Abstract
There are divergences between neuropathic pain and visceralgia in terms of the duration, location, and character of hyperalgesia. It is generally recognized that nociceptive receptors, including P2X receptors, may play different roles in nociceptive mechanisms. The different roles of P2X1–7 receptors have not been fully understood both in neuropathic pain and visceral hyperalgesia. In order to explore the different expressions of P2X1–7 receptors in these two hyperalgesia models, the lumbosacral dorsal root ganglion (DRG) neurons from rat sciatic nerve chronic constriction injury (CCI) model and neonatal colorectal distention (NCRD) model were studied (both the primary nociceptive neuron afferents of those two models projected to the same segment of spinal cord). Both immunohistochemistry (IHC) technique and real-time fluorescence quantitative polymerase chain reaction (RT-PCR) technology were applied to analyze the protein expression levels and nucleic acid of P2X1–7 receptors. We found that except P2X2 and P2X3, the expression levels of P2X1 and P2X5 receptors increased in neuropathic pain while those expression levels of P2X4, P2X6, and P2X7 receptors increased in visceral pain. Our results also suggested that in addition to P2X2/3 heteromeric, other P2X subunits may also involved in generation heteromeric such as P2X1/5 and/or P2X2/5 in neuropathic pain and P2X4/6 and/or P2X4/7 in visceral pain.
Keywords: Neuropathic pain, Visceralgia, DRG, P2X-Rs, Expression characteristics
Introduction
Peripheral neuropathic pain exhibits persistent or paroxysmal stimulus-independent pain such as acute precise shooting or lancinating [1]. Visceral hyperalgesia exists in functional bowel and other visceral disorders with multiple characteristics such as chronic indistinct dull pain. It differs from neuropathic pain in terms of the duration, location, and character of the hyperalgesia [2].
Accumulated studies aim to characterize and clarify the different mechanisms of neuropathic pain and visceral hyperalgesia, including the expression of receptors involved in nociception. For example, many studies found that potential vanilloid type 1 (TRPV1) channels activate and express in neuropathic pain models [3]. Besides the TRPV1 channels [4], TRPV3 channels [5] and TRPV4 channels [6] may also contribute to the initiation and maintenance of visceral hyperalgesia. It suggests that different subtypes of other nociceptive ion channels or receptors may play different roles in neuropathic pain and visceralgia, respectively.
P2X receptors are other important nociceptive candidates [7]. So far, seven P2X receptor subunits, designated as P2X1–7, have been identified. These receptors were assembled as either homotrimeric or heterotrimeric receptors and widely distributed in different organs and tissues of the body. Many facts showed that the P2X receptors were closely related to the generation and transmission of pain and inflammation nociceptive signals [8]. Specifically, the P2X3 homomeric and P2X2/3 heteromeric were involved in the generation and transmission of nociception of neuropathic [9] and visceral pain [10]. Although various roles of P2X1–7 receptors in sensory neurons have been studied for many years, the differential roles have not been fully understood either in neuropathic pain or visceral hyperalgesia.
In order to explore the roles of P2X1–7 receptors involved in neuropathic and visceral pain comprehensively, we adopted the rat sciatic nerve chronic constriction injury (CCI) model [11] and neonatal colorectal distention (NCRD) model [12], which represent the neuropathic and visceral pain models, respectively. Their primary nociceptive neuron afferents were projected to the same segment of spinal cord. The messenger RNA (mRNA) and protein expression levels of P2X1–7 receptors in lumbosacral L4–S4 dorsal root ganglion (DRG) neurons were investigated by means of real-time fluorescence quantitative polymerase chain reaction (RT-PCR) and immunohistochemistry (IHC) techniques, respectively.
Material and method
Animals
These experiments were approved by Animal Care and Use Committee of Jianhan University in accordance with the regulations of the US National Institutes of Health. Clean grade male Spraque-Dawley (SD) rats (180–240-g body weight), and female SD pregnant rats (350–400-g body weight, each female rat can produce 10–12 rats), got from Wuhan University Center for Animal Experiment (SCXK, Hupeh 2008–0004). Rats were housed in plastic cages containing wood chip bedding and maintained on a 12:12-h light-dark cycle and were given ad libitum access to food and water.
The rat chronic neuropathic pain model establishment and behavior assessment
A chronic neuropathic pain rat model was successfully produced in adult rats by placing loosely constrictive ligatures around the common sciatic nerve [11].
Briefly, adult male SD rats were anesthetized by injection of chloral hydrate (400 mg/kg, intraperitoneal) and then were placed on a platform in prone position. After the common sciatic nerve exposing, about 7 mm of nerve was freed of adhering tissue and four ligatures (4.0 chromic gut) were tied loosely with about 1-mm spacing (CCI group) proximal to the sciatica’s trifurcation. Several groups of control rats were used, some were not operated upon (control group), and others received bilateral sham procedures (sham CCI group, sciatic exposure without ligation).
The animals were inspected during the first 14 postoperative days. During this inspective period, each rat was placed upon a table and carefully observed for 1 to 2 min by a blind test. The gait, the posture of the affected hind paw, and the extent of spontaneous pain behavior of model rat were rated [13]. The pain behavior rating score was significantly different from control group.
Evaluation standards of spontaneous pain behavior rating score:
0 score, no obvious behavioral change;
1 score, claw slightly flexed;
2 score, claw obvious buckling and rollover outward;
3 score, lateral, medial margin of claw parts contact with the glass plate but not bearing;
4 score, animal foot lift, not in contact with the glass plate;
5 score, in addition to lift the foot, licking, and biting toe.
The chronic visceral hypersensitivity model establishment and behavior assessment
A chronic visceral hypersensitivity was produced successfully in adult rats by NCRD method [12].
Male SD rats (8 days old) were divided into three groups with different treatments. Rats in NCRD group received colorectal distention (CRD) on a daily basis between the ages of 8 and 21 days. The distention was repeated twice (separated by 30 min) within 1 h. Rats in sham NCRD group were gently held and touched on the perineal area daily between the ages of 8 and 21 days. The rats in each group were housed in cages with their mothers. No treatment, procedure, or further intervention was done by the investigator during postnatal day 21. Adult male rat behavioral responses to CRD were assessed in all groups by measuring the abdominal withdrawal reflex (AWR) with a semi-quantitative score or by measuring the threshold intensity of CRD that elicits an expression contraction in the abdominal wall musculature.
Measurement of the AWR score consists of visual observation of the animal response to graded CRD (20, 40, 60, and 80 mmHg). Measuring the threshold intensity of CRD consisted of recording the stimulus intensity that evokes a visually identifiable contraction of the abdominal wall (AWR score was 3). Test trail was performed by a blind test. The AWR score and the threshold intensity of CRD were significantly different from control group.
0 score, no behavioral response to CRD;
1 score, brief head movement followed by immobility;
2 score, contraction of abdominal muscles;
3 score, lifting of abdomen;
4 score, body arching and lifting of pelvic structures.
RT-PCR
After behavior testing, SD rats were anesthetized by injection of chloral hydrate (400 mg/kg, i.p.), and then, the corresponding segments (L4–S4) of DRG were separated immediately. Total RNA was isolated from ganglia by the TRIzol reagent (Invitrogen, USA; 50–100-mg/ml usage). RNA was quantified by a BioPhotometer (Eppendorf, Germany) and diluted to 1 μg/μl. RNA was reverse transcripted according to the manufacturer’s instructions of QuantiTect Reverse Transcription Kit (QIAGEN, Germany) by MyCycler Thermal Cycler (Bio-Rad, USA). For quantitation of PCR cDNA, ganglion sample was run in a 25 μl PCR reaction with 2× QuantiTect SYBR Green PCR Master Mix (QIAGEN, Germany) and gene-specific primers (either primer pairs for P2X-Rs (1–7) or primer pair for a housekeeping gene β-actin (QIAGEN, Germany); the expected PCR product amplicon sizes were 100–250 bp. Thermal cycling was performed in IQ5 Multicolor Real-Time PCR Detection System (Bio-Rad, USA), consisting of 95 °C for 15 min followed by 45 cycles of 95 °C for 10 s and 58 °C for 30 s, then the step of 55–95 °C for 15 min for getting the melting curves in order to detect the purity of PCR products (V = 0.06 s/1 °C). P2X expressions in each ganglia were quantified using the comparative Ct method [14]. Ct values were taken at a threshold of fluorescence. P2X expression in each ganglia was quantified using the comparative C(T) method, where Ct(P2X) = numbers of PCR cycles for P2X primers to reach threshold, Ct(β-actin) = number of PCR cycles for β-actin primer to reach the same threshold, ΔCt = Ct(P2X) − Ct(β-actin), and ΔΔCt = ΔCt(X) − ΔCt(Z) (where ΔCt(X) = CCI or CRD group ganglion sample ΔCt and ΔCt(Z) = control group ganglion sample). The relative quantity of P2X-Rs in each ganglion sample was then expressed as a multiple of ganglion sample Z, P2X-RsN = 2−ΔΔCt.
IHC staining
After behavior testing, SD rats were anesthetized by injection of chloral hydrate (400 mg/kg, i.p.) and then were systemically perfused by 4 % paraformaldehyde. The corresponding segments (L4–S4) of DRG were carefully separated after fixation. After paraffin embedding, DRG paraffin tissue blocks were cut into 4-μm-thick slices. After dewaxing and rehydration treatments, microwave antigen retrieval, and endogenous peroxidase blocking, slices were incubated with the primary antibodies in 10 % normal goat serum at 4 °C overnight. Subsequently, the sections were incubated with horseradish peroxidase (HRP)-marked goat anti-rabbit or donkey anti-goat IgG in PBS for 1 h and then reacted with color at room temperature, timely terminating the color reaction, hematoxylin counterstain, dehydration, and transparention and observing under the microscope (Olympus, Japan) and taking photograph. The primary antibodies were rabbit anti-P2X1–5, 7 antibodies, at 1:200 proportional dilution (Alomone Labs, Israel) [15], and goat anti-P2X6 antibody, at 1:100 dilution (Santa Cruz, USA). The second antibodies were HRP-conjugated goat anti-rabbit and donkey anti-goat IgG (1:1000, Santa Cruz Inc., USA).
Data analysis
Average values were expressed as the mean ± SE, with n equals to the number of rats studied. Statistical significance of results was analyzed with one-way ANOVA.
Results
The rat chronic neuropathic pain model establishment and behavior assess
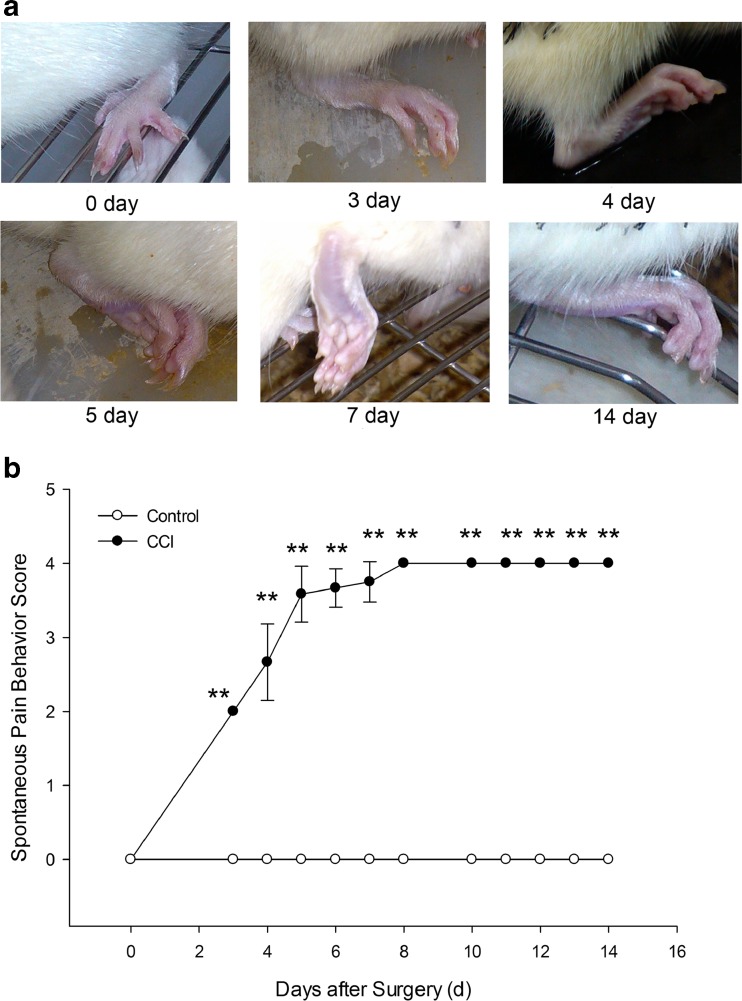
Hyperalgesic responses were evident on the second postoperative day and lasted for over 2 months as previously described [11, 13]. We recorded the progression of gait and the posture of the affected hind paw in model rats by photos (Fig. 1a). Spontaneous pain behavior score had been noted; the result demonstrated that there was a significant difference (P < 0.01) between control group and CCI group, indicating that a successful model was established on the third day of posttreatment (Fig. 1b).
Fig. 1.
Rat spontaneous pain behavior recording photos (a) and rating score (b) after CCI surgery. Figure 1a is the rat affected hind paw photos of spontaneous pain behavior recording at 0, 3, 4, 5, 7, and 14 days. Figure 1b is the postoperative spontaneous pain behavior rating score. The score began to increase from the third day and reach the peak on the seventh day. It could sustain for 14 days until drawing the materials from rats. There was a especially significant difference between control groups and CCI group. **P < 0.01, compare to control group, n = 6
The rat chronic visceral hypersensitivity model establishment and behavior assess
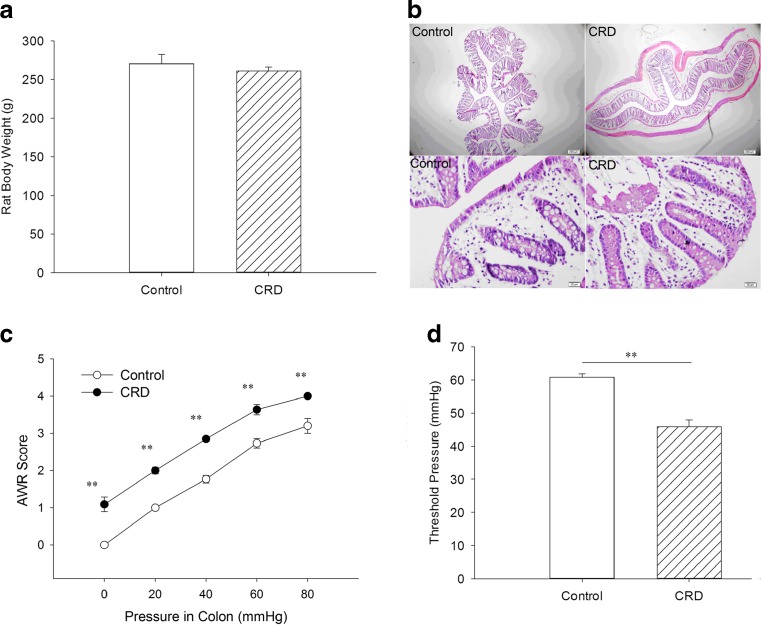
NCRD in neonatal rats can produce a chronic visceral hypersensitivity in adult rats. We built this chronic visceral pain model in rats successfully according to classic literature method [12]. The rat body weight (Fig. 2a), the colon wall slice pathological morphology, and inflammatory infiltration performance (stained by hematoxylin and eosin (H&E); Fig. 2b) were studied. The result showed that there were no significant differences between the control and NCRD groups. Measurement of the AWR response to graded CRD (20, 40, 60, and 80 mmHg) assign as AWR scores (Fig. 2c). We also measured the threshold intensity of CRD (usually in AWR = 3) that elicited an expression contraction in the abdominal wall musculature (Fig. 2d). There were significant differences (P < 0.01) between the control and NCRD groups in terms of the graded CRD AWR score and the threshold intensity of CRD.
Fig. 2.
The difference of rat body weight (a), colon wall slice pathological morphology (b), the graded CRD AWR score (c), and the threshold intensity of CRD (d) between adult rat chronic visceral hypersensitivity model and control group. Figure 2a and b showed that the rat body weight and the colon wall slice pathological morphology and inflammatory infiltration performance (stained by H&E, up 40×, below 400×) have no significant differences between the control and NCRD groups. Figure 2c and d revealed that there were extremely significant differences between the control and NCRD groups in terms of the graded CRD AWR score and the threshold intensity of CRD. **P < 0.01, compare to control group, n = 6
P2X1–7 receptors of mRNA levels of rat L4–S4 DRG neurons between two pain models
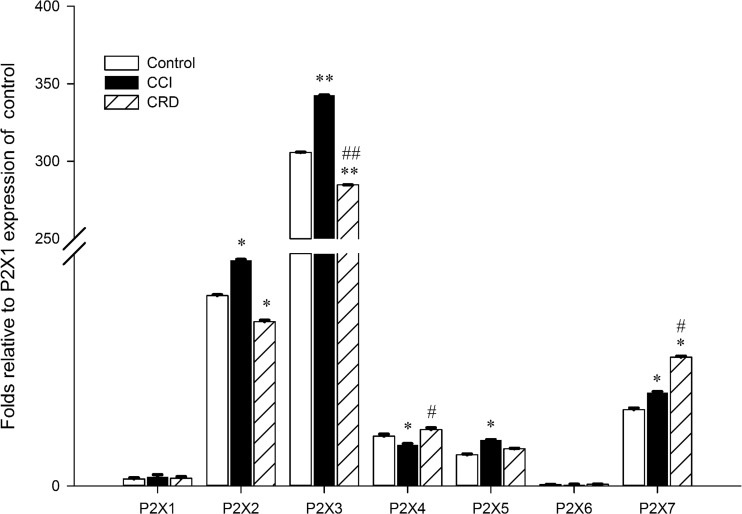
The mRNA expressions of the seven P2X receptor subtypes in L4–S4 rat DRG neurons in neuropathic pain and visceral pain models were compared (Fig. 3). We found that all P2X receptor mRNAs were expressed in DRG neurons of the control, CCI, and CRD groups, although the expression of P2X6 receptor was much lower compared to other P2X receptors. These results were similar to previous studies [16, 17]. There was no significant difference in the expression of P2X1 receptor between CCI and CRD groups. The mRNA expression levels of P2X2, 3, and 5 were much higher in CCI group than those in CRD groups (32 ± 0.12, 343 ± 0.13, and 6.5 ± 0.08 vs 23 ± 0.2, 285 ± 0.03, and 5.3 ± 0.07, folds compared to the expression levels of P2X1 receptors, P < 0.05), especially for the P2X3 (P < 0.01). The expression levels of the P2X4 and 7 receptors were significantly lower in CCI group than that in CRD groups (5.8 ± 0.19 and 13.3 ± 0.11 vs 8.1 ± 0.25 and 18.3 ± 0.16, folds compared to the expression levels of P2X1 receptors, P < 0.05).
Fig. 3.
Expressions of P2X1–7 receptors of mRNA in rat DRG in two pain models (neuropathic pain and visceral pain models); quantitative PCR data showing the P2X1–7 receptors of mRNA expression fold levels relative to control group P2X1 in two pain models. *P < 0.05, compare to control group; **P < 0.01, compare to control group; #P < 0.05, compare to CCI group; ##P < 0.01, compare to CCI group, n = 6 and independent reactions for each sample
Expressions of P2X1–7 receptors in L4–S4 rat DRG neurons between two pain models
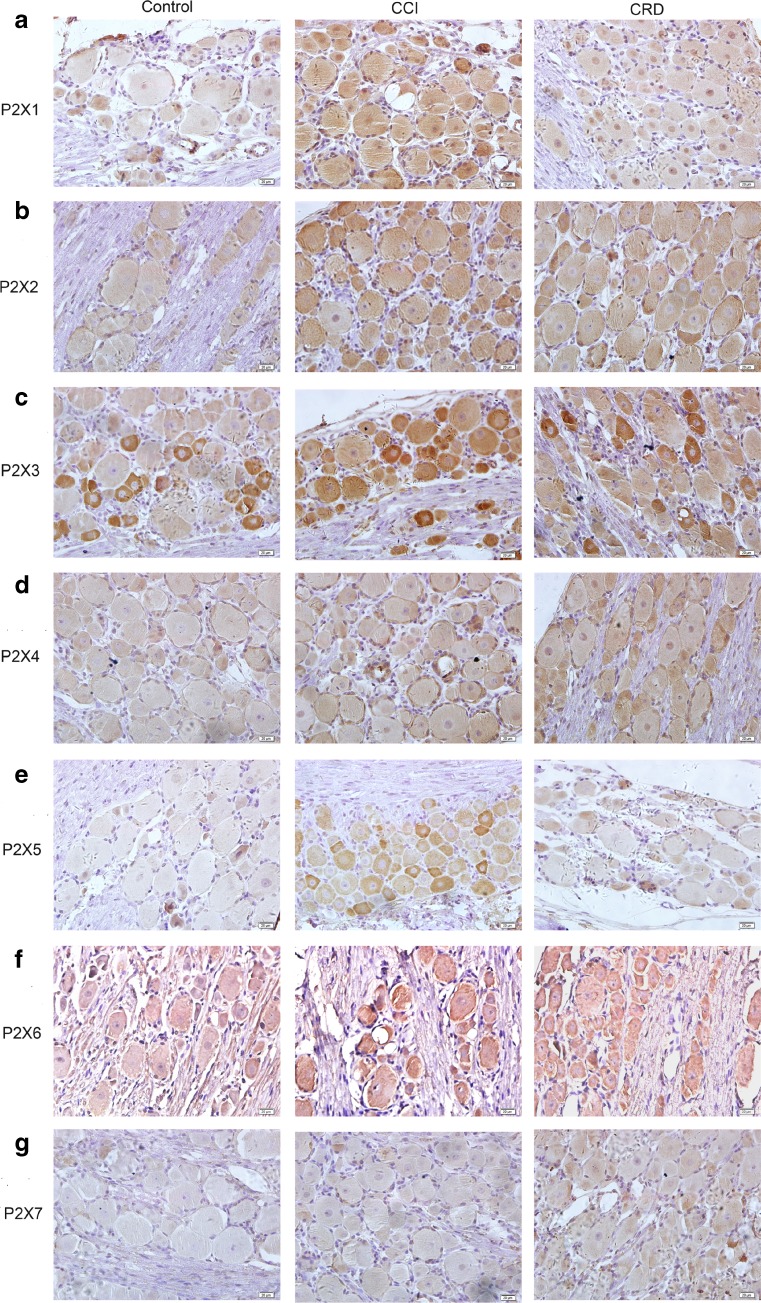
The expression characteristics of the seven P2X receptors on L4–S4 rat DRG neurons in neuropathic pain and visceral pain models were observed (Table 1 and Fig. 4a–g). Immunoreactively positive and negative neurons were counted to calculate the proportion of positive neurons. We found that all P2X receptors were expressed in DRG neurons of the control, CCI, and CRD groups just like other reports [18, 19] except the P2X7 receptor which was only found in CRD group. The expressions of P2X1, P2X2, P2X3, and P2X5 receptors were the most obvious in CCI group and the more obvious in CRD group than that in control group. Interestingly, the P2X3 and P2X5 expressions were stained more intensely in CCI group than that of control and CRD groups (Table 1 and Fig. 4a–c, e). The expression order of the P2X4, P2X6, and P2X7 (Table 1 and Fig. 4d, f, g) receptor subtypes was lower in CCI group than that in CRD group.
Table 1.
Localization of P2X1–7 receptor immunoreactivities in rat dorsal root ganglion (DRG) of different nociceptive pain models
| P2X1 | P2X2 | P2X3 | P2X4 | P2X5 | P2X6 | P2X7 | ||
|---|---|---|---|---|---|---|---|---|
| Control | L M S |
± ± + |
+ + + |
+ + ++ |
± ± + |
+ + + |
+ + + |
− − − |
| CCI | L M S |
++ ++ ++ |
+++ +++ +++ |
+++ +++ ++++ |
+ + + |
++ ++ +++ |
++ ++ ++ |
± ± ± |
| CRD | L M S |
+ + + |
++ ++ ++ |
++ ++ ++ |
++ ++ ++ |
+ + + |
++ ++ ++ |
+ + + |
+++ very strong signal, ++ strong signal, + moderate signal, ± just detectable, − undetectable. L, M, and S representing the cell diameter are greater than 50 μm, between 20 and 50 μm, less than, or equal to 20 μm, respectively
Fig. 4.
Localization of P2X1–7 receptor immunoreactivities in rat DRG in different pain models (control, neuropathic pain, and visceral pain models). a–g P2X1–7 receptor immunoreactivities in rat DRG of control, CCI, and CRD groups (400×)
Discussion
Neuropathic pain differs from visceral pain in terms of the duration, location, and character of the hyperalgesia [2]. Many studies found that TRPV1 channels activate and express in neuropathic pain models [3]. But, TRPV3 channels [5] and TRPV4 channels [6] may also contribute to the initiation and maintenance of visceral hyperalgesia. This indicated that those subtypes of P2X1–7 receptors might express differentially between neuropathic pain and visceralgia. The rat sciatic nerve CCI model [11] and NCRD model [12] properly represented the neuropathic pain and visceral pain research models, respectively. We adopted these models because the primary nociceptive neuron afferents of them were projected to the same segment of spinal cord. It was helpful to explore the different expressions of P2X receptors in the same segment of spinal cord. Our study compared the expressions of P2X1–7 receptors on native sensory neurons between neuropathic and visceral pain models comprehensively.
Studies have reported that besides P2X3 receptor, mRNA transcripts of P2X1, P2X2, P2X4, P2X5, P2X6, and P2X7 receptors have also been detected in DRG [16, 17]. P2X1–6 receptors were labeled with polyclonal antibodies in the DRG neuron [18, 19]. Our present study showed that the expressions of P2X1–7 receptor subtypes (with the protein and mRNA levels) were widespread in DRG of neuropathic pain and visceral pain models, which were in agreement with the results obtained in the previous experiments [16–19], although there were some different expressions between mRNA and protein levels, which perhaps were induced by their different expression progress.
Many evidence suggest that P2X2 and P2X3 receptors play an important role in the generation and transduction of sensory nociceptive signals [7, 9]. The P2X3 homomeric and P2X2/3 heteromeric were involved in the generation and transmission of nociception both in neuropathic [9] and visceral pain models [10]. But, more observations found other meaningful phenomena. Some scholars found that the expression of P2X3 in neuropathic pain did not change [20] or decrease [21]. More interestingly, the receptor expression and functionality experiments revealed a decrease in fast-desensitizing P2X3 receptors in rat DRG neurons following spinal nerve ligation, but certain subsets of DRG neurons maintain normal P2X3 receptor expression and function and these remaining receptors may contribute to the P2X3 receptor-mediated neuropathic pain [21]. In addition, the spinal nerve ligation experiments also showed that the expressions of P2X3 and P2X6 decreased while the expression of P2X5 increased [22]. The recent study found that P2X3- and P2X5-labeled rat DRG neurons, regardless of the number, cell morphology, or cell markers, were extremely similar, suggesting that P2X5 was also possibly related to peripheral pain [17]. Our results showed that expressions of P2X2 and P2X3 receptors increased obviously in neuropathic pain model just as previously reported [7, 9]. Interestingly, we found that the expressions of P2X1 and P2X5 receptors were much higher in neuropathic pain model than that in visceral pain model.
Other research suggested that the expression of P2X4 receptor in the dorsal horn of the thoracic cord increased in the visceral pain model [23] and that the expressions of P2X7 receptors were increased in the jejunum of postinflammatory visceral hypersensitivity mice [24] and haemorrhagic cystitis mice model [25], respectively. Our results showed that except the P2X2 and P2X3 receptors, the expressions of P2X4 and P2X7 receptors increased obviously in visceral pain model.
Furthermore, there were many direct and indirect evidence demonstrating that the two subunits can co-assemble receptors, such as P2X2/3, P2X2/6, P2X1/2, P2X1/4, P2X4/5, P2X4/6, P2X4/7, and P2X1/5 [8]. Our experiment results (except the P2X2 and P2X3-Rs, P2X1 and P2X5-Rs increased in neuropathic pain, while P2X4, P2X6, and P2X7-Rs increased in visceral pain) suggested that in addition to P2X2/3 heteromeric, other P2X subunits may also involved in generation of heteromeric such as P2X1/5 and/or P2X2/5 in neuropathic pain and P2X4/6 and/or P2X4/7 in visceral pain.
Existing studies reported that P2X1 and P2X3 receptors undergo fast desensitization (mediated fast pain) while slow desensitization (mediated slow pain) were observed in P2X2, P2X4, P2X5, and P2X7 receptors by ATP application [26]. But when co-expressed, they produce different desensitizing currents once activated, such as P2X2/3 and P2X1/5 (producing faster-desensitizing currents), while P2X1/4, P2X4/5, P2X4/6, and P2X4/7 are producing more slowly desensitizing currents [8]. Our results suggested that those fast-desensitizing P2X homomeric and/or heteromeric receptors increased in neuropathic pain, while those slowly desensitizing P2X homomeric and/or heteromeric receptors increased in visceral pain. The current results were consistent with the clinical characteristics of the two pain models, respectively. This phenomenon suggested that the differential expressions of P2X receptors subtypes may be one of the reasons in the diversity between neuropathic pain and visceral pain.
ATP is thought to be an important mediator of nociception and acts through two different purinoceptor families, P2X and P2Y receptor families. Similar to the P2X receptor, some subtypes of P2Y receptors, such as P2Y1 and P2Y2 receptors, are expressed in rat DRG neurons [17]. In addition, it has been reported that P2Y1, P2Y4, P2Y6, P2Y12, and P2Y13 receptors are expressed in rat spinal cord [17]. These results suggest that P2Y receptors may also play an important role in the development of neuropathic pain [17]. Among P2X and P2Y receptors, many studies revealed that P2X2 and P2X3 receptors have a substantial contribution to ATP-mediated nociception [7, 9], and A-317491, the first non-nucleotide antagonist blocking P2X3 homomeric and P2X2/3 heteromeric channels, has been shown to be antinociceptive in rat models of neuropathic pain [27, 28]. In addition, a selective P2X7 receptor antagonist A-740003 has also been reported to reduce neuropathic pain in the rat [29]. Our present results with many other reports suggest that in addition to P2X2, P2X3, and P2X7 receptors, other purinoceptors, including homomeric and heteromeric receptors, may also contribute to ATP-mediated nociception. Therefore, it would be also important to develop antagonists not only targeting P2X2, P2X3, and P2X7 receptors but also targeting other purinoceptors which are involved in pain sensation for clinic pain management including neuropathic pain and visceral pain.
Conclusions
Our present findings suggested that in addition to the P2X2 and P2X3 receptors, the P2X1 and P2X5 subunits were also involved in the generation of the nociceptive in neuropathic pain while the P2X4, P2X6, and P2X7 receptors may relate to visceral pain more intensely. The studies also suggested that in addition to P2X2/3 heteromeric, other P2X subunits may also generate heteromeric such as P2X1/5 and/or P2X2/5 in neuropathic pain and P2X4/6 and/or P2X4/7 in visceral pain. This is the first direct evidence to explore the expression differentials of P2X1–7 receptors on the same segments of spinal cord in both neuropathic pain and visceral pain models, comprehensively.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81371235 to C.-Y.L. and No. 81473442 to L.C.) and Wuhan Science and Technology Foundation (No. 2014060101010040 to C.-Y.L.).
References
- 1.Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353(9168):1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 2.Gebhart GF. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications IV. Visceral afferent contributions to the pathobiology of visceral pain. Am J Physiol Gastrointest Liver Physiol. 2000;278(6):G834–G838. doi: 10.1152/ajpgi.2000.278.6.G834. [DOI] [PubMed] [Google Scholar]
- 3.Palazzo E, et al. Moving towards supraspinal TRPV1 receptors for chronic pain relief. Mol Pain. 2010;6:66. doi: 10.1186/1744-8069-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christianson JA, et al. Development, plasticity and modulation of visceral afferents. Brain Res Rev. 2009;60(1):171–186. doi: 10.1016/j.brainresrev.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ueda T, et al. TRPV3, a thermosensitive channel is expressed in mouse distal colon epithelium. Biochem Biophys Res Commun. 2009;383(1):130–134. doi: 10.1016/j.bbrc.2009.03.143. [DOI] [PubMed] [Google Scholar]
- 6.Mochizuki T, et al. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem. 2009;284(32):21257–21264. doi: 10.1074/jbc.M109.020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnstock G. Purinergic mechanisms and pain—an update. Eur J Pharmacol. 2013;716(1–3):24–40. doi: 10.1016/j.ejphar.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 8.Murrell-Lagnado RD, Qureshi OS. Assembly and trafficking of P2X purinergic receptors (review) Mol Membr Biol. 2008;25(4):321–331. doi: 10.1080/09687680802050385. [DOI] [PubMed] [Google Scholar]
- 9.North RA, Jarvis MF. P2X receptors as drug targets. Mol Pharmacol. 2013;83(4):759–769. doi: 10.1124/mol.112.083758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnstock G. Purinergic mechanosensory transduction and visceral pain. Mol Pain. 2009;5:69. doi: 10.1186/1744-8069-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 12.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119(5):1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 13.Mao J, et al. Intrathecal MK-801 and local nerve anesthesia synergistically reduce nociceptive behaviors in rats with experimental peripheral mononeuropathy. Brain Res. 1992;576(2):254–262. doi: 10.1016/0006-8993(92)90688-6. [DOI] [PubMed] [Google Scholar]
- 14.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 15.Brass D, et al. Using antibodies against P2Y and P2X receptors in purinergic signaling research. Purinergic Signal. 2012;8(Suppl 1):61–79. doi: 10.1007/s11302-011-9278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collo G, et al. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996;16(8):2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi K, Yamanaka H, Noguchi K. Expression of ATP receptors in the rat dorsal root ganglion and spinal cord. Anat Sci Int. 2013;88(1):10–16. doi: 10.1007/s12565-012-0163-9. [DOI] [PubMed] [Google Scholar]
- 18.Vulchanova L, et al. Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology. 1997;36(9):1229–1242. doi: 10.1016/S0028-3908(97)00126-3. [DOI] [PubMed] [Google Scholar]
- 19.Xiang Z, Bo X, Burnstock G. Localization of ATP-gated P2X receptor immunoreactivity in rat sensory and sympathetic ganglia. Neurosci Lett. 1998;256(2):105–108. doi: 10.1016/S0304-3940(98)00774-5. [DOI] [PubMed] [Google Scholar]
- 20.Biggs JE, et al. P2X(3) expression is not altered by lingual nerve injury. Neurosci Lett. 2008;441(1):110–114. doi: 10.1016/j.neulet.2008.05.118. [DOI] [PubMed] [Google Scholar]
- 21.Kage K, et al. Alteration of dorsal root ganglion P2X3 receptor expression and function following spinal nerve ligation in the rat. Exp Brain Res. 2002;147(4):511–519. doi: 10.1007/s00221-002-1263-x. [DOI] [PubMed] [Google Scholar]
- 22.Kim C, Chung JM, Chung K. Changes in the gene expression of six subtypes of P2X receptors in rat dorsal root ganglion after spinal nerve ligation. Neurosci Lett. 2003;337(2):81–84. doi: 10.1016/s0304-3940(02)01302-2. [DOI] [PubMed] [Google Scholar]
- 23.Qin M, et al. The changes of P2X4 receptor expression in nucleus of tractus solitarius and the dorsal horn of thoracic cord after visceral pain induced by acetic acid. Chin J Neuroanatom. 2006;5:15. [Google Scholar]
- 24.Keating C, et al. P2X7 receptor-dependent intestinal afferent hypersensitivity in a mouse model of postinfectious irritable bowel syndrome. J Immunol. 2011;187(3):1467–1474. doi: 10.4049/jimmunol.1100423. [DOI] [PubMed] [Google Scholar]
- 25.Martins JP, et al. The role of P2X7 purinergic receptors in inflammatory and nociceptive changes accompanying cyclophosphamide-induced haemorrhagic cystitis in mice. Br J Pharmacol. 2012;165(1):183–196. doi: 10.1111/j.1476-5381.2011.01535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82(4):1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 27.McGaraughty S, et al. Effects of A-317491, a novel and selective P2X3/P2X2/3 receptor antagonist, on neuropathic, inflammatory and chemogenic nociception following intrathecal and intraplantar administration. Br J Pharmacol. 2003;140(8):1381–1388. doi: 10.1038/sj.bjp.0705574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang WS, et al. Electroacupuncture and A-317491 depress the transmission of pain on primary afferent mediated by the P2X3 receptor in rats with chronic neuropathic pain states. J Neurosci Res. 2014;92(12):1703–1713. doi: 10.1002/jnr.23451. [DOI] [PubMed] [Google Scholar]
- 29.Honore P, et al. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther. 2006;319(3):1376–1385. doi: 10.1124/jpet.106.111559. [DOI] [PubMed] [Google Scholar]