Abstract
Tunable properties of multi-arm poly(ethylene glycol) (PEG) hydrogel, crosslinked by Michael-type addition, support diverse applications in tissue engineering. Bioactive modification of PEG is achieved by incorporating integrin binding sequences, like RGD, and crosslinking with tri-functional protease sensitive crosslinking peptide (GCYKNRGCYKNRCG), which compete for the same reactive groups in PEG. This competition leads to a narrow range of conditions that support sufficient crosslinking density to provide structural control. Kinetics of hydrogel formation plays an important role in defining the conditions to form hydrogels with desired mechanical and biological properties, which have not been fully characterized. In this study, we explored how increasing PEG functionality from 4 to 8-arms and the concentration of biological moieties, ranging from 0.5mM to 3.75mM, affected the kinetics of hydrogel formation, storage modulus, and swelling after the hydrogels were allowed to form for 15 or 60minutes. Next, human bone marrow stromal cells were encapsulated and cultured in these modified hydrogels to investigate the combined effect of mechano-biological properties on phenotypes of encapsulated cells. While the molar concentration of the reactive functional groups (-vinyl sulfone) was identical in the conditions comparing 4 and 8-arm PEG, the 8-arm PEG formed faster, allowed a greater degree of modification, and was superior in three-dimensional culture. The degrees of swelling and storage modulus of 8-arm PEG were less affected by the modification compared to 4-arm PEG. These findings suggest that 8-arm PEG allows a more precise control of mechanical properties that could lead to a larger spectrum of tissue engineering applications.
Keywords: PEG hydrogels, Multi-arm, Michael-type addition, Mechanical properties, RGD, 3D culture
INTRODUCTION
Naturally derived and synthetic hydrogels have been extensively exploited in biomedical materials research due to their abilities in directing cellular behavior and function1,2. Natural hydrogels, such as collagen3 and fibrin4, are compatible with live cells, support cell attachment, migration and remodeling; however, the mechanical and biological properties of these materials are closely tied. For example, altering the Matrigel or collagen concentration in hydrogels changes the stiffness of the gel and also affects the concentration of bioactive factors, such as integrin binding and protease sensitive sites. This interdependence of mechanical properties and the biological activity in natural hydrogels makes it challenging to prepare matrices suitable for the diverse requirements of biomedical applications. In contrast to natural hydrogels, biologically inert synthetic hydrogels, such as poly(ethylene glycol) (PEG), have tunable physical properties independent of their biological activity5-10. These properties are important for regulating cellular differentiation and function11-13 and can be manipulated through PEG hydrogel composition, solid concentration, and crosslinking chemistry.
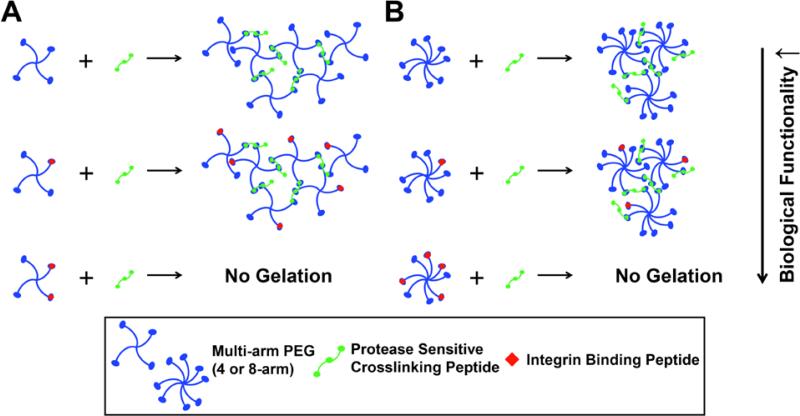
PEG hydrogels are prepared using Michael-type addition (MTA) chemistry, where cysteine-containing peptides react with the unsaturated groups of the multi-arm PEG14,15. The biocompatible chemistry of the MTA reaction allows bioactive modification of the inert PEG hydrogels through the incorporation of integrin binding sequences, like RGD, followed by crosslinking with protease sensitive peptides. Modification of PEG with monofunctional RGD peptides promotes cell adhesion, and crosslinking with di- or trifunctional protease sensitive peptides allow migration and proliferation of the encapsulated cells5,8,16,17. Specifically for tissue engineering applications, cysteine containing peptides react with the unsaturated groups of the end-functionalized multi-arm PEG to form PEG hydrogels14,15. As a result, the modification with RGD, or any other monofunctional peptide, leaves fewer “free” reactive arms available for crosslinking (Fig.1). We have demonstrated previously that adding a third reactive site to bifunctional crosslinking peptides permits continuation of the hydrogel network, improving crosslinking density18 (Fig.1). Despite the improved crosslinking density of hydrogels prepared with 4-arm PEG and tri-functional crosslinkers, there are still limitations on the extent of modification with RGD. Furthermore, a higher concentration of monofunctional peptides results in a greater degree of swelling, which contributes to dilution of the incorporated bioactive molecule concentrations in the hydrogel19.
Figure 1. Crosslinking mechanism for RGD modified multi-arm PEG and YKNR crosslinking peptides.
This schematic shows the crosslinking kinetics between (A) 4-arm PEG and (B) 8-arm PEG that are modified with integrin binding peptides, RGD, and trifunctional protease sensitive crosslinking peptide, YKNR, through Michael-type addition. Intermolecular reactions and the presence of a third binding site allowed continuation of the network formation. However, bioactive modification with integrin binding peptides, RGD, affected crosslinking kinetics by binding to the reactive sites on the PEG molecules, preventing network growth at those binding sites. L-Cysteine hydrochloride monohydrate (L-Cys) was substituted on a molar basis as a biologically inactive surrogate molecule for RGD.
The crosslinking kinetics and macroscopic properties of MTA PEG hydrogels depend on pH, PEG solid concentration, and the presence of charged amino acid residues15,20. However, it has not been fully explored how the PEG functionality and the consequences of bioactive modification with monofunctional peptides affect the crosslinking kinetics, mechanical properties, and the behavior of encapsulated cells. In this study, we investigated the effects of increasing the number of reactive groups around the core of the PEG macromer from 4 to 8 on the macroscopic properties of the hydrogel, such as swelling and storage modulus. The total molar concentration of the reactive functional groups in the conditions comparing 4 and 8-arm PEG was identical (e.g. 10mM [–VS] for 5% 4 and 8-arm PEGs), yet we demonstrated that a greater local concentration of reactive arms around the core of the PEG macromer resulted in improved crosslinking efficiency and reduced network defects stemming from incomplete gelation. These findings support our hypothesis that PEG macromers with increased functionality allow a greater degree of bioactive modification without compromising the mechanical properties of the hydrogel and result in an improved hydrogel design for various biomedical applications.
EXPERIMENTAL SECTION
1. Hydrogel Materials
Hydrogels for mechanical and physical characterization studies were prepared with 4 and 8-arm PEG vinyl sulfone (PEG-VS, 20kDa and 40kDa, >99% purity, Jenkem Technology). The hydrogels were formed via MTA with plasmin sensitive tri-functional crosslinking peptides A- GCYK↓NRGCYK↓NRCG (YKNR) (1663.91 g mol−1, >90% purity, GenScript, cleavage site indicated by ↓) for all experiments. The stoichiometric ratio of -VS to thiol (-SH) groups was kept constant at 1:1 ratio for all experiments. PEG hydrogels were modified with the integrin binding peptide GCGYGRGDSPG (RGD) (1067.10 g mol−1, GenScript) to allow attachment of the encapsulated cells. For experiments without cells, L-Cysteine hydrochloride monohydrate (L-Cys) (98.5-101.0%, F.W. 175.64 g mol−1, Fisher BioReagents) was substituted on a molar basis as a biologically inactive model compound for RGD7. All methods and conditions for 4 and 8-arm PEG acrylate (PEG-A, 20kDa and 40kDa, >99% purity, Jenkem Technology) hydrogels were identical.
2. Rheology – Storage Modulus and the Rate Constant of Gel Formation
The storage modulus (G’) and the rate constant of gel formation were studied using AR-G2 rheometer (TA Instruments, New Castle, DE) equipped with 20mm parallel plates and a Peltier stage was utilized for mechanical testing. We compared 4 and 8-arm PEG-VS hydrogels (5% w/v) crosslinked with YKNR peptide and modified with varying concentration of L-Cys, ranging from 0 to 3mM. After 100μl of the PEG solution was mixed with YKNR peptide, the mixture was directly pipetted between the rheometer's plates and each test was conducted at 37°C for a 60min time sweep at 10% strain and 1 rad/sec. The rate constant of gel formation was calculated from G’ curves by using the curve fit tool in MATLAB (Natick, MA). A non-linear regression was performed using the equation:
The storage modulus at the initial time, t=0, is defined as Y0. In the above equation, the term Plateau is the storage modulus, G’, after complete gelation, K represents the rate constant for gel formation in unit of reciprocal time, and t represents time.
3. Swelling of the PEG hydrogels
Hydrogels (4 and 8-arm PEG) at various final concentrations (4 – 10% w/v) were crosslinked with YKNR and modified with L-Cys at concentrations ranging from 0 to 3.75mM. Hydrogels were prepared by dissolving each precursor in 0.05M HEPES buffer at pH 7.4. For example, to make 100μl of PEG-8A 5% gel with 1.25mM L-Cys, 5mg of PEG-8VS/PEG-8A was dissolved in 45μl of HEPES buffer and mixed with 0.022mg of L-Cys in 10μl of HEPES buffer. Larger volumes of stock solutions were prepared to minimize weighing error. L-Cys was allowed to bind to the PEG macromer's reactive sites at 37°C for 15min. Next, 0.49mg of YKNR was dissolved in 40μl HEPES buffer at a 1:1 molar ratio of the functional groups in PEG. Afterwards, the YKNR solution was pipetted into the modified PEG solution and mixed, vortexed, and pipetted into 20μl gels. After 1min, the reaction was quenched and PEG gels were submerged in 15ml of MilliQ water for 24h. After 24h, excess water was removed and the gels were weighed. The mass swelling ratio (Qm) was calculated by dividing the mass of the swollen hydrogels (ms) by the mass of the dry gel components (md)21,22.
4. Elastically Active Chain Concentration
Elastically active chain concentration in the PEG network was calculated by using the modified Flory-Rehner equation that shows a direct relation between the PEG precursor weight percent and associated Qm15.
In the Flory-Rehner equation, X1, polymer-solvent interaction parameter, is equal to 0.43 for PEG in water, Vd is the polymer volume in the dry state, and v1 is the molar volume of water in cm3/mol. v2,s is the equilibrium polymer volume fraction after swelling has occurred (1/Qm)23, and v2,c is the polymer volume fraction at crosslinking.
5. HS-5 Green Fluorescent Protein (GFP) Transfection and Culture
Human bone marrow stromal cell line, HS-5 cells (ATCC) were cultured in DMEM (Dulbecco's modified Eagle's medium, ATCC) supplemented with 10% fetal bovine serum (FBS) (Biowest) and 1% PenStrep (Lonza). The cells were cultured in T-75 flasks in 5% CO2 atmosphere at 37°C and split in a 1:5 ratio every 3-4 days. For confocal fluorescent imaging HS-5 cells were transfected with Green Fluorescent Protein (GFP)24. Briefly, 24 hours prior to transfection, HS-5 cells were seeded and cultured in 6 well plates. Once cell confluency reached 60% a transfection reagent Fugene 6 (Promega), was first mixed with serum-free Opti-Mem media (Invitrogen) and sequentially with DNA (pLenti-GIII-GFP) (Applied Biological Materials) according to the manufacturer's instructions. This mixture was carefully added dropwise and then incubated at 37°C for 24 – 48 hours. After verifying HS-5 was successfully transfected with GFP, transfected cells were selected and cultured in media supplemented with puromyocin (1μg/ml) (Sigma-Aldrich).
6. HS-5 Cell Encapsulation and 3D Culture in PEG Hydrogels
Prior to in situ crosslinking and encapsulation, HS-5 cells were suspended at a concentration of 2 × 106 cells/mL and centrifuged for 5min at 300G to remove excess media. In order to investigate the effects of RGD concentration on 3D cell behavior without inducing changes in hydrogel mechanical properties, the total concentration of monofunctional cysteine groups composed of L-Cys and RGD peptide was kept constant at 1.25mM. We prepared 5% PEG-4VS or PEG-8VS by dissolving PEG in the appropriate amount of 0.05M HEPES Buffer at pH 7.4, followed by modification with 1.25mM RGD or 0.25mM RGD plus 1mM L-Cys. The monofunctional agents were allowed to bind to the PEG macromers at 37°C for 15min. Then, the cell pellet was reconstituted with modified 4-arm or 8-arm PEG and formed gels by the addition of trifunctional plasmin sensitive crosslinking peptide, YKNR. The stoichiometric ratio VS:SH was kept 1:1 for all experiments. Each 50μl gel was crosslinked between two glass slides at 37°C for 15min and flipped at 7min to prevent cell sedimentation and clustering. After 15min, gels were placed in a 48 well plate and covered with 400μl of DMEM. Media was changed every 2 days and cells were cultured up to 12 days. All images were taken using Leica DMI3000B and a confocal microscope Nikon A1 was used for imaging GFP transfected HS-5 cells.
7. DAPI Staining and Cell Viability Assay
Cells were stained with DAPI (Invitrogen) and LIVE/DEAD kit (488/570) (Invitrogen) at Day 12 to evaluate cell viability as described by the manufacturer's protocol. DAPI stain was added directly to cells in full media and incubated for 15 min. Spheroid formation was identified by multiple nuclei staining. All images were taken using Leica DMI3000B.
8. Statistical Analysis
All statistical analyses were performed using GraphPad Prism (GraphPad Prism Software, La Jolla, CA). Data are reported as mean ± standard deviation of measurements, and statistical analyses were performed with Two-way ANOVA followed by Bonferroni post-test.
RESULTS
1. Storage Modulus of 5% PEG Hydrogels
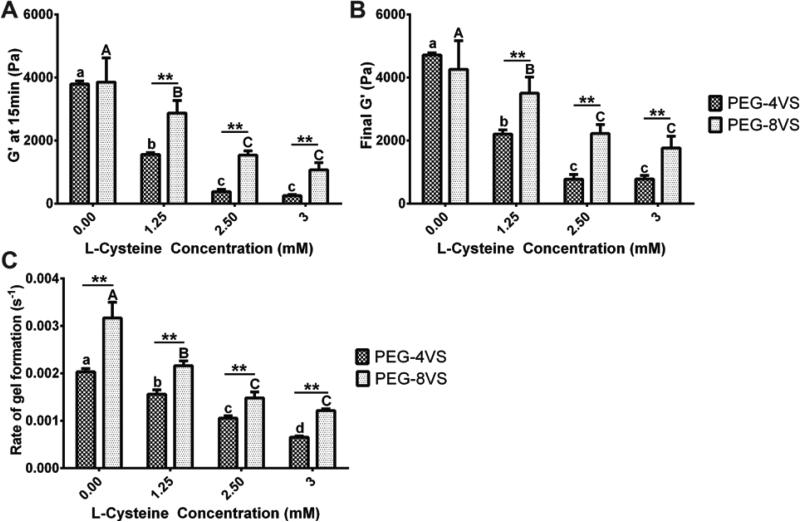
First, we studied how the addition of 1.25, 2.5, and 3mM L-Cys affected the storage modulus (G’) compared to unmodified hydrogels (0mM L-Cys). Addition of up to 3mM L-Cys to 5% 4-arm PEG hydrogels allowed complete gelation, which was determined to be the maximal concentration of the monofunctional peptide. The storage modulus of unmodified 4 and 8-arm PEG hydrogels showed no significant difference after 15min of gelation (Fig.2A) or after 60min (Fig.2B). However, upon the addition of L-Cys, storage moduli at 15min and at 60min of 4-arm PEG hydrogels were significantly lower compared to those of 8-arm PEG gels modified with L-Cys (Fig.2A,B). The final G’ of 4-arm PEG hydrogels modified with 3mM L-Cys modification only attained 0.8kPa compared to the 1.8kPa of 8-arm PEG gels, and 4.7kPa for unmodified 4-arm PEG hydrogels (Fig.2B). Modification with L-Cys had a pronounced negative effect on gelation and storage modulus of 4-arm PEG hydrogels, especially when the reaction was quenched after 15min. In that case, the storage modulus of 4-arm PEG hydrogels modified with 1.25mM L-Cys reached only 1.6kPa compared to the 2.9kPa of 8-arm PEG hydrogels, and 3.8kPa for unmodified 4-arm PEG hydrogels (Fig.2A). This negative effect was also observed for the G’ of 8-arm PEG hydrogels with the addition of L-Cys; yet, less pronounced compared to 4-arm PEG hydrogels.
Figure 2. The effect of the L-Cysteine concentration on storage modulus and rate constant of gel formation.
Time sweep tests were performed at 1 rad/sec and 10% strain to measure storage modulus (G’) of 5% 4 and 8-arm PEGs modified with 0, 1.25, 2.5 and 3mM L-Cysteine (A) after 15min and (B) after 60min of gelation. There were no significant differences between G’ of unmodified 4 and 8-arm PEGs (0mM L-Cys). However, G’ of 4 and 8-arm PEG (A) at 15min and (B) at 60min was significantly different at all other L-Cys concentrations. (C) Rate constant of gel formation of 5% 4 and 8-arm PEGs were significantly different at all conditions. All data is reported as mean ± S.D. (n = 3). Significance was determined by a two-way ANOVA followed by Bonferroni post-tests with p < 0.05. Double asterisks (**) denoted significance between PEG-4VS and PEG-8VS at all L-Cys concentration. Different lower (4-arm) and uppercase (8-arm) letter represent statistically significant difference within modified PEG-4VS and PEG-8VS conditions.
2. Rate Formation of PEG Hydrogels
We performed a time sweep test to determine the constant of gelation rate and investigate how the addition of bioactive moieties affects the crosslinking kinetics. Overall, the rate constant of gel formation was dependent on the number of arms available for crosslinking. The rate constant of gel formation of the 8-arm PEG hydrogel was significantly higher than those of 4-arm PEG hydrogel at all L-Cys concentrations (Fig.2C). The incorporation of L-Cys in both 4 and 8-arm PEG hydrogels resulted in a decrease in the rate constant of gel formation and the decrease was more significant when L-Cys concentration increased. For example, the incorporation of 3mM L-Cys caused the rate constant of gel formation to decrease one order of magnitude (from 2.0 × 10−3 sec−1 to 6.5 × 10−4 sec−1) for 4-arm PEG hydrogels. The decrease in the rate constant of gel formation of 8-arm PEG hydrogels also occurred; however, it was less significant compared to 4-arm PEG hydrogels.
3. Swelling of PEG-VS Hydrogels
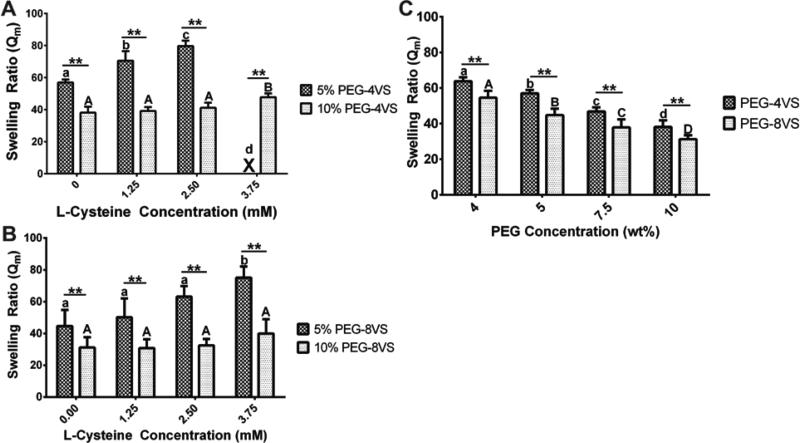
4 and 8-arm PEG hydrogels modified with increasing concentrations of L-Cys demonstrated significantly increased swelling ratios and a reduced concentration of elastically active chains (Fig.S1B); however, the swelling ratio of 8-arm PEG hydrogels was less affected by the incorporation of L-Cys than relative to 4-arm PEG hydrogels (Fig.3B). In 5% PEG hydrogels with 1.25mM L-Cys modification, 4-arm PEG hydrogels had a swelling ratio 1.4 times greater than 8-arm PEG hydrogels. When no L-Cys was added or at a L-Cys concentration of 1.25mM, there was no significant difference in the swelling ratio in 5% 8-arm PEG hydrogels in contrast to 5% 4-arm PEG hydrogels. Furthermore, 8-arm PEG hydrogels supported efficient network crosslinking up to 3.75mM L-Cys modification (Fig.3B) when 4-arm PEG hydrogels could form with up to 3mM L-Cys content (Fig.3A).
Figure 3. The effects of L-Cysteine and PEG-VS concentration on swelling ratio.
Swelling ratios were compared between (A) 5% and 10% 4-arm PEG and (B) 8-arm PEG modified with 0, 1.25, 2.5 and 3.75mM L-Cys. Swelling ratios of 5% 4-arm PEG hydrogels were significantly different from those of 10% 4-arm PEGs at all L-Cys concentrations. Swelling ratios of 5% 4-arm PEGs with 0mM L-Cys were significantly different from those of 5% 4-arm PEGs modified with 1.25 and 2.5mM L-Cys. However, swelling ratios of 10% 4-arm PEGs with 3.75mM L-Cys was only significantly different from those of all 10% 4-arm PEGs. (P<0.001) An 'X' denotes conditions that did not form gels. (B) For 8-arm PEG hydrogels, swelling ratios of 5% 8-arm PEGs were significantly different from those of 10% 8-arm PEGs at all L-Cys concentrations. However, there was no significant difference among any 10% 8-arm PEGs at all L-Cys concentrations (P<0.01) (C) Swelling ratios of 4-arm PEGs were significantly different from those of 8-arm PEGs at all PEG concentrations. All data is reported as mean ± standard deviation (n = 8). Significance was determined by a two-way ANOVA followed by Bonferroni post-tests. Triple asterisks (***) denoted significance between PEG-4VS and PEG-8VS at all L-Cys concentration (p < 0.001). Different lower (4-arm) and uppercase (8-arm) letter represented statistically significant difference within modified PEG-4VS and PEG-8VS conditions (p < 0.05).
We also compared the equilibrium mass swelling ratio of 4 and 8-arm PEG-VS hydrogels with varying solid concentrations from 4 to 10% w/v when the crosslinking reaction was quenched after 15min. Gels with PEG concentrations of less than 4% did not form after 15min, and gels with PEG concentration above 10% were not investigated due to the small mesh size and high storage modulus, which are less suitable for applications involving cells6. We found that a higher concentration of PEG resulted in less swelling for both 4 and 8-arm PEG hydrogels (Fig.3C). For example, the lowest Qm values, which are indicative of higher crosslinking density, were obtained for unmodified 4 and 8-arm at the 10% PEG concentrations. Even though both 4 and 8-arm PEG conditions had the same molar concentrations of reactive groups, significantly lower Qm values were observed in 8-arm PEG hydrogels compared to 4-arm PEG hydrogels at all PEG concentrations. Similar trends were observed for concentration of elastically active chains calculated by using the Flory-Rehner equation (Fig.S1A).
4. Material Properties Comparisons Between 5% PEG-VS and PEG-A Hydrogel
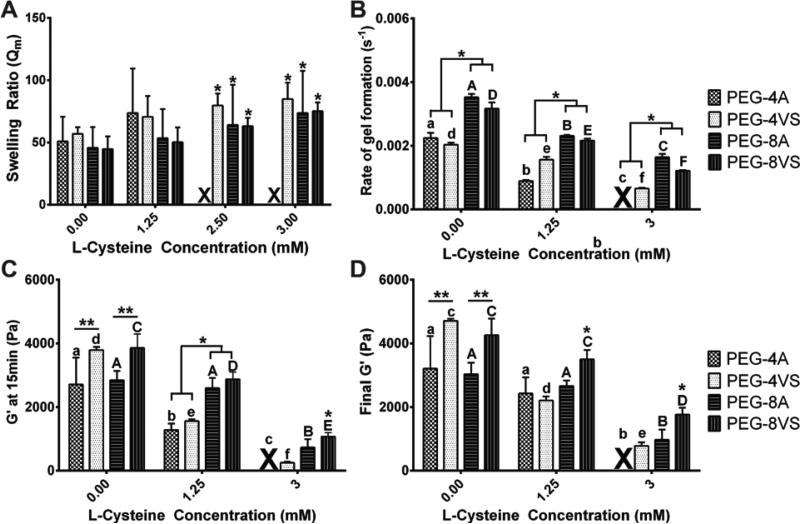
Different reactive end groups, such as acrylate (PEG-A) and vinylsulfone (PEG-VS), have the ability affect the mechanical properties of PEG MTA hydrogels. We investigated whether PEG functionality and the degree of modification demonstrate similar trends across various end groups of PEG in crosslinking efficiency and macroscopic properties. The PEG-VS and PEG-A macromers are identical in structure besides their reactive end groups. The considerable differences in resulting material properties among different MTA reactive groups arise from differences in electronegativity of the end groups affecting the crosslinking efficiency25. The main difference between the two functional end groups was the range of possible L-Cys modification in 5% 4-arm PEG hydrogels. PEG-4A hydrogels could form with a maximum of 1.25mM when PEG-4VS supported efficient network crosslinking up to 3mM L-Cys modification (Fig.4A). Overall, both PEG-8VS and PEG-8A demonstrated more efficient crosslinking compared to either 4-arm PEGs. As an example, the rate constant of gel formation of both PEG-8VS and PEG-8A was significantly higher than those of both PEG-4VS and PEG-4A at all L-Cys concentrations (Fig.4B). Also G’ of both 4-arm PEG hydrogels modified with 1.25mM L-Cys at 15min was significantly lower than G’ of both 8-arm PEG hydrogels modified with 1.25mM L-Cys at 15min (Fig.4C). However, there was no significant difference between any measurements of swelling ratio (Fig.4A). Overall, the trend of an increase in swelling ratio and a decrease in storage modulus with the addition of L-Cys was similar in PEG-A. Modification with L-Cys had a less pronounced negative effect on gelation and storage modulus of both PEG-8VS and PEG-8A compared to PEG-4VS and PEG-4A, especially when the reaction was quenched after 15min (Fig.4C).
Figure 4. Comparison of PEG-VS and PEG-A.
(A) Swelling ratio, (B) the rate constant of gel formation, storage modulus after 15min of gelation (C) and after 60min (D) were compared between 5% 4-arm PEG and 8-arm PEG hydrogels (PEG-VS and PEG-A) modified with 0, 1.25, and 3mM L-Cys. For swelling ratio, there was no statistical difference between PEG-VS and PEG-A at all conditions. However, PEG-4A gels could form with a maximum of 1.25mM when PEG-4VS supported efficient network crosslinking up to 3mM modification. The rate constant of gel formation of both PEG-8VS and PEG-8A was significantly higher than those of PEG-4VS and PEG-4A at all L-Cys concentration. G’ of both unmodified 8-arm PEGs after 15min and 60min was significantly higher from G’ of both unmodified 4-arm PEGs; however, upon addition of L-Cys, there were no significant differences between G’ of PEG-8A and both 4-arm PEGs. An 'X' denotes conditions that did not form gels. All data is reported as mean ± S.D. (A) n = 8, (B – D) n = 3. Significance was determined by a two-way ANOVA followed by Bonferroni post-tests. A single asterisk (*) denoted significance among groups of PEG-VS and PEG-A (both 4 and 8-arm) modified with a same L-Cys concentration. Different lower (4-arms) and uppercase (8-arms) letter represented statistically significant difference within modified PEG-VS and PEG-A groups (both 4 and 8-arm) (p < 0.05).
5. PEG Functionality and the Bioactive Modification Affect Cellular Growth and Behavior
PEG hydrogels with the same solid concentrations, but modified with different RGD concentrations, exhibit different mechanical properties. Here, we encapsulated and cultured HS-5 cells in 5% 4 and 8-arm PEG hydrogels modified with 0.25mM and 1.25mM RGD (Fig.5). To isolate the effect of the concentration of biological cues, we incorporated 1mM L-Cys to PEG hydrogels modified with 0.25mM RGD to mimic the same mechanical properties of PEG gels modified with 1.25mM RGD by blocking the same number of reactive arms. These hydrogels were crosslinked with the plasmin sensitive tri-functional crosslinking peptide composed of fibrin-derived sequences, YKNR, to allow proteolytic degradation18.
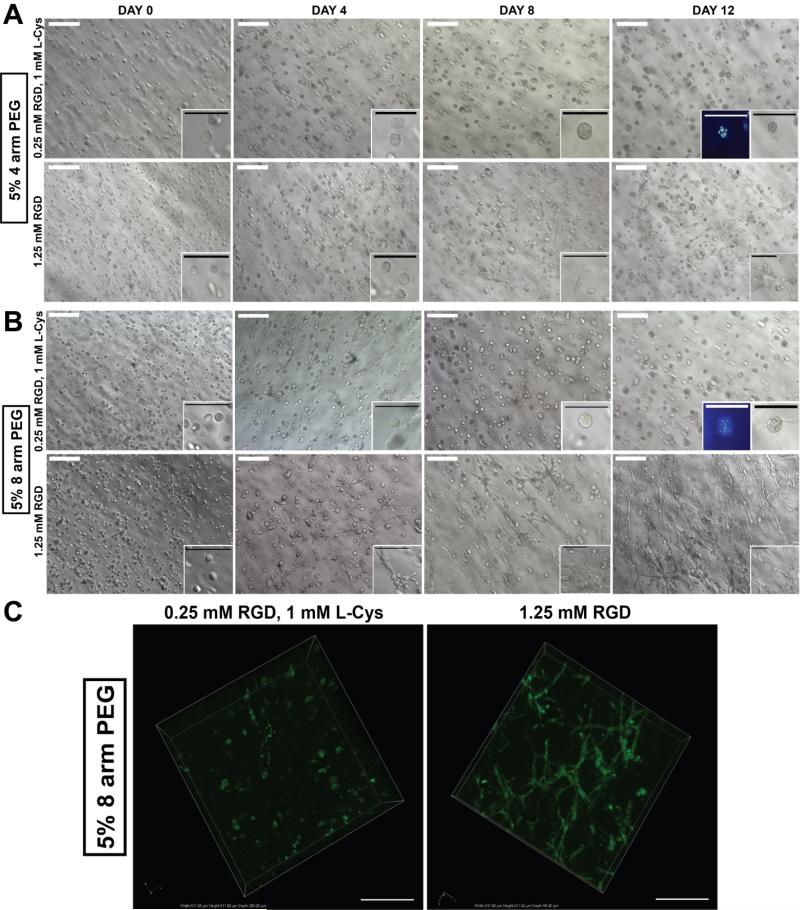
Figure 5. The effect of the RGD concentration on human bone marrow stromal cells growth and the network formation.
Stromal cells derived from human bone marrow (HS-5) [2 × 106 cells/mL] were encapsulated in 5% (A) 4-arm and (B) 8-arm PEG-VS modified with 0.25mM RGD and 1.25mM RGD and cultured up to Day 12. HS-5 cultured in 5% 8-arm PEGVS modified with 1.25mM RGD formed more interconnected and complete network. In contrast, HS-5 cultured in 4-arm PEG-VS and 8-arm PEG-VS modified with 0.25mM RGD + 1mM L-Cysteine formed spheroids confirmed by multiple nuclei observed using DAPI staining due to insufficient cell adhesion. Scale bar: White = 200μm, Black/DAPI = 100μm (C) GFP transfected Human bone marrow stromal cells (GFP HS-5) [2 × 106 cells/mL gels] were encapsulated in 5% 8-arm PEG-VS modified with 0.25mM RGD + 1mM L-Cysteine and 1.25mM RGD and cultured up to Day 12. GFP HS-5 cultured in 5% 8-arm PEG-VS modified with 1.25mM RGD formed more interconnected and complete network. Images were taken using a confocal microscope Nikon A1. Scale bar: 100μm.
HS-5 cells cultured in 5% 8-arm PEG hydrogels modified with 1.25mM RGD exhibited fibroblast-like spindle morphology starting at Day 4 and stromal networks began to form through affixing with other adjacent cell populations by Day 12 (Fig.5B). In 5% 4-arm PEG hydrogels modified with 1.25mM RGD, HS-5 cells started to express fibroblast-like spindle morphology at Day 8; however, stromal networks were not as dense nor interconnected as those observed in 5% 8-arm PEG modified with 1.25mM RGD (Fig.5A). Despite a less dense network, spheroid formation was still observed in 5% 4 and 8-arm PEG gels modified with 0.25mM RGD, which was confirmed by multiple nuclei DAPI staining (Fig.5A,B). In addition, although a reduction in the concentration of RGD limited cell network growth, cells were viable in gels modified with 0.25mM RGD (Fig.S2).
DISCUSSION
In this study, we aimed to investigate how the modification of PEG macromers with monofunctional groups affects the material properties. Considering that there are equal molar concentrations of reactive end groups participating in the reaction, we hypothesized that a greater number of arms per molecule core would allow a greater degree of bioactive modification without affecting the mechanical properties of the hydrogel. Interestingly, the crosslinking kinetics and the storage modulus of unmodified 4 and 8-arm PEG gels were not affected by the spatial distribution of arms around the core of the PEG macromer. In contrast, incorporation of biological cues dramatically affected the storage modulus and the crosslinking kinetics of modified 4-arm PEG gels compared to the 8-arm PEG gels. The rate constant of gel formation of 4 and 8-arm PEG gels was significantly different at all L-Cys concentrations and the most significant differences were observed during the first 15min of crosslinking. These results indicate that increasing the spatial distribution of arms around the core of the PEG macromer attenuates the negative effect of modification on the crosslinking kinetics and storage modulus.
We also investigated whether PEG functionality and the degree of modification demonstrate similar trends across various end groups of PEG in crosslinking efficiency and macroscopic properties. The PEG-VS and PEG-A macromers are identical in structure besides their reactive end groups; however, the considerable differences in resulting material properties were observed. The significant differences in the gel formation kinetics and the storage modulus of PEG-VS and PEG-A resulted from the differences in reactivity of the end groups. A sulfone group of PEG-VS has a higher electron withdrawing capability than a carbonyl group of PEG-A, making the vinyl moiety more electron deficient, and more reactive with the electron rich thiol group compared to the acrylate26. Due to more reactive vinyl sulfone moiety, unmodified PEG-VS demonstrates higher storage modulus after 15 and 60min compared to PEG-A regardless of the number of reactive end groups. Also, the bioactive modification had a less pronounced negative effects on the properties of PEG-VS compared to PEG-A due to the same reason. Importantly, increasing bioactive modification resulted in similar trends of decreased storage modulus, reduced rate of gel formation, and increased swelling in both PEG-A and PEG-VS hydrogels.
According to the MTA chemistry of the hydrogel formation, the number of reactive arms, elastically inactive loop formation, and entropy are related to this phenomenon. First, the formation of elastically inactive loops is more critical in PEG with lower functionality because the effective crosslink functionality must be greater than two in order for networks between any elastically active chains to occur20. Second, based on previous studies involving Monte Carlo simulation of many-chain star polymers27,28, an increase in the number of arms causes an expansion of the arms due to the influence of three-body effects in the central regions of the “core”. As a result, when the number of arms is high, the segment density near the core is much higher than in the periphery, as described by the model of Daoud and Cotton29. Therefore, this core effect reduces the interpenetration and overlap of other macromer's arms; thus, there is a higher probability for a greater number of the end functional groups to be exposed at a given time for efficient crosslinking to occur30. Finally, the mobile tip of the chain encounters an entropic potential barrier that penalizes deep fluctuations needed to bring the tip to the tethering point31. As the number of arms on the macromer increases, there is progressively less space left for the local fluctuation of the chains29, meaning that 8-arm PEG would encounter a lower entropic potential barrier compared to 4-arm PEG.
Bioactive modification of PEG hydrogels is done by incorporating integrin binding sequences like RGD; however, monofunctional RGD occupies the same reactive sites on the PEG molecules used for the crosslinking reaction, thus affecting its material properties. In this study, we measured the equilibrium swelling ratio (Qm) of 4 and 8-arm PEG hydrogels because Qm correlates with crosslinking density and serves as an indication of overall hydrogel network structural defects, such as multiple primary loops and dangling ends15,20. Qm is also relevant in biological applications since high swelling is known to mitigate the effects of incorporated RGD due to dilution32. Overall, a greater PEG solid concentration resulted in less swelling and better network formation regardless of the number of arms. Even with the presence of added biological cues, 8-arm PEG hydrogels had significantly lower Qm values and demonstrated a greater range of modification compared to 4-arm PEG at all concentrations. Lower Qm values of modified 8-arm PEG hydrogels compared to those of 4-arm PEG hydrogels indicated a more densely crosslinked networks, which also correlated with a higher storage modulus. Similarly, the calculations using the Flory-Rehner equation of the concentration of elastically active chains predicted more elastically active chains 8-arm PEG at all biologically relevant PEG solid concentrations (4 – 10% w/v) than 4-arm PEG (Fig.S1A). Both experimental data and theoretical calculations confirmed how functionality is directly related to effective crosslinking and the concentration of elastically active chains. This implies that additional types and concentrations of biological cues, such as growth factors, can be incorporated into 8-arm PEG hydrogels for further modification7,8,33.
To investigate how the changes in macroscopic properties induced by bioactive modification affect cellular behavior and function, we encapsulated and cultured HS-5 cells in 4 and 8-arm PEG hydrogels. Cell growth and stromal network formation were observed in 8-arm PEG hydrogels modified with 1.25mM RGD. We also found that HS-5 cells cultured in both 4-arm and 8-arm PEG hydrogels modified with 0.25mM RGD formed spherical multicellular aggregates (spheroids). Spheroid formations were previously described in anchorage-dependent cell lines on non-adherent surfaces or in bio-inert polymers34,35. Multiple reports have shown that spheroid formation is directly related to cell-cell contacts that regulate cell survival and growth, which was shown to be dependent on integrin-mediated cell contacts and RGD peptides36,37. As a result, we concluded that 0.25mM was an insufficient RGD concentration to promote appropriate cell adhesion and growth. More importantly, HS-5 cells cultured in 4-arm PEG hydrogels modified with 1.25mM RGD did not form as dense and interconnected stromal networks as they did in the 8-arm PEG modified with 1.25mM RGD. The 4-arm PEG gel, in this case, had a swelling ratio 1.4 times greater than the 8-arm PEG, demonstrating the negative effect of swelling on cell growth and matrix remodeling. The more swollen matrix of the 4-arm PEG hydrogel was inferior because swelling is known to mitigate the effects of incorporated RGD due to dilution32 (e.g. 1.25mM RGD gets diluted to 0.34mM after swelling for 4-arm PEG gels). These results demonstrate that bioactive modification not only affects the PEG hydrogel's macroscopic properties, but also regulates cellular behavior and function.
CONCLUSIONS
Bioactive modification affected the mechanical properties of multi-arm PEG gels, such as storage modulus and swelling, as a consequence of the macroscopic network changes. These changes were dependent on PEG functionality (4 vs. 8-arm) and PEG solid concentrations, which are directly related to the concentration of elastically active chains and the overall amount of binding sites. Overall, 8-arm PEG allowed a greater degree of modification and its macroscopic properties and crosslinking kinetics were less affected by the act of modification or quenching compared to 4-arm PEG. These advantages provide a finely tuned design for various biological applications with broad range of bioactive modification.
Supplementary Material
ACKNOWLEDGMENTS
The authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. JK gratefully acknowledges the Tissue Engineering and Regeneration (TEAM) Grant (NIH T32 DE007057).
Footnotes
Supporting Information
Concentration of elastically active chains, and LIVE/DEAD assay.
References
- 1.Peppas NA, Langer R. New challenges in biomaterials. Science. 1994 Mar 25;263(5154):1715–1720. doi: 10.1126/science.8134835. [DOI] [PubMed] [Google Scholar]
- 2.Hubbell JA. Biomaterials in tissue engineering. Biotechnology (N Y) 1995 Jun;13(6):565–576. doi: 10.1038/nbt0695-565. [DOI] [PubMed] [Google Scholar]
- 3.Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J, et al. Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol. 2009 Oct;20(8):931–941. doi: 10.1016/j.semcdb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005 Aug;3(8):1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 5.Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998 Feb;39(2):266–276. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci U S A. 2003 Apr 29;100(9):5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pratt AB, Weber FE, Schmoekel HG, Muller R, Hubbell JA. Synthetic extracellular matrices for in situ tissue engineering. Biotechnol Bioeng. 2004 Apr 5;86(1):27–36. doi: 10.1002/bit.10897. [DOI] [PubMed] [Google Scholar]
- 8.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005 Jan;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 9.van de Wetering P, Metters AT, Schoenmakers RG, Hubbell JA. Poly(ethylene glycol) hydrogels formed by conjugate addition with controllable swelling, degradation, and release of pharmaceutically active proteins. J Control Release. 2005 Feb 16;102(3):619–627. doi: 10.1016/j.jconrel.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Weiss MS, Bernabe BP, Shikanov A, Bluver DA, Mui MD, Shin S, et al. The impact of adhesion peptides within hydrogels on the phenotype and signaling of normal and cancerous mammary epithelial cells. Biomaterials. 2012 May;33(13):3548–3559. doi: 10.1016/j.biomaterials.2012.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006 Aug 25;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 12.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010 Jun;9(6):518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burdick JA, Murphy WL. Moving from static to dynamic complexity in hydrogel design. Nat Commun. 2012;3:1269. doi: 10.1038/ncomms2271. [DOI] [PubMed] [Google Scholar]
- 14.Morpurgo M, Veronese FM, Kachensky D, Harris JM. Preparation of characterization of poly(ethylene glycol) vinyl sulfone. Bioconjug Chem. 1996 May-Jun;7(3):363–368. doi: 10.1021/bc9600224. [DOI] [PubMed] [Google Scholar]
- 15.Lutolf MP, Hubbell JA. Synthesis and physicochemical characterization of end-linked poly(ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules. 2003 May-Jun;4(3):713–722. doi: 10.1021/bm025744e. [DOI] [PubMed] [Google Scholar]
- 16.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003 Nov;24(24):4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 17.Fittkau MH, Zilla P, Bezuidenhout D, Lutolf MP, Human P, Hubbell JA, et al. The selective modulation of endothelial cell mobility on RGD peptide containing surfaces by YIGSR peptides. Biomaterials. 2005 Jan;26(2):167–174. doi: 10.1016/j.biomaterials.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Shikanov A, Smith RM, Xu M, Woodruff TK, Shea LD. Hydrogel network design using multifunctional macromers to coordinate tissue maturation in ovarian follicle culture. Biomaterials. 2011 Apr;32(10):2524–2531. doi: 10.1016/j.biomaterials.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh SP, Schwartz MP, Lee JY, Fairbanks BD, Anseth KS. A peptide functionalized poly(ethylene glycol) (PEG) hydrogel for investigating the influence of biochemical and biophysical matrix properties on tumor cell migration. Biomater Sci. 2014 Jul 1;2(7):1024–1034. doi: 10.1039/C4BM00022F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metters A, Hubbell J. Network formation and degradation behavior of hydrogels formed by Michael-type addition reactions. Biomacromolecules. 2005 Jan-Feb;6(1):290–301. doi: 10.1021/bm049607o. [DOI] [PubMed] [Google Scholar]
- 21.Sokic S, Papavasiliou G. Controlled proteolytic cleavage site presentation in biomimetic PEGDA hydrogels enhances neovascularization in vitro. Tissue Eng Part A. 2012 Dec;18(23-24):2477–2486. doi: 10.1089/ten.tea.2012.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizzi SC, Hubbell JA. Recombinant protein-co-PEG networks as cell-adhesive and proteolytically degradable hydrogel matrixes. Part I: Development and physicochemical characteristics. Biomacromolecules. 2005 May-Jun;6(3):1226–1238. doi: 10.1021/bm049614c. [DOI] [PubMed] [Google Scholar]
- 23.Metters AT, Anseth KS, Bowman CN. Fundamental studies of a novel, biodegradable PEG-b-PLA hydrogel. Polymer. 2000;(41):3993–4004. [Google Scholar]
- 24.Kim J, Wu B, Niedzielski SM, Hill MT, Coleman RM, Ono A, et al. Characterizing natural hydrogel for reconstruction of three-dimensional lymphoid stromal network to model T-cell interactions. J Biomed Mater Res A. 2015 Feb 3; doi: 10.1002/jbm.a.35409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phelps EA, Enemchukwu NO, Fiore VF, Sy JC, Murthy N, Sulchek TA, et al. Maleimide cross-linked bioactive PEG hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv Mater. 2012 Jan 3;24(1):64–70. doi: 10.1002/adma.201103574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chatani S, Nair DP, Bowman CN. Relative reactivity and selectivity of vinyl sulfones and acrylates towards the thiol-Michael addition reaction and polymerization. Polymer Chemistry. 2013;4(4):1048–1055. [Google Scholar]
- 27.Molina LA, Freire JJ. Monte Carlo Simulation of Many-Chain Star Polymer Solutions. Macromolecules. 1999;32:499–505. [Google Scholar]
- 28.Cecca AD, Freire JJ. Monte Carlo Simulation of Star Polymer Systems with the Bond Fluctuation Model. Macromolecules. 2002;35:2851–2858. [Google Scholar]
- 29.Daoud M, Cotton JP. Star shaped polymers : a model for the conformation and its concentration dependence. J Physics. 1982;43:531–538. [Google Scholar]
- 30.Pakula T, Vlassopoulos D, Fytas G, Roovers J. Structure and Dynamics of Melts of Multiarm Polymer Stars. Macromolecules. 1998;31:8931–8940. [Google Scholar]
- 31.Shanbhag S, Larson RG. Chain retraction potential in a fixed entanglement network. Phys Rev Lett. 2005 Feb 25;94(7):076001. doi: 10.1103/PhysRevLett.94.076001. [DOI] [PubMed] [Google Scholar]
- 32.Jongpaiboonkit L, King WJ, Murphy WL. Screening for 3D environments that support human mesenchymal stem cell viability using hydrogel arrays. Tissue Eng Part A. 2009 Feb;15(2):343–353. doi: 10.1089/ten.tea.2008.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seliktar D, Zisch AH, Lutolf MP, Wrana JL, Hubbell JA. MMP-2 sensitive, VEGF-bearing bioactive hydrogels for promotion of vascular healing. J Biomed Mater Res A. 2004 Mar 15;68(4):704–716. doi: 10.1002/jbm.a.20091. [DOI] [PubMed] [Google Scholar]
- 34.Koide N, Sakaguchi K, Koide Y, Asano K, Kawaguchi M, Matsushima H, et al. Formation of multicellular spheroids composed of adult rat hepatocytes in dishes with positively charged surfaces and under other nonadherent environments. Exp Cell Res. 1990 Feb;186(2):227–235. doi: 10.1016/0014-4827(90)90300-y. [DOI] [PubMed] [Google Scholar]
- 35.Ijima H, Nakazawa K, Mizumoto H, Matsushita T, Funatsu K. Formation of a spherical multicellular aggregate (spheroid) of animal cells in the pores of polyurethane foam as a cell culture substratum and its application to a hybrid artificial liver. J Biomater Sci Polym Ed. 1998;9(7):765–778. doi: 10.1163/156856298x00136. [DOI] [PubMed] [Google Scholar]
- 36.Korff T, Augustin HG. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol. 1998 Nov 30;143(5):1341–1352. doi: 10.1083/jcb.143.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bates RC, Edwards NS, Yates JD. Spheroids and cell survival. Crit Rev Oncol Hematol. 2000 Nov-Dec;36(2-3):61–74. doi: 10.1016/s1040-8428(00)00077-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.