Abstract
Gestational diabetes mellitus (GDM) associates with increased L-arginine transport and extracellular concentration of adenosine in human umbilical vein endothelial cells (HUVECs). In this study we aim to determine whether insulin reverses GDM-increased L-arginine transport requiring adenosine receptors expression in HUVECs. Primary cultured HUVECs from full-term normal (n = 38) and diet-treated GDM (n = 38) pregnancies were used. Insulin effect was assayed on human cationic amino acid transporter 1 (hCAT1) expression (protein, mRNA, SLC7A1 promoter activity) and activity (initial rates of L-arginine transport) in the absence or presence of adenosine receptors agonists or antagonists. A1 adenosine receptors (A1AR) and A2AAR expression (Western blot, quantitative PCR) was determined. Experiments were done in cells expressing or siRNA-suppressed expression of A1AR or A2AAR. HUVECs from GDM exhibit higher maximal transport capacity (maximal velocity (Vmax)/apparent Michaelis Menten constant (Km), Vmax/Km), which is blocked by insulin by reducing the Vmax to values in cells from normal pregnancies. Insulin also reversed the GDM-associated increase in hCAT-1 protein abundance and mRNA expression, and SLC7A1 promoter activity for the fragment −606 bp from the transcription start point. Insulin effects required A1AR, but not A2AAR expression and activity in this cell type. In the absence of insulin, GDM-increased hCAT-1 expression and activity required A2AAR expression and activity. HUVECs from GDM pregnancies exhibit a differential requirement of A1AR or A2AAR depending on the level of insulin, a phenomenon that represent a condition where adenosine or analogues of this nucleoside could be acting as helpers of insulin biological effects in GDM.
Keywords: Diabetes, Insulin, Adenosine receptor, Fetal, Endothelium
Introduction
Gestational diabetes mellitus (GDM) characterizes by abnormal maternal D-glucose metabolism and altered insulin signaling in the fetoplacental circulation [1–3]. GDM also associates with increased uptake of the cationic amino acid L-arginine [4], the substrate for nitric oxide (NO) synthesis by the endothelial NO synthase (eNOS) [5], in human umbilical vein endothelial cells (HUVECs), changes referred as GDM-associated fetoplacental endothelial dysfunction [3, 6]. The latter is reinforced with findings showing that uptake of the endogenous nucleoside adenosine, a potent vasodilator in the human fetoplacental vasculature [7, 8] and other vascular beds [9–11], is also reduced in HUVECs from GDM pregnancies [8, 12].
Reduced adenosine transport leads to increased extracellular levels of adenosine in HUVECs primary cultures from GDM pregnancies, a finding that agrees with the elevated adenosine plasma level detected in human umbilical vein blood from GDM pregnancies [8, 13]. We reported that activation of A2A adenosine receptors (A2AAR) is required for the increase caused by insulin on L-arginine transport via the human cationic amino acid transporter 1 (hCAT-1), a Na+- and pH-independent membrane transporter (apparent Km ~100 μM) [14, 15], and eNOS activity in HUVECs from normal pregnancies [6, 12]. Additionally, insulin restores GDM-reduced adenosine transport [15] in this cell type. However, whether insulin effect requires activation of adenosine receptors (ARs) in the fetoplacental endothelium from GDM pregnancies is unknown [3, 11]. We hypothesize that insulin will reverse the GDM-associated increase in hCAT-1 expression and activity requiring expression and activation of ARs in HUVECs. Our results show that increased L-arginine transport in HUVECs from GDM pregnancies is reversed by insulin to values in cells from normal pregnancies via a mechanism where A1AR play a role.
Materials and methods
Human umbilical cords and study groups
Umbilical cords were collected after delivery from 38 full-term normal or 38 full-term GDM pregnancies. Ethnicity of patients included in this study was Hispanic. The investigation conforms to the principles outlined in the Declaration of Helsinki. Ethics Committee approval from the Faculty of Medicine of the Pontificia Universidad Católica de Chile and informed consent of patients (all of them from the Hospital Clínico UC in Santiago de Chile) were obtained. Patients between the 24–28 weeks of gestation with basal glycemia >5 mM (>90 mg/dL, i.e., overnight starvation) and with >7.9 mM (>140 mg/dL) at 2 h after an oral glucose load (75 g) were diagnosed as gestational diabetes (according with the Perinatal Guide 2014 report from the Health Ministry of Chile) and subjected to dietary treatment with 1500 kcal/day and a maximum of 200 g per day carbohydrates (Table 1). The homeostasis model assessment for insulin resistance (HOMA-IR) was calculated from:
where Insulin is in micro unit per millilitre and Glucose is basal glycemia in millimole per litre [16, 17]. Insulin sensitivity (IS) was derived from these values by (expressed in %). Additionally, β cell function (β, expressed in %) was estimated from:
Table 1.
Clinical characteristics of women with normal or GDM pregnancies and newborns
| Variables | Normal pregnancies (n = 38) | GDM pregnancies (n = 38) |
|---|---|---|
| Maternal variables | ||
| Age (years) | 28 ± 6 (18–38) | 31 ± 6 (20–38) |
| Height (cm) | 159 ± 7 (157–162) | 161 ± 9 (157–163) |
| Weight (kg) | ||
| 24–28 wg | 52 ± 1.3 (50–55) | 53 ± 1.2 (50–55) |
| 38–40 wg | 61 ± 1.1† (55–65) | 63 ± 1.1† (59–66) |
| BMI (kg/m2) | ||
| 24–28 wg | 20.6 ± 0.2 (20.3–21.0) | 20.5 ± 0.47 (20.3–20.7) |
| 38–40 wg | 24.1 ± 0.1 (22.3–24.8) | 24.3 ± 0.44 (23.9–24.8) |
| Systolic blood pressure (mmHg) | ||
| 24–28 wg | 101 ± 4 (98–107) | 103 ± 5 (97–106) |
| 38–40 wg | 106 ± 6 (95–112) | 110 ± 3 (101–112) |
| Glycosylated hemoglobin A1c | ||
| 24–28 wg (% of total) [mmol/mol] | 4.1 ± 0.3 (3.6–4.7) [21.3 ± 1.6 (18.7–24.4)] | 4.1 ± 0.2 (3.8–4.6) [21.3 ± 1.0 (19.7–23.9)] |
| 38–40 wg (% of total) [mmol/mol] | 4.0 ± 0.3 (3.3–5.0) [20.2 ± 1.5 (16.7–25.3)] | 6.1 ± 0.1*† (5.6–6.9) [41.0 ± 0.7 (37.7–46.4)] |
| Glycemia basal at delivery (mmol/L) | 4.4 ± 0.4 (4.1–5.1) | 4.6 ± 0.5 (3.9–5.6) |
| OGTT (mmol/L) | ||
| Glycemia basal | 4.5 ± 0.4 (3.9–5.2) | 4.7 ± 0.4 (4.1–5.2) |
| Glycemia 2 h after glucose | 5.1 ± 0.5 (3.7–6.1) | 9.8 ± 1.2* (8.1–11.9) |
| Plasma insulin (μU/mL) | 5.1 ± 0.1 (4.2–5.4) | 7.9 ± 1.5* (6.2–12.1) |
| HOMA-IR | 1.00 ± 0.05 (0.77–1.22) | 1.62 ± 0.16* (1.37–1.97) |
| HOMA-IS (%) | 101 ± 5 (82–130) | 62 ± 6* (51–73) |
| HOMA-β (%) | 114 ± 7 (64–140) | 143 ± 14* (115–310) |
| Newborn variables | ||
| Sex (female/male) | 12/16 | 13/15 |
| Gestational age (weeks) | 38.1 ± 0.3 (38–40) | 38.2 ± 0.2 (38–39) |
| Birth weight (g) | 3087 ± 67 (2417–3451) | 4299 ± 63* (3571–4731) |
| Height (cm) | 50 ± 2.2 (47–54) | 51 ± 3.3 (48–54) |
| Ponderal index (g/cm3 × 100) | 2.46 ± 0.02 (1.7–7.4) | 3.44 ± 0.02* (2.9–6.0) |
| Umbilical vein D-glucose (mmol/L) | 3.8 ± 0.5 (3.1–4.4) | 4.4 ± 0.5 (4.2–4.8) |
| Umbilical vein insulin (μU/mL) | 6.1 ± 0.7 (5.5–7.0) | 11.6 ± 0.6* (8.9–13.1) |
| HOMA-IR | 1.03 ± 0.09 (0.76–1.36) | 2.27 ± 0.12* (1.66–2.80) |
| HOMA-IS (%) | 97 ± 12 (74–132) | 44 ± 4* (34–60) |
Data are presented as mean ± SD (range). All women included in this study were Hispanic. GDM patients were treated with diet. OGTT was measured between 24 and 28 wg. HOMA-IR, HOMA-IS, and HOMA-β (β cell function) were estimated as described in “Materials and methods”
OGTT oral glucose tolerance test, BMI body mass index, HOMA-IR homeostasis model assessment for insulin resistance, HOMA-IS homeostasis model assessment for insulin sensitivity, wg weeks of gestation
*P < 0.05 versus values in Normal pregnancies; †P < 0.05 versus values at 24–28 weeks of gestation in Normal or GDM pregnancies
Cell culture
Confluent HUVECs primary cultures (37 °C, 5 % CO2) were cultured in medium 199 (M199; Gibco Life Technologies, Carlsbad, CA, USA) up to passage 3 as described [6]. The culture medium was supplemented with 0.04 nM (~5.1 μU/mL) or 0.07 nM (~7.9 μU/mL) insulin for normal or GDM pregnancies, respectively [8]. Experiments were performed in the absence (referred as ‘without insulin’ or ‘in the absence of insulin’) or presence (referred as ‘with insulin’ or ‘in the presence of insulin’) of exogenous 1 nM insulin for 8 h. Cells were also exposed to 30 nM (2R,3R,4S,5R)-2-[6-(cyclopentylamino)purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol (CPA, A1AR agonist) (Sigma, Atlanta, GA, USA), 30 nM 8-cyclopentyl-1,3-dipropylxanthine (DPCPX, A1AR antagonist) (Sigma), 30 nM 2-[p-(2-carbonyl-ethyl)-phenylethylamino]-5′-N-ethylcarboxamidoadenosine (CGS-21680, A2AAR agonist) (Sigma), 10 nM 4-(2-[7-amino-2-[2-furyl]-[1, 2, 4]triazolo[2,3-a]{1,3,5}triazin-5-yl-amino]ethyl)phenol (ZM-241385, A2AAR antagonist) (Sigma), 100 nM 2-[[6-Amino-3,5-dicyano-4-[4-(cyclopropylmethoxy)phenyl]-2-pyridinyl]thio]-acetamide (BAY60-6583, A2BAR agonist) (Tocris Bioscience, Bristol, UK), 30 nM N-(4-cyanophenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)phenoxy]-acetamide (MRS-1754, A2BAR antagonist) (Tocris Bioscience), 30 nM 1-[2-chloro-6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-ß-D-ribofuranuronamide (2-Cl-IB-MECA, A3AR agonist) (Tocris Bioscience), and 30 nM 3-propyl-6-ethyl-5[(ethylthio)carbonyl]-2-phenyl-4-propyl-3-pyridinecarboxylate (MRS-1523, A3AR antagonist) (Tocris Bioscience) [6].
L-Arginine transport
Overall 0–1000 μM L-arginine transport (3 μCi/mL L-[3H]arginine (NEN, Dreieich, FRG), 1 min incubation, 37 °C) was measured as described [6]. Briefly, transport assays were performed in Krebs ((mM): NaCl 131, KCl 5.6, NaHCO3 25, NaH2PO4 1, Hepes 20, CaCl2 2.5, MgCl2 1 (pH 7.4, 37 °C)) in cells preincubated (12–14 h) in phosphate-buffered saline (PBS) solution ((mM): 130 NaCl, 2.7 KCl, 0.8 Na2HPO4, 1.4 KH2PO4 (pH 7.4, 4 °C)) containing 0.25 % newborn and 0.25 % fetal calf sera. PBS and Krebs solutions were also supplemented with 2 U/mL adenosine deaminase 1. ATP, ADP, AMP, or adenosine was not detected in PBS or Krebs solutions as assayed by high-performance light chromatography (not shown) as described [12]. Cell monolayers were rinsed with ice-cold Krebs to terminate tracer uptake. Radioactivity in formic acid cell digests was determined by liquid scintillation counting, and uptake was corrected for D-[3H]mannitol (NEN) disintegrations per minute (d.p.m.) in the extracellular space [6].
Initial rate for transport (i.e., linear uptake up to 1 min) was derived from slope of linear phase of 100 μM L-arginine transport. Values for L-arginine transport were adjusted to the one phase exponential association equation considering the least squares fit:
where vi is initial velocity, Vm is mayor velocity at a given time (t) and L-arginine concentration, and e and k are constants. Overall L-arginine transport at initial rates was adjusted to the Michaelis-Menten hyperbola plus a nonsaturable, linear component as described [6]. Saturable L-arginine transport kinetic parameters maximal velocity (Vmax) and apparent Michaelis-Menten constant (Km) of transport were calculated as described [6]. The relative contribution of GDM, insulin, or ARs to the saturable L-arginine transport kinetic parameters was estimated from the maximal transport capacity (Vmax/Km) values for L-arginine transport by:
where CVmax and CKm are the kinetics parameters for L-arginine transport in control conditions in cells from normal or GDM pregnancies, and XVmax and XKm are kinetics parameters of L-arginine transport in HUVECs from normal or GDM pregnancies exposed to different experimental conditions [6, 8].
Trans-stimulation experiments for saturable L-arginine transport (100, 250, 500 μM) were performed in cells incubated (2 h) with Krebs solution containing 10 mM L-lysine. Incubation medium was removed and replaced by L-lysine-free Krebs and transport was determined as above [18].
Reverse transcription and quantitative RT-PCR
Experiments were performed using a Step One real time PCR system (Applied Biosystem, CA, USA) in a reaction mix containing 0.2 μM primers and master mix provided in the brilliant SYBR green qPCR Master Mix (Applied Biosystem, CA, USA) as described [6]. Hot Start Taq DNA polymerase was activated (15 min, 95 °C), and assays included a 95 °C denaturation (15 s), annealing (20 s) at 54 °C (hCAT-1 and 28S), and extension at 72 °C (hCAT-1 and 28S, 10 s). Fluorescent product was detected after 3-s step to 5 °C below the product melting temperature (Tm). Product specificity was confirmed by agarose gel electrophoresis (2 % w/v) and melting curve analysis. The product Tm values were 79.1 °C for hCAT-1 and 86.7 °C for 28S. hCAT-1 and 28S standards were prepared as described [6]. Oligonucleotide primers are as follows: hCAT-1 (sense) 5′-GAGTTAGATCCAGCAGACCA-3′, hCAT-1 (anti-sense) 5′-TGTTCACAATTAGCCCAGAG-3′, 28S (sense) 5′-TTGAAAATCCGGGGGAGAG-3′, 28S (anti-sense) 5′-ACATTGTTCCAACATGCCAG-3′. Expected size products for hCAT-1 (151 bp) and 28S (105 bp) were confirmed in PCR experiments. The 28S rRNA number of copies was unaltered (P > 0.05, n = 16) in all experimental conditions (not shown).
Western blotting
Proteins (70 μg) separated by polyacrylamide gel (10 %) electrophoresis were probed with a primary polyclonal goat anti-hCAT-1 (1:500), goat anti-A1AR (1:1000), mouse anti-A2AAR (1:1000) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), or rabbit anti-A2BAR (1:1000) or anti-A3AR (1:1000) (Abcam, Cambridge, UK), or monoclonal mouse anti-β-actin (1:3000) (Sigma Aldrich, St Louis, MO, USA) antibodies [6]. Proteins were detected by enhanced chemiluminescence in a ChemiDoc-It® 510 Imagen System (UVP, LCC Upland, CA, USA) and quantified by densitometry.
HCAT-1 promoter cloning
Genomic DNA was isolated using the Wizard SV Genomic DNA Purification System (Promega, Madison, WI, USA). The sequences −1606 and −650 bp from the transcription start point of the SLC7A1 gene (GenBank: AL596114) were PCR-amplified using Elongase Enzyme System (Invitrogen) and cloned into pGL3-basic reporter system [6, 18]. The pGL3–hCAT-1 reporter constructs generated were pGL3–hCAT-1−1606 and pGL3–hCAT-1−650.
Transient transfection
Sub-confluent (60–80 %) HUVECs primary cultures were resuspended in serum-free M199. Aliquots of cell suspension (0.5 mL, 3.2 × 106 cells/mL) were mixed with 10 μg of pGL3–hCAT1−1606 or pGL3–hCAT1−650 constructs, pGL3-Basic (empty pGL3 vector), pGL3-Control (Simian Virus 40 promoter (SV40) pGL3 vector), and the internal transfection control vector pRL-TK expressing Renilla luciferase (Promega) [6]. After electroporation (300 V, 700 μF, 5–10 ms) (Gene Pulser II System, BioRad, CA, USA), cells were cultured (48 h) in M199 containing 2 % FCS. Transfection efficiency was estimated by transfection of the pEGFP-N3 vector (Clontech, Mountain View, CA, USA) and fluorescent cells were counted under an inverted fluorescent microscope (Leica DMIL; Wetzlar, Germany).
Luciferase assay
Firefly and Renilla luciferase activity was measured using Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) in a Sirius luminometer (Berthold Detection System; Oak Ridge, TN, USA) [6].
Adenosine receptors suppression
Since the pharmacological approach used in this study suggested that A2BAR and A3AR may not be involved in the effect of insulin, we focused in generating cells knockdown for A1AR and A2AAR. Suppression of A1AR and A2AAR expression was done using the commercially available short interference RNAs (siRNA) Adenosine A1-R siRNA(h) and Adenosine A2A-R siRNA(h) (Santa Cruz Biotechnology) following manufacturer’s instructions (http://datasheets.scbt.com/siRNA_protocol.pdf). Cells knockdown for A1AR (KDA1AR) and A2AAR (KDA2AAR) were generated.
Statistical analysis
Values are mean ± SD (range) or SEM, with n = 38 different cell cultures (3–4 replicates) from normal or GDM pregnancies. The normality of the data was determined with Kolmogorov-Smirnov test. Comparisons between two and more groups were performed by means of Student’s unpaired t test and analysis of variance (ANOVA), respectively. If the ANOVA demonstrated a significant interaction between variables, post hoc analyses were performed by the multiple-comparison Bonferroni correction test. P < 0.05 was considered statistically significant. The statistical software GraphPad Instat 3.0b and Graphpad Prism 7.0a.65 (GraphPad Software Inc., San Diego, CA, USA) were used for data analysis. P < 0.05 was considered statistically significant.
Results
Patients and newborns
Normal or GDM pregnancies were singleton and pregnant women were normotensive, nonsmoking, non-alcohol or drug consuming, and without intrauterine infection or any other medical or obstetrical complications (Table 1). Newborns to GDM pregnancies were heavier at birth, with higher umbilical vein insulin and HOMA-IR, but lower HOMA-IS compared with normal pregnancies.
L-Arginine transport
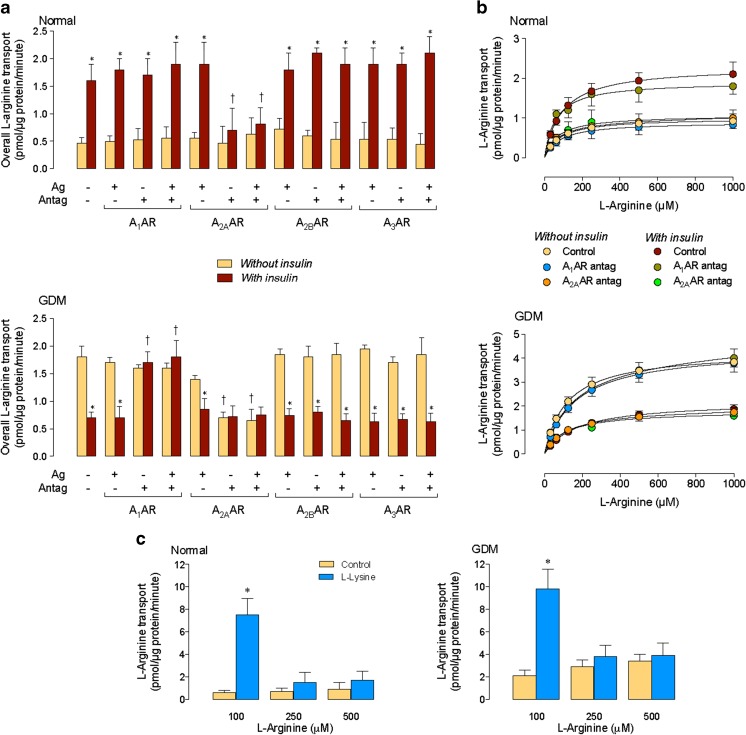
In the absence of insulin, overall transport of L-arginine was unaltered by ARs agonists or antagonists in cells from normal pregnancies (Fig. 1a). Insulin increased overall transport in this cell type, an effect blocked by an A2AAR antagonist. In the absence of insulin, overall L-arginine transport was higher in cells from GDM compared with normal pregnancies. GDM effect was reversed by an A2AAR antagonist to values in cells from normal pregnancies. Insulin reversed GDM-increase in transport to values in normal pregnancies, an effect blocked by an A1AR antagonist.
Fig. 1.
Involvement of adenosine receptors on GDM and insulin effect on L-arginine transport. a Overall (100 μM) L-arginine transport in HUVECs from normal (Normal) or gestational diabetes mellitus (GDM) pregnancies incubated without or with insulin (1 nM, 8 h) in the absence (−) or presence (+) of A1AR, A2AAR, A2BAR, or A3AR agonists (Ag) or antagonists (Antag). b Saturable L-arginine transport as in a in cells incubated without (Control) or with A1AR or A2AAR antagonists. c L-Arginine transport (1 min) in cells preloaded with a solution without L-lysine (Control) or with 1 mM L-lysine (L-Lysine) (see “Materials and methods”). In a, *P < 0.05 vs. corresponding values in Without insulin. †P < 0.05 vs. corresponding values in the absence of Ago and Antag. In c, *P < 0.05 vs. all other values. Values are mean ± SEM (n = 38)
Overall L-arginine transport kinetics was semisaturable (not shown), and non-saturable transport component (i.e., KD) was similar in normal or GDM pregnancies (Table 2) and unaltered by insulin or agonists or antagonists for ARs. However, insulin increased the vi of transport in normal pregnancies, an effect blocked by the A2AAR antagonist. In the absence of insulin, GDM-increase in vi was blocked by the A2AAR antagonist. Insulin reversed GDM effect on vi, an effect blocked by the A1AR antagonist (Table 2).
Table 2.
Kinetic parameters for L-arginine transport in HUVECs from normal or GDM pregnancies
| Saturable transport | Overall transport | ||||
|---|---|---|---|---|---|
| V max (pmol/μg protein/ min) | K m (μM) | V max/K m (pmol/μg protein/min/(μM)) | K D (pmol/μg protein/min/(μM)) | v i (pmol/μg protein/ 0.5 s) | |
| Normal pregnancies | |||||
| Without insulin | |||||
| Control | 1.2 ± 0.2 | 89 ± 20 | 0.0135 ± 0.0027 | 0.0011 ± 0.0004 | 0.006 ± 0.0001 |
| A1AR ag | 1.2 ± 0.1 | 103 ± 21 | 0.0117 ± 0.0016 | 0.0012 ± 0.0005 | 0.006 ± 0.0001 |
| A1AR antag | 1.0 ± 0.2 | 77 ± 40 | 0.0130 ± 0.0047 | 0.0011 ± 0.0004 | 0.005 ± 0.0002 |
| A1AR ag/antag | 1.1 ± 0.3 | 79 ± 26 | 0.0139 ± 0.0042 | 0.0012 ± 0.0003 | 0.006 ± 0.0002 |
| A2AAR ag | 1.1 ± 0.2 | 108 ± 15 | 0.0102 ± 0.0016 | 0.0011 ± 0.0003 | 0.005 ± 0.0001 |
| A2AAR antag | 1.0 ± 0.1 | 99 ± 33 | 0.0101 ± 0.0022 | 0.0010 ± 0.0003 | 0.007 ± 0.0001 |
| A2AAR ag/antag | 1.3 ± 0.4 | 87 ± 24 | 0.0149 ± 0.0044 | 0.0013 ± 0.0007 | 0.008 ± 0.0003 |
| A2BAR ag | 1.5 ± 0.3 | 98 ± 21 | 0.0153 ± 0.0031 | 0.0012 ± 0.0004 | 0.008 ± 0.0002 |
| A2BAR antag | 1.2 ± 0.1 | 91 ± 16 | 0.0132 ± 0.0017 | 0.0011 ± 0.0004 | 0.006 ± 0.0001 |
| A2BAR ag/antag | 1.1 ± 0.3 | 87 ± 21 | 0.0126 ± 0.0032 | 0.0012 ± 0.0006 | 0.006 ± 0.0002 |
| A3AR ag | 0.9 ± 0.2 | 62 ± 23 | 0.0145 ± 0.0043 | 0.0012 ± 0.0005 | 0.006 ± 0.0002 |
| A3AR antag | 1.3 ± 0.1 | 97 ± 21 | 0.0134 ± 0.0020 | 0.0013 ± 0.0004 | 0.007 ± 0.0001 |
| A3AR ag/antag | 1.2 ± 0.3 | 96 ± 31 | 0.0125 ± 0.0036 | 0.0012 ± 0.0005 | 0.006 ± 0.0002 |
| With insulin | |||||
| Control | 2.3 ± 0.1* | 93 ± 22 | 0.0247 ± 0.0035* | 0.0012 ± 0.0004 | 0.012 ± 0.0002* |
| A1AR ag | 2.7 ± 0.1* | 104 ± 21 | 0.0260 ± 0.0031* | 0.0012 ± 0.0004 | 0.013 ± 0.0003* |
| A1AR antag | 2.2 ± 0.1* | 88 ± 20 | 0.0250 ± 0.0035* | 0.0011 ± 0.0005 | 0.011 ± 0.0003* |
| A1AR ag/antag | 2.2 ± 0.2* | 81 ± 16 | 0.0272 ± 0.0039* | 0.0012 ± 0.0006 | 0.012 ± 0.0003* |
| A2AAR ag | 2.9 ± 0.2* | 137 ± 20 | 0.0212 ± 0.0023* | 0.0010 ± 0.0004 | 0.011 ± 0.0002* |
| A2AAR antag | 0.9 ± 0.4† | 95 ± 29 | 0.0095 ± 0.0036† | 0.0011 ± 0.0004 | 0.004 ± 0.0002† |
| A2AAR ag/antag | 1.1 ± 0.2† | 82 ± 27 | 0.0134 ± 0.0034† | 0.0013 ± 0.0005 | 0.007 ± 0.0002† |
| A2BAR ag | 1.7 ± 0.1* | 95 ± 21 | 0.0179 ± 0.0025* | 0.0010 ± 0.0005 | 0.011 ± 0.0002* |
| A2BAR antag | 2.3 ± 0.2* | 110 ± 29 | 0.0209 ± 0.0031* | 0.0013 ± 0.0003 | 0.012 ± 0.0002* |
| A2BAR ag/antag | 1.9 ± 0.3* | 89 ± 21 | 0.0213 ± 0.0043* | 0.0013 ± 0.0005 | 0.011 ± 0.0003* |
| A3AR ag | 2.5 ± 0.1* | 84 ± 12 | 0.0298 ± 0.0027* | 0.0012 ± 0.0005 | 0.014 ± 0.0003* |
| A3AR antag | 2.2 ± 0.1* | 88 ± 18 | 0.0250 ± 0.0033* | 0.0011 ± 0.0003 | 0.011 ± 0.0002* |
| A3AR ag/antag | 2.4 ± 0.3* | 99 ± 18 | 0.0242 ± 0.0038* | 0.0013 ± 0.0006 | 0.013 ± 0.0003* |
| GDM pregnancies | |||||
| Without insulin | |||||
| Control | 4.3 ± 0.3 | 120 ± 31 | 0.0358 ± 0.0059 | 0.0013 ± 0.0003 | 0.021 ± 0.004 |
| A1AR ag | 4.3 ± 0.3 | 143 ± 33 | 0.0301 ± 0.0045 | 0.0014 ± 0.0004 | 0.021 ± 0.004 |
| A1AR antag | 4.2 ± 0.3 | 125 ± 25 | 0.0336 ± 0.0045 | 0.0012 ± 0.0002 | 0.019 ± 0.003 |
| A1AR ag/antag | 4.1 ± 0.5 | 119 ± 34 | 0.0345 ± 0.0071 | 0.0013 ± 0.0003 | 0.020 ± 0.004 |
| A2AAR ag | 4.0 ± 0.3 | 150 ± 35 | 0.0267 ± 0.0041 | 0.0012 ± 0.0001 | 0.016 ± 0.002 |
| A2AAR antag | 2.1 ± 0.5† | 135 ± 42 | 0.0156 ± 0.0043† | 0.0012 ± 0.0001 | 0.009 ± 0.002† |
| A2AAR ag/antag | 2.2 ± 0.4† | 127 ± 26 | 0.0173 ± 0.0034† | 0.0011 ± 0.0003 | 0.010 ± 0.002† |
| A2BAR ag | 4.3 ± 0.3 | 134 ± 33 | 0.0321 ± 0.0051 | 0.0012 ± 0.0001 | 0.018 ± 0.002 |
| A2BAR antag | 4.1 ± 0.3 | 120 ± 34 | 0.0342 ± 0.0060 | 0.0012 ± 0.0002 | 0.019 ± 0.003 |
| A2BAR ag/antag | 3.9 ± 0.6 | 129 ± 37 | 0.0302 ± 0.0067 | 0.0012 ± 0.0004 | 0.017 ± 0.004 |
| A3AR ag | 4.1 ± 0.3 | 142 ± 53 | 0.0289 ± 0.0064 | 0.0012 ± 0.0001 | 0.017 ± 0.003 |
| A3AR antag | 4.2 ± 0.3 | 135 ± 43 | 0.0311 ± 0.0061 | 0.0012 ± 0.0001 | 0.018 ± 0.003 |
| A3AR ag/antag | 4.0 ± 0.5 | 121 ± 31 | 0.0331 ± 0.0064 | 0.0013 ± 0.0003 | 0.020 ± 0.004 |
| With insulin | |||||
| Control | 2.2 ± 0.5* | 172 ± 40 | 0.0128 ± 0.0029* | 0.0013 ± 0.0003 | 0.009 ± 0.002* |
| A1AR ag | 2.2 ± 0.4* | 142 ± 35 | 0.0155 ± 0.0033* | 0.0014 ± 0.0040 | 0.011 ± 0.012* |
| A1AR antag | 4.4 ± 0.3† | 162 ± 46 | 0.0272 ± 0.0048† | 0.0012 ± 0.0002 | 0.017 ± 0.003† |
| A1AR ag/antag | 4.1 ± 0.4† | 149 ± 35 | 0.0275 ± 0.0047† | 0.0013 ± 0.0003 | 0.018 ± 0.003† |
| A2AAR ag | 1.9 ± 0.3* | 134 ± 31 | 0.0142 ± 0.0028* | 0.0012 ± 0.0001 | 0.008 ± 0.001* |
| A2AAR antag | 1.9 ± 0.4* | 163 ± 95 | 0.0117 ± 0.0046* | 0.0012 ± 0.0001 | 0.007 ± 0.002* |
| A2AAR ag/antag | 2.2 ± 0.4* | 135 ± 25 | 0.0163 ± 0.0030* | 0.0013 ± 0.0003 | 0.010 ± 0.002* |
| A2BAR ag | 2.2 ± 0.2* | 142 ± 37 | 0.0155 ± 0.0027* | 0.0012 ± 0.0001 | 0.009 ± 0.001* |
| A2BAR antag | 2.0 ± 0.3* | 173 ± 72 | 0.0116 ± 0.0033* | 0.0012 ± 0.0001 | 0.007 ± 0.002* |
| A2BAR ag/antag | 2.2 ± 0.3* | 165 ± 25 | 0.0133 ± 0.0019* | 0.0014 ± 0.0005 | 0.010 ± 0.002* |
| A3AR ag | 2.3 ± 0.2* | 162 ± 34 | 0.0142 ± 0.0021* | 0.0012 ± 0.0001 | 0.009 ± 0.001* |
| A3AR antag | 2.3 ± 0.3* | 163 ± 53 | 0.0141 ± 0.0032* | 0.0012 ± 0.0001 | 0.009 ± 0.002* |
| A3AR ag/antag | 2.2 ± 0.2* | 152 ± 25 | 0.0145 ± 0.0018* | 0.0011 ± 0.0005 | 0.008 ± 0.002* |
Data are presented as mean ± SEM (n = 38). L-Arginine transport (0–1000 μM, 1 min, 37 °C) was measured in HUVECs from normal (Normal) or gestational diabetes mellitus (GDM) pregnancies exposed (8 h) to culture medium without (Without insulin) or with (With insulin) insulin (1 nM). Transport assays were done in the absence (Control) or presence of the A1AR agonist CPA (30 nM, A1AR ag), antagonist DPCPX (30 nM, A1AR antag), or both (A1AR ag/antag), the A2AAR agonist CGS-21680 (30 nM, A2AAR ag), antagonist ZM-241385 (10 nM, A2AAR antag), or both (A2AAR ag/antag), the A2BAR agonist BAY60-6583 (100 nM, A2BAR agonist), antagonist MRS-1754 (30 nM, A2BAR antag), or both (A2BAR ag/antag), the A3AR agonist 2-Cl-IB-MECA (30 nM, A3AR ag), antagonist MRS-1523 (30 nM, A3AR antag) or both (A3AR ag/antag) (see “Materials and methods”). Maximal velocity (V max) and apparent Michaelis-Menten constant (K m) of saturable transport were calculated assuming a single Michaelis-Menten hyperbola. V max/K m represents maximal L-arginine transport capacity. The lineal phase of overall transport of L-arginine (K D) was obtained from transport data fitted to a Michaelis-Menten equation increased in a lineal component. Initial velocity (v i) was calculated for 0.5 s with 100 μM L-arginine transport
*P < 0.05 versus corresponding values in Without insulin; †P < 0.05 versus corresponding Control values
After subtracting the KD from overall transport, the resulting saturable transport of L-arginine (Fig. 1b) coursed with higher Vmax and Vmax/Km values, without variations in the apparent Km in cells from normal pregnancies exposed to insulin (Table 2). Similar results were found in cells from GDM pregnancies in the absence of insulin. However, insulin reversed GDM-increase in Vmax and Vmax/Km to values in normal pregnancies. The A2AAR antagonist blocked insulin effect in normal pregnancies. In addition, this antagonist blocked GDM effect on saturable transport in the absence of insulin. However, the A1AR antagonist blocked insulin effect on saturable transport in GDM pregnancies. Parallel experiments show that saturable transport of 100, but not 250 or 500 μM L-arginine, was trans-stimulated by L-lysine reaching similar values in both conditions (Fig. 1c).
HCAT-1 expression
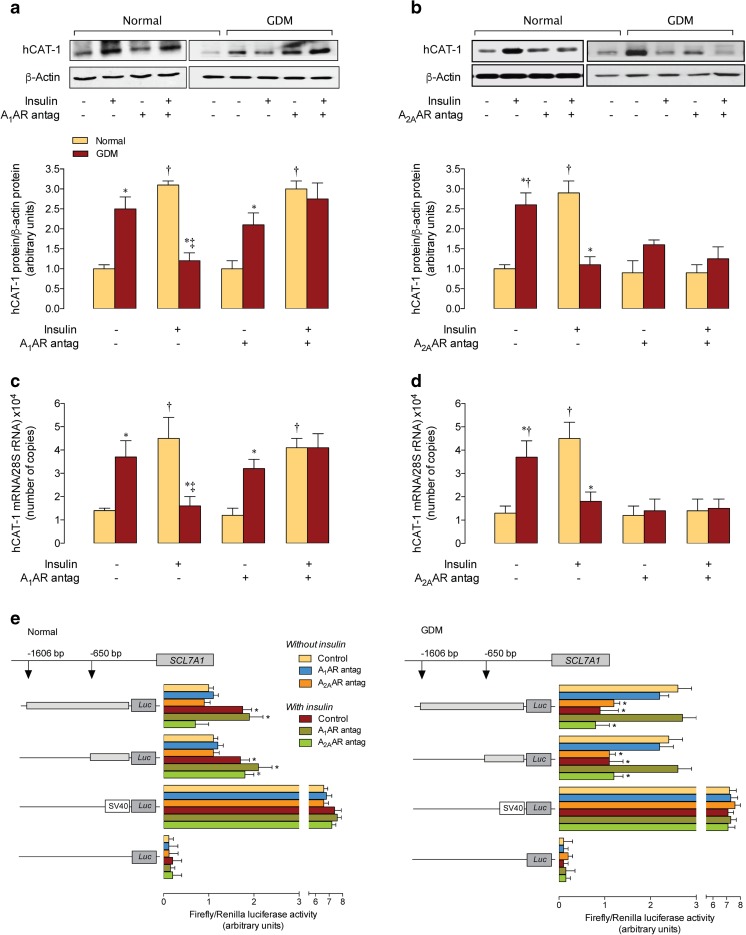
Insulin increased hCAT-1 protein abundance in HUVECs from normal pregnancies to values in GDM (Fig. 2a), but reversed GDM-increase in hCAT-1 to values in normal pregnancies in absence of this hormone. Insulin effect in normal pregnancies was unaltered by the A1AR antagonist, but this antagonist blocked this hormone’s effect in GDM. However, the A1AR antagonist in the absence of insulin did not alter the GDM-increased hCAT-1 protein abundance. Insulin effect in cells from GDM was unaltered by an A2AAR antagonist; however, this antagonist alone blocked the GDM-increase in hCAT-1 (Fig. 2b). Insulin increased hCAT-1 protein abundance in normal pregnancies was blocked by A2AAR antagonist, but this antagonist did not alter hCAT-1 protein abundance in the absence of insulin. Similar responses to insulin were obtained for hCAT-1 mRNA expression in both cell types (Fig. 2c, d).
Fig. 2.
Involvement of A1AR and A2AAR on GDM and insulin effect on hCAT-1 expression. a Western blot for hCAT-1 protein abundance in HUVECs from normal (Normal) or gestational diabetes mellitus (GDM) pregnancies incubated in the absence (−) or presence (+) insulin (1 nM, 8 h) and/or A1 adenosine receptor (A1AR) antagonist (Antag). Lower panel, hCAT-1/β-actin ratio densitometries normalized to 1 in cells from normal pregnancies in the absence of insulin or the antagonist. b hCAT-1 protein abundance as in a for A2AAR antagonist. c hCAT-1 mRNA expression as in a for A1AR antagonist. d hCAT-1 mRNA expression as in b for A2AAR antagonist. e Luciferase (Luc) reporter constructs containing two truncations of SLC7A1 promoter (−1606 and −650 bp from the transcription start point) were transfected in HUVECs from normal or GDM pregnancies, along with Renilla reporter plasmid, and assayed for Firefly and Renilla luciferase activity, respectively. Results depict ratio of Firefly/Renilla luciferase activity. After 36 h of transfection, cells were incubated for further 8 h without (Without insulin) or with (With insulin) insulin (1 nM) in the absence (Control) or presence of A1AR or A2AAR antagonists. Cells were also transfected with the empty pGL3-basic vector or pGL3-control vector (SV40 pGL3) as negative or positive controls, respectively (see “Materials and methods”). In a and c, *P < 0.05 vs. corresponding values in Normal. †P < 0.05 vs. values in the absence of insulin or A1AR antagonist. ‡P < 0.05 vs. all other corresponding values. In b and d, *P < 0.05 vs. corresponding values in Normal. †P < 0.05 vs. all other corresponding values. In e, *P < 0.05 vs. vs. all other values except between themselves in the corresponding promoter constructs. Values are mean ± SEM (n = 38)
SLC7A1 promoter activity
Reporter luciferase activity in cells from normal pregnancies transfected with pGL3–hCAT-1−1606 or pGL3–hCAT-1−650 constructs was lower compared with GDM in the absence of insulin (Fig. 2e). Insulin increased the reporter activity for both constructs in normal pregnancies. Insulin effect was unaltered by the A1AR antagonist, but blocked by the A2AAR antagonist in cells transfected with the pGL3–hCAT-1−1606, but not with the pGL3–hCAT-1−650 construct. In the absence of insulin, GDM-increased reporter activity for both constructs was reversed only by the A2AAR antagonist. However, insulin reversed the GDM increase in the reporter activity for both constructs, an effect that was abolished by the A1AR antagonist and unaltered by the A2AAR antagonist.
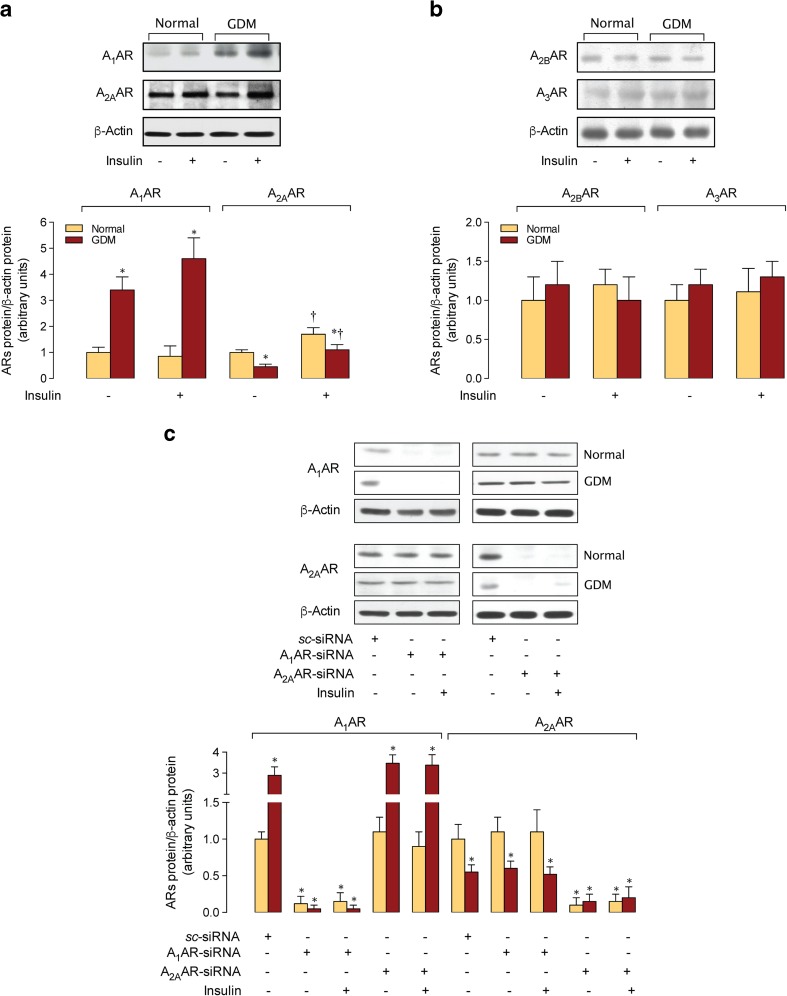
Adenosine receptors expression
A1AR protein abundance was higher in cells from GDM compared with normal pregnancies and was unaltered by insulin in both cell types (Fig. 3a). On the contrary, A2AAR was lower in cells from GDM compared with normal pregnancies, and insulin increased this ARs protein abundance in both cell types. However, A2BAR or A3AR protein abundance was unaltered by insulin in cells from normal or GDM pregnancies.
Fig. 3.
Adenosine receptors expression. a Western blot for A1AR (37 kDa), A2AAR (45 kDa), A2BAR (37 kDa), and A3AR (55 kDa) protein abundance in HUVECs from normal (Normal) or gestational diabetes mellitus (GDM) pregnancies incubated in the absence (−) or presence (+) of insulin (1 nM, 8 h). Lower panel, Adenosine receptors (ARs)/β-actin ratio densitometries normalized to 1 in cells from normal pregnancies in the absence of insulin. b Western blot for A1AR and A2AAR protein abundance in HUVECs as in A in non-transfected (−) or transfected (+) cells with siRNA against A1AR (A1AR-siRNA) or A2AAR (A2AAR-siRNA) (see “Materials and methods”). Scramble siRNA (sc-siRNA) was used as control. Lower panel, A1AR/β-actin or A2AAR/β-actin ratio densitometries normalized to 1 in cells transfected with sc-siRNA from normal or GDM pregnancies in the absence of insulin. In a, *P < 0.05 vs. corresponding values in Normal, †P < 0.05 vs. corresponding values in the absence of insulin in A2AAR. In b, *P < 0.05 vs. all other corresponding values in sc-siRNA. Values are mean ± SEM (n = 29)
Adenosine receptors suppression and L-arginine transport
Protein abundance for A1AR and A2AAR was abolished in KDA1AR and KDA2AAR cells, respectively, from normal or GDM pregnancies (Fig. 3b). Insulin increase in Vmax, Vmax/Km, and vi for L-arginine transport was unaltered in KDA1AR, but blocked in KDA2AAR cells from normal pregnancies (Table 3). Changes in Vmax/Km caused by insulin in normal or GDM pregnancies were paralleled by similar changes in hCAT-1 protein abundance in KDA1AR (Fig. 4a) or KDA2AAR (Fig. 4b) cells. In addition, insulin effect in hCAT-1 mRNA expression (Fig. 4c) was similar to hCAT-1 protein abundance and activity in both cell types.
Table 3.
Involvement of A1AR and A2AAR on L-arginine transport in HUVECs
| V max (pmol/μg protein/min) | K m (μM) | V max/K m (pmol/μg protein/min/(μM)) | |
|---|---|---|---|
| Normal pregnancies | |||
| Without insulin | |||
| Control | 1.3 ± 0.3 | 91 ± 18 | 0.0142 ± 0.0030 |
| KDA1AR | 1.1 ± 0.3 | 99 ± 19 | 0.0111 ± 0.0026 |
| KDA2AAR | 1.1 ± 0.2 | 97 ± 32 | 0.0113 ± 0.0028 |
| With insulin | |||
| Control | 2.6 ± 0.2* | 99 ± 24 | 0.0263 ± 0.0042* |
| KDA1AR | 2.5 ± 0.3* | 92 ± 22 | 0.0272 ± 0.0049* |
| KDA2AAR | 1.2 ± 0.2† | 89 ± 27 | 0.0135 ± 0.0032† |
| GDM pregnancies | |||
| Without insulin | |||
| Control | 3.9 ± 0.4* | 97 ± 25 | 0.0402 ± 0.0053* |
| KDA1AR | 3.4 ± 0.3* | 93 ± 23 | 0.0366 ± 0.0083* |
| KDA2AAR | 1.4 ± 0.4† | 87 ± 35 | 0.0161 ± 0.0090† |
| With insulin | |||
| Control | 1.5 ± 0.4 | 88 ± 29 | 0.0170 ± 0.0051 |
| KDA1AR | 4.2 ± 0.6*†‡ | 83 ± 27 | 0.0506 ± 0.0118*†‡ |
| KDA2AAR | 1.1 ± 0.3 | 97 ± 26 | 0.0113 ± 0.0031 |
Data are presented as mean ± SEM (n = 29). L-Arginine transport (0–1000 μM, 1 min, 37 °C) was measured in HUVECs from normal (Normal pregnancies) or gestational diabetes mellitus (GDM pregnancies) pregnancies exposed (8 h) to culture medium without (Without insulin) or with (With insulin) insulin (1 nM). Transport assays were done in cells expressing A1AR and A2AAR (Control), cells knockdown for A1AR (KDA1AR) or for A2AAR (KDA2AAR) as described in “Materials and methods.” Maximal velocity (V max) and apparent Michaelis-Menten constant (K m) of saturable transport were calculated assuming a single Michaelis-Menten hyperbola. V max/K m represents maximal L-arginine transport capacity
*P < 0.05 versus corresponding values in Normal pregnancies Without insulin; †P < 0.05 versus corresponding Control; ‡P < 0.03 versus corresponding values in KDA2AAR
Fig. 4.
GDM and insulin effect on hCAT-1 expression in A1AR and A2AAR knockdown cells. a Western blot for hCAT-1 protein abundance in HUVECs in the absence (−) or presence (+) of insulin (1 nM, 8 h) in non-transfected (−) or transfected (+) cells with siRNA against A1AR (KDA1AR). Lower panel, hCAT-1/β-actin ratio densitometries normalized to 1 in cells transfected with sc-siRNA from normal or GDM pregnancies in the absence of insulin. b Western blot for hCAT-1 protein abundance with siRNA against A2AAR (KDA2AAR) as in A. c hCAT-1 mRNA expression in KDA1AR or KDA2AAR cells as in A. d Luciferase (Luc) reporter construct pGL3-hCAT-1−1606 of SLC7A1 promoter transfected in KDA1AR or KDA2AAR cells, along with Renilla reporter plasmid. After 36 h of transfection, cells were incubated without (−) or with (+) insulin (1 nM, 8 h) (see “Materials and methods”). e Luciferase (Luc) reporter construct SLC7A1 promoter transfected in KDA1AR or KDA2AAR cells as in d. In a, *P < 0.05 vs. all other values except between themselves, †P < 0.05 vs. all other corresponding values in GDM. In b, *P < 0.05 vs. all other values. †P < 0.05 vs. all other values except between themselves. In c–e, *P < 0.05 vs. all other values in Normal except between themselves. †P < 0.05 vs. all other values in GDM except between themselves. ‡P < 0.05 vs. corresponding values in Normal. Values are mean ± SEM (n = 29)
Insulin increase in pGL3–hCAT-1−1606 construct reporter activity was abolished in KDA2AAR, but unaltered in KDA1AR cells from normal pregnancies (Fig. 4d). However, insulin increase in pGL3–hCAT-1−650 construct reporter activity was unaltered in KDA2AAR or KDA1AR cells from normal pregnancies. Insulin reversed GDM-increase in pGL3–hCAT-1−1606 or pGL3–hCAT-1−650 constructs reporter activity, an effect blocked in KDA1AR, but unaltered in KDA2AAR cells. However, in the absence of insulin GDM-increased constructs, reporter activity was unaltered in KDA2AAR or KDA1AR cells.
Discussion
This study shows that GDM-associated increase in L-arginine transport is mediated by hCAT-1 in HUVECs and is reversed by insulin to values in cells from normal pregnancies via a mechanism requiring expression and activity of A1AR. This effect of insulin includes reestablishment of hCAT-1 maximal transport capacity (Vmax/Km), and protein abundance and mRNA expression. Insulin restored SLC7A1 (for hCAT-1) gene expression, likely due to activation of a promoter region located between −650 bp from the transcription start point of this gene. In the absence of insulin, GDM-increased hCAT-1 expression and activity depends on A2AAR expression. Thus, GDM effect on hCAT-1 expression and activity results from differential activation of A1AR in the presence, but A2AAR in the absence of insulin in HUVECs.
L-Arginine transport in GDM
L-Arginine transport is mediated by more than one transport system in HUVECs, from where the very-high affinity system y+L (Km ~1 μM), and the system y+ family members hCAT-1 (Km ~100 μM) and hCAT-2B (Km ~250 μM) [6, 14, 15, 18] play crucial roles. In HUVECs from GDM, the overall L-arginine transport was semisaturable with a non-saturable L-arginine transport component (KD) detected from L-arginine concentrations >250 μM, suggesting that L-arginine transport was likely mediated by at least two membrane transport mechanisms in this cell type. The possibility that hCAT-1 mediates L-arginine transport is likely since the apparent Km value for saturable transport is within the range of this transport system in HUVECs [6, 18] and other cell types [14, 15, 19, 20]. In addition, L-arginine transport was trans-stimulated by ~7-fold only when a concentration of L-arginine (100 μM in this study) was close to the Km for hCAT-1. Since trans-stimulation of hCAT-1-mediated L-arginine transport by L-lysine is known to be higher (~5–10 fold) than hCAT-2B-mediated transport (~2–3 fold) [14, 15, 19–21], involvement of hCAT-2B transport activity in HUVECs from GDM pregnancies is unlikely.
The increased Vmax/Km for saturable transport in cells from GDM was paralleled by similar changes in hCAT-1 protein abundance. Thus, increased transport could result from higher bioavailability of this type of membrane transporters in HUVECs from GDM pregnancies. This phenomenon could be due to higher SLC7A1 expression since the promoter activity for the region from −1606 bp to the transcription start point for this gene and the hCAT-1 mRNA expression were increased in a similar proportion (~2.6-fold) to the increase detected for Vmax/Km and hCAT-1 protein abundance in cells from GDM pregnancies.
Adenosine receptors involvement on insulin effect
We previously reported that insulin increased the Vmax/Km for L-arginine transport and hCAT-1 expression in HUVECs from normal pregnancies [6]. In the present study, insulin reverses the GDM-associated increase in Vmax/Km and hCAT-1 expression in HUVECs. This apparent contradictory effect of insulin on L-arginine transport agrees with previous reports showing a similar dual effect of this hormone on membrane transport mechanisms, including the human equilibrative nucleoside transporters 1 (hENT1) and hENT2 for adenosine uptake in HUVECs [8, 12] and human placental microvascular endothelial cells (hPMECs) [22] from normal or GDM pregnancies. Thus, a dual effect of insulin is not restricted to L-arginine transport via hCATs. Interestingly, insulin did not alter the KD in cells from normal or GDM pregnancies, suggesting that a parallel plasma membrane transport mechanism to hCAT-1 is either unaltered or minimally altered reaching a negligible influence on overall transport by this hormone. The latter is supported by previous findings showing that insulin did not alter the protein abundance of hCAT-2A/B (i.e., low affinity, high capacity hCAT-2A and high affinity, low capacity hCAT-2B) in HUVECs from normal pregnancies [6]. Additionally, it is reported that insulin increases CAT-1, but not CAT-2B mRNA level and CAT-1-mediated L-arginine transport in rat neonatal ventricular myocites [23]. Thus, a selective phenomenon regarding modulation of hCAT1 and hCAT-2B by insulin is likely in human placenta endothelium.
Protein abundance for A1AR is higher, but A2AAR is lower in cells from GDM compared with normal pregnancies in the absence of insulin, suggesting that these two ARs subtypes play a role in HUVECs under this pathological condition. Since GDM-increased Vmax/Km for transport was blocked by an A2AAR antagonist in the absence of insulin, this ARs subtype could be acting as hCAT-1 activator. Extracellular level of adenosine in primary cultures of HUVECs [4, 24], hPMECs [22], and in the umbilical vein blood [6, 8, 22, 25] from GDM pregnancies is higher (~1 μM) than in normal pregnancies (~0.4 μM). Since A2AAR affinity for adenosine (~0.3 μM) [10, 26] is within this range of the adenosine concentration detected in GDM, an activated state of these receptors is expected. However, we cannot rule out the possibility that the increase in the extracellular concentration of adenosine could lead to adenosine-reduced expression of A2AAR in HUVECs from GDM. Additionally, as a potential limitation of our study, it is feasible that adenosine release from HUVECs after 1 min of incubation (time used for transport assay measurements), even in the presence of adenosine deaminase 1, was enough to saturate A1AR and A2AR and cause a basal increase in L-arginine transport in this cell type. Since HUVECs from GDM pregnancies are shown to express hENT1 and hENT2 accounting for equilibrative adenosine transport [8, 12], and because hENT1 expression and activity is downregulated in HUVECs from GDM [8, 12], we speculate on the possibility that a potential efflux of adenosine via hENT2 from HUVECs is likely. Even when the affinity of A1AR and A3AR for adenosine are ~0.1 and ~0.3 μM, respectively [10], agonists or antagonists for these ARs subtypes did not alter GDM-effect on transport, suggesting that their involvement in this phenomenon is unlikely.
Insulin reverses GDM-associated increase in L-arginine transport most likely via a mechanism that requires A1AR since an antagonist for A1AR, but not other ARs antagonists, blocked insulin effect. Thus, a change in the phenotype in HUVECs from GDM caused by insulin regarding these receptor subtypes is plausible. The latter could result from a change in the metabolic state of this type of cells. Supporting this possibility is the recently proposed change caused by insulin from a preferential mitogenic phenotype (i.e., activated p44/44mapk/activated Akt ratio >1) to a preferential metabolic phenotype (i.e., activated p44/44mapk/activated Akt ratio <1) in HUVECs from GDM pregnancies [8]. Since A1AR activation leads to p44/42mapk and Akt-mediated signaling, but A2AAR activation leads to p44/42mapk signaling [10, 26], a preferential A1AR-associated, Akt-dependent metabolic phenotype could be caused by insulin in HUVECs from GDM. This could be a phenomenon potentiated in cells from GDM pregnancies since a lower A2AAR protein abundance is detected in this cell type, thus further reducing the possibility of an A2AAR-associated, p44/42mapk-dependent mitogenic phenotype. Interestingly, insulin increases the A2AAR protein abundance in both cell types, which could be interpreted as a more preferential mitogenic phenotype. However, considering that this hormone did not alter A1AR protein abundance in cells from normal pregnancies, but increased its protein abundance in GDM, a predominant A2AAR-associated, normal-like phenotype (A2AAR/A1AR ratio ~1.7) induced by insulin is seen in cells from normal pregnancies. Since A2AAR/A1AR ratio was ~1.3 in cells from GDM, a minor role of A2AAR with a major role of A1AR in the response to insulin in HUVECs is likely.
Since suppression of A1AR expression (KDA1AR cells), but not in KDA2AAR cells, abolished the changes caused by insulin in GDM, A1AR role on insulin effects is directly supported. Interestingly, in the absence of insulin, the GDM effect on hCAT-1 expression and activity was reversed only in KDA2AAR cells, complementing the results obtained with the use of an A2AAR antagonist in cells expressing this ARs subtype, or when an A2AAR agonist was used in KDA2AAR cells. Insulin effect on L-arginine transport in GDM depends on an A1AR-dependent activation of SLC7A1 expression at the promoter region −650 bp from the transcription start point. Previous studies show that this region of the SLC7A1 promoter contains at least four consensus sites between −177 and −105 bp from the transcription start point for the general transcription factor specific protein 1 (Sp1), and that Sp1 binding to these sites is increased by insulin in cells from normal pregnancies [18]. Thus, Sp1 as well as other transcription factors associated with the control of expression of membrane transporters families, such as C/EBP homologous protein 10 (CHOP) binding to SLC7A1 in C6 rat glioma cells [27], could be responsible of insulin modulation of hCAT-1 expression in cells from GDM pregnancies. There is no information addressing a role for Sp1 as a cell signaling mechanism triggered by activation of ARs [10, 11, 26]. Thus, we do not rule out the possibility that biological effects of insulin could require adenosine acting via A1AR as a helper or positive regulator via Sp1 activation in HUVECs from GDM pregnancies. Since umbilical blood insulin level in GDM pregnancies is higher (~1.9-fold) compared with normal pregnancies, and because a larger concentration of insulin was required to reverse GDM increase in L-arginine transport in vitro, the observed increase in the plasma insulin at the fetal circulation may be not enough to restore GDM-associated alterations in the human fetoplacental circulation. The latter could also result from fetal insulin resistance as suggested by an elevated HOMA-IR value (~2.2-fold versus normal pregnancies) detected in this group of newborns, agreeing with previous reports in GDM [8, 22, 28].
In summary, GDM is associated with increased L-arginine transport in HUVECs, which results from higher hCAT-1 expression and activity. Insulin reverses GDM-associated effects on transport requiring activation and higher expression of A1AR in this cell type. In the absence of insulin, GDM effect on hCAT-1 expression and activity is dependent on A2AAR expression and activation (see Fig. 5). A simple interpretation of our results is that insulin increases adenosine extracellular concentration in cells from normal pregnancies leading to predominant activation of A2AAR to mediate insulin increase in L-arginine transport. However, cells from GDM pregnancy show induction of A1AR with a predominant response resulting from activation of this type of receptor to mediate insulin decrease in L-arginine transport. Thus, a differential modulatory effect of insulin is played by adenosine receptors in endothelial cells from the macrovasculature in the human placenta from GDM. Since this GDM-associated fetal hyperinsulinemia is a condition that seems not to be enough to restore umbilical vein endothelial function, and because a state of less sensitivity to insulin in GDM fetoplacental vasculature is feasible, a higher concentration of insulin could be required to actually see a beneficial effect of this hormone. Insulin therapy is a protocol applied in pregnant women with GDM that are not responsive to diet and/or exercise to normalize their glycemia. However, this approach associates with a risk of the offspring to develop adulthood diseases such as diabetes mellitus type 2 [29, 30] and of the mother to course with GDM in a future pregnancy [31]. Thus, we emphasize the need of a therapeutical approach considering the potential metabolic modulation of circulating adenosine level and/or adenosine receptors activation/inactivation complementing insulin therapy protocol in pregnant women with GDM to restore fetoplacental endothelial dysfunction for the benefit of the mother and the newborn [3].
Fig. 5.
Proposed involvement of A1AR on insulin-reversal of GDM increased L-arginine transport in human umbilical vein endothelial cells. In the presence of an extracellular level of insulin similar to that detected in the human umbilical vein blood in gestational diabetes mellitus (GDM) (0.07 nM insulin (see Table 1)), the human cationic amino acid transporter 1 (hCAT-1) protein abundance and availability at the plasma membrane of human umbilical vein endothelial cells (HUVECs) result in an increase ( ) in transport of L-arginine (orange arrow) compared with cells from normal pregnancies. This phenomenon is maintained by a higher expression of the SLC7A1 gene for hCAT-1 leading to higher hCAT-1 mRNA expression and protein abundance. GDM effect is caused by adenosine activation of A2AAR, but not A1AR leading to increased activity of transcription factors (TFs ?) on the −650 bp from the transcription start point fragment of the SLC7A1 promoter under this environmental condition. Insulin activation of insulin receptors (IRs) does not contributes to the modulation of expression and activity of hCAT-1 in this cell type. Higher extracellular insulin concentration (1 nM) reverses (
) in transport of L-arginine (orange arrow) compared with cells from normal pregnancies. This phenomenon is maintained by a higher expression of the SLC7A1 gene for hCAT-1 leading to higher hCAT-1 mRNA expression and protein abundance. GDM effect is caused by adenosine activation of A2AAR, but not A1AR leading to increased activity of transcription factors (TFs ?) on the −650 bp from the transcription start point fragment of the SLC7A1 promoter under this environmental condition. Insulin activation of insulin receptors (IRs) does not contributes to the modulation of expression and activity of hCAT-1 in this cell type. Higher extracellular insulin concentration (1 nM) reverses ( ) GDM-associated changes in hCAT-1 expression (SLC7A1 promoter activity, mRNA and protein abundance) and activity (narrow orange arrow) to values in cells from normal pregnancies. Insulin biological effect requires activation of A1AR, but not A2AAR, with this subtype of adenosine receptors acting as helper or positive regulator of insulin biological actions in HUVECs from GDM pregnancies
) GDM-associated changes in hCAT-1 expression (SLC7A1 promoter activity, mRNA and protein abundance) and activity (narrow orange arrow) to values in cells from normal pregnancies. Insulin biological effect requires activation of A1AR, but not A2AAR, with this subtype of adenosine receptors acting as helper or positive regulator of insulin biological actions in HUVECs from GDM pregnancies
Acknowledgments
Authors thank Mrs Amparo Pacheco and Mrs Ninoska Muñoz from CMPL, Pontificia Universidad Católica de Chile, for excellent technical and secretarial assistance, respectively.
Abbreviations
- GDM
Gestational diabetes mellitus
- NO
Nitric oxide
- eNOS
Endothelial NO synthase
- HUVECs
Human umbilical vein endothelial cells
- hPMECs
Human placental microvascular endothelial cells
- ARs
Adenosine receptors
- A1AR
A1 adenosine receptors
- A2AAR
A2A adenosine receptors
- A2BAR
A2B adenosine receptors
- A3AR
A3 adenosine receptors
- hCAT-1
Human cationic amino acid transporter 1
- hENT1
Human equilibrative nucleoside transporters 1
- siRNA
Short interference RNAs
- KDA1AR
A1AR knockdown cells
- KDA2AAR
A2AAR knockdown cells
- CHOP
C/EBP homologous protein 10.
Compliance with ethical standards
Funding
This works was supported by Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT 1150377, 1150344, and 11150083), Chile.
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, Hod M, Kitzmiller JL, Kjos SL, Oats JN, Pettitt DJ, Sacks DA, Zoupas C. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30:251–260. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 2.Colomiere M, Permezel M, Riley C, Desoye G, Lappas M. Defective insulin signaling in placenta from pregnancies complicated by gestational diabetes mellitus. Eur J Endocrinol. 2009;160:567–578. doi: 10.1530/EJE-09-0031. [DOI] [PubMed] [Google Scholar]
- 3.Sobrevia L, Salsoso R, Sáez T, Sanhueza C, Pardo F, Leiva A. Insulin therapy and fetoplacental vascular function in gestational diabetes mellitus. Exp Physiol. 2015;100:231–238. doi: 10.1113/expphysiol.2014.082743. [DOI] [PubMed] [Google Scholar]
- 4.Vásquez G, Sanhueza F, Vásquez R, González M, San Martín R, Casanello P, Sobrevia L. Role of adenosine transport in gestational diabetes-induced L-arginine transport and nitric oxide synthesis in human umbilical vein endothelium. J Physiol. 2004;560:111–122. doi: 10.1113/jphysiol.2004.068288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calabrò RS, Gervasi G, Bramanti P. L-Arginine and vascular diseases: lights and pitfalls! Acta Biomed. 2014;85:222–228. [PubMed] [Google Scholar]
- 6.Guzmán-Gutiérrez E, Westermeier F, Salomón C, González M, Pardo F, Leiva A, Sobrevia L. Insulin-increased L-arginine transport requires A2A adenosine receptors activation in human umbilical vein endothelium. PLoS One. 2012;7:e41705. doi: 10.1371/journal.pone.0041705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Read MA, Boura AL, Walters WA. Vascular actions of purines in the foetal circulation of the human placenta. Br J Pharmacol. 1993;110:454–460. doi: 10.1111/j.1476-5381.1993.tb13832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westermeier F, Salomón C, Farías M, Arroyo P, Fuenzalida B, Sáez T, Salsoso R, Sanhueza C, Guzmán-Gutiérrez E, Pardo F, Leiva A, Sobrevia L. Insulin requires normal expression and signalling of insulin receptor A to reverse gestational diabetes-reduced adenosine transport in human umbilical vein endothelium. FASEB J. 2015;29:37–49. doi: 10.1096/fj.14-254219. [DOI] [PubMed] [Google Scholar]
- 9.Eltzschig HK. Adenosine: an old drug newly discovered. Anesthesiology. 2009;111:904–915. doi: 10.1097/ALN.0b013e3181b060f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fredholm BB. Adenosine—a physiological or pathophysiological agent? J Mol Med (Berl) 2014;92:201–206. doi: 10.1007/s00109-013-1101-6. [DOI] [PubMed] [Google Scholar]
- 11.Antonioli L, Blandizzi C, Csóka B, Pacher P, Haskó G. Adenosine signalling in diabetes mellitus—pathophysiology and therapeutic considerations. Nat Rev Endocrinol. 2015;11:228–241. doi: 10.1038/nrendo.2015.10. [DOI] [PubMed] [Google Scholar]
- 12.Westermeier F, Salomón C, González M, Puebla C, Guzmán-Gutiérrez E, Cifuentes F, Leiva A, Casanello P, Sobrevia L. Insulin restores gestational diabetes mellitus-reduced adenosine transport involving differential expression of insulin receptor isoforms in human umbilical vein endothelium. Diabetes. 2011;60:1677–1687. doi: 10.2337/db11-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maguire MH, Szabó I, Valkó IE, Finley BE, Bennett TL. Simultaneous measurement of adenosine and hypoxanthine in human umbilical cord plasma using reversed-phase high-performance liquid chromatography with photodiode-array detection and on-line validation of peak purity. J Chromatogr B Biomed Sci Appl. 1998;707:33–41. doi: 10.1016/S0378-4347(97)00581-1. [DOI] [PubMed] [Google Scholar]
- 14.Devés R, Boyd CA. Transporters for cationic amino acids in animal cells: discovery, structure, and function. Physiol Rev. 1998;78:487–545. doi: 10.1152/physrev.1998.78.2.487. [DOI] [PubMed] [Google Scholar]
- 15.Mann GE, Yudilevich DL, Sobrevia L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev. 2003;83:183–252. doi: 10.1152/physrev.00022.2002. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Wang YH, Wu HH, Ding H, Li Y, Wang ZH, Li F, Zhang JP. Changes of insulin resistance and β-cell function in women with gestational diabetes mellitus and normal pregnant women during mid- and late pregnant period: a case–control study. J Obstet Gynaecol Res. 2013;39:647–652. doi: 10.1111/j.1447-0756.2012.02009.x. [DOI] [PubMed] [Google Scholar]
- 18.González M, Gallardo V, Rodríguez N, Salomón C, Westermeier F, Guzmán-Gutiérrez E, Abarzúa F, Leiva A, Casanello P, Sobrevia L. Insulin-stimulated L-arginine transport requires SLC7A1 gene expression and is associated with human umbilical vein relaxation. J Cell Physiol. 2011;262:2916–2924. doi: 10.1002/jcp.22635. [DOI] [PubMed] [Google Scholar]
- 19.Chin-Dusting JP, Willems L, Kaye DM. L-arginine transporters in cardiovascular disease: a novel therapeutic target. Pharmacol Ther. 2007;116:428–436. doi: 10.1016/j.pharmthera.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Rajapakse NW, Johnston T, Kiriazis H, Chin-Dusting J, Du XJ, Kaye DM. Augmented endothelial L-arginine transport ameliorates pressure overload induced cardiac hypertrophy. Exp Physiol. 2015 doi: 10.1113/EP085250. [DOI] [PubMed] [Google Scholar]
- 21.Closs EI, Scheld JS, Sharafi M, Förstermann U. Substrate supply for nitric-oxide synthase in macrophages and endothelial cells: role of cationic amino acid transporters. Mol Pharmacol. 2000;57:68–74. [PubMed] [Google Scholar]
- 22.Salomón C, Westermeier F, Puebla C, Arroyo P, Guzmán-Gutiérrez E, Pardo F, Leiva A, Casanello P, Sobrevia L. Gestational diabetes reduces adenosine transport in human placental microvascular endothelium, an effect reversed by insulin. PLoS One. 2012;7:e40578. doi: 10.1371/journal.pone.0040578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simmons WW, Closs EI, Cunningham JM, Smith TW, Kelly RA. Cytokines and insulin induce cationic amino acid transporter (CAT) expression in cardiac myocytes. Regulation of L-arginine transport and no production by CAT-1, CAT-2A, and CAT-2B. J Biol Chem. 1996;271:11694–11702. doi: 10.1074/jbc.271.20.11694. [DOI] [PubMed] [Google Scholar]
- 24.Ethier MF, Chander V, Dobson JG., Jr Adenosine stimulates proliferation of human endothelial cells in culture. Am J Physiol. 1993;265:H131–H138. doi: 10.1152/ajpheart.1993.265.1.H131. [DOI] [PubMed] [Google Scholar]
- 25.Yoneyama Y, Suzuki S, Sawa R, Yoneyama K, Power GG, Araki T. Increased plasma adenosine concentrations and the severity of preeclampsia. Obstet Gynecol. 2002;100:1266–1270. doi: 10.1016/S0029-7844(02)02247-0. [DOI] [PubMed] [Google Scholar]
- 26.Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—an update. Pharmacol Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang CC, Chiribau CB, Majumder M, Chiang CM, Wek RC, Kelm RJ, Jr, Khalili K, Snider MD, Hatzoglou M. A bifunctional intronic element regulates the expression of the arginine/lysine transporter Cat-1 via mechanisms involving the purine-rich element binding protein A (Pur alpha) J Biol Chem. 2009;284:32312–32320. doi: 10.1074/jbc.M109.024471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q, Huang R, Yu B, Cao F, Wang H, Zhang M, Wang X, Zhang B, Zhou H, Zhu Z. Higher fetal insulin resistance in Chinese pregnant women with gestational diabetes mellitus and correlation with maternal insulin resistance. PLoS One. 2013;8:e59845. doi: 10.1371/journal.pone.0059845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norman RJ, Wang JX, Hague W. Should we continue or stop insulin sensitizing drugs during pregnancy? Curr Opin Obstet Gynecol. 2004;16:245–250. doi: 10.1097/00001703-200406000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Verier-Mine O. Outcomes in women with a history of gestational diabetes. Screening and prevention of type 2 diabetes. Literature review. Diabetes Metab. 2010;36:595–616. doi: 10.1016/j.diabet.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Löbner K, Knopff A, Baumgarten A, Mollenhauer U, Marienfeld S, Garrido-Franco M, Bonifacio E, Ziegler AG. Predictors of postpartum diabetes in women with gestational diabetes mellitus. Diabetes. 2006;55:792–797. doi: 10.2337/diabetes.55.03.06.db05-0746. [DOI] [PubMed] [Google Scholar]