Abstract
Erythropoietin (EPO) has both erythropoietic and tissue-protective properties. The EPO analogues carbamylated EPO (CEPO) and pyroglutamate helix B surface peptide (pHBSP) lack the erythropoietic activity of EPO but retain the tissue-protective properties that are mediated by a heterocomplex of EPO receptor (EPOR) and the β common receptor (βCR). We studied the action of EPO and its analogues in a model of wound healing where a bovine aortic endothelial cells (BAECs) monolayer was scratched and the scratch closure was assessed over 24 h under different oxygen concentrations. We related the effects of EPO and its analogues on repair to their effect on BAECs proliferation and migration (evaluated using a micro-Boyden chamber). EPO, CEPO and pHBSP enhanced scratch closure only at lower oxygen (5%), while their effect at atmospheric oxygen (21%) was not significant. The mRNA expression of EPOR was doubled in 5% compared with 21% oxygen, and this was associated with increased EPOR assessed by immunofluorescence and Western blot. By contrast, βCR mRNA levels were similar in 5% and 21% oxygen. EPO and its analogues increased both BAECs proliferation and migration, suggesting that both may be involved in the reparative process. The priming effect of low oxygen tension on the action of tissue-protective cytokines may be of relevance to vascular disease, including atherogenesis and restenosis.
INTRODUCTION
Erythropoietin (EPO) promotes erythropoiesis via ligation and homodimerization of EPOR (1–3). Recent data show that EPO is expressed in several tissues and has multiple tissue-protective and reparative activities, being a prototypic tissue-protective cytokine (4,5). These properties of EPO have been investigated in preclinical models of ischemic, traumatic and inflammatory injuries and diverse models of vascular disease (6–8).
Injury of the vascular endothelium represents a critical feature in the early stages of vascular disease (9–11). Hypoxia is associated with endothelial injury and dysfunction, and also stimulates EPO production. In fact, EPO derived from vascular endothelial cells appears to be important in protecting the endothelium against ischemic injury (12–14), possibly through its effects on endothelial cell proliferation, apoptosis and differentiation, as well as via the induction of angiogenesis (15–17).
Recent studies have shown that the protective effects of EPO are mediated by a tissue-protective receptor, which is distinct from the conventional homodimeric EPOR. This tissue-protective receptor is a heterodimeric complex composed of EPOR and the common β subunit of receptors for GM-CSF, IL-3 and IL-5 (βCR, also known as CD131) (9,18–21).
As a tissue-protective cytokine, EPO has hematopoietic effects that may be undesirable, increasing the hematocrit, and possibly increasing the risk of cardiovascular complications including hypertension and thrombosis (22,23). A new generation of EPO analogues that are tissue-protective but not erythropoietic have therefore been developed. These compounds bind to the EPOR-βCR heterodimeric complex but not the EPOR homodimer, and therefore may represent a potentially safer and more effective intervention for the treatment of vascular disease (12,24,25).
Carbamylated EPO (CEPO) is tissue-protective in several models in vivo, yet is not erythropoietic because it does not bind to the classical homodimeric EPOR (26). More recently, an 11-amino acids helix B surface peptide that mimics the tertiary structure of EPO has been developed that has tissue-protective properties without stimulating hematopoiesis (27,28).
EPO and its nonerythropoietic analogues have been shown to be protective in models of cardiovascular injury (29,30) and to promote wound healing in the skin (27,28). The aim of this study was to investigate the potential of EPO and its analogues in aortic endothelial cells at low oxygen concentrations, as these may be particularly relevant to vascular disease, including atherosclerosis. For this purpose, we investigated the protective effect of EPO, CEPO and pHBSP in an in vitro model of wound healing in bovine aortic endothelial cells (BAECs) in low (5%) and atmospheric (21%) oxygen concentrations. We also studied the effects of EPO and its analogues on BAEC proliferation and migration, two processes that are important in wound closure in this model. The results reported here indicate that oxygen concentration may be an important factor in determining susceptibility to tissue-protective cytokines.
MATERIALS AND METHODS
All chemicals were from Sigma-Aldrich unless otherwise stated. The peptide (pHBSP, or ARA290; pyroglu-EQLERALNSS) and its scrambled form (scr-pHBSP; pyroglu-LSEARNQSEL) were from Araim Pharmaceuticals.
Cell Culture
Bovine aortic endothelial cells (BAECs) were obtained from the European Collection of Cell Cultures (ECACC) and used between passages 4 and 12. The cells were cultured in Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin (final concentration 100 IU/mL), and were cultured at 37°C in a humidified atmosphere containing 5% CO2 and 21% oxygen.
Scratch Assay
The scratch assay was the term used for the endothelial cell injury model. The conditions of this model were initially optimized by culturing the cells after injury in culture media containing different concentrations of FBS (0%, 1% and 10%) over a period of 0, 24, 48 and 72 h. The optimized condition of 1% FBS and a 24 h incubation were used to study the effect of EPO and its analogues at varying concentrations (0 to 100 ng/mL) under 21% oxygen and 5% oxygen, either acute (24 h after injury) or chronic (1 wk before injury and 24 h after injury).
For the scratch assay, the cells were seeded into 12-well plates at a seeding density of 1 × 105 cell/mL and cultured in normal medium until confluent. A scratch was made in the cell monolayer using a P1000 blue plastic pipette tip (Starlab Ltd.) creating a cell free area of approximately 1.5-mm width. The cells were then washed three times with PBS to remove any loose cell debris, then medium containing 1% FBS with or without EPO, CEPO or pHBSP was added. A peptide with a scrambled sequence of amino acids (scr-pHBSP) was used at the same concentration to ensure that the effects of the amino acid sequence in pHBSP were specific. The defined area of the wound was photographed under an inverted microscope (Olympus CKX41) at 4× magnification with a Micropix 5 megapixel color CMOS digital camera. The position of the wound image was standardized each time against a horizontal line drawn on the base of the plate passing through the center of each well. All the images were quantified using ImageJ software (NIH, rsb.info.nih.gov/ij).
Real-time qPCR
Cells were seeded into 24-well plates and cultured until 80% confluent, at which point one plate was incubated under 21% oxygen while a matched plate was incubated under 5% oxygen for 24 h prior to RNA extraction.
Cells were lysed using TRIzol (Invitrogen, Life Technologies) and RNA was then extracted and purified. RNA quality and concentration were determined using a NanoDrop ND-1000 (NanoDrop Technologies) (31).
Reverse transcription and real-time quantitative PCR (qPCR) were carried out on RNA samples for EPOR, βCR, VEGF and β2-microglobulin (a housekeeping gene not affected by change in oxygen levels), using TaqMan gene expression assays (Applied Biosystems/Life Technologies) as previously reported (32). For gene expression quantification, the comparative threshold cycle (ΔΔCt) method was used following manufacturer’s guidelines. Results were normalized to β2-microglobulin expression and expressed as arbitrary units using one of the normoxic samples as a calibrator. VEGF, a hypoxia-induced gene, was measured as a positive control to validate the method and conditions used for the experiment.
Western Blot
BAECs were seeded into 24 well plates at a seeding density of 1 × 105 cell/mL and cultured until 80% confluent, at which point one plate was incubated under 21% oxygen while a matched plate was incubated under 5% oxygen for 24 h. Cells were then lysed in 80 μL lysis buffer (25 mmol/L Tris HCl pH 7. 6, 0.1% SDS, 1% deoxycholate, 1% NP40, 0.5 mol/L EDTA, 40 mmol/L EGTA and protease inhibitors). Lysates were then centrifuged at 11,000g for 15 min at 4°C and the supernatant was collected. Protein concentrations were quantified using a BCA reagent kit (Pierce Biotechnology). Thirty μg of cellular proteins were separated on a 10% SDS-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane (Amersham/GE Healthcare Life Sciences). After blocking with 5% skimmed milk (for EPOR detection) or 3% bovine serum albumin (BSA) for βCR detection for 1 h, membranes were incubated with various primary antibodies overnight, followed by HRP-conjugated secondary antibodies for 1 h at room temperature. EPOR was detected using goat anti-EPOR (W-20) (Santa Cruz Biotechnology, Heidelberg, Germany) at 1:200 dilution and anti-goat secondary antibody (A8919) (Sigma-Aldrich, Dorset, UK) at 1:10,000 dilution. βCR was detected using rabbit anti-βCR( N-20) (Santa Cruz Biotechnology, Heidelberg, Germany) at 1:200 dilution and anti-rabbit secondary antibody (A0545) (Sigma-Aldrich, Dorset, UK) at 1:5,000 dilution. GAPDH was used as a loading control and detected using rabbit monoclonal GAPDH antibodies (14C10) (Cell Signaling Technology) at 1:1000 dilution and anti-rabbit secondary antibody A0545 (Sigma-Aldrich, Dorset, UK) at 1:20,000 dilution. Protein bands were visualized by exposing membranes developed with the ECL reagent (Amersham/GE Healthcare Life Sciences, Little Chalfont, Buckinghamshire, UK) to chemiluminescence film (Hyperfilm ECL) (Amersham/GE Healthcare Life Sciences).
Immunofluorescence
BAECs were cultured on poly-L-ornithine–coated coverslips until confluent. Cells were scratched and cultured under 21% or 5% oxygen for 24 h prior to fixation. Cells were then washed with PBS and fixed with 4% paraformaldehyde for 15 min at room temperature. Fixed cells were then washed with PBS and treated with 1% Triton X-100 in PBS (Sigma-Aldrich) for 10 min at room temperature to permeabilize the plasma membrane. After blocking the cells using 10% donkey serum and 0. 3% Triton X-100 for 1 h at room temperature, they were incubated with goat anti-EPOR and rabbit anti-βCR at 1:100 dilution in 1% donkey serum at room temperature for 1 h. Donkey anti-goat IgG-FITC (sc-2024, Santa Cruz Biotechnology, Heidelberg, Germany) and donkey anti-rabbit IgG-CFL 647 (sc-362291, Santa Cruz Biotechnology, Heidelberg, Germany) (1:100 dilution each) were the secondary antibodies used to detect EPOR and βCR respectively. Cellular nuclei were stained with Prolong Gold antifade reagent with DAPI (P36941) (Life Technologies). Images were obtained using a confocal laser scanning microscope (Leica TCS SP8) at 20× or 100× magnification. Fluorescence signal was quantified by calculating corrected total cell fluorescence (CTCF). CTCF was calculated to minimize any autofluorescence from the background using the following formula:
Cell Viability Assay
Cell viability was evaluated using the trypan blue exclusion method. Cells were seeded into 96-well plates at a density of 1 × 104 cells/mL (0.15 mL/well) in culture medium. After 24 h, medium was removed and replaced by 150 μL fresh medium containing different concentrations of EPO, CEPO, pHBSP or scr-pHBSP (0, 1 and 10 ng/mL) and incubated for 0, 24 and 48 h. At each time point, trypan blue was added and stained (dead) and unstained (living) cells were counted. Results were expressed as viable cell count/mL.
Migration Assay
A micro-Boyden chamber assay (NeuroProbe) was used to assess the effect of EPO, CEPO, pHBSP and scr-pHBSP at various concentrations (0, 0. 1, 1, and 10 ng/mL) on cell migration under 21% or 5% oxygen using a 48-well micro Boyden chamber as previously described (33). Cells were cultured under 21% or 5% oxygen for 24 h prior to the experiment. Cells were allowed to migrate for 4 h. The cells that migrated through an 8-μm-pore-size polycarbonate, PVP-free filter membrane were then stained using Diff-Quick stain (Gamidor Tech Services LTD) and counted under 40× magnification microscope.
Statistical Analysis
All data were analyzed using GraphPad Prism 4 software. Differences in treatment (with or without EPO or its analogues) were tested for significance using one-way analysis of variance (ANOVA) followed by a Bonferroni correction for multiple comparisons post-test. A t test was used to compare the expression of EPOR or βCR under different oxygen levels.
All supplementary materials are available online at www.molmed.org.
RESULTS
Low Oxygen Tension Is Required for Stimulation of Wound Closure by EPO and Its Analogues
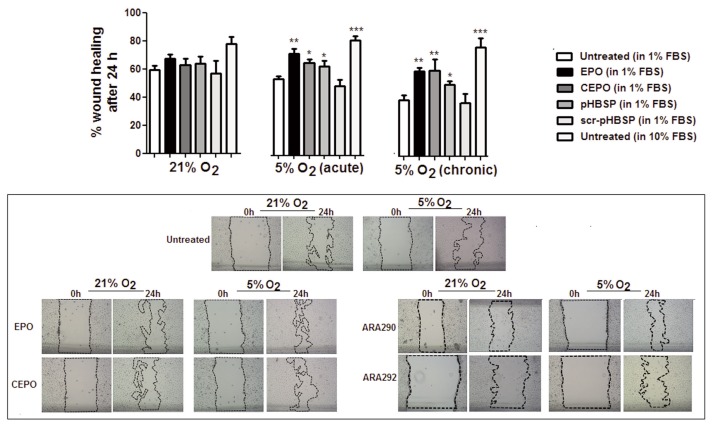
After preliminary experiments testing various conditions, EPO, CEPO and pHBSP were used at a concentration of 1 ng/mL in culture medium containing 1% FBS as this concentration yielded optimum results after an incubation period of 24 h at 5% oxygen level. This effect was not observed at 21% oxygen (Supplementary Figure S1). Using 10% FBS caused a rapid and almost complete repair, and therefore it would have been impossible to detect a reparative action of a test compound at this serum concentration.
As shown in Figure 1, EPO, CEPO and pHBSP significantly improved scratch closure 24 h after treatment in BAECs cultured under acute (24 h, during the culture with the test compound) or chronic (1 wk preexposure) exposure to 5% oxygen. The effect of 10% FBS is shown as a positive control. However, EPO and its analogues showed no significant improvement on wound closure in cells maintained in 21% oxygen. No significant change was observed after treatment with the scrambled peptide (scr-pHBSP) under either 21% or 5% oxygen.
Figure 1.
Low oxygen tension stimulates wound closure induced by EPO and its analogues in a scratch assay model in BAECs. EPO and its analogues (CEPO and pHBSP) enhanced wound closure in BAECs when incubated in 5% oxygen for 24 h (acute) and when incubated in 5% oxygen for 1 wk prior to injury and 24 h after injury (chronic) but not under 21% oxygen at a concentration of 1 ng/mL. The scrambled peptide (scr-pHBSP) showed no effect on wound closure in BAECs under 21% and 5% oxygen. Results are expressed as % wound healing after 24 h. Each data point represent the mean value ± SEM (n = 6), *p < 0.05, **p < 0.01, ***p < 0.01. Representative examples of the scratch assay images with the scratch area outlined under 21% and acute 5% oxygen for untreated cells and cells treated with EPO or its analogues are also shown (lower panel).
EPOR Expression Is Increased in BAECs under Low Oxygen
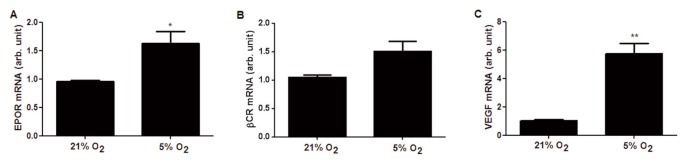
Quantitative PCR was used to compare the gene expression of EPOR and βCR under different oxygen levels. As shown in Figure 2, EPOR mRNA levels were significantly increased by two-fold in 5% oxygen, while those for βCR did not change significantly. VEGF was used as a positive control representing a hypoxia-inducible gene, and its expression increased by six-fold under our experimental conditions.
Figure 2.
Low oxygen tension increases gene expression of EPOR but not of the β common receptor (βCR) in BAECs. Cells were cultured under 21% or 5% oxygen for 24 h, then EPOR (A) and βCR (B) expression was analyzed by quantitative PCR (qPCR). (C) VEGF, a known hypoxia-induced gene, was measured as a positive control. The data are expressed as arbitrary units versus the samples incubated in 21% oxygen and are expressed as the mean ± SEM of nine samples. *p < 0.05, p** < 0.01.
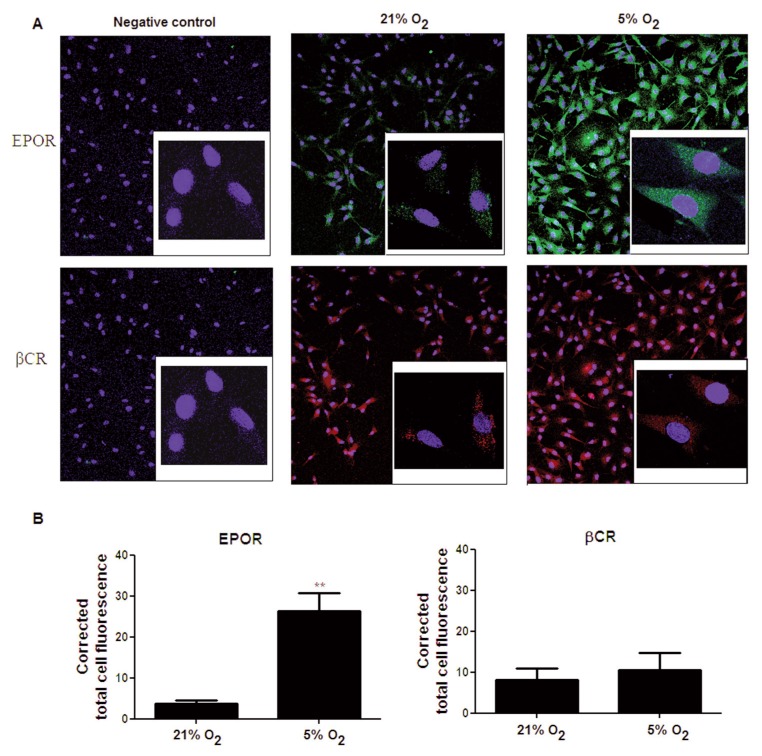
To assess the significance of the observed induction of EPOR mRNA, EPOR and βCR proteins were also studied using immunofluorescence and Western blot. In immunofluorescence experiments, the negative control (no antibody against the protein of interest) showed no significant staining for EPOR (green) or βCR (red), indicating the specificity of the antibody used (Figure 3A). Without changes to the settings of the confocal microscope, to allow a direct comparison, we detected a strong signal for EPOR when cells where incubated in 5% oxygen compared with a weak signal when they were cultured under 21% oxygen (Figure 3A). No change in the signal for βCR expression was observed in either 21% or 5% oxygen. Semiquantitative image analysis of the signals showed a significant increase in the intensities of EPOR in hypoxia compared with normoxia, but no difference in the signal intensity of βCR (Figure 3B).
Figure 3.
Low oxygen tension increases the protein expression of EPOR but not of the β common receptor. (A) Immunostaining of BAECs cultured under 21% oxygen or 5% oxygen (24 h incubation) with anti-EPOR and anti-βCR antibodies, which were then examined by confocal microscopy. Nuclei were stained with DAPI. Images shown are at 20× magnification with insets representing a 100× magnification of the cells. Negative control images represent cells that have not been treated with the antibodies against the protein of interest. Quantitative analysis of EPOR and βCR expression is shown in (B) represented as corrected total cell fluorescence (arbitrary unit). Each data point represents the mean ± SEM (n = 3), **p < 0.01.
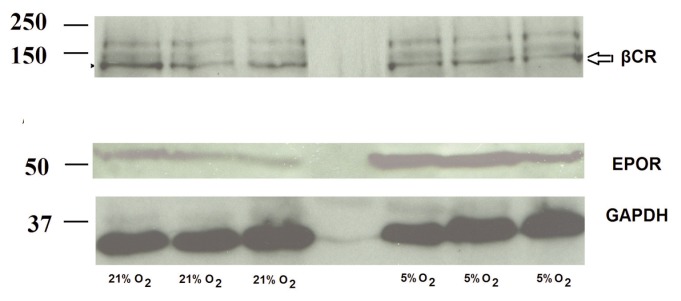
Western blot analysis showed a very similar pattern for the expression of both proteins, with EPOR protein, but not βCR, being increased in 5% oxygen compared with 21% oxygen (Figure 4).
Figure 4.
EPOR and not βCR protein expression increases in BAECs under low oxygen conditions. Western blot analysis of BAECs cultured under 21% oxygen and 5% oxygen for 24 h, showing the expression of EPOR (56 kDa) and βCR (130 kDa). GAPDH (37 kDa) was used as loading control for the samples.
EPO and Its Tissue-Protective Analogues Induce BAECs Proliferation
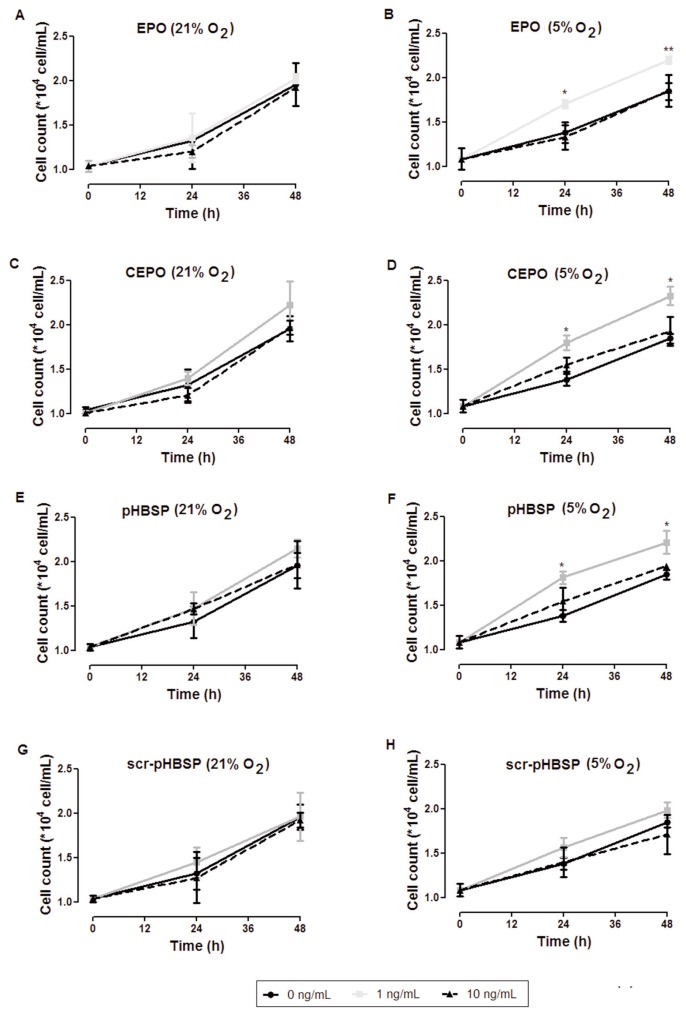
Figure 5 shows cell proliferation (assessed by counting viable cells as described in Materials and Methods) in cells exposed to EPO, CEPO, pHBSP or scr-pHBSP under 21% oxygen (left panel) or 5% oxygen (right panel). While in 21% oxygen there was no significant change in cell proliferation between treated and untreated cells (Figures 5A, C, E), in 5% oxygen, a significant proliferative effect of EPO and its analogues was observed after 24 h (Figures 5B, D, F). Treatment with scr-pHBSP did not significantly increase cell proliferation under any oxygen concentration (Figures 5G, H). We obtained similar results using the MTT assay as a different method for assessing proliferation, based on detection of cell metabolism (Supplementary Figure S2).
Figure 5.
Low oxygen tension induces the effect of EPO and its tissue-protective analogues on BAECs proliferation. BAECs were treated with EPO, CEPO, pHBSP and scr-pHBSP at different concentrations (0, 1 and 10 ng/mL) then incubated under 21% oxygen (left panel) or 5% oxygen (right panel) for 24 h. The effect of EPO (A,B), CEPO (C,D), pHBSP (E,F) and scr-pHBSP (G,H) on BAECs proliferation was analyzed by trypan blue exclusion method. Each data point represent mean ± SEM (n = 3), *p < 0.05 and ** p < 0.01.
EPO and Its Tissue-Protective Analogues Induce BAECs Migration
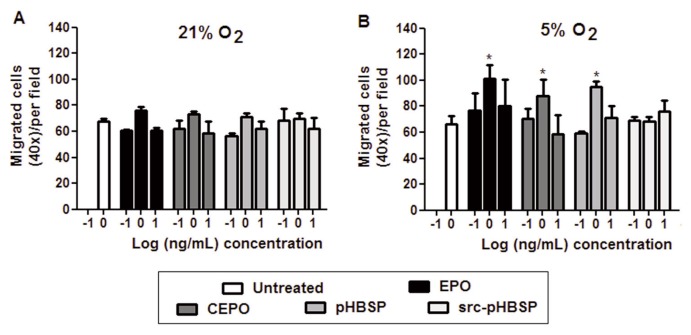
The possible chemotactic effect of EPO and its analogues was studied in a standard cell migration assay using a micro-Boyden chamber. In 21% oxygen, EPO, CEPO and pHBSP had no significant effect on cell migration (Figure 6A) but they stimulated cell migration in 5% oxygen (Figure 6B). It can be noted from the same figure that scr-pHBSP had no effect under either conditions.
Figure 6.
Low oxygen tension induces the effect of EPO and its tissue-protective analogues on BAEC migration. BAECs were incubated under 21% oxygen (A) or 5% oxygen (B) for 24 h. Effect of EPO, CEPO, pHBSP and scr-pHBSP at different concentrations (0–10 ng/mL) on BAEC migration was assessed using a Boyden chamber chemotactic assay. Each data point represents the mean value ± SEM (n = 5 for untreated and EPO, n = 3 for CEPO, pHBSP and scr-pHBSP), *p < 0.05 and ** p < 0.01.
DISCUSSION
Several studies have demonstrated the efficacy of tissue-protective molecules, including EPO, CEPO and pHBSP, in various models of wound healing such as burn injury (34) and cutaneous punch wound (27,35), A case report on the effect of EPO on the healing of skin ulcers was reported (36) and a multicenter clinical trial is ongoing with EPO in burn and scald injuries (37). Whilst the possible wound healing actions of EPO have been investigated in other contexts, most of these studies have focused on skin wounds and their inflammatory and microvascular complications.
Here we show that at an oxygen concentration of 5%, aortic endothelial cells are more responsive to the regenerative action of EPO than when they are maintained at an oxygen concentration of 21%, and this effect is shared by nonerythropoietic EPO derivatives or tissue-protective peptides. Our findings may be of relevance to the response to ischemic injury and to vascular diseases associated with lower oxygenation. The comparison of the effects of EPO with its nonerythropoietic analogues sheds light on the mechanisms involved in the protective effects of EPO and its analogues in the vascular endothelium. The fact that low oxygen tension augments the responsiveness of cells to the reparative effect of EPO, at least in part via upregulation of EPOR expression, is consistent with the increased EPOR expression reported in the ischemic brain (38), and provides additional evidence supporting the hypothesis that EPOR is implicated in the protective action of EPO. However, the effects of CEPO in this model indicate that the reparative actions are not mediated by the classical EPOR homodimer that is involved in the erythropoietic action of EPO (26). Our results are compatible with the hypothesis that, in some cases, tissues may not be fully responsive to EPO due to the lack of one of the subunits of the tissue-protective receptor heterocomplex (7). However they become responsive following injury (12,39), exposure to inflammatory cytokines such as tumor necrosis factor-α (TNF-α) (40) or hypoxia (41,42), with the expression of the EPOR subunit being a limiting factor. This finding may reconcile, at least in part, the fact that EPO exerts its activity (in this case, tissue protection) on other cells than erythroblasts with the observation that EPOR expression is very low on nonerythroid cells. It also explains the protective activity of EPO in various models of organ hypoxia.
The effective concentration of EPO used in our in vitro model was 1 ng/mL (Supplementary Figure S1), a lower concentration than reported in some previous studies, in which a concentration range (8 to 40 ng/mL) was used for in vitro studies (32,43–45). One possible explanation could be an increased responsiveness to EPO under lower oxygen concentrations. This might be due to the observed upregulation of EPOR in low oxygen. However, low oxygen induces several factors in addition to EPO, including VEGF and heat shock proteins, which, in turn, could increase EPOR expression (40,46,47). It is therefore possible that low oxygen tension induces other mediators that synergize with EPO or its analogues by several mechanisms. In our experimental model, even higher concentration of EPO, up to 100 ng/mL did not increase healing significantly, although there was a trend for a dose-dependent increase. It may be that slightly different experimental conditions might explain the healing effect observed by others at atmospheric oxygen concentrations.
Our data also shed light on the mechanisms underlying the reparative action of tissue-protective cytokines. Several mechanisms could play a role in these effects of EPO, including angiogenesis, modulating inflammation, promoting cell migration or proliferation, and mobilizing endothelial progenitor cells. Angiogenesis is a major physiological response to ischemia that involves a sequence of events including cell proliferation, migration and differentiation of ECs; it is regulated by several proangiogenic growth factors, including VEGF, basic fibroblast growth factor (bFGF), nitric oxide (NO) and angiopoietin-1 (48,49).
In the context of vascular wound healing and within the limitations of the experimental model used here, EPO might act by promoting both cell proliferation and migration, which are essential for initiating therapeutic angiogenesis. The induction of cell proliferation and migration in arterial endothelial cells is in agreement with earlier reports on the effects on mesenchymal stem cells (33) and bovine aortic endothelial cells (44), but these studies did not focus on oxygen tension and its effect on cell migration and proliferation. Other studies looked at the effect of hypoxia on enhancing cell migration with EPO but did not investigate the responses to the EPO analogues (50–52). The regrowth and repair of the vascular endothelium appears to be an important factor limiting the accelerated atherosclerosis observed in some models of arterial injury (53).
EPO has other, potentially deleterious, effects on the vascular endothelium, including the induction of procoagulant or inflammatory factors expression, such as E- and P-selectin, plasminogen activator inhibitor (PAI) and vascular cell adhesion molecule-1 (VCAM-1). These may contribute to the prothrombotic effects that have been reported for EPO, and are not observed with CEPO or the small-molecular weight peptide pHBSP. Of note, pHBSP has been engineered specifically to activate the body’s natural repair system following injury via activation of antiinflammatory, tissue-protective and reparative signaling pathways (7,9,26,27,54).
CONCLUSION
Our study shows the efficacy of EPO and its analogues in promoting repair of aortic endothelial cells primed by low oxygen tension. These effects were probably mediated by effects on cell migration and proliferation. Future studies using in vivo models of vascular injury will determine whether EPO analogues may be of value in human vascular disease.
Supplemental Data
ACKNOWLEDGMENTS
Supported by European Regional Development Fund, Project “Peptide Research Network of Excellence,” to P Ghezzi.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare they have no competing interests as defined by Molecular Medicine or other interests that might be perceived to influence the results and discussion reported in this paper.
Cite this article as: Heikal L, et al. (2015) Low oxygen tension primes aortic endothelial cells to the reparative effect of tissue-protective cytokines. Mol. Med. 21:709–16.
REFERENCES
- 1.Jelkmann W. Erythropoietin: structure, control of production, and function. Physiol Rev. 1992;72:449–89. doi: 10.1152/physrev.1992.72.2.449. [DOI] [PubMed] [Google Scholar]
- 2.Watowich SS, Hilton DJ, Lodish HF. Activation and inhibition of erythropoietin receptor function- Role of receptor dimerization. Mol Cell Biol. 1994;14:3535–49. doi: 10.1128/mcb.14.6.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 4.Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005;6:484–94. doi: 10.1038/nrn1687. [DOI] [PubMed] [Google Scholar]
- 5.Mengozzi M, Ermilov P, Annenkov A, Ghezzi P, Pearl F. Definition of a family of tissue-protective cytokines using functional cluster analysis: a proof-of-concept study. Front Immunol. 2014;5:115. doi: 10.3389/fimmu.2014.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grasso G, Sfacteria A, Cerami A, Brines M. Erythropoietin as a tissue-protective cytokine in brain injury: What do we know and where do we go? Neuroscientist. 2004;10:93–8. doi: 10.1177/1073858403259187. [DOI] [PubMed] [Google Scholar]
- 7.Brines M, et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci U S A. 2004;101:14907–12. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marzo F, et al. Erythropoietin in heart and vessels: focus on transcription and signalling pathways. J Thromb Thrombolysis. 2008;26:183–7. doi: 10.1007/s11239-008-0212-3. [DOI] [PubMed] [Google Scholar]
- 9.Brines M, Cerami A. The receptor that tames the innate immune response. Mol Med. 2012;18:486–96. doi: 10.2119/molmed.2011.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Congote LF, Sadvakassova G, Dobocan MC, Di-Falco MR, Li Q. Erythropoietin-dependent endothelial proteins: Potential use against erythropoietin resistance. Cytokine. 2010;51:113–8. doi: 10.1016/j.cyto.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Varani J, Ward PA. Mechanisms of endothelial cell injury in acute inflammation. Shock. 1994;2:311–9. doi: 10.1097/00024382-199411000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Sanchis-Gomar F, Perez-Quilis C, Lippi G. Erythropoietin receptor (EpoR) agonism is used to treat a wide range of disease. Mol Med. 2013;19:62–4. doi: 10.2119/molmed.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Estrada OM, Rodriguez-Millan E, Gonzalez-de Vicente E, Reina M, Vilaro S, Fabre M. Erythropoietin protects the in vitro blood-brain barrier against VEGF-induced permeability. Eur J Neurosci. 2003;18:2538–44. doi: 10.1046/j.1460-9568.2003.02987.x. [DOI] [PubMed] [Google Scholar]
- 14.Chong ZZ, Kang JQ, Maiese K. Angiogenesis and plasticity: Role of erythropoietin in vascular systems. J Hematother Stem Cell Res. 2002;11:863–71. doi: 10.1089/152581602321080529. [DOI] [PubMed] [Google Scholar]
- 15.Anagnostou A, et al. Erythropoietin receptor messenger-RNA expression in human endothelial cells. Proc Natl Acad Sci U S A. 1994;91:3974–8. doi: 10.1073/pnas.91.9.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noguchi CT, Wang L, Rogers HM, Teng R, Jia Y. Survival and proliferative roles of erythropoietin beyond the erythroid lineage. Expert Rev Mol Med. 2008;10:1–23. doi: 10.1017/S1462399408000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahlmann FH, et al. Erythropoietin regulates endothelial progenitor cells. Blood. 2004;103:921–6. doi: 10.1182/blood-2003-04-1284. [DOI] [PubMed] [Google Scholar]
- 18.Sautina L, et al. Induction of nitric oxide by erythropoietin is mediated by the beta common receptor and requires interaction with VEGF receptor 2. Blood. 2010;115:896–905. doi: 10.1182/blood-2009-04-216432. [DOI] [PubMed] [Google Scholar]
- 19.Nangaku M. Tissue protection by erythropoietin: new findings in a moving field. Kidney Int. 2013;84:427–9. doi: 10.1038/ki.2013.140. [DOI] [PubMed] [Google Scholar]
- 20.Dumont F, Bischoff P. Non-erythropoietic tissue-protective peptides derived from erythropoietin: WO2009094172. Expert Opin Ther Pat. 2010;20:715–23. doi: 10.1517/13543771003627464. [DOI] [PubMed] [Google Scholar]
- 21.Broxmeyer HE. Erythropoietin: multiple targets, actions, and modifying influences for biological and clinical consideration. J Exp Med. 2013;210:205–8. doi: 10.1084/jem.20122760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet. 2007;369:381–8. doi: 10.1016/S0140-6736(07)60194-9. [DOI] [PubMed] [Google Scholar]
- 23.Wun T, Law L, Harvey D, Sieracki B, Scudder SA, Ryu JK. Increased incidence of symptomatic venous thrombosis in patients with cervical carcinoma treated with concurrent chemotherapy, radiation, and erythropoietin. Cancer. 2003;98:1514–20. doi: 10.1002/cncr.11700. [DOI] [PubMed] [Google Scholar]
- 24.Coleman TR, et al. Cytoprotective doses of erythropoietin or carbamylated erythropoietin have markedly different procoagulant and vasoactive activities. Proc Natl Acad Sci U S A. 2006;103:5965–70. doi: 10.1073/pnas.0601377103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bohr S, et al. Modulation of cellular stress response via the erythropoietin/CD131 heteroreceptor complex in mouse mesenchymal-derived cells. J Mol Med (Berl) 2015;93:199–210. doi: 10.1007/s00109-014-1218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leist M, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–42. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- 27.Brines M, et al. Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc Natl Acad Sci U S A. 2008;105:10925–30. doi: 10.1073/pnas.0805594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erbayraktar Z, Erbayraktar S, Yilmaz O, Cerami A, Coleman T, Brines M. Nonerythropoietic tissue protective compounds are highly effective facilitators of wound healing. Mol Med. 2009;15:235–41. doi: 10.2119/molmed.2009.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiordaliso F, et al. A nonerythropoietic derivative of erythropoietin protects the myocardium from ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2005;102:2046–51. doi: 10.1073/pnas.0409329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueba H, et al. Suppression of coronary atherosclerosis by helix B surface peptide, a nonerythropoietic, tissue-protective compound derived from erythropoietin. Mol Med. 2013;19:195–202. doi: 10.2119/molmed.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mengozzi M, et al. Erythropoietin-induced changes in brain gene expression reveal induction of synaptic plasticity genes in experimental stroke. Proc Natl Acad Sci U S A. 2012;109:9617–22. doi: 10.1073/pnas.1200554109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cervellini I, Annenkov A, Brenton T, Chernajovsky Y, Ghezzi P, Mengozzi M. Erythropoietin (EPO) increases myelin gene expression in CG4 oligodendrocyte cells through the classical EPO receptor. Mol Med. 2013;19:223–9. doi: 10.2119/molmed.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zwezdaryk KJ, et al. Erythropoietin, a hypoxia-regulated factor, elicits a pro-angiogenic program in human mesenchymal stem cells. Exp Hematol. 2007;35:640–52. doi: 10.1016/j.exphem.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 34.Bohr S, et al. Alternative erythropoietin-mediated signaling prevents secondary microvascular thrombosis and inflammation within cutaneous burns. Proc Natl Acad Sci U S A. 2013;110:3513–8. doi: 10.1073/pnas.1214099110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padgett DA, Marucha PT, Sheridan JF. Restraint stress slows cutaneous wound healing in mice. Brain Behav Immun. 1998;12:64–73. doi: 10.1006/brbi.1997.0512. [DOI] [PubMed] [Google Scholar]
- 36.Ferri C, et al. Recombinant human erythropoietin stimulates vasculogenesis and wound healing in a patient with systemic sclerosis complicated by severe skin ulcers. Clin Exp Dermatol. 2010;35:885–7. doi: 10.1111/j.1365-2230.2010.03847.x. [DOI] [PubMed] [Google Scholar]
- 37.Guenter CI, et al. A multi-center study on the regenerative effects of erythropoietin in burn and scalding injuries: study protocol for a randomized controlled trial. Trials. 2013;14:124. doi: 10.1186/1745-6215-14-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernaudin M, et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:643–51. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Moore E, Bellomo R. Erythropoietin (EPO) in acute kidney injury. Ann Intensive Care. 2011;1:3. doi: 10.1186/2110-5820-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, et al. Tumor necrosis factor alpha primes cerebral endothelial cells for erythropoietin-induced angiogenesis. J Cereb Blood Flow Metab. 2011;31:640–7. doi: 10.1038/jcbfm.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beleslin-Cokic BB, Cokic VP, Yu XB, Weksler BB, Schechter AN, Noguchi CT. Erythropoietin and hypoxia stimulate erythropoietin receptor and nitric oxide production by endothelial cells. Blood. 2004;104:2073–80. doi: 10.1182/blood-2004-02-0744. [DOI] [PubMed] [Google Scholar]
- 42.Beleslin-Cokic BB, et al. Erythropoietin and hypoxia increase erythropoietin receptor and nitric oxide levels in lung microvascular endothelial cells. Cytokine. 2011;54:129–35. doi: 10.1016/j.cyto.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trincavelli ML, et al. Regulation of erythropoietin receptor activity in endothelial cells by different erythropoietin (EPO) derivatives: an in vitro study. Int J Mol Sci. 2013;14:2258–81. doi: 10.3390/ijms14022258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su K-H, et al. β common receptor integrates the erythropoietin signaling in activation of endothelial nitric oxide synthase. J Cell Physiol. 2011;226:3330–9. doi: 10.1002/jcp.22678. [DOI] [PubMed] [Google Scholar]
- 45.Cokic BBB, Cokic VP, Suresh S, Wirt S, Noguchi CT. Nitric oxide and hypoxia stimulate erythropoietin receptor via MAPK kinase in endothelial cells. Microvasc Res. 2014;92:34–40. doi: 10.1016/j.mvr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Velly L, Pellegrini L, Guillet B, Bruder N, Pisano P. Erythropoietin 2nd cerebral protection after acute injuries: A double-edged sword? Pharmacol Ther. 2010;128:445–59. doi: 10.1016/j.pharmthera.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Xu B, Dong G-H, Liu H, Wang Y-Q, Wu H-W, Jing H. Recombinant human erythropoietin pretreatment attenuates myocardial infarct size: a possible mechanism involves heat shock Protein 70 and attenuation of nuclear factor-kappaB. Ann Clin Lab Sci. 2005;35:161–8. [PubMed] [Google Scholar]
- 48.Nakao T, Shiota M, Tatemoto Y, Izumi Y, Iwao H. Pravastatin induces rat aortic endothelial cell proliferation and migration via activation of PI3K/Akt/mTOR/p70 S6 kinase signaling. J Pharmacol Sci. 2007;105:334–41. doi: 10.1254/jphs.fp0070682. [DOI] [PubMed] [Google Scholar]
- 49.Cooke JP. NO and angiogenesis. Atheroscler Suppl. 2003;4:53–60. doi: 10.1016/s1567-5688(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 50.Gammella E, Leuenberger C, Gassmann M, Ostergaard L. Evidence of synergistic/additive effects of sildenafil and erythropoietin in enhancing survival and migration of hypoxic endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2013;304:L230–9. doi: 10.1152/ajplung.00112.2012. [DOI] [PubMed] [Google Scholar]
- 51.Liu N, Tian J, Cheng J, Zhang J. Effect of erythropoietin on the migration of bone marrow-derived mesenchymal stem cells to the acute kidney injury microenvironment. Exp Cell Res. 2013;319:2019–27. doi: 10.1016/j.yexcr.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Lester RD, Jo M, Campana WM, Gonias SL. Erythropoietin promotes MCF-7 breast cancer cell migration by an ERK/mitogen-activated protein kinase-dependent pathway and is primarily responsible for the increase in migration observed in hypoxia. J Biol Chem. 2005;280:39273–7. doi: 10.1074/jbc.M509446200. [DOI] [PubMed] [Google Scholar]
- 53.Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in absence of endothelium. Lab Invest. 1983;49:327–33. [PubMed] [Google Scholar]
- 54.Bennett CL, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299:914–24. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.