Abstract
Military recruits and elite athletes are susceptible to stress fracture injuries. Genetic predisposition has been postulated to have a role in their development. The P2X7 receptor (P2X7R) gene, a key regulator of bone remodelling, is a genetic candidate that may contribute to stress fracture predisposition. The aim of this study is to evaluate the putative contribution of P2X7R to stress fracture injury in two separate cohorts, military personnel and elite athletes. In 210 Israeli Defense Forces (IDF) military conscripts, stress fracture injury was diagnosed (n = 43) based on symptoms and a positive bone scan. In a separate cohort of 518 elite athletes, self-reported medical imaging scan-certified stress fracture injuries were recorded (n = 125). Non-stress fracture controls were identified from these cohorts who had a normal bone scan or no history or symptoms of stress fracture injury. Study participants were genotyped for functional SNPs within the P2X7R gene using proprietary fluorescence-based competitive allele-specific PCR assay. Pearson’s chi-squared (χ2) tests, corrected for multiple comparisons, were used to assess associations in genotype frequencies. The variant allele of P2X7R SNP rs3751143 (Glu496Ala—loss of function) was associated with stress fracture injury, whilst the variant allele of rs1718119 (Ala348Thr—gain of function) was associated with a reduced occurrence of stress fracture injury in military conscripts (P < 0.05). The association of the variant allele of rs3751143 with stress fractures was replicated in elite athletes (P < 0.05), whereas the variant allele of rs1718119 was also associated with reduced multiple stress fracture cases in elite athletes (P < 0.05). The association between independent P2X7R polymorphisms with stress fracture prevalence supports the role of a genetic predisposition in the development of stress fracture injury.
Keywords: P2X7 receptor, Bone, Stress fracture injury
Introduction
The lifetime incidence of stress fracture injury in military recruits and elite athletes has been reported to range from 1 to 24 % [1, 2] and can present at various skeletal sites, most commonly in the lower limbs [3]. Stress fractures are typically caused by excessive repeated mechanical loading applied in a rhythmic, sub-threshold manner [4], although the exact pathophysiology is not fully understood [5]. Inadequate bone remodelling can contribute to the development of stress fracture injury [6], alongside a number of environmental risk factors, including diet and nutrition, training status, training environment and individual biomechanics [7, 8]. A genetic contribution to stress fracture risk is likely given that certain individuals present with multiple stress fractures at various skeletal sites [9], comparable stress fracture injuries occurring in monozygotic twins [10], high stress fracture recurrence rates [11], variable stress fracture incidence in military recruits who are exposed to equivalent training loads [12] and candidate gene studies investigating stress fracture prevalence [13–17].
The highly polymorphic purinergic P2X7 receptor (P2X7R) is a potential candidate to mediate stress fracture susceptibility due to previous associations with bone phenotypes [18, 19]. P2X7R is expressed by osteoblasts and osteoclasts in vitro [20], with receptor activation causing distinct cellular responses that include apoptosis [18] and increased cell permeability in osteoclasts [18] and osteoblasts [21]. These roles for P2X7R are supported by P2X7R knockout (KO) murine models [22], where decreased bone mass [23], inflammatory response [24] and mechanical loading-induced intercell signalling [19] have been reported in the KO compared to wild-type (WT) animals. However, when whole-exome sequencing was employed to investigate genetic associations with stress fracture injury in military recruits, no significant associations with P2X7R gene were shown [17]. The reason for this may be due to the relatively modest sample size (cases = 34, controls = 60) resulting in insufficient power to detect associations with stress fracture injury. Alternatively, the methodology for exome capture or sequencing may have been sub-optimal to capture and cover the P2X7R gene region.
Overall, 15 functional SNPs (http://www.ncbi.nlm.nih.gov/snp access date 12 February 2015; for SNP locations [18]), leading to either the gain or loss of function of the P2X7R protein, have been identified and several SNPs have been associated with bone phenotypic alterations including bone loss [25, 26], lower bone mineral density (BMD) at the hip and lumbar spine [26, 27], fracture risk [18] and overall osteoporosis risk [27, 28].
To date, no studies have specifically investigated P2X7R SNPs in relation to stress fracture injury prevalence. We hypothesised that functional SNPs in P2X7R may play a role in stress fracture pathogenesis. Twelve functional SNPs, believed to be associated with bone phenotypes, were analysed in a cohort of military recruits with stress fractures; five of these SNPs were selected based on the results from the military cohort and previous literature related to P2X7R SNPs and bone phenotypes. The five SNPs were then validated in an independently recruited, ethnically distinct cohort of elite athletes with stress fractures.
Methods
Military participants
Two-hundred and ten active duty Israel Defense Force (IDF) soldiers (197 men and 13 women; see Table 1 for participant characteristics), who were referred to the Central Orthopaedic Clinic of the IDF with symptoms of stress fracture injury, volunteered to participate in the study. Stress fracture injuries were diagnosed following evaluation by an orthopaedic surgeon and a bone scan based on the standard protocol and criteria adopted in the IDF [29]. Participants were classified as either having no evidence of acute stress fracture injury or having acute stress fractures based on previously described criteria [30]. Due to the high rate of low-grade bone stress lesions (bone marrow oedema and/or periostitis without cortical fracture) and to facilitate focussing on clinically relevant stress fractures, bone stress lesions without fracture and metatarsal stress fractures were excluded from the study. Participants classified as not having stress fractures, confirmed by bone scan, were considered control participants. The study was approved by the Israeli Medical Corps Ethics Committee, and each participant provided written informed consent prior to their involvement in the study.
Table 1.
Characteristics of military personnel and elite athletes with and without radiologically confirmed stress fracture injuries
| Subject characteristics | Stress fracture (n = 43) | Non-stress fracture (n = 167) | P value |
|---|---|---|---|
| Military (males and females) | |||
| Age (year) | 20.3 ± 1.6 | 18.9 ± 0.5 | 0.12 |
| Height (m) | 1.76 ± 0.05 | 1.77 ± 0.07 | 0.77 |
| Body mass (kg) | 70.44 ± 7 | 72.12 ± 10.09 | 0.34 |
| BMI (kg/m2) | 22.6 ± 1.8 | 23.2 ± 2.6 | 0.19 |
| Smoking (n and % of smokers) | 11 (25 %) | 62 (37 %) | 0.07 |
| Ethnicity (non-Ashkenazi and Ashkenazi) | 36 % non-Ashkenazi and 64 % Ashkenazi | 45 % non-Ashkenazi and 55 % Ashkenazi | 0.10 |
| Military (males only) | Stress fracture (n = 41) | Non-stress fracture (n = 157) | |
| Age (year) | 20.3 ± 1.6 | 19.1 ± 0.4 | 0.16 |
| Height (m) | 1.76 ± 0.05 | 1.77 ± 0.07 | 0.45 |
| Body mass (kg) | 70.78 ± 7 | 72.6 ± 11.1 | 0.15 |
| BMI (kg/m2) | 22.6 ± 1.9 | 23.2 ± 2.7 | 0.11 |
| Smoking (n and % of smokers) | 10 (24 %) | 57 (36 %) | 0.08 |
| Ethnicity (non-Ashkenazi and Ashkenazi) | 39 % non-Ashkenazi and 61 % Ashkenazi | 46 % non-Ashkenazi and 56 % Ashkenazi | 0.18 |
| Elite athlete (males and females) | Stress fracture (n = 125) | Non-stress fracture (n = 376) | P value |
| Age (year) | 27.7 ± 7.5 | 24.4 ± 5.4 | 0.01** |
| Height (m) | 1.82 ± 10 | 1.81 ± 8.3 | 0.43 |
| Body mass (kg) | 77.3 ± 14.5 | 77.8 ± 10.5 | 0.29 |
| BMI (kg/m2) | 23.2 ± 2.7 | 23.7 ± 2.2 | 0.07 |
| Age at elite (year) | 18.2 + 4.2 | 17 ± 2.2 | 0.01* |
| Training (h/week) | 20 ± 11.3 | 18.2 ± 10.1 | 0.12 |
| Alcohol consumption (units/week) | 5.2 ± 6.9 | 4.1 ± 6.1 | 0.15 |
| Elite athlete (males only) | Stress fracture (n = 125) | Non-stress fracture (n = 376) | |
| Age (year) | 27.2 ± 6.9 | 24.2 ± 5.5 | 0.01** |
| Height (m) | 1.85 ± 7.2 | 1.82 ± 7.1 | 0.01** |
| Body mass (kg) | 82.9 ± 10.6 | 79.6 ± 9.4 | 0.01** |
| BMI (kg/m2) | 24.1 ± 2.1 | 23.9 ± 2.1 | 0.46 |
| Age at elite (years) | 18.2 ± 4.3 | 17 ± 2.2 | 0.01* |
| Training (h/week) | 21.6 ± 11.9 | 18.2 ± 10.5 | 0.01** |
| Alcohol consumption (units/week) | 5.6 ± 7.3 | 4.2 ± 6.2 | 0.12 |
* Denotes a significance level of P<0.05, ** P<0.01
Elite athlete participants
In total, 518 elite athletes (449 men and 69 women; Table 1) volunteered to participate in this study forming the Stress Fracture Elite Athlete (SFEA) cohort. Participants were recruited from the UK and North America and were mainly white Caucasian (83.2 % in the stress fracture cases and 79.9 % in the non-stress fracture controls). Those suffering a stress fracture confirmed by medical imaging (computerised tomography (CT), magnetic resonance imaging (MRI), X-rays or bone scan) were cases, and those never having had a stress fracture or indicative symptoms formed the control group. In 17 athletes, there was a lack of stress fracture history clarity (e.g. reports of stress lesions and suspected stress fractures that were not confirmed by medical imaging), and thus, these participants were removed from the statistical analysis. Due to the low number of female subjects, the male athletes were stratified into a male only cohort and cases of multiple stress fractures (more than one discrete occurrence at any site) for the purposes of analyses. Information on the SFEA cohort has been reported previously [31]. Ethical approval was granted by the Nottingham Trent University Ethical Review Committee, and each participant provided written informed consent prior to their involvement in the study.
Data collection in both cohorts was in line with the ethical standards set by the 1964 Declaration of Helsinki.
DNA extraction
From military participants, DNA was extracted from peripheral blood leukocytes using the PureGene1 Kit (Gentra Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions. From the elite athletes, genomic DNA was derived from saliva deposited into a 5-mL collection tube and subsequently mixed with 2 mL of preservative in accordance with manufacturer’s guidelines (Norgen Biotek Corp., Saliva DNA Collection Kit, Thorold, Canada).
Genotyping
Selection of functional SNPs was based upon previous studies, reporting significant associations between the P2X7R gene and bone phenotypes [18, 25–28]. In both cohorts, genotyping of the P2X7R gene were performed by LGC Genomics (Herts, UK) using proprietary fluorescence-based competitive allele-specific polymerase chain reaction assay. Twelve SNPs were examined in military conscripts, and five were downselected for analysis in the elite athletes based on prior research detailing an association with bone phenotypes (Table 2). LGC Genomics laboratory staff was blinded to the clinical status (case or control) of the genotyped individuals.
Table 2.
Call rate and minor allele frequency (MAF) of SNPs analysed in stress fracture and non-stress fracture groups
| rs number | Polymorphism | Amino acid change | MAF | Call rate (%) |
|---|---|---|---|---|
| Military cohort | ||||
| rs35933842 | 151 + 1G-T | Null allele | 0.02 | 93 |
| rs17525809 | 253T-C | Val76Ala | 0.06 | 90 |
| rs28360447 | 474G-A | Gly150Arg | 0.01 | 86 |
| rs208294 | 489C-T | His155Tyr | 0.44 | 73 |
| rs7958311 | 835G-A | Arg270His | 0.21 | 65 |
| rs7958316 | 853G-A | Arg276His | 0.03 | 81 |
| rs28360457 | 946G-A | Arg307Gln | 0.01 | 94 |
| rs1718119 | 1068G-A | Ala348Thr | 0.35 | 88 |
| rs2230911 | 1096C-G | Thr357Ser | 0.06 | 80 |
| rs2230912 | 1405A-G | Gln460Arg | 0.13 | 88 |
| rs3751143 | 1513A-C | Glu496Ala | 0.21 | 90 |
| rs1653624 | 1729T-A | Ile568Asn | 0.01 | 94 |
| Elite athlete cohort | ||||
| rs208294 | 489C-T | His155Tyr | 0.42 | 97 |
| rs1718119 | 1068G-A | Ala348Thr | 0.39 | 91 |
| rs2230912 | 1405A-G | Gln460Arg | 0.17 | 95 |
| rs3751143 | 1513A-C | Glu496Ala | 0.16 | 94 |
| rs1653624 | 1729T-A | Ile568Asn | 0.05 | 97 |
Statistical analysis
Statistical analyses were performed using statistical package SPSS version 21.0 (SPSS Inc., Chicago, IL, USA). Student’s t test was used for analysis of descriptive variables. For all data, the Pearson’s chi-squared (χ2) test was used to assess associations in genotype frequencies and to assess the observed frequency of each genotype with what would be expected in accordance with Hardy-Weinberg equilibrium. The Benjamini and Hochberg false discovery rate test [32] was applied in order to account for multiple comparisons of SNPs in both studies. Acceptable level of significance was classified as P < 0.05.
Results
P2X7R SNPs and stress fracture occurrence
Information for the SNPs genotyped along with the minor allele frequencies and call rates for both studies are shown in Table 2. All SNPs (from both studies) were shown to be in Hardy-Weinberg equilibrium, except for rs2230912 and rs3751143 (P < 0.05) in the military cohort.
Military cohort
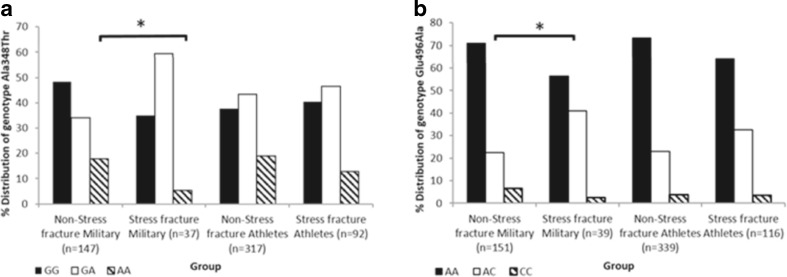
Analyses of 12 P2X7R SNPs in the military cohort showed that the frequency of the gain of function A allele of SNP rs1718119 (combined and male only; Fig. 1) and the loss of function C allele of SNP rs3751143 (male only) were significantly associated with stress fracture injury occurrence (P < 0.05; Table 3). Analysis of rs3751143 in men only showed that individuals carrying at least one loss of function alleles (C) were more likely to suffer from stress fractures (P = 0.04) (Fig. 1 and Table 3). Homozygosity for the A allele of rs1718119 was underrepresented in the non-stress fracture control population (5.4 % in stress fracture sufferers compared to 17.7 % in the non-stress fractures cohort) (P = 0.01) (Table 3). No other SNP was significantly associated with stress fracture occurrence (P > 0.05). After correcting for multiple comparisons using the false discovery rate (FDR) test (Benjamini and Hochberg [32]), none of the findings remained significant (P > 0.05).
Fig. 1.
a Distribution of rs1718119 alleles in military personnel and elite athlete stress fracture and non-stress fracture groups. Asterisk indicates significance at P < 0.05 in comparison to the matched non-stress fracture group. b Distribution of rs3751143 alleles in military personnel and elite athlete stress fracture and non-stress fracture groups. Asterisk indicates significance at P < 0.05 in comparison to the matched non-stress fracture group
Table 3.
P2X7R genotype distribution (percentage) and allele frequency of stress fracture cases and controls in military personnel and elite athletes
| All | Men | |||||
|---|---|---|---|---|---|---|
| Military | ||||||
| Stress fracture n (%) | Non-stress fracture n (%) | Stress fracture n (%) | Non-stress fracture n (%) | |||
| rs35933842 | ||||||
| GG | 36 (95) | 153 (98) | 35 (97) | 144 (97) | ||
| GT | 2 (5) | 4 (2) | 1 (3) | 4 (3) | ||
| TT | 0 | 0 | 0 | 0 | ||
| 38 | 157 | 36 | 148 | |||
| P = | 0.38 | P = | 0.98 | |||
| Allele frequency | ||||||
| G | 0.97 | 0.99 | 0.99 | 0.99 | ||
| T | 0.03 | 0.01 | 0.01 | 0.01 | ||
| P = | 0.29 | P = | 0.98 | |||
| rs17525809 | ||||||
| TT | 32 (82) | 132 (89) | 32 (84) | 123 (89) | ||
| TC | 7 (18) | 17 (11) | 6 (16) | 15 (11) | ||
| CC | 0 | 0 | 0 | 0 | ||
| 39 | 149 | 38 | 138 | |||
| P = | 0.28 | P = | 0.41 | |||
| Allele frequency | ||||||
| T | 0.91 | 0.94 | 0.92 | 0.95 | ||
| C | 0.09 | 0.57 | 0.08 | 0.05 | ||
| P = | 0.21 | P = | 0.34 | |||
| rs28360447 | ||||||
| GG | 37 (100) | 142 (99) | 35 (100) | 133 (99) | ||
| GA | 0 | 2 (1) | 0 | 2 (1) | ||
| AA | 0 | 0 | 0 | 0 | ||
| 37 | 144 | 35 | 135 | |||
| P = | 0.64 | P = | 0.47 | |||
| Allele frequency | ||||||
| G | 1.00 | 0.99 | 1.00 | 0.99 | ||
| A | 0 | 0.07 | 0 | 0.07 | ||
| P = | 0.47 | P = | 0.47 | |||
| rs208294 | ||||||
| CC | 14 (39) | 33 (28) | 13 (38) | 28 (26) | ||
| CT | 16 (44) | 63 (53) | 16 (47) | 58 (54) | ||
| TT | 6 (17) | 22 (19) | 5 (15) | 21 (20) | ||
| 36 | 118 | 34 | 107 | |||
| P = | 0.46 | P = | 0.21 | |||
| Allele frequency | ||||||
| C | 0.33 | 0.37 | 0.34 | 0.37 | ||
| T | 0.67 | 0.64 | 0.66 | 0.63 | ||
| P = | 0.41 | P = | 0.49 | |||
| rs7958311 | ||||||
| GG | 27 (75) | 60 (60) | 25 (74) | 55 (60) | ||
| GA | 8 (22) | 33 (33) | 8 (23) | 32 (35) | ||
| AA | 1 (3) | 7 (7) | 1 (3) | 4 (4) | ||
| 36 | 100 | 34 | 91 | |||
| P = | 0.16 | P = | 0.4 | |||
| Allele frequency | ||||||
| G | 0.86 | 0.77 | 0.85 | 0.78 | ||
| A | 0.10 | 0.24 | 0.15 | 0.22 | ||
| P = | 0.06 | P = | 0.14 | |||
| rs7958316 | ||||||
| GG | 35 (95) | 127 (95) | 33 (94) | 116 (94) | ||
| GA | 2 (5) | 7 (5) | 2 (6) | 7 (6) | ||
| AA | 0 | 0 | 0 | 0 | ||
| 37 | 134 | 35 | 123 | |||
| P = | 0.97 | P = | 1 | |||
| Allele frequency | ||||||
| G | 0.97 | 0.97 | 0.97 | 0.97 | ||
| A | 0.03 | 0.03 | 0.03 | 0.03 | ||
| P = | 0.96 | P = | 0.99 | |||
| rs28360457 | ||||||
| GG | 37 (100) | 158 (99) | 35 (100) | 147 (99) | ||
| GA | 0 | 2 (1) | 0 | 2 (1) | ||
| AA | 0 | 0 | 0 | 0 | ||
| 37 | 160 | 35 | 149 | |||
| P = | 0.13 | P = | 0.49 | |||
| Allele frequency | ||||||
| G | 1.00 | 0.99 | 1.00 | 0.99 | ||
| A | 0 | 0.01 | 0 | 0.01 | ||
| P = | 0.5 | P = | 0.49 | |||
| rs1718119 | ||||||
| GG | 13 (35) | 71 (48) | 12 (34) | 66 (49) | ||
| GA | 22 (60) | 50 (34) | 21 (60) | 45 (33) | ||
| AA | 2 (5) | 26 (20) | 2 (6) | 25 (18) | ||
| 37 | 147 | 35 | 136 | |||
| P = | 0.01* | P = | 0.01* | |||
| Allele frequency | ||||||
| G | 0.65 | 0.65 | 0.64 | 0.65 | ||
| A | 0.35 | 0.35 | 0.36 | 0.35 | ||
| P = | 0.94 | P = | 0.89 | |||
| rs2230911 | ||||||
| CC | 33 (89) | 114 (88) | 32 (91) | 106 (89) | ||
| CG | 4 (11) | 16 (12) | 3 (9) | 13 (11) | ||
| GG | 0 | 0 | 0 | 0 | 0 | |
| 37 | 130 | 35 | 119 | |||
| P = | 0.8 | P = | 0.69 | |||
| Allele frequency | ||||||
| C | 0.95 | 0.94 | 0.96 | 0.94 | ||
| GG | 0.05 | 0.06 | 0.04 | 0.06 | ||
| P = | 0.79 | P = | 0.66 | |||
| rs2230912 | ||||||
| AA | 27 (69) | 110 (75) | 25 (68) | 101 (75) | ||
| GA | 12 (31) | 36 (25) | 12 (32) | 34 (25) | ||
| GG | 0 | 0 | 0 | 0 | ||
| 39 | 146 | 37 | 135 | |||
| P = | 0.44 | P = | 0.38 | |||
| Allele frequency | ||||||
| A | 0.85 | 0.88 | 0.84 | 0.87 | ||
| G | 0.15 | 0.12 | 0.16 | 0.13 | ||
| P = | 0.41 | P = | 0.35 | |||
| rs3751143 | ||||||
| AA | 22 (56) | 107 (71) | 22 (60) | 97 (69) | ||
| AC | 16 (41) | 34 (22) | 15 (40) | 33 (24) | ||
| CC | 1 (3) | 10 (7) | 0 | 10 (7) | ||
| 39 | 151 | 37 | 140 | |||
| P = | 0.05* | P = | 0.04* | |||
| Allele frequency | ||||||
| A | 0.77 | 0.82 | 0.80 | 0.81 | ||
| CC | 0.23 | 0.18 | 0.20 | 0.19 | ||
| P = | 0.23 | P = | 0.77 | |||
| rs1653624 | ||||||
| TT | 36 (92) | 158 (99) | 35 (95) | 147 (99) | ||
| TA | 3 (8) | 1 (1) | 2 (5) | 1 (1) | ||
| AA | 0 | 0 | 0 | 0 | ||
| 39 | 159 | 37 | 148 | |||
| NA | NA | |||||
| Allele frequency | ||||||
| T | 0.96 | 1.00 | 0.97 | 1.00 | ||
| A | 0.04 | 0.00 | 0.03 | 0.00 | ||
| NA | NA | |||||
| Elite athletes | ||||||
| rs208294 | ||||||
| CC | 120 (98) | 348 (95) | 94 (98) | 312 (95) | ||
| CT | 2 (2) | 16 (4) | 2 (2) | 14 (4) | ||
| TT | 0 | 1 (0) | 0 | 1 (0) | ||
| 122 | 365 | 96 | 327 | |||
| P = | 0.32 | P = | 0.52 | |||
| Allele frequency | ||||||
| C | 0.98 | 0.98 | 0.99 | 0.98 | ||
| T | 0.02 | 0.03 | 0.01 | 0.02 | ||
| P = | 0.09 | P = | 0.21 | |||
| rs1718119 | ||||||
| GG | 47 (40) | 128 (38) | 36 (40) | 112 (37) | ||
| GA | 54 (47) | 147 (43) | 41 (45) | 130 (43) | ||
| AA | 15 (13) | 64 (19) | 14 (15) | 60 (20) | ||
| 116 | 339 | 91 | 302 | |||
| P = | 0.34 | P = | 0.63 | |||
| Allele frequency | ||||||
| G | 0.64 | 0.59 | 0.62 | 0.59 | ||
| A | 0.36 | 0.41 | 0.38 | 0.41 | ||
| P = | 0.18 | P = | 0.34 | |||
| rs2230912 | ||||||
| AA | 78 (67) | 243 (68) | 61 (66) | 211 (66) | ||
| GA | 37 (32) | 107 (30) | 31 (33) | 99 (31) | ||
| GG | 1 (1) | 9 (2) | 1 (1) | 9 (3) | ||
| 116 | 359 | 93 | 319 | |||
| P = | 0.53 | P = | 0.6 | |||
| Allele frequency | ||||||
| A | 0.83 | 0.83 | 0.82 | 0.82 | ||
| G | 0.17 | 0.17 | 0.18 | 0.18 | ||
| P = | 0.8 | P = | 0.34 | |||
| rs3751143 | ||||||
| AA | 75 (64) | 261 (73) | 62 (67) | 240 (76) | ||
| AC | 38 (33) | 82 (23) | 26 (28) | 65 (21) | ||
| CC | 4 (3) | 13 (4) | 4 (4) | 12 (4) | ||
| 117 | 356 | 92 | 317 | |||
| P = | 0.12 | P = | 0.27 | |||
| Allele frequency | ||||||
| A | 0.80 | 0.85 | 0.82 | 0.86 | ||
| C | 0.20 | 0.15 | 0.19 | 0.14 | ||
| P = | 0.05* | P = | 0.08 | |||
| rs1653624 | ||||||
| TT | 119 (98) | 339 (93) | 94 (99) | 303 (93) | ||
| TA | 2 (2) | 25 (7) | 1 (1) | 22 (7) | ||
| AA | 0 (0) | 0 (0) | 0 | 0 | ||
| 121 | 364 | 95 | 325 | |||
| P = | NA | P = | NA | |||
| Allele frequency | ||||||
| T | 100 | 0.97 | 0.99 | 0.97 | ||
| A | 0 | 0.03 | 0.01 | 0.03 | ||
| P = | NA | P = | NA | |||
P values are the result of comparisons between genotype and allele frequencies between the stress fracture and non-stress fracture cohorts
*Significant difference when cases were compared to controls (P < 0.05)
Elite athletes
Analyses of five P2X7R SNPs in the SFEA cohort showed that whilst rs1718119 was not associated with single stress fracture prevalence (P = 0.34) in elite athletes (Fig. 1), a significant association with multiple stress fracture injury in comparison to the non-stress fracture cohort was shown (P = 0.01; Table 4). Homozygotes for the A allele of rs1718119 were present in only 2.4 % of the multiple stress fracture cohort in comparison to 18.9 % of the non-stress fracture group. The frequency of the A allele was also significantly decreased in the multiple stress fracture group (P < 0.01). The frequency of the C allele of rs3751143 was associated with stress fracture prevalence (P = 0.05; Table 3). No association with stress fracture injury prevalence was seen in rs208294, rs2230912 or rs1653624 (Table 3). Combining homozygotes for the rare allele with heterozygotes showed no differences in either cohort for all SNPs investigated (data not shown). After correcting for multiple comparisons using the FDR test, none of the data remained significant (P > 0.05).
Table 4.
P2X7R genotype distribution and allele frequency in elite athletes with multiple stress fractures
| SNP | Genotype | Multiple stress fracture (%) |
|---|---|---|
| rs208294 | CC | 47 (98) |
| TC | 1 (2) | |
| TT | 0 | |
| P = | 0.7 | |
| Allele frequency | C | 0.99 |
| T | 0.01 | |
| P = | 0.37 | |
| rs1718119 | GG | 23 (50) |
| GA | 21 (46) | |
| AA | 2 (4) | |
| P = | 0.04* | |
| Allele frequency | G | 0.64 |
| A | 0.36 | |
| P = | <0.01* | |
| rs2230912 | AA | 32 (70) |
| GA | 14 (30) | |
| GG | 0 (0) | |
| P = | 0.55 | |
| Allele frequency | A | 0.84 |
| G | 0.16) | |
| P = | 0.64 | |
| rs3751143 | AA | 31 (67) |
| AC | 13 (28) | |
| CC | 2 (4) | |
| P = | 0.66 | |
| Allele frequency | A | 0.82 |
| C | 0.19 | |
| P = | 0.58 | |
| rs1653624 | TT | 47 (98) |
| TA | 1 (2) | |
| AA | 0 (0) | |
| P = | NA | |
| Allele frequency | T | 0.99 |
| A | 0.01 | |
| P = | NA |
P values are the result of comparisons between genotype and allele frequencies between the multiple stress fracture and non-stress fracture cohorts
*Significance at P < 0.05 in comparison to the non-stress fracture elite athlete group
Discussion
The findings from these two distinct populations are the first to demonstrate an independent association between stress fracture injury and functional polymorphisms (rs3751143 and rs1718119—linkage disequilibrium was not shown in either cohort) in the P2X7R gene. These independent associations are confirmed in two ethnically distinct populations and under conditions of different aetiologies of injury, although it should be noted that these associations were diminished following testing for multiple comparisons. The rs3751143 and rs1718119 SNPs have, however, been consistently shown to be associated with bone phenotypes and clinical outcomes in older populations [18, 25, 27, 28]. The mechanisms by which sequence variants in P2X7R may be involved in stress fracture injury occurrence are unknown, although rs3751143 and rs1718119 have been shown to have effects on receptor functioning [18, 33] and human bone adaptations [28]. These effects on bone remodelling and cellular processes are plausible contributors to the putative association shown here between these variants and stress fracture injury occurrence.
P2X7Rs are expressed in all bone cells, and for this reason, the specific mechanisms of how the P2X7Rs influence stress fracture injury is difficult to distinguish and is most likely multi-factorial. The pathophysiology of stress fracture injury is related to the bone failing to adapt to repetitive loading cycles causing damage to bone micro-architecture and resultant bone weakness [8]. As a result of allelic variations in P2X7R SNPs, impairment in sensitivity to mechanical loading may cause genotype specific alterations in mechanotransduction [19].
Whilst the bone phenotypic consequences of allelic variations in the rs3751143 and rs1718119 are reported in osteoporotic or older individuals, this has not been explored in young populations with stress fracture injury. It is highly likely that the aetiology of stress fracture injury would be different to the aetiology of bone disease, which may lead to each SNP being associated with different phenotypic outcomes. In rs3751143, the loss of function variant also has bone phenotypic consequences. In vitro, the rs3751143 variant is associated with osteoclast apoptosis [18], reduced pore formation [34] and reduced pro-inflammatory cytokine secretion [35]. In vivo, lower lumbar spine BMD [28] and an increased risk of fracture have been shown [18]. The rs1718119 polymorphism results in increased receptor functioning related to monocyte activation and increases interleukin-1 alpha and beta release from monocytes and macrophages [33]. Recent in vivo studies have shown that the rs1718119 variant may also be related to bone phenotypes, including increased BMD in middle aged (≥50 years) and osteoporotic men and women [27, 28] and a reduced susceptibility to vertebral fracture in post-menopausal women and osteoporotic men and women [25, 28].
Although the rs3751143 and rs1718119 SNPs were independently associated with increased stress fracture injury prevalence in both cohorts, other SNPs within the P2X7R gene were not, despite evidence that rs28360447, rs28360457, rs2230912 and rs1653624 are associated with bone phenotypic changes [25, 28, 33]. The lack of association may be due to the variability in the anatomical sites where stress fractures were sustained; it is possible that specific SNPs might influence stress fracture risk at different anatomical sites. There might also have been minimal or no effect of these SNPs on stress fracture injury predisposition compared with the previously highlighted associations with osteoporosis and fragility fractures [18, 27, 28].
Although the number of genotyped individuals was higher than previously published studies exploring genetic associations with stress fracture prevalence [13–17], there are some limitations to this study. After correcting for multiple comparisons, the results did not remain significant. However, the conservative nature of multiple comparison testing increases the occurrence of a type II error. It is very unlikely that the current findings occurred by chance as they have been replicated in completely separate cohorts, and the direction of the effect is consistent with previous in vivo and in vitro research [18, 25–28] investigating P2X7R SNPs and bone phenotypes. rs2230912 and rs3751143 were not in Hardy-Weinberg equilibrium in the military cohort. Since the genotyping method is reliable, genotyping error is very unlikely. The reasons for the disequilibrium may include non-random mating or chance findings. Since the direction of association between rs3751143 and stress fracture injury was the same in the military population, elite athletes and previous bone phenotypic studies [34, 35], the disequilibrium seems unlikely to have influenced the outcome. The call rates (Table 2) for the alleles are lower than expected; this is due to an inadequate type and/or volume of sample being provided rather than SNP measurement issues.
In the athlete cohort, the stress fracture groups were older at sample collection and at the age of becoming elite compared with the non-stress fracture control group (Table 1). Despite this, the average age of the non-stress fracture group was higher than the average age at which stress fracture injury occurred (19.9 ± 3.9 years). The age at reaching elite status being higher in the stress fracture group (Table 1) raises the possibility that the skeletal adaptations that take place as a result of increased training at a younger age [36, 37] are beneficial for stress fracture prevention [38]. However, excessive increases in training loads have also been related to stress fracture occurrence [39], making it difficult to draw a definitive cause of injury from the present study. The retrospective study design may have introduced recall bias; however, this would be minimal as stress fracture injuries have a measurable impact in time of absence from training. Future studies should investigate P2X7R SNP associations with specific anatomical locations of stress fracture injury and attempt to control for variables, such as age and training parameters.
In conclusion, two functional SNPs within P2X7R gene are independently associated with stress fracture injury in two separate cohorts of healthy, exercising individuals, although the significant associations did not persist after performing multiple comparison testing. The precise mechanism by which these mutations may influence stress fracture risk is unknown but may include decreased sensitivity of bone to mechanical loading or decreased osteoclast apoptosis. The preliminary results of this trend for an association between gain of function and loss of function polymorphisms in the P2X7R gene and stress fracture risk need to be validated in a larger stress fracture population.
Acknowledgments
We are grateful to all participants for taking part in the study.
Disclosure statement
The authors have nothing to disclose.
Contributor Information
Ian Varley, Email: ian.varley@ntu.ac.uk.
Julie P. Greeves, Email: julie.greeves143@mod.uk
Craig Sale, Email: craig.sale@ntu.ac.uk.
Eitan Friedman, Email: eitan.friedman@sheba.health.gov.il.
Daniel S. Moran, Email: dani.moran@sheba.health.gov.il
Ran Yanovich, Email: ran.yanovich@sheba.health.gov.il.
Peter J. Wilson, Email: P.Wilson@liverpool.ac.uk
Alison Gartland, Email: a.gartland@sheffield.ac.uk.
David C. Hughes, Email: david.hughes02@ntu.ac.uk
Trent Stellingwerff, Email: tstellingwerff@csipacific.ca.
Craig Ranson, Email: cranson@cardiffmet.ac.uk.
William D. Fraser, Email: W.Fraser@uea.ac.uk
James A. Gallagher, Email: J.A.Gallagher@liverpool.ac.uk
References
- 1.Fredericson M, Chew K, Ngo J, Cleek T, Kiratli J, Cobb K. Regional bone mineral density in male athletes: a comparison of soccer players, runners and controls. Br J Sports Med. 2007;41:664–668. doi: 10.1136/bjsm.2006.030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gam A, Goldstein L, Karmon Y, Mintser I, Grotto I, Guri A, Goldberg A, Ohana N, Onn E, Levi Y, Bar-Dayan Y. Comparison of stress fractures of male and female recruits during basic training in the Israeli anti-aircraft forces. Mil Med. 2005;170:711–712. doi: 10.7205/MILMED.170.8.710. [DOI] [PubMed] [Google Scholar]
- 3.Gaeta M, Minutoli F, Scribano E, Ascenti G, Vinci S, Bruschetta D, Magaudda L, Blandino A. CT and MR imaging findings in athletes with early tibial stress injuries: comparison with bone scintigraphy findings and emphasis on cortical abnormalities. Radiology. 2005;235:553–561. doi: 10.1148/radiol.2352040406. [DOI] [PubMed] [Google Scholar]
- 4.McBryde AM. Stress fractures in runners. Clin Sports Med. 1985;4:737–752. [PubMed] [Google Scholar]
- 5.Warden SJ, Hurst JA, Sanders MS, Turner CH, Burr DB, Li J. Bone adaptation to a mechanical loading program significantly increases skeletal fatigue resistance. J Bone Miner Res. 2005;20:809–816. doi: 10.1359/JBMR.041222. [DOI] [PubMed] [Google Scholar]
- 6.Schaffler MB, Radin EL, Burr DB. Long-term fatigue behaviour of compact bone at low strain magnitude and rate. Bone. 1990;11:321–326. doi: 10.1016/8756-3282(90)90087-F. [DOI] [PubMed] [Google Scholar]
- 7.Bennell KL, Matheson G, Meeuwisse W, Brukner P. Risk factors for stress fractures. Sports Med. 1999;28:91–122. doi: 10.2165/00007256-199928020-00004. [DOI] [PubMed] [Google Scholar]
- 8.Warden SJ, Davis IS, Fredericson M. Management and prevention of bone stress injuries in long distance runners. J Orthop Sports Phys Ther. 2014;44:749–765. doi: 10.2519/jospt.2014.5334. [DOI] [PubMed] [Google Scholar]
- 9.Lambros G, Alder D. Multiple stress fractures of the tibia in a healthy adult. Am J Orthop. 1997;26:687–688. [PubMed] [Google Scholar]
- 10.Singer A, Ben-Yehuda O, Ben-Ezra Z, Zaltzman S. Multiple identical stress fractures in monozygotic twins. Case report. J Bone Joint Surg. 1990;72:444–445. [PubMed] [Google Scholar]
- 11.Gehrmann RM, Renard RL. Current concepts review: stress fractures of the foot. Foot Ankle Int. 2006;27:577–750. doi: 10.1177/107110070602700919. [DOI] [PubMed] [Google Scholar]
- 12.Giladi M, Milgrom C, Kashtan H, Stein M, Chisin R, Dizian R. Recurrent stress fractures in military recruits. One-year follow-up of 66 recruits. J Bone Joint Surg. 1986;68:439–441. doi: 10.1302/0301-620X.68B3.3733811. [DOI] [PubMed] [Google Scholar]
- 13.Korvala J, Hartikka H, Pihlajamäki H, Solovieva S, Ruohola JP, Sahi T, Barral S, Ott J, Ala-Kokko L, Männikkö M. Genetic predisposition for femoral neck stress fractures in military conscripts. BMC Genet. 2010;21(11):95. doi: 10.1186/1471-2156-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chatzipapas C, Boikos S, Drosos GI, Kazakos K, Tripsianis G, Serbis A, Stergiopoulos S, Tilkeridis C, Verettas DA, Stratakis CA. Polymorphisms of the vitamin D receptor gene and stress fractures. Horm Metab Res. 2009;41:635–640. doi: 10.1055/s-0029-1216375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yanovich R, Milgrom R, Friedman E, Moran DS. Androgen receptor CAG repeat size is associated with stress fracture risk: a pilot study. Clin Orthop Relat Res. 2011;469(10):2925–2931. doi: 10.1007/s11999-011-1805-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yanovich R, Friedman E, Milgrom R, Oberman B, Freedman L, Moran DS. Candidate gene analysis in Israeli soldiers with stress fractures. J Sports Sci Med. 2012;11:147–155. [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman E, Moran DS, Ben-Avraham D, Yanovich R, Atzmon G. Novel candidate genes putatively involved in stress fracture predisposition detected by whole-exome sequencing. Genet Res (Camb) 2014;96:e004. doi: 10.1017/S001667231400007X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohlendorff SD, Tofteng CL, Jensen JE, Petersen S, Civitelli R, Fenger M, Abrahamsen B, Hermann AP, Eiken P, Jørgensen NR. Single nucleotide polymorphisms in the P2X7 gene are associated to fracture risk and to effect of estrogen treatment. Pharmacogenet Genomics. 2007;17:555–567. doi: 10.1097/FPC.0b013e3280951625. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Liu D, Ke HZ, Duncan RL, Turner CH. The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J Biol Chem. 2005;280:42952–42959. doi: 10.1074/jbc.M506415200. [DOI] [PubMed] [Google Scholar]
- 20.Gartland A, Buckley KA, Bowler WB, Gallagher JA. Blockade of the pore-forming P2X7 receptor inhibits formation of multinucleated human osteoclasts in vitro. Calcif Tissue Int. 2003;73:361–369. doi: 10.1007/s00223-002-2098-y. [DOI] [PubMed] [Google Scholar]
- 21.Gartland A, Hipskind RA, Gallagher JA, Bowler WB. Expression of a P2X7 receptor by a subpopulation of human osteoblasts. J Bone Miner Res. 2001;16:846–856. doi: 10.1359/jbmr.2001.16.5.846. [DOI] [PubMed] [Google Scholar]
- 22.Gartland A, Buckley KA, Hipskind RA, Perry MJ, Tobias JH, Buell G, Chessell I, Bowler WB, Gallagher JA. Multinucleated osteoclast formation in vivo and in vitro by P2X7 receptor-deficient mice. Crit Rev Eukaryot Gene Expr. 2003;13:243–253. doi: 10.1615/CritRevEukaryotGeneExpr.v13.i24.150. [DOI] [PubMed] [Google Scholar]
- 23.Ke HZ, Qi H, Weidema AF, Zhang Q, Panupinthu N, Crawford DT, Grasser WA, Paralkar VM, Li M, Audoly LP, Gabel CA, Jee WS, Dixon SJ, Sims SM, Thompson DD. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol Endocrinol. 2003;17:1356–1367. doi: 10.1210/me.2003-0021. [DOI] [PubMed] [Google Scholar]
- 24.Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, Gabel CA. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;15(168):6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 25.Jørgensen NR, Husted LB, Skarratt KK, Stokes L, Tofteng CL, Kvist T, Jensen JE, Eiken P, Brixen K, Fuller S, Clifton-Bligh R, Gartland A, Schwarz P, Langdahl BL, Wiley JS. Single-nucleotide polymorphisms in the P2X7 receptor gene are associated with post-menopausal bone loss and vertebral fractures. Eur J Hum Genet. 2012;20:675–681. doi: 10.1038/ejhg.2011.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gartland A, Skarratt KK, Hocking LJ, Parsons C, Stokes L, Jørgensen NR, Fraser WD, Reid DM, Gallagher JA, Wiley JS. Polymorphisms in the P2X7 receptor gene are associated with low lumbar spine bone mineral density and accelerated bone loss in post-menopausal women. Eur J Hum Genet. 2012;20:559–564. doi: 10.1038/ejhg.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wesselius A, Bours MJ, Henriksen Z, Syberg S, Petersen S, Schwarz P, Jørgensen NR, van Helden S, Dagnelie PC. Association of P2X(7) receptor polymorphisms with bone mineral density and osteoporosis risk in a cohort of Dutch fracture patients. Osteoporos Int. 2013;24:1235–1246. doi: 10.1007/s00198-012-2059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Husted LB, Harsløf T, Stenkjær L, Carstens M, Jørgensen NR, Langdahl BL. Functional polymorphisms in the P2X7 receptor gene are associated with osteoporosis. Osteoporos Int. 2013;24:949–959. doi: 10.1007/s00198-012-2035-5. [DOI] [PubMed] [Google Scholar]
- 29.Moran DS, Evans RK, Hadad E. Imaging of lower extremity stress fracture injuries. Sports Med. 2008;38:345–356. doi: 10.2165/00007256-200838040-00005. [DOI] [PubMed] [Google Scholar]
- 30.Zwas ST, Elkanovitch R, Frank G. Interpretation and classification of bone scintigraphic findings in stress fractures. J Nucl Med. 1987;28:452–457. [PubMed] [Google Scholar]
- 31.Varley I, Hughes DC, Greeves JP, Stellingwerff T, Ranson C, Fraser WD, Sale C. RANK/RANKL/OPG pathway: genetic associations with stress fracture period prevalence in elite athletes. Bone. 2015;71:131–136. doi: 10.1016/j.bone.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300. [Google Scholar]
- 33.Stokes L, Fuller SJ, Sluyter R, Skarratt KK, Gu BJ, Wiley JS. Two haplotypes of the P2X(7) receptor containing the Ala-348 to Thr polymorphism exhibit a gain-of-function effect and enhanced interleukin-1beta secretion. J Fed Am Soc Exp Biol. 2010;24:2916–2927. doi: 10.1096/fj.09-150862. [DOI] [PubMed] [Google Scholar]
- 34.Gu W, Schlichthörl G, Hirsch JR, Engels H, Karschin C, Karschin A, Derst C, Steinlein OK, Daut J. Expression pattern and functional characteristics of two novel splice variants of the two-pore-domain potassium channel TREK-2. J Physiol. 2002;539(Pt 3):657–668. doi: 10.1113/jphysiol.2001.013432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sluyter R, Shemon AN, Wiley JS (2004) Glu496 to Ala polymorphism in the P2X7 receptor impairs ATP-induced IL-1 beta release from human monocytes. J Immunol 172:399–405. [DOI] [PubMed]
- 36.Lorentzon M, Mellstrom D, Ohlsson C. Association of amount of physical activity with cortical bone size and trabecular volumetric BMD in young adult men: the GOOD study. J Bone Miner Res. 2005;20:1936–1943. doi: 10.1359/JBMR.050709. [DOI] [PubMed] [Google Scholar]
- 37.Tobias JH, Steer CD, Mattocks CG, Riddoch C, Ness AR. Habitual levels of physical activity influence bone mass in 11-year-old children from the United Kingdom: findings from a large population-based cohort. J Bone Miner Res. 2007;22:101–109. doi: 10.1359/jbmr.060913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tenforde AS, Fredericson M. Influence of sports participation on bone health in the young athlete: a review of the literature. Phys Med Rehabil. 2011;3:861–867. doi: 10.1016/j.pmrj.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 39.Bennell KL, Malcolm SA, Thomas SA. Risk factors for stress fractures in track and field athletes: a 12 month prospective study. Am J Sports Med. 1996;24:810–818. doi: 10.1177/036354659602400617. [DOI] [PubMed] [Google Scholar]