Abstract
Abstract
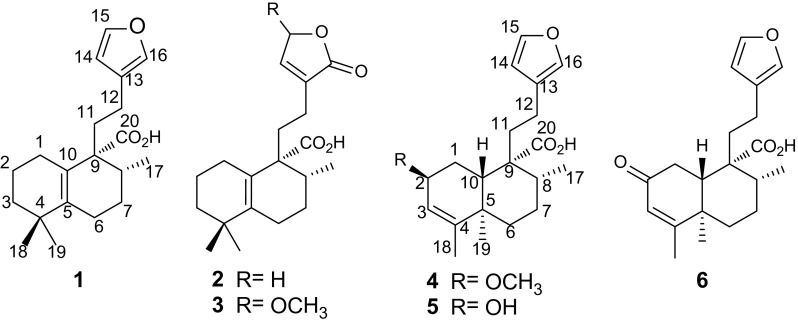
Three new halimane-type diterpenoids formosins A–C (1–3), and three clerodane-type diterpenoids formosins D–F (4–6), were isolated from the twigs of Excoecaria formosana. Their structures were assigned on the basis of spectroscopic data analysis. Compounds 1 and 4 showed moderate anti-microbial activities against Bacillus subtilis (MIC = 50 and 50 μg/mL, respectively). Compound 6 exhibited moderate anti-microbial activities against two strains of Helicobacter pylori (Hp-SS1 and ATCC 43504) with MIC values of 50 and 50 μg/mL, respectively.
Graphical abstract
Electronic supplementary material
The online version of this article (doi:10.1007/s13659-016-0086-6) contains supplementary material, which is available to authorized users.
Keywords: Excoecaria formosana, Halimane-type, Clerodane-type, Diterpenoid, Anti-microbial
Introduction
The genus Excoecaria (Euphorbiaceae) compromising 40 species, are widely distributed in Africa and East Asia [1]. Several plants in this genus have been used in folk medicine to treat psoriasis, dermatitis and pruritus [2–4]. The characteristic of this plant genus is the poisonous milk latex, which causes skin blister [5]. Chemical investigations on this plant genus have led to the isolation of structurally diverse compounds with significant biological activities including anti-tumor promoting, anti-ulcer, and anti-microbial activities [6–10]. In the current study, three new halimane-type diterpenoids formosins A–C (1–3), and three clerodane-type diterpenoids formosins D–F (4–6), were isolated from the twigs of Excoecaria formosana. Presented herein are the isolation, structural characterization, and biological evaluation of these compounds.
Results and Discussion
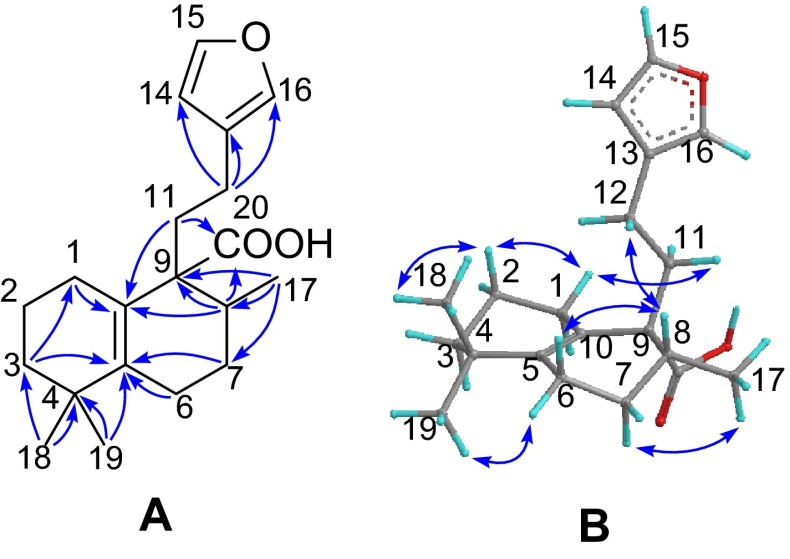
Compound 1, a white powder, gave a molecular formula C20H28O3 as determined by the (+)-HRESIMS ion at m/z 339.1937 [M + Na]+ (calcd 339.1931) requiring seven degrees of unsaturation. The IR absorptions (3000–2800 cm−1, broad band) and (1695 cm−1) showed the presence of a carboxylic group. The diagnostic NMR data (Tables 1, 2) suggested the presence of a β-substituted furan ring (δH 6.27, 7.22, and 7.34), a persubstituted double bond, and a carboxylic group (δC 181.5). These functionalities accounted for five out of the seven indices of hydrogen deficiency, requiring the presence of two additional rings in the structure of 1. The aforementioned data suggested that compound 1 is a halimane-type diterpenoid and is structurally related to crotohalimaneic acid [11]. The planar structure of 1 was deduced by 2D NMR spectra. In the HMBC spectrum (Fig. 1), two tertiary methyls (δH 1.00, and 1.04, each 3H, s) correlating with C-3, C-4 and C-5 were attached to C-4. The multiple HMBC correlations of H-3, H-7, and H3-19/C-5 (δC 141.3); H-1, H-8, and H-11/C-10 (δC 125.2); and H3-17/C-7, C-8, and C-9 indicated the presence of a typical Δ5(10) double bond and 8-Me (C-17). The β-substituted furan ring and carboxylic group were located at C-12 and C-9 by the HMBC correlations of H-12/C-13, C-14 and C-16; and H-8 and H-11/C-20, respectively. The relative configuration of 1 was established by the ROESY experiment (Fig. 1). The ROESY cross-peaks of H3-18/H-2β, H-2β/H-1β, H-1β/H-11, and H-12/H-8 indicated that they are co-facial and randomly assigned in a β-configuration. In consequence, H3-19 and H3-17 were α-oriented by the ROESY correlations of H3-19/H-6α and H3-17/H-7α. The structure of 1 (formosin A) was herein elucidated as shown.
Table 1.
1H NMR data for compounds 1–6 in CDCl3 at 400 MHz
| Position | 1 (mult., J in Hz) | 2 (mult., J in Hz) | 3 (mult., J in Hz) | 4 (mult., J in Hz) | 5 (mult., J in Hz) | 6 (mult., J in Hz) |
|---|---|---|---|---|---|---|
| 1 | α 1.92 (m) | α 1.86 (m) | α 1.90 (m) | α 1.76 (m) | α 1.67 (m) | α 2.89 (dd, 17.9, 14.5) |
| β 1.68 (m) | β 1.64 (m) | β 1.68 (m) | β 2.22 (m) | β 2.32 (m) | β 2.66 (dd, 17.9, 3.1) | |
| 2 | 1.64 (m) | 1.60 (m) | 1.60 (m) | 3.61 (m) | 4.19 (m) | |
| 3 |
α 1.36 (td, 12.5, 5.4) |
α 1.35 (td, 12.1, 4.3) |
α 1.36 (m) | 5.43 (d, 4.7) | 5.27 (br s) | 5.78 (s) |
| β 1.50 (m) | β 1.46 (m) | β 1.52 (m) | ||||
| 6 | α 2.12 (m) | α 2.12 (m) | α 2.16 (m) | α 1.79 (m) | α 1.80 (m) | α 2.16 (m) |
| β 1.94 (m) | β 1.92 (m) | β 1.94 (m) | β 1.29 (m) | β 1.21 (m) | β 1.40 (m) | |
| 7 | α 1.52 (m) | α 1.52 (m) | α 1.56 (m) | α 2.11 (m) | α 2.11 (m) | α 2.24 (m) |
| β 1.70 (m) | β 1.66 (m) | β 1.72 (m) | β 1.47 (m) | β 1.47 (m) | β 1.56 (m) | |
| 8 | 1.78 (m) | 1.73 (m) | 1.75 (m) | 1.61 (m) | 1.55 (m) | 1.62 (m) |
| 10 | 1.95 (m) | 1.62 (m) | 1.90 (m) | |||
| 11 | 1.98 (m) | 1.98 (m) | 2.00 (m) | 1.94 (m) 2.23 (m) |
1.92 (m) 2.25 (m) |
1.94 (m) 2.18 (m) |
| 12 | 2.10 (m) 2.27 (m) |
2.02 (m) 2.15 (m) |
2.03 (m) 2.18 (m) |
2.32 (m) 2.57 (m) |
2.34 (m) | 2.33 (m) |
| 14 | 6.27 (br s) | 7.17 (t, 1.6) | 6.83 (br s) | 6.28 (br s) | 6.28 (br s) | 6.26 (br s) |
| 15 | 7.34 (br s) | 4.77 (d, 1.8) | 5.74 (br s) | 7.34 (br s) | 7.35 (br s) | 7.34 (br s) |
| 16 | 7.22 (br s) | 7.22 (br s) | 7.23 (br s) | 7.22 (br s) | ||
| 17 | 0.95 (d, 6.7) | 0.94 (d, 6.7) | 0.94 (d, 5.6) | 1.16 (d, 6.8) | 1.15 (d, 6.9) | 1.17 (d, 6.8) |
| 18 | 1.04 (s) | 1.04 (s) | 1.03 (s) | 1.65 (s) | 1.62 (s) | 1.90 (s) |
| 19 | 1.00 (s) | 1.00 (s) | 0.99 (s) | 0.91 (s) | 0.99 (s) | 1.08 (s) |
| 2-OMe | 3.34 (s) | |||||
| 15-OMe | 3.49 (s) |
Table 2.
13C NMR data for compounds 1–6 in CDCl3 at 100 MHz
| Carbons | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1 | 19.5 | 19.4 | 19.4 | 24.6 | 30.7 | 36.5 |
| 2 | 28.2 | 28.0 | 28.1 | 74.3 | 69.4 | 199.9 |
| 3 | 39.4 | 39.3 | 39.3 | 120.6 | 125.1 | 125.8 |
| 4 | 34.8 | 34.8 | 34.8 | 149.2 | 146.5 | 171.4 |
| 5 | 141.3 | 141.7 | 142.2 | 39.3 | 39.2 | 40.2 |
| 6 | 25.1 | 25.0 | 25.0 | 36.8 | 37.1 | 36.2 |
| 7 | 27.5 | 27.4 | 27.4 | 27.1 | 27.0 | 26.7 |
| 8 | 33.6 | 33.4 | 33.5 | 36.8 | 36.9 | 36.7 |
| 9 | 54.9 | 54.6 | 54.6 | 49.5 | 49.4 | 49.8 |
| 10 | 125.2 | 125.3 | 125.1 | 42.5 | 46.1 | 46.1 |
| 11 | 31.0 | 28.7 | 28.4 (28.5) | 33.7 | 33.7 | 33.4 |
| 12 | 18.8 | 19.5 | 19.5 | 17.2 | 17.4 | 17.8 |
| 13 | 125.7 | 134.5 | 138.9 | 124.8 | 124.3 | 123.9 |
| 14 | 111.0 | 143.9 | 141.5 | 110.9 | 110.8 | 110.7 |
| 15 | 142.7 | 70.2 | 102.5 | 142.8 | 142.9 | 142.9 |
| 16 | 138.5 | 174.3 | 171.3 | 138.5 | 138.6 | 138.7 |
| 17 | 17.4 | 17.4 | 17.4 | 16.5 | 16.6 | 16.3 |
| 18 | 26.6 | 26.6 | 26.6 | 18.1 | 17.9 | 19.2 |
| 19 | 29.0 | 28.9 | 28.9 | 16.0 | 17.4 | 16.0 |
| 20 | 181.5 | 180.4 | 180.0 | 182.6 | 181.8 | 181.3 |
| 2-OMe | 56.3 | |||||
| 15-OMe | 56.9 (57.0) |
Fig. 1.
a Selected HMBC, and b ROESY correlations of 1
Compound 2 had the molecular formula of C20H28O4, as determined by the 13C NMR data and the (+)-HRESIMS ion at m/z 355.1839 [M + Na]+ (calcd 355.1880), which is 16 mass units more than that of 1. Comparison of its NMR spectroscopic data (Tables 1, 2) with those of 1 revealed they are structural analogues with the obvious difference being the presence of an α,β-unsaturated-γ-lactone moiety instead of the β-substituted furan ring. It was confirmed by the NMR data (δH 4.77 and 7.17; δC 70.2, 134.5, 143.9, and 174.3), as well as the key HMBC correlations from H-12 to C-13, C-14, and C-16 (Figure S13, Supporting Information). Thus, the structure of 2 (formosin B) was determined as shown.
Compound 3 displayed a molecular formula of C21H30O5 as established by the (+)-HRESIMS at m/z 385.1991 [M + Na]+ (calcd 385.1991) and the 13C NMR data. Analysis of the NMR data (Tables 1, 2) of 3 showed many similarities to those of 2. The only difference was the presence of an additional methoxy group (δH 3.49, s, 3H), which was located at C-15 to form the acetal motif, which was confirmed by the downfield shifted C-15 (ΔδC 32.3) as compared to that of 2. Compound 3 was obtained as a pair of inseparable C-15 epimers, which exhibited several pairs of very close carbon resonances in the 13C NMR spectrum (Figure S19, Supplementary Material). Therefore, the structure of 3 (formosin C) with its relative configuration was confirmed as depicted by the HMBC and ROESY spectra (Figures S21 and S22, Supplementary Material).
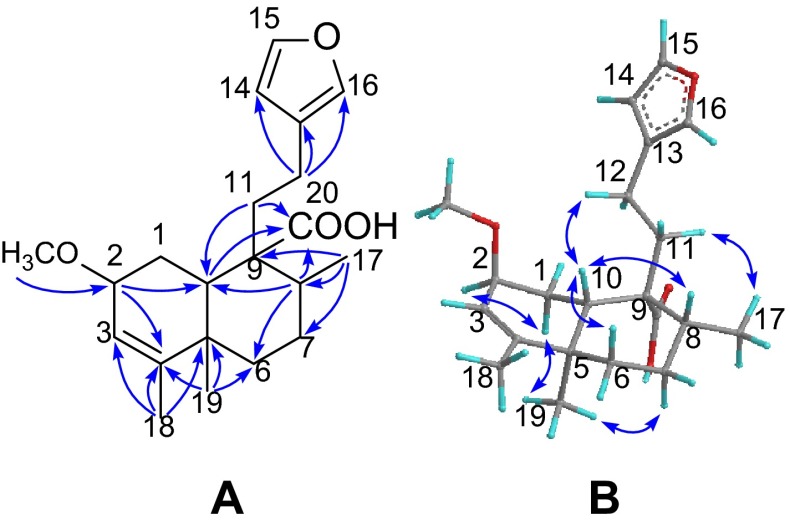
Compound 4 was obtained as a white powder with a molecular formula of C21H30O4 as established by the 13C NMR data and the (+)-HRESIMS ion at m/z 369.1970 [M + Na]+ (calcd 369.2036), demanding seven degrees of unsaturation. The IR absorption bands (3000–2800 cm−1, broad band; and 1695 cm−1) showed the presence of a carboxylic group. The characteristic NMR signals for a β-furan ring, a trisubstituted double bond, a methoxyl and a carboxylic groups were observed from the 1H and 13C NMR spectroscopic data analysis (Tables 1, 2). Comprehensive analysis of the NMR spectra of 4 revealed its structure is highly related with that of junceic acid [12] with a clerodane-type diterpenoid backbone. The only difference was the presence of an additional methoxyl group in 4, which was placed at C-2 by the HMBC correlation (Fig. 2) from CH3O (δH 3.34) to C-2 (δC 74.3). The carboxylic group was attached to C-9 via the HMBC correlations from H-8, H-10 and H-11 to C-20 (δC 182.6). The key HMBC cross-peaks from H3-18 to C-3 (δC 120.6), C-4 (δC 149.2) and C-5, and from H3-19 to C-4 revealed the presence of Δ3 double bond. The relative configuration of 4 was established by the ROESY experiment (Fig. 2). The ROESY correlations of H-10/H-6β and H-10/H-8 indicated that H-8 and H-10 are co-facial and randomly assigned in a β-configuration. Consequently, H3-19 and H-2 were thus assigned to be α-directed by the ROESY correlations of H3-19/H-1α, H3-19/H-7α, and H-2/H-1α. Therefore, the structure of 4 (formosin D) was established as depicted.
Fig. 2.
a Selected HMBC, and b ROESY correlations of 4
Compound 5 possessed a molecular formula C20H28O4 based on the 13C NMR data and the (+)-HRESIMS ion at m/z 355.1885 [M + Na]+ (calcd 355.1888), which is 14 mass units less than that of 4. Detailed analysis of the NMR data (Tables 1, 2) of 5 revealed that its structure is closely related with that of 4 with the only difference being the absence of the methyl etherification, which is consistent with the molecular formula. The structure of 5 (formosin E) with the relative configuration was further confirmed by HMBC and ROESY spectra (Figures S39 and S40, Supplementary Material).
Compound 6, named formosin F, exhibited a sodiated molecular ion at m/z 353.1724 [M + Na]+ (calcd 353.1723) in the (+)-HRESIMS, consistent with a molecular formula of C20H26O4, which was supported by the 13C NMR data. Comparison of the NMR data (Tables 1, 2) of 6 with those of 5 revealed they are structural analogues. The main difference was the presence of a keto group at C-2 in 6 instead of the hydroxy group in the latter, which was confirmed by the HMBC correlations (Figure S42, Supporting Information) from H-3 and H-10 to C-2 (δC 199.9). The structure of 6 was thus determined as shown.
The new isolates were tested for anti-microbial activities against a panel of microbes in vitro by the microdilution method [13, 14]. Compounds 1 and 4 exhibited moderate activity against Bacillus subtilis ATCC 6633 with MIC values of 50 and 50 μg/mL, respectively, where magnolol was used as the positive control (MIC = 12.5 μg/mL). Compound 6 showed moderate antibacterial activities against two strains Helicobacter pylori (Hp-SS1 or ATCC 43504) with MIC values of 50 and 50 μg/mL, respectively, and metronidazole was used as the positive control (MIC = 0.312 and 128 μg/mL, respectively).
Experimental Section
General Experimental Procedures
Optical rotations were determined on a Perkin-Elmer 341 polarimeter. UV spectra were recorded on a Shimadzu UV-2550 spectrophotometer. IR spectra were acquired on a Perkin-Elmer 577 spectrometer. NMR spectra were measured on a Bruker AM-400 spectrometer with TMS as internal standard. ESIMS and HRESIMS were performed on a Bruker Daltonics Esquire 3000 plus and a Waters-Micromass Q-TQF Ultima Global mass spectrometer, respectively. Semi-preparative HPLC was performed on a Waters 1525 binary pump system with a Waters 2489 detector (210 nm) and equipped with a YMC-Pack ODS-A (250 × 10 mm, S-5 μm). Silica gel (200–300 mesh, Qingdao Haiyang Chemical Co., Ltd.), C18 reversed-phase (RP-18) silica gel (20–45 μm, Fuji Silysia Chemical Ltd.), CHP20P MCI gel (75–150 μm, Mitsubishi Chemical Corporation), and Sephadex LH-20 gel (Amersham Biosciences) were used for column chromatography (CC). Pre-coated silica gel GF254 plates (Qingdao Haiyang Chemical Co., Ltd.) were used for TLC detection. All solvents used for CC were of analytical grade (Shanghai Chemical Reagents Co., Ltd.), and solvents used for HPLC were of HPLC grade (J & K Scientific Ltd.).
Plant Material
The twigs of E. formosana were collected from Sanya city of Hainan Province, the People’s Republic of China, and authenticated by Prof. S.-M. Huang, Department of Biology, Hainan University. A voucher specimen has been deposited in Shanghai Institute of Materia Medica, Chinese Academy of Sciences (accession number: SMEF-2006-1Y).
Extraction and Isolation
The air-dried, powdered twigs of E. formosana (6.0 kg) was extracted three times with 95 % EtOH at room temperature to give a crude extract (290 g), which was partitioned between EtOAc and H2O. The EtOAc-soluble fraction (85 g) was subjected to passage over an MCI gel column (MeOH/H2O, 3:7–9:1) to afford fractions A–G. Fraction C (25.7 g) was separated over a silica gel column eluted with gradient mixtures of petroleum ether–acetone (35:1–1:1, v/v) to afford major fractions C1-C6. Fraction C3 (3.4 g) was separated on a reversed-phase C18 silica gel column (MeOH/H2O, 55–100 %) to yield three major portions (C3a–C3c), and each of those was purified by a semi-preparative HPLC (60 % CH3CN in H2O, 3 mL/min) to yield compounds 1 (20 mg), 2 (10 mg), and 6 (100 mg), respectively. Fraction C4 (515 mg) was purified by a semi-preparative HPLC (55 % CH3CN in H2O, 3 mL/min) to give compound 4 (15 mg). Fraction C6 (1.5 g) was separated on a column of Sephadex LH-20, and then purified by a semi-preparative HPLC (50 % CH3CN in H2O, 3 mL/min) to yield compound 5 (8 mg). Fraction E (11.4 g) was chromatographed on a silica gel column eluted with petroleum ether-ethyl acetate (25:1–1:4, v/v) to afford subfractions E1–E4. Fraction E2 (217 mg) was separated on a reversed-phase column containing C18 silica gel (MeOH/H2O, 70–100%) to yield three fractions E2a–E2c. Fraction E2b (35 mg) was separated by a semi-preparative HPLC (70 % CH3CN in H2O, 3 mL/min) to yield compound 3 (8 mg).
Formosin A (1)
White powder; +123 (c 0.10, MeOH); UV (MeOH) λmax (log ε) 206 (4.19) nm; IR (KBr) νmax 3444, 3000–2500, 1695, 1622, 1458, 1257, 1024, 600 cm−1; 1H NMR (CDCl3), see Table 1 and 13C NMR (CDCl3) see Table 2; (+)-ESIMS m/z 339.2 [M + Na]+; (+)-HRESIMS m/z 339.1937 [M + Na]+ (calcd for C20H28O3Na, 339.1931).
Formosin B (2)
White powder; +212 (c 0.10, MeOH); UV (MeOH) λmax (log ε) 207 (3.92); IR (KBr) νmax 3435, 3000–2500, 1753, 1695, 1456, 1205, 1070, 756 cm−1; 1H NMR (CDCl3), see Table 1 and 13C NMR (CDCl3) see Table 2; (+)-ESIMS m/z 355.2 [M + Na]+; (+)-HRESIMS m/z 355.1839 [M + Na]+ (calcd for C20H28O4Na, 355.1880).
Formosin C (3)
White powder; +22 (c 0.10, MeOH); UV (MeOH) λmax (log ε) 211 (3.58); IR (KBr) νmax 3425, 3000–2500, 1726, 1693, 1659, 1468, 1178, 1065, 600 cm−1; 1H NMR (CDCl3), see Table 1 and 13C NMR (CDCl3) see Table 2; (+)-ESIMS m/z 385.2 [M + Na]+; (+)-HRESIMS m/z 385.1991 [M + Na]+ (calcd for C21H30O5Na, 385.1991).
Formosin D (4)
White powder; −164 (c 0.025, MeOH); UV (MeOH) λmax (log ε) 204 (4.21); IR (KBr) νmax 3427, 3000–2500, 1695, 1452, 1254, 1082 cm−1; 1H NMR (CDCl3), see Table 1 and 13C NMR (CDCl3) see Table 2; (+)-ESIMS m/z 369.2 [M + Na]+; (+)-HRESIMS m/z 369.1970 [M + Na]+ (calcd for C21H30O4Na, 369.2036).
Formosin E (5)
White powder; −20 (c 0.05, MeOH); UV (MeOH) λmax (log ε) 199 (3.89); IR (KBr) νmax 3419, 3000–2500, 1695, 1452, 1383, 1244, 1026, 874, 600 cm−1; 1H NMR (CDCl3), see Table 1 and 13C NMR (CDCl3) see Table 2; (+)-ESIMS m/z 355.2 [M + Na]+; (+)-HRESIMS m/z 355.1885 [M + Na]+ (calcd for C20H28O4Na, 355.1888).
Formosin F (6)
White powder; −175 (c 0.10, MeOH); UV (MeOH) λmax (log ε) 237 (4.86); IR (KBr) νmax 3000–2500, 1703, 1633, 1435, 1381, 1219, 1026, 874 cm−1; 1H NMR (CDCl3), see Table 1 and 13C NMR (CDCl3) see Table 2; (+)-ESIMS m/z 331 [M + H]+; (+)-HRESIMS m/z 353.1724 [M + Na]+ (calcd for C20H26O4Na, 353.1723).
Antimicrobial Activity Assay
The in vitro antibacterial activities against Bacillus subtilis ATCC 6633 were tested by applying the protocols described in our previous research [13].
Antibacterial tests against Helicobacter pylori strains (Hp-SS1 or ATCC 43504 strain) were carried out in vitro according to the protocols described previously [14].
Electronic supplementary material
Supplementary material 1 (PDF 3792 kb) Electronic supplementary material The online version of this article (doi:) contains supplementary material, which is available to authorized users
Acknowledgments
Financial support from the National Natural Science Foundation (Grant No. U1302222; 81321092) of the People’s Republic of China is gratefully acknowledged. We thank Prof. S.-M. Huang of Hainan University for the identification of the plant material.
Compliance with Ethical Standards
Conflict of Interest
The authors declare no conflict of interest.
Footnotes
Bing-Dong Lin and Bin Zhou have contributed equally to this work.
References
- 1.Ma JS. Zhongguo Zhiwu Zhi. Beijing: Science Press; 1997. p. 8. [Google Scholar]
- 2.Giang PM, Son PT, Matsunami K, Otsuka H. Chem. Pharm. Bull. 2005;53:1600–1603. doi: 10.1248/cpb.53.1600. [DOI] [PubMed] [Google Scholar]
- 3.Ning DS, Peng LY, Lv SH, Li DP, Pan ZH. Nat. Prod. Res. 2015;29:524–528. doi: 10.1080/14786419.2014.953498. [DOI] [PubMed] [Google Scholar]
- 4.Wang ZC, Lin YM, Feng DQ, Ke CH, Lin P, Yan CL, Chen JD. Molecules. 2009;14:414–422. doi: 10.3390/molecules14010414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumarasinghe S, Seneviratne R. Australas. J. Dermatol. 1998;39:275–276. doi: 10.1111/j.1440-0960.1998.tb01492.x. [DOI] [PubMed] [Google Scholar]
- 6.Konishi T, Yamazoe K, Konoshima T, Maoka T, Fujiwara Y, Miyahara K. J. Nat. Prod. 2003;66:108–111. doi: 10.1021/np020321j. [DOI] [PubMed] [Google Scholar]
- 7.Konishi T, Konoshima T, Fujiwara Y, Kiyosawa S. J. Nat. Prod. 2000;63:344–346. doi: 10.1021/np990366t. [DOI] [PubMed] [Google Scholar]
- 8.Agoramoorthy G, Chandrasekaran M, Venkatesalu V, Hsu M. Braz. J. Microbiol. 2007;38:739–742. doi: 10.1590/S1517-83822007000400028. [DOI] [Google Scholar]
- 9.Thirunavukkarasu P, Ramkumar L, Ramanathan T. Global. J. Pharmacol. 2009;3:123–126. [Google Scholar]
- 10.Konishi T, Takasaki M, Tokuda H, Kiyosawa S, Konoshima T. Biol. Pharm. Bull. 1998;21:993–996. doi: 10.1248/bpb.21.993. [DOI] [PubMed] [Google Scholar]
- 11.Roengsumran S, Pornpakakul S, Muangsin N, Sangvanich P, Nhujak T, Singtothong P, Chaichit N, Puthong S, Petsom A. Planta Med. 2004;70:87–89. doi: 10.1055/s-2004-815466. [DOI] [PubMed] [Google Scholar]
- 12.Asakawa Y, Toyota M, Ueda A. Phytochemistry. 1990;29:2165–2167. doi: 10.1016/0031-9422(90)83030-5. [DOI] [Google Scholar]
- 13.Yang SP, Yue JM. Bioorg. Med. Chem. Lett. 2001;11:3119–3122. doi: 10.1016/S0960-894X(01)00633-3. [DOI] [PubMed] [Google Scholar]
- 14.Yin S, Fan CQ, Dong L, Yue JM. Tetrahedron. 2006;62:2569–2575. doi: 10.1016/j.tet.2005.12.041. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (PDF 3792 kb) Electronic supplementary material The online version of this article (doi:) contains supplementary material, which is available to authorized users