TLR4-cathepsin V complex in response to HMGB1 that is inhibited by SQV (Figure 3F). The expression levels of TLR4, MyD88 and IRAK4 in the immunoprecipitations were similar and not modified by SQV treatment (Figure 3E).
Figure 3.
SQV inhibits the interaction between cathepsin V and the TLR4-Myddosome receptor signaling complex. (A,B) HEK293 cells transfected with HA tagged TLR4 were immunoprecipitated for HA:TLR4 or cathepsin V followed by subsequent cathepsin V and or TLR4:HA immunobloting. (C) Recombinant human TLR4 and cathepsin V binding was measured by surface plasmon resonance. SQV at 0.625, 1.25, 2.5, 5 μmol/L dose dependently inhibited binding of TLR4 to cathepsin V. (D) Human monocyte-derived macrophages were pre-treated with SQV (30 μmol/L for 1h) followed by the addition of HMGB1 for 30 and 60 min. Representative immunoblot of immunoprecipitated cathepsin V or MYD88 blotted against TLR4 and IRAK4. (E) Representative immunoblot of TLR4, IRAK4, cathepsin V and MYD88 total protein expression used as input for the experiments in (D). (F) Histogram of quantitation of multiple experiments from (D). (G) Effect of SQV on HMGB1-induced IRAK-1 protein expression compared with β-actin as determined by Western blot analysis.
Recent studies have shown that HIV-protease inhibitors can act as inhibitors of the 20S core particle of the 26S proteasome and the total 26S proteasome (24). Thus, we also tested the effect of the compounds on proteasome activity. We found only weak inhibition of this target by SQV in our screen (Supplementary Table 2). Moreover, in our confirmatory assay SQV and SID showed no inhibition, while 334 had only a weak effect at higher concentration (Supplementary Figure S4A). Proteasome inhibitors are also potent blockers of the HMGB1- induced inflammatory cytokine production, and complete inhibition of the proteasome exerts similar protection to that of SID, SQV and 334 (Supplementary Figures S4B, C). Furthermore, both SID and SQV act synergistically with the proteasome inhibitor MG132 to block the HMGB1-induced TNF-α production (Supplementary Figures S4D, E) and thus the antiinflammatory mode of action of these compounds is independent of their proteasome activity. In TLR4-driven cellular responses, the 26S proteasome is required for signaling (for example, NF-κB activation), which is downstream of the signaling intermediates IRAK1. IRAK1 is rapidly degraded after TLR stimulation (25,26). To determine whether SQV acts upstream of NF-κB, we assessed degradation of IRAK1 in human monocyte-derived macrophages exposed to HMGB1 for 30 and 60 min in presence or absence of SQV. HMGB1 treatment led to the loss of IRAK1, indicating its activation by HMGB1 exposure as shown in the representative Western blot (Figure 3G) with a quantitation of multiple experiments presented in the histogram (Supplemental Figure S3). These findings support the conclusion that at least one of the targets for the inhibitor is proximal to this signaling intermediate.
SQV Protects in Models Known to be Driven by the HMGB1-TLR4 Axis
Antibodies to HMGB1 are protective in polymicrobial sepsis even as a posttreatment strategy consistent with the known role of HMGB1 as a late mediator of the lethality in this condition (27). Here we show that the administration of SQV or 334 given daily for 3 d starting at 24 h after initiation of sepsis using a model of cecal ligation and puncture (CLP) significantly reduced mortality in mice (Figures 4A, B). Dosage was determined based on dose-dependent toxicity shown in Supplemental Figure S5. We have previously reported that early HMGB1-dependent TLR4 activation significantly contributes to inflammation-associated organ damage in the (I/R) model of sterile inflammation (8). Pretreatment with SQV (5 mg/kg bodyweight) (28) significantly reduced liver damage in a murine model of warm liver (I/R) involving 60 min of ischemia followed by 6 h of reperfusion, indicated by a significant reduction in ALT levels (Figure 4C). The dramatic inhibition of liver necrosis using SQV was confirmed histologically and the percent necrosis quantitated in each image shows a strong reduction in necrosis in the liver of SQV-treated mice (Figure 4D). Therefore, SQV and 334 exert potent protection in models of known HMGB1-mediated immunopathology.
DISCUSSION
The interaction of the danger-associated molecular pattern molecule (DAMP) HMGB1 with pattern recognition receptors, including TLR4, drives inflammation in many acute and chronic conditions (1). This led us to screen for inhibitors of the TLR4-mediated pathway activated by disulfide HMGB1. Using TNF-α production by macrophages as a gold standard to assess the response to disulfide HMGB1, we identified first generation HIV PIs from a screen of pharmacologically active and clinically used compounds and subsequently identified ST033438 (334) from a screen of known protease inhibitors (18). Furthermore, we discovered that cathepsin V is a unique mammalian target for both SQV and 334. Our studies also uncovered an unexpected role for cathepsin V in TLR4 signaling, raising the possibility that cathepsin V inhibitors could be used to target DAMP-induced inflammation.
SQV was introduced into clinical practice in 1995 as an inhibitor of the HIV protease involved in viral replication (29). However, SQV and other first generation HIV-PIs have been shown to reduce immunopathology and tissue damage in several experimental models including sepsis (30), pancreatitis (31) and hypoxia-induced pulmonary arterial hypertension (32). A recent clinical trial showed that a 6-month course of SVQ improves proteinurea in steroid-resistant nephrotic syndrome (33). From these experimental and clinical studies it was conceivable that a secondary, mammalian protease target exists for this class of antiviral drugs. SQV has been shown to suppress activity of the 20S core particle of the 26S proteasome as well as total 26S proteasome (25,26). By showing that SQV blocks cathepsin V, we provide another distinct mammalian target for first generation HIV-PI. The importance of cathepsin V as a target in the HMGB1-TLR4 pathway is further supported by our findings that SID26681509, a known cathepsin inhibitor and 334, a novel pro-tease inhibitor, block both disulfide HMGB1-induced TNF-α production and cathepsin V activity.
The role of cathepsins in disease and as critical regulators of innate immunity is slowly emerging (34). Cathepsins are a family of multifunctional proteases that include eleven cysteine cathepsins in humans. In general, cathepsins are synthesized as proenzymes that are expressed in lysosomes and are optimally activated under acidic conditions to cleave a wide array of protein targets (35). Cathepsins B, K, L and S have been shown to be expressed mainly by macrophages. Functions attributed to cysteine cathepsins include cell death and inflammasome signaling (36), antigen presentation (37), collagen turnover in bone and cartilage (38), vascular remodeling (39) and neuropeptide and hormone processing (40). Interestingly, endosomal TLRs, including TLR3, 7 and 9 require cleavage that can involve cathepsins B, K and H for ligand recognition and activation (41–48). In the setting of rheumatoid arthritis involving TLR4 (49), psoriasis-like lesions involving TLR7 (50) and collagen-induced arthritis involving TLR9 (51), cathepsins have been shown to influence disease. Less is known about the mammalian cathepsin V, which is expressed in humans, pigs, cows, dogs and sheep but not in mice, where cathepsin L represents the murine homologue (37,52). While initial biodistribution studies only demonstrated expression of cathepsin V in testis, thymus and within the corneal epithelium (53), it has since been detected in skin (54), endothelial cells (55), breast and colorectal carcinomas (56) and human PBMCs (55). Polymorphisms of cathepsin V have been associated with diabetes and early onset myasthenia gravis (57). Absence of cathepsin V in mice (carrying the cathepsin L homologue) limits our in vivo murine models in establishing a direct link to cathepsin V. We do show, however, that SQV protects the TLR4-HMGB1-induced pathology. This is further confirmed using the protease inhibitor compound 334. We can thus not rule out other targets of SQV, but are presenting solid data here demonstrating that cathepsin V is one target. Our present findings show that cathepsin V is expressed in primary human monocytes isolated from blood (Supplementary Figure S6) and in a human monocytic cell line where it binds directly with TLR4. This interaction, which is already present in unstimulated cells, is intensified by HMGB1 treatment and blocked by SQV implicating cathepsin V in the formation of the human TLR4 signaling complex. Support for a proximal target for SQV in HMGB1-induced TLR4 signaling is provided by showing that SQV blocks the interaction of MyD88 with the TLR4 signaling complex and IRAK1 degradation; steps involved in the formation and function of the myddosome (58). These results support the view that DAMP-and pathogen-associated molecular pattern molecule (PAMP)-mediated responses are already distinguished at the receptor level even when the signals are transmitted through the same toll-like receptor.
CONCLUSION
In summary, the current studies identify cathepsin V as a novel target in DAMP-driven inflammation and TLR4 signaling. The demonstration that an FDA-approved drug with a well established safety and toxicity profile functions as a cathepsin V inhibitor may open the door for therapeutic repurposing approaches for acute and chronic inflammation.
Supplemental Data
ACKNOWLEDGMENTS
We would like to thank Patricia Loughran for providing the histology and Rick Shapiro for technical advice on experiments. This work was supported by grants to TRB P50GM053789 and GM098446 to Huan Yang.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Cite this article as: Pribis JP, et al. (2015) The HIV protease inhibitor saquinavir inhibits HMGB1-driven inflammation by targeting the interaction of cathepsin V with TLR4/MyD88. Mol. Med. 21:749–57.
REFERENCES
- 1.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–62. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving auto-immune and inflammatory disease. Nat Rev Rheumatol. 2012;8:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 3.Schiraldi M, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012;209:551–63. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wahamaa H, et al. High mobility group box protein 1 in complex with lipopolysaccha-ride or IL-1 promotes an increased inflammatory phenotype in synovial fibroblasts. Arthritis Res Ther. 2011;13:R136. doi: 10.1186/ar3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang D, Billiar TR, Lotze MT. A Janus tale of two active high mobility group box 1 (HMGB1) redox states. Mol Med. 2012;18:1360–2. doi: 10.2119/molmed.2012.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang H, Antoine DJ, Andersson U, Tracey KJ. The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol. 2013;93:865–73. doi: 10.1189/jlb.1212662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antoine DJ, Harris HE, Andersson U, Tracey KJ, Bianchi ME. A systematic nomenclature for the redox states of high mobility group box (HMGB) proteins. Mol Med. 2014;20:135–7. doi: 10.2119/molmed.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsung A, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–43. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang R, et al. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med. 2006;12:105–14. doi: 10.2119/2006-00010.Yang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy RM, et al. Systemic inflammation and remote organ injury following trauma require HMGB1. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1538–44. doi: 10.1152/ajpregu.00272.2007. [DOI] [PubMed] [Google Scholar]
- 11.Nadatani Y, et al. High mobility group box 1 promotes small intestinal damage induced by nonsteroidal anti-inflammatory drugs through Toll-like receptor 4. Am J Pathol. 2012;181:98–110. doi: 10.1016/j.ajpath.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa EN, et al. Contribution of high-mobility group box-1 to the development of ventilator-induced lung injury. Am J Respir Crit Care Med. 2006;174:400–7. doi: 10.1164/rccm.200605-699OC. [DOI] [PubMed] [Google Scholar]
- 13.Kruger B, et al. Islet-expressed TLR2 and TLR4 sense injury and mediate early graft failure after transplantation. Eur J Immunol. 2010;40:2914–24. doi: 10.1002/eji.201040601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maroso M, et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16:413–9. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- 15.Bauer EM, et al. High mobility group box 1 contributes to the pathogenesis of experimental pulmonary hypertension via activation of Toll-like receptor 4. Mol Med. 2012;18:1509–18. doi: 10.2119/molmed.2012.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong Xda E, et al. High mobility group box I (HMGB1) release from tumor cells after treatment: implications for development of targeted chemoimmunotherapy. J Immunother. 2007;30:596–606. doi: 10.1097/CJI.0b013e31804efc76. [DOI] [PubMed] [Google Scholar]
- 17.Kim S, et al. Signaling of high mobility group box 1 (HMGB1) through toll-like receptor 4 in macrophages requires CD14. Mol Med. 2013;19:88–98. doi: 10.2119/molmed.2012.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gero D, et al. Identification of pharmacological modulators of HMGB1-induced inflammatory response by cell-based screening. PloS One. 2013;8:e65994. doi: 10.1371/journal.pone.0065994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang H, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsung A, et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in non-parenchymal cells. J Immunol. 2005;175:7661–8. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 21.Yang H, et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A. 2010;107:11942–7. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myers MC, Shah PP, Diamond SL, Huryn DM, Smith AB., 3rd Identification and synthesis of a unique thiocarbazate cathepsin L inhibitor. Bioorg Med Chem Lett. 2008;18:210–4. doi: 10.1016/j.bmcl.2007.10.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah PP, et al. Kinetic characterization and molecular docking of a novel, potent, and selective slow-binding inhibitor of human cathepsin L. Mol Pharmacol. 2008;74:34–41. doi: 10.1124/mol.108.046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piccinini M, et al. The HIV protease inhibitors nelfinavir and saquinavir, but not a variety of HIV reverse transcriptase inhibitors, adversely affect human proteasome function. Antivir Ther. 2005;10:215–23. [PubMed] [Google Scholar]
- 25.Kawagoe T, et al. Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat Immunol. 2008;9:684–91. doi: 10.1038/ni.1606. [DOI] [PubMed] [Google Scholar]
- 26.Lin SC, Lo YC, Wu H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature. 2010;465:885–90. doi: 10.1038/nature09121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 28.Pacifici R, Di Carlo S, Bacosi A, Pichini S, Zuccaro P. Cytokine production in saquinavir treated mice. Int J Immunopharmacol. 1997;19:243–8. doi: 10.1016/s0192-0561(97)00031-3. [DOI] [PubMed] [Google Scholar]
- 29.Kitchen VS, et al. Safety and activity of saquinavir in HIV infection. Lancet. 1995;345:952–5. doi: 10.1016/s0140-6736(95)90699-1. [DOI] [PubMed] [Google Scholar]
- 30.Weaver JG, Rouse MS, Steckelberg JM, Badley AD. Improved survival in experimental sepsis with an orally administered inhibitor of apoptosis. FASEB J. 2004;18:1185–91. doi: 10.1096/fj.03-1230com. [DOI] [PubMed] [Google Scholar]
- 31.Singh VP, et al. Nelfinavir/ritonavir reduces acinar injury but not inflammation during mouse caerulein pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1040–6. doi: 10.1152/ajpgi.90642.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gary-Bobo G, et al. Effects of HIV protease inhibitors on progression of monocrotaline- and hypoxia-induced pulmonary hypertension in rats. Circulation. 2010;122:1937–47. doi: 10.1161/CIRCULATIONAHA.110.973750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coppo R, et al. Saquinavir in steroid-dependent and -resistant nephrotic syndrome: a pilot study. Nephrol Dial Transplant. 2012;27:1902–10. doi: 10.1093/ndt/gfs035. [DOI] [PubMed] [Google Scholar]
- 34.Cesen MH, Pegan K, Spes A, Turk B. Lysosomal pathways to cell death and their therapeutic applications. Exp Cell Res. 2012;318:1245–51. doi: 10.1016/j.yexcr.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Turk V, et al. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim Biophys Acta. 2012;1824:68–88. doi: 10.1016/j.bbapap.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3:E255–63. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- 37.Bromme D, Li Z, Barnes M, Mehler E. Human cathepsin V functional expression, tissue distribution, electrostatic surface potential, enzymatic characterization, and chromosomal localization. Biochemistry. 1999;38:2377–85. doi: 10.1021/bi982175f. [DOI] [PubMed] [Google Scholar]
- 38.Novinec M, Lenarcic B. Cathepsin K: a unique collagenolytic cysteine peptidase. Biol Chem. 2013;394:1163–79. doi: 10.1515/hsz-2013-0134. [DOI] [PubMed] [Google Scholar]
- 39.Sjoberg S, Shi GP. Cysteine protease cathepsins in atherosclerosis and abdominal aortic aneurysm. Clin Rev Bone Miner Metab. 2011;9:138–47. doi: 10.1007/s12018-011-9098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Funkelstein L, Hook V. The novel role of cathepsin L for neuropeptide production illustrated by research strategies in chemical biology with protease gene knockout and expression. Methods Mol Biol. 2011;768:107–25. doi: 10.1007/978-1-61779-204-5_5. [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto F, et al. Cathepsins are required for Toll-like receptor 9 responses. Biochem Biophys Res Commun. 2008;367:693–9. doi: 10.1016/j.bbrc.2007.12.130. [DOI] [PubMed] [Google Scholar]
- 42.Park B, Brinkmann MM, Spooner E, Lee CC, Kim YM, Ploegh HL. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat Immunol. 2008;9:1407–14. doi: 10.1038/ni.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Creasy BM, McCoy KL. Cytokines regulate cysteine cathepsins during TLR responses. Cell Immunol. 2011;267:56–66. doi: 10.1016/j.cellimm.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sepulveda FE, et al. Critical role for asparagine endopeptidase in endocytic Toll-like receptor signaling in dendritic cells. Immunity. 2009;31:737–48. doi: 10.1016/j.immuni.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 45.Toscano F, et al. Cleaved/associated TLR3 represents the primary form of the signaling receptor. J Immunol. 2013;190:764–73. doi: 10.4049/jimmunol.1202173. [DOI] [PubMed] [Google Scholar]
- 46.Ewald SE, Engel A, Lee J, Wang M, Bogyo M, Barton GM. Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. J Exp Med. 2011;208:643–51. doi: 10.1084/jem.20100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ewald SE, et al. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456:658–62. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pla A, Pascual M, Renau-Piqueras J, Guerri C. TLR4 mediates the impairment of ubiquitin-proteasome and autophagy-lysosome pathways induced by ethanol treatment in brain. Cell Death Dis. 2014;5:e1066. doi: 10.1038/cddis.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirabara S, Kojima T, Takahashi N, Hanabayashi M, Ishiguro N. Hyaluronan inhibits TLR-4 dependent cathepsin K and matrix metalloproteinase 1 expression in human fibroblasts. Biochem Biophys Res Commun. 2013;430:519–22. doi: 10.1016/j.bbrc.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Hirai T, et al. Cathepsin K is involved in development of psoriasis-like skin lesions through TLR-dependent Th17 activation. J Immunol. 2013;190:4805–11. doi: 10.4049/jimmunol.1200901. [DOI] [PubMed] [Google Scholar]
- 51.Asagiri M, et al. Cathepsin K-dependent toll-like receptor 9 signaling revealed in experimental arthritis. Science. 2008;319:624–7. doi: 10.1126/science.1150110. [DOI] [PubMed] [Google Scholar]
- 52.Hagemann S, et al. The human cysteine protease cathepsin V can compensate for murine cathepsin L in mouse epidermis and hair follicles. Eur J Cell Biol. 2004;83:775–80. doi: 10.1078/0171-9335-00404. [DOI] [PubMed] [Google Scholar]
- 53.Adachi W, et al. Isolation and characterization of human cathepsin V: a major proteinase in corneal epithelium. Invest Ophthalmol Vis Sci. 1998;39:1789–96. [PubMed] [Google Scholar]
- 54.Chen N, Seiberg M, Lin CB. Cathepsin L2 levels inversely correlate with skin color. J Invest Dermatol. 2006;126:2345–7. doi: 10.1038/sj.jid.5700409. [DOI] [PubMed] [Google Scholar]
- 55.Keegan PM, Surapaneni S, Platt MO. Sickle cell disease activates peripheral blood mononuclear cells to induce cathepsins k and v activity in endothelial cells. Anemia. 2012;2012:201781. doi: 10.1155/2012/201781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Santamaria I, Velasco G, Cazorla M, Fueyo A, Campo E, Lopez-Otin C. Cathepsin L2, a novel human cysteine proteinase produced by breast and colorectal carcinomas. Cancer Res. 1998;58:1624–30. [PubMed] [Google Scholar]
- 57.Viken MK, et al. Polymorphisms in the cathepsin L2 (CTSL2) gene show association with type 1 diabetes and early-onset myasthenia gravis. Hum Immunol. 2007;68:748–55. doi: 10.1016/j.humimm.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 58.Flannery S, Bowie AG. The interleukin-1 receptor-associated kinases: critical regulators of innate immune signalling. Biochem Pharmacol. 2010;80:1981–91. doi: 10.1016/j.bcp.2010.06.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.