Abstract
Neonatal brain hypoxic ischemia (HI) often results in long-term motor and cognitive impairments. Post-ischemic inflammation greatly effects outcome and adenosine receptor signaling modulates both HI and immune cell function. Here, we investigated the influence of adenosine A1 receptor deficiency (A1R−/−) on key immune cell populations in a neonatal brain HI model. Ten-day-old mice were subjected to HI. Functional outcome was assessed by open locomotion and beam walking test and infarction size evaluated. Flow cytometry was performed on brain-infiltrating cells, and semi-automated analysis of flow cytometric data was applied. A1R−/− mice displayed larger infarctions (+33 %, p < 0.05) and performed worse in beam walking tests (44 % more mistakes, p < 0.05) than wild-type (WT) mice. Myeloid cell activation after injury was enhanced in A1R−/− versus WT brains. Activated B lymphocytes expressing IL-10 infiltrated the brain after HI in WT, but were less activated and did not increase in relative frequency in A1R−/−. Also, A1R−/− B lymphocytes expressed less IL-10 than their WT counterparts, the A1R antagonist DPCPX decreased IL-10 expression whereas the A1R agonist CPA increased it. CD4+ T lymphocytes including FoxP3+ T regulatory cells, were unaffected by genotype, whereas CD8+ T lymphocyte responses were smaller in A1R−/− mice. Using PCA to characterize the immune profile, we could discriminate the A1R−/− and WT genotypes as well as sham operated from HI-subjected animals. We conclude that A1R signaling modulates IL-10 expression by immune cells, influences the activation of these cells in vivo, and affects outcome after HI.
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-015-9482-3) contains supplementary material, which is available to authorized users.
Keywords: Brain hypoxic-ischemia, Neuroimmunomodulation, Neonatology, Adenosine A1 receptor, Cellular immunity, Statistical data interpretation
Introduction
Inflammation greatly affects pathogenesis and outcome after neonatal brain injury [1, 2]. Thus, infections and inflammation may aggravate subsequent hypoxic ischemic (HI) encephalopathy [1]. To study the interplay between key parts of the immune system, we have developed a flow cytometry-based method to investigate immune populations previously suggested to influence outcome after neonatal HI [3] and demonstrated that brain inflammation is sustained for months after HI with involvement of both innate and adaptive immunity [3].
Adenosine is a known regulator of immunity and has substantial effects on other systems involved in brain homeostasis, including vascular reactivity, temperature control, and cell work [4]. Adenosine typically reduces inflammation by Gs-coupled adenosine A2A and A2B receptor signaling that stimulates formation of cAMP [5]. However, adenosine A1 and A3 receptors are coupled to Gi, mediating inhibition of cAMP [5] and thus potentially promoting an opposing effect. A balance between the receptor subtypes dependent on expression pattern and affinity for adenosine is therefore suggested to affect immunological phenotype [4]. It is known that neonatal hypoxic ischemia alters adenosine deaminase levels and A1 receptor levels [6, 7], but the impact of adenosine A1 signaling on immune cell function has not been evaluated in the post ischemic neonatal brain.
Notably, caffeine, a competitive antagonist of adenosine A1, A2A, and A2B receptors, is used in neonates against apnea of prematurity and has become a candidate for neuroprotection [8]. This amplifies the need for increased knowledge about the impact of adenosine receptor signaling in neonatal brain injury to better understand the effects modulatory therapy may have. Therefore, we examined if adenosine A1 receptor deficiency in neonatal brain HI alters brain injury and the subsequent immune response.
Materials and methods
Animals and experimental groups
All experiments were approved by the regional ethics committee, Stockholms norra djurförsöksetiska nämnd, in accordance with local institutional guidelines. Animals were randomized to treatment group (sham or HI), and all evaluations were performed by investigators blinded to treatment and genotype.
Adenosine A1R−/− congenic mice of C57/bl6 background described in detail previously [9] and wild-type (WT) C57/bl6 mice, of either sex, specific pathogen free (SPF) with free access to pelleted food were used. Pups were kept with their mother except during surgery and hypoxia. Animals were euthanized by injection of 240 mg/kg sodium pentobarbiturate i.p. and cardiac perfusion with 12 mL phosphate-buffered saline (PBS) to remove intravascular blood cells.
Hypoxic ischemic brain injury
HI and sham operation was performed at postnatal day 10, n = 47 and n = 21 respectively. We used a modified version of the Vannucci model [10] as described previously [11, 12], with unilateral electrocoagulation at 8 W of the right carotid artery via midline neck incision under isoflurane sedation and local bupivacaine infiltration. Pups rested 1 h with the dam after surgery, prior to 1 h of 10 % O2 in 90 % N2 at 36 °C skin temperature.
Immunohistochemistry
Brains were collected after HI in WT and A1R−/− (n = 47 and n = 21, respectively) at day 24 after behavioral evaluation. Three levels of each brain were collected, corresponding to bregma 1.32, −1.64, and −2.92 mm in the adult mouse brain using cryostat 10–12-μm sections. Tissue sections were fixed for 10 min in 4 % paraformaldehyde. Endogenous peroxidase was blocked by 0.3 % H2O2 in 3 % Normal Horse Serum for 10 min. Microtubule associated protein-2 (MAP-2) staining VECTOR® M.O.M.™ Immunodetection Kit and VECTASTAIN® Elite ABC-Peroxidase Kit were used according to the manufacturer’s specifications. Additional primary antibodies used were B220 (BD Biosciences, 557390; 1:1000) and IL-10 (Abcam, ab9969; 1:1000). The enzymatic coloration of immunoreactivity was performed by simultaneous full immersion in 3,3′-diaminobenzidine, DAB for all slides of the same marker to enable comparison. Imaging was performed with a Nikon eclipse E800 microscope with an Olympus DP70 camera with DP controller and version 3.1.1.267 acquisition software. ImageJ version 1.47t was used to analyze positive staining with color thresholding to select positive staining. IL-10 staining was evaluated for whole brain, whereas co-localization of B220 and IL-10 was investigated in the infarction zone. Percent MAP-2 stained area was manually selected in each hemisphere of brain slice, with investigators blinded to genotype and treatment. Infarction size was measured, and atrophy was calculated as tissue loss excluding the infarction compared to contralateral hemisphere.
Behavioral tests
Behavioral assessments were done at 24 days of age, 2 weeks after HI in WT and A1R−/−n = 47 and n = 21, respectively). Open field test (Kungsbacka Mät- och Reglerteknik AB, Fjärås, Sweden) was used to investigate explorative behavior during 30 min. The animals were taken directly from their home litters and put in the open field box without further handling. In addition, the beam walking test, where mice walk three times back and forth on a beam (10-mm wide, 600-mm long), was performed directly after the open field test. Mice were handled with care to assure a calm explorative state of mind in the animal and the number of slips with the hind limbs recorded in a blinded manner when possible.
Telemetry
The Somedic metabolic system (Somedic; Hörby, Sweden) with implantable Minimitters was used to record VCO2, body temperature, heart rate, simultaneously at 3-min intervals in adult mice WT and A1R−/− (n = 7 and n = 4, respectively). Briefly, the animal was anesthetized with isoflurane, the Minimitter implanted into the peritoneal cavity and the leads sutured in a lead II position across the heart. Analgesia was ensured by local application of 5 % Xylocaine (AstraZeneca, Södertälje, Sweden), and subcutaneous injection of Temgesic (0.3 mg kg−1, Schering-Plough Europe, Brussels, Belgium) after the implantation. Each mouse was allowed at least 7 days of recovery before recording was initiated.
For hypoxic challenge, mice were first allowed to acclimatize in the airtight ventilation chamber with a constant flow of air at 20 °C for 2 h, and after which oxygen concentration was lowered to 10 % and kept constant at this level for 1 h.
FACS analysis
Randomization to sham or HI operation was performed at postnatal day 10 and samples were analyzed at day 17 in sham A1R−/− (n = 9) and WT (n = 6) and in HI A1R−/− (n = 16) and WT (n = 3). For B lymphocyte dynamics in WT, separate samples were analyzed at day 11 (n = 6), day 12 (n = 7), day 17 (n = 6), day 24 (n = 6), day 100 (n = 7), and day 200 (n = 3). The immune activation in WT after HI was equal to that previously reported, and statistical power to detect alterations was calculated from our previous data [3]. Day 17 was chosen to detect both innate and adaptive activation [3]. Gross morphology was assessed with the following scoring system: 1 = no injury, 2 = observable tissue loss, 3 = small plaques, 4 = large plaques, and 5 = cystic lesions, n = 5. Cell preparation was performed as described previously [3]. For antibodies used, see Supplemental Table I. Flow cytometric data was collected on a FACS Aria (BD Biosciences).
Automated gating has been proposed as a solution to inconsistent results of manually analyzed flow cytometry data [13]. Even if there are attempts to standardize lymphocyte gating [14], there is no current standard for tissue resident immune cells since the conformation, ramification, and marker distribution within these cells become substantially altered. Thus, we deployed a data-driven automated gating approach that still allowed us to compare the results with known cell populations described with manual one- or two-dimensional gating in the literature (Supplemental figure II).
Flow cytometry analysis was made using FlowCore package for R [15]. Obvious debris in brain was removed using a polygon gate in forward and side scatter. This filter was manually set to exclude maximal values outside the cytometer’s range and events smaller than the lymphocyte cloud. Thus, all events of a size congruent with intact cells were included, since cell size is substantially more varied in the brain than in the spleen or lymph nodes. A curvfilter was used to filter out immune cell populations, and not a normal distribution filter, as infiltrating populations not necessarily fulfill the assumption of normality. Dead cells were excluded using DAPI stain. Since the brain in an unchallenged state is a fairly immune-privileged site [16], there were often no clear positive populations in controls, why the negative population was selected as reference and cells with higher expression than that were regarded as positive cells. Median fluorescent intensities of the selected populations were used to discriminate the positive and negative populations. The same filter settings were used for each marker in all brain samples. After automated batching, all gate settings were visually confirmed and no manual adjustments were required. 2.5 × 10 [6] events were collected from each brain hemisphere. The temporal activation of B220+ B lymphocytes experiments were done separately and analyzed as described previously [3].
To investigate IL-10 production in brain-infiltrating leukocytes after HI (n = 5), cell suspensions from homogenized brain were prepared as described above. EasySep™ Mouse FITC Positive Selection Kit was used according to the manufacturer’s specifications to purify CD45+ leukocytes. To stimulate IL-10 production, cells were incubated 5 h in complete RPMI (10 % FCS, 1 % glutamine, 1 % PeSt, 50 μM b-ME) culture medium containing PMA (50 ng/ml, final concentration), ionomycin (500 ng/ml final), LPS (10 mg/ml final), and monensin (2 mM final) [17]. LIVE/DEAD Blue VD (Life Technologies) and Fc-block (1:100) (Biolegend) was used to exclude dead cells and block unspecific binding. Please see Supplemental figure 1 for antibodies used. After staining of surface antigens BD Cytofix/Cytoperm (BD Biosciences) was used to fixate and permeabilize cells for IL-10 detection. Data was acquired on an LSR FortessaII (BD Biosciences) and analyzed with FlowJo X 10.0.7r2 software.
Splenocyte production of IL-10 was investigated with flow cytometric staining as described above in cells from WT (n = 3) and A1R−/− (n = 3) spleens in the presence or absence of adenosine 66 nM A1R agonist (CPA), 66 nM A1R antagonist and two concentrations 100 nM and 20 nM of the pan adenosine agonist NECA compared to PBS. A balanced D-optimal experimental design with 100 % estimated D-efficiency was used and analyzed with linear regression in R version 3.2.2 (2015-08-14) package ggplot2 (1.0.1).
Statistical analysis
Mann-Whitney U test was used to analyze data on morphology and behavior data. ANOVA with Neuman-Keuls post hoc test when appropriate, was used to investigate key immune cell populations previously identified [3]. Chi-square test was used to compare mortality. A principal component analysis (PCA) with the NIPALS algorithm was used when analyzing flow cytometry data. Expectation-maximization (EM) cluster analysis was used to predict genotype and treatment group. Correlation analysis was made with multiple linear regression or linear regression. Statistical calculations were made in StatSoft, Inc. (2011) STATISTICA, version 10 or R.
Results
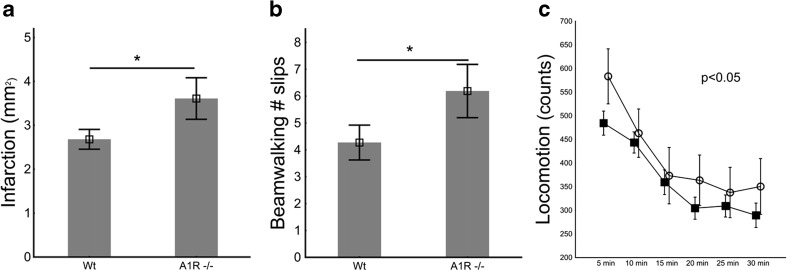
Adenosine A1 receptor-deficient mice (A1R−/−) displayed 44 % larger infarctions 1 week after HI (Fig. 1a) than WT controls. There was a nonsignificant trend towards higher mortality during hypoxia in A1R−/− than WT (22.7 vs. 7.7 %). A1R−/− had significantly increased numbers of slips in the beam walking test and increased locomotion in the open field test (Fig. 1b, c), which correlated with infarction size with a coefficient of 0.52 (p < 0.001) and 0.37 (p < 0.01), respectively. Thus, we concluded that A1R−/− mice suffer from more severe injury after HI.
Fig. 1.
Morphology and behavioral outcome in A1R−/− and WT mice after HI brain injury (n = 21 and n = 47, respectively). a A1R−/− mice had significantly larger infarctions than WT, measured as the area of lost MAP-2 immunohistochemical staining; b increased numbers of slips in beamwalking tests; and c increased locomotion in open field tests (open circles) compared to WT (filled squares). As measured by Mann-Whitney U test (a and b) and ANOVA repeated measurements analysis (c), respectively. Bars represent SE and *p < 0.05
Temperature and heart rate regulation is affected by adenosine A1 receptors [18] and reduction of cell work has been reported in many cell types and organ systems [4]. To assess the impact of A1R deficiency on respiration, temperature, and circulation, we used a telemetry system which recorded temperature, heart rate, and CO2 production during 1 h normoxia and subsequent hypoxia. During this time, no significant differences in those parameters were observed between WT and A1R−/− mice (Supplemental figure 1).
Since adenosine modulates local immune responses and inflammation is a risk factor for adverse outcome after HI [1], we hypothesized that the local A1 receptor-mediated regulation of immune cells may contribute to infarct formation.
Innate immunity
We found a significant increase in the number of CD11b-expressing cells in brains from HI-subjected A1R−/− mice compared to WT (p = 0.01, F = 6.8). Neither CD11c+ antigen presenting cells nor Ly-6G+ granulocyte populations displayed any genotype-dependent changes (data not shown).
Adaptive immunity
B lymphocytes
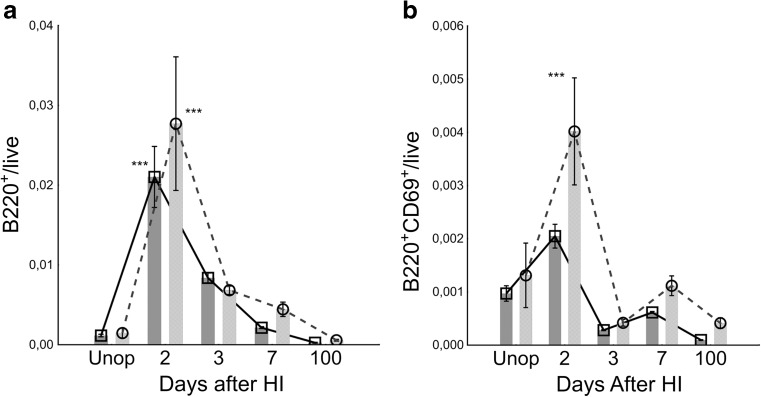
In order to study the kinetics of B lymphocytes, we first investigated WT mice subjected to HI. We found a rapid and highly significant increase of B220+ B lymphocytes infiltrating both the affected and unaffected hemisphere (Fig. 2a). The number returned to baseline 100 days after HI. In parallel, the number of activated B lymphocytes increased as measured by the very early activation antigen, CD69 (Fig. 2b). Notably, the number of CD69-expressing cells significantly increased preferentially in the damaged hemisphere 2 days after HI and the number of activated (CD69+) B lymphocytes decreased over time (Fig. 2b).
Fig. 2.
Temporal B lymphocyte brain infiltration and activation pattern in WT C57/Bl6 mice after HI. a Number of brain-infiltrating B220+ B lymphocytes in the infarcted ipsilateral hemisphere (circles, light grey bars) and undamaged contralateral hemisphere (squares, dark grey bars). Two days after HI, a highly significant influx of B lymphocytes was observed which then declined. b The number of activated CD69+ B lymphocytes increased 2 days after HI. n = 6 day 11, n = 7 day 12, n = 6 day 17, n = 6 day 24, n = 7 day 100, and n = 3 day 200. Error bars indicate SE. Data analysis performed by manual gating. ***p < 0.001
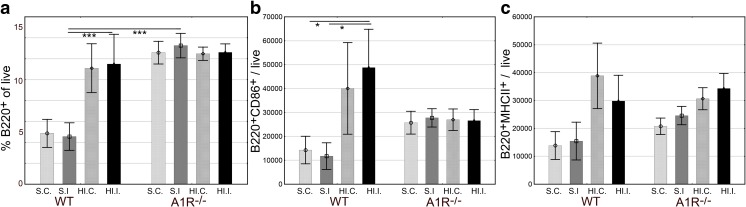
Next, we compared brains from A1R−/− and WT mice subjected to HI or sham procedures. Sham-operated A1R−/− mice displayed significantly increased numbers of brain-infiltrating B220+ B lymphocytes compared to sham-operated WT (Fig. 3a). Surprisingly, the relative number of infiltrating B lymphocytes in A1R−/− mice did not increase as in WT after the HI procedure (Fig. 3a). Upon activation, B lymphocytes upregulate expression of the co-stimulatory molecule CD86, a molecule providing the important signal 2 for T lymphocyte activation [19]. In WT brains, the number of B lymphocytes expressing CD86 increased significantly (Fig. 3b), substantiating the finding of increased CD69+ activation (Fig. 2b). In A1R−/−, however, the frequency of CD86+ B lymphocytes did not increase after HI (Fig. 3b). In contrast, the increase in MHC class II (ANOVA p = 0.002 for treatment), presenting extracellular antigens, after HI was similar in A1R−/− compared to WT controls (Fig. 3c), indicating intact function.
Fig. 3.
Differential B lymphocyte response to brain injury in A1R−/− mice 7 days after HI. S.C. sham contralateral hemisphere, S.I. sham ipsilateral hemisphere, HI.C. HI contralateral hemisphere, HI.I. HI ipsilateral hemisphere. a There was a robust B lymphocyte response in WT animals with increased percentage of live brain-infiltrating cells 1 week after HI. In A1R−/− mice, B lymphocytes failed to respond to the HI insult, although sham-operated animals had a significantly higher B lymphocyte infiltration than WT controls. b The number of CD86+ B lymphocytes increased after HI, however, only in the WT and not in the A1R−/−. c Antigen presenting function of B lymphocytes from A1R−/− mice appeared similar as the HI-induced change in MHC class II expression was comparable to WT (ANOVA p = 0.002 for treatment). Error bars indicate SE. A1R−/− sham-operated group n = 9, A1R−/− HI group n = 16, WT sham-operated group n = 6, WT HI group n = 3. *p < 0.05 and ***p < 0.001
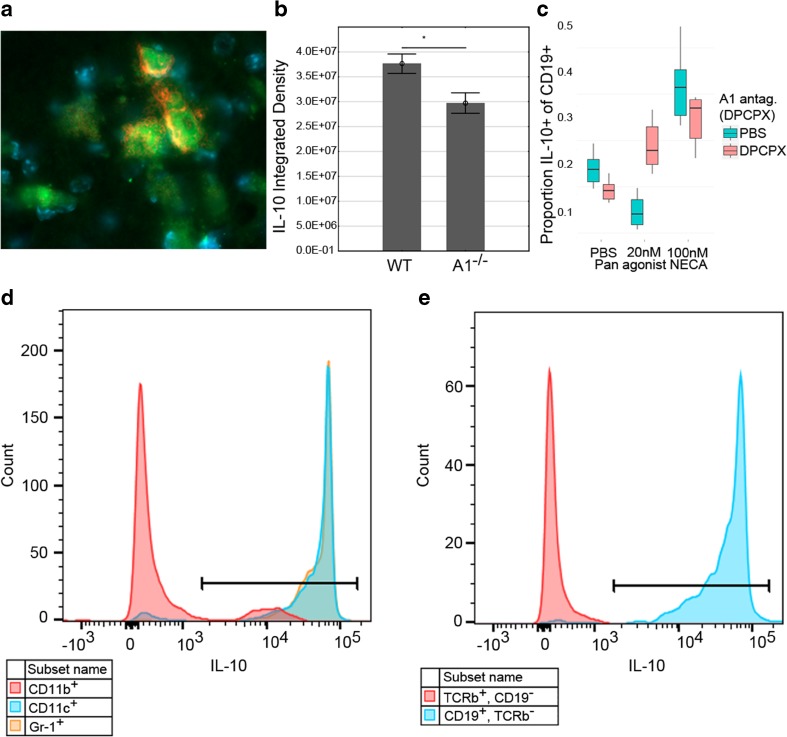
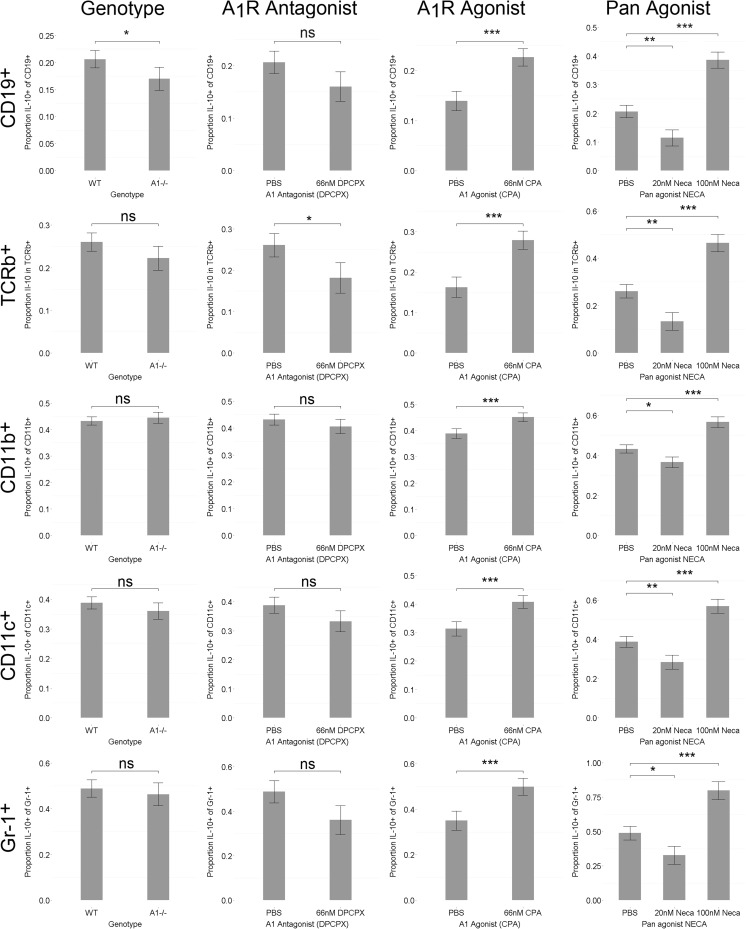
Decreased activation in B lymphocytes might intuitively suggest neuroprotection; however, our findings imply the opposite since A1−/− mice acquire larger lesions, and thus, the influx of B lymphocytes into WT brains could represent a protective B lymphocyte subpopulation. Therefore, we investigated IL-10 production in HI brains, a hallmark cytokine characteristic for regulatory B lymphocytes [20]. One and two weeks after HI, the majority of brain-infiltrating B lymphocytes produced IL-10 (Fig. 4a, e), supporting an anti-inflammatory role of these cells. Up to 97 % of the B lymphocytes expressed IL-10 in brains with high damage score in gross morphology as measured by flow cytometry. The IL-10+ percentage was lower in less-injured animals due to background presence of CD19+IL-10− cells that appeared unaffected by brain injury. On average, 59 % of CD19+ brain-infiltrating cells expressed IL-10 (n = 5). In addition, the majority of brain-infiltrating CD11c+ and Gr-1+ cells (up to 94 and 97 %, respectively) produced IL-10 after HI, whereas the corresponding numbers were 14 and 1 % of CD11b+ and TCRβ+ cells, respectively in the animals with the most severe gross morphology (Fig. 4d, e).
Fig. 4.
IL-10 production in the brain after HI brain injury measured by immunoreactivity. a Brain-infiltrating B lymphocytes from A1R−/− (displayed) and WT mice produced IL-10 coherent with regulatory B lymphocyte phenotype 2 weeks after HI. B220+ B lymphocytes (red), IL-10 (green), and nuclear DAPI staining (blue) 100× magnification. b There was significantly less total IL-10 in brains of A1R −/− than in WT, respectively. n = 21 for A1R −/− and n = 47 for WT. Analyzed with Student’s T test. *p < 0.05 arbitrary units. Error bars indicate SE. c At 20 nM pan adenosine receptor agonist NECA (a concentration similar to physiological adenosine concentrations), IL-10 production decreased in all investigated immune populations. In contrast, at100 nM NECA (comparable to brain adenosine concentration during HI), IL-10 levels increased. The presence of the A1R antagonist DPCPX disrupted the biphasic response, here exemplified as proportion IL-10+ of CD19+ B lymphocytes. Box and whisker plots indicate IQR ± 1.5 IQR. n = 3 for both genotypes used. d Representative flow cytometry data from WT brain of an animal with large infarction after HI show that CD11c+ and GR-1+ cells produced IL-10 (most of them double positive (CD11c+Gr-1+)) while most CD11b+ cells did not. e Similarly, most CD19+ B lymphocytes produced IL-10, whereas almost no IL10+ TCRβ+ T lymphocytes were found
When comparing the overall brain hemisphere IL-10 expression levels by densiometric evaluation of immune-reactivity, brains from A1R−/− mice expressed significantly lower IL-10 levels as compared to WT (Fig. 4b). To functionally test if A1R signaling affects IL-10 production, we stimulated splenocytes from WT and A1R−/− animals to produce IL-10 in the presence or absence of A1R antagonist DPCPX and A1R agonist CPA. Indeed A1R−/− B lymphocytes displayed a significantly decreased proportion IL-10 producing CD19+ B lymphocytes, paralleled by a similar, although not significant (p = 0.11), trend in the presence of the A1R antagonist (Fig. 5). Inversely, the A1R agonist significantly increased the proportion IL-10+ cells not only in B lymphocytes, but in all investigated immune populations (Fig. 5). Furthermore, the unselective adenosine receptor agonist NECA decreased the proportion IL-10+ cells at low (20 nM) concentration while there was an increase at high concentration (100 nM). The 20 nM concentration of NECA was selected to represent basal levels and the 100 nM concentration is within the reported concentrations of the endogenous agonist adenosine after HI [21]. Intriguingly, this biphasic pattern in all IL-10+ cells was disrupted by antagonism of A1R indicating that A1R regulation of IL-10 expression is dependent on agonist concentration (exemplified by CD19+ cells, Fig. 4c).
Fig. 5.
IL-10 production in indicated immune cell populations in WT (n = 3) and in the A1R−/− (n = 3) assessed by flow cytometry after stimulation with PMA, ionomycin, and LPS in the presence or absence of A1R agonist (CPA) or antagonist (DPCPX) and/or the influence of 20 or 100 nM of the pan adenosine receptor agonist NECA with monensin (2 mM) added during the last 5 h of culture. Error bars indicate SE. *p < 0.05, **p < 0.01, ***p < 0.001
T helper and T regulatory lymphocytes
There were no significant alterations in CD4+ T helper cells in the A1R−/− brains compared to WT (Fig. 6a, b). Since A1R−/− mice displayed less IL-10, we investigated if regulatory T lymphocytes were affected by A1R deletion. Brains from A1R−/− mice demonstrated no significant alterations in CD4+FoxP3+ T regulatory cells compared to WT (data not shown). Thus, T regulatory cells cannot explain the altered outcome in the A1R−/−.
Fig. 6.
T lymphocyte response in the brains of A1R−/− and WT mice after HI. S.C. sham-operated contralateral hemisphere, S.I. sham-operated ipsilateral hemisphere, HI.C. HI contralateral hemisphere, HI.I. HI ipsilateral hemisphere. a, b The CD4+ T helper response to brain HI was unaffected by genotype 1 week after the lesion (ANOVA p < 0.01 for treatment). c Cytotoxic CD8+ T lymphocytes in A1R−/− brains were unable to respond to HI in contrast to WT. d The number of CD8+CD69+ brain-infiltrating T lymphocytes was unaffected by HI in the A1R−/−. Error bars indicate SE. A1R−/− sham-operated group n = 9, A1R−/− HI group n = 16, WT sham-operated group n = 6, WT HI group n = 3. *p < 0.05 and ***p < 0.001
Brain-infiltrating cytotoxic CD8+ T lymphocytes (Fig. 6c) and the activation of these cells, as measured by the very early activation antigen CD69 (Fig. 6d), increased significantly in WT animals after HI. To our surprise, the number of CD8+ (Fig. 6c) and CD8+CD69+ (Fig. 6d) T lymphocyte numbers remained unaffected by HI in A1R−/− brains. In summary, the CD4+ T lymphocyte response to HI in A1R−/− mice was largely unaffected, whereas CD8+ T lymphocytes appeared unable to respond to the injury in A1R−/− mice.
Classification of genotype and treatment from immune patterns
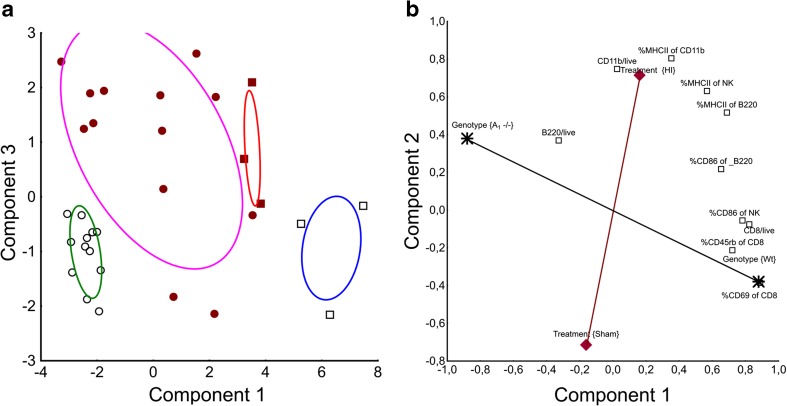
We noted distinct patterns of immune cell activation in A1R−/− and WT. Since many of the factors included in the study are mechanistically entangled, we used a principal component analysis to reduce the dimensionality and discriminate between the main effects, as described previously [22]. Three significant principal components described 69 % of the variance in the damaged and contralateral hemisphere. Under the assumption that the components describes distinct underlying factors, component 1 best described genotype effects and component 3 best described treatment effects since these factors most influenced loadings on each component, respectively (Fig. 7a). Component 2 displayed approximately equal loadings for genotype and treatment. HI and A1R−/− genotype displayed positive loadings on this component whereas sham and WT had negative. Thus, brain immune activation in A1R−/− is more similar to HI than WT, which is coherent with our finding of more severe injury in the A1R−/− animals.
Fig. 7.
Principal component analysis (PCA) of the main factors affecting overall variability in the data. x- and y-axis represent loadings on each component respectively. a When components 1 and 3 were plotted against each other (see “Results” for details), treatment (sham or HI) and genotypes (A1R−/− to WT) could be discriminated, identifying a specific immune profile for each of the four combinations. Expectation-maximization (EM) clustering could correctly classify genotype and treatment from immune cell profile alone in 90 % of the cases; ovals represent 95 % CI for each cluster. Open black circles are sham-operated A1R−/−, filled red circles HI exposed A1R−/−, open black squares sham-operated WT, and filled red squares are HI exposed WT. b To visualize the separate influence of treatment and genotype on major immune populations in the damaged brain, we plotted the two first components from a consecutive PCA including only cell populations significantly affected by genotype, together representing 66 % of the total variation. As the components are uncorrelated, any line passing origo is equally valid as axis, and the black axis (line) from A1R−/− to WT describes the genotype effect. Immune populations that affects outcome due to deletion of A1R would be expected to correlate both with genotype and injury, similar to the %CD86+B220+ population. CD11b expression is mainly dependent on injury size and CD8+ T lymphocytes preferably dependent on genotype as suggested by the PCA. No variables correlated positively with sham operation. A1R−/− sham-operated group n = 9, A1R−/− HI group n = 16, WT sham-operated group n = 6, WT HI group n = 3
In order to illustrate the capability of the model, we attempted to classify genotype and treatment group belonging (sham or HI) from the immune parameters alone. If the immune pattern observed was due to random variation, it is extremely unlikely that genotype and treatment groups could be classified from the immune pattern seen. Thus, failure to group the cases into the correct treatment and genotype groups would falsify the hypothesis of a causative relationship. When plotting individual animals against components 1 and 3, it was possible to accurately distinguish A1R−/− from WT mice (genotype) and treatment (HI or sham; Fig. 7a). To investigate whether these clusters could be mathematically separated, a generalized EM clustering was applied to components 1 and 3. Ninety percent of the cases were classified correctly. Two of the three misclassified cases were HI treated and had negative loading on component 3 similar to sham-operated animals. Not all HI-treated animals obtain brain injury after HI, and those two cases likely have minimal or no damage to the brain. We conclude that the model is adequately able to discriminate between genotype and injury size-induced effects.
Of note, genotype had no influence (loading) on component 3 (Fig. 7a), and therefore, this component was uncorrelated to genotype effects. Instead, variations in injury size within the HI model best describe the variation in component 3, since this is the major source of uncontrolled variation in our dataset. Thus, we exploited this finding and assumed that component 3 could be used as an approximation of injury size. Given this assumption, the fraction of CD86 expressing B220+ B cells in the brain of A1R−/− animals after HI correlated negatively to injury (p = 0.03, r2 = 0.36). For comparison, CD8+ T lymphocytes displayed a nonsignificant trend towards a positive correlation with injury.
To investigate the immune populations in the damaged brain specifically, we performed a second PCA rendering new components (Fig. 7b). To limit noise and increase resolution, we only included immune populations significantly affected by treatment and/or genotype.
The new first two components explained 66 % of the variance. Treatment (sham or HI)-induced effects were related to innate immunity, and genotype effects were related to adaptive immunity such as CD8+ T lymphocytes (Fig. 7b), confirmed by multiple regression (p < 0.001, b* = −0.74 for treatment and p = 0.38, b* = 0.11 for genotype). Activated CD86+B220+ B lymphocytes were related to both HI and genotype (p < 0.001, b* = −0.55 for genotype and p = 0.04, b* = 0.3 for treatment).
Together, the PCA and regression analysis indicate that immune activation profile is influenced by the combination of A1R−/−genotype and HI treatment and not by differences in injury size (between genotypes) alone.
Discussion
The major findings were that A1 receptor deficiency in neonatal mice is associated with larger brain damage after hypoxic ischemia, worse neuromotor outcome, and altered post-ischemic activation of immune cell populations.
Adenosine A1 receptors have profound effects on heart rate, temperature [18], and metabolism [4] under certain conditions. It is also well known that temperature markedly affects the impact of an ischemic insult [23]. Therefore, it was possible that the differences in ischemic injury could be related to differences in body temperature. However, there were no significant alterations in temperature, heart rate, and CO2 production before or during hypoxia in A1R−/− mice.
Previously, an adult global brain ischemia model showed an increased neuronal loss after treatment with A1 receptor antagonist but no alteration in brain injury in the A1R−/− compared to WT mice [24]. In a model of HI in neonatal rats, A1 receptor antagonism during or after neonatal ischemic brain injury did not affect lesion size compared with controls; however, treatment given before HI increased mortality [25]. The apparent inconsistency may be due to differences between the models [26], species, and maturation stages. A1 receptors are known to inhibit excitatory neurotransmission and removing this brake can have negative effects in hypoxia [9]. It is not impossible that such an effect could have contributed to the alteration in outcome and that this in turn has influenced the immune response. However, our statistical analysis strongly suggests that this is not the only explanation and that genotype has a more direct influence on the immune reaction to HI.
There were large differences between genotypes in post-ischemic immune activation. The increase of CD11b+ cells was greater in A1R−/− brains than in WT, and an amplified CD11b+cell response may contribute to the larger injury seen in A1R−/− mice [27, 28].
Few in vitro studies provide direct evidence that A1R signaling critically influences macrophage function while in vivo studies do [4]. Therefore, an indirect mechanism involving other cell types is implied, rather than a direct A1R signaling effect on CD11b+ cells.
We and others have recently shown that the adaptive immune system including T lymphocytes is activated long after a brain injury [3, 29]. Of note, mice without mature B and T lympocytes are protected against brain injury [29]. Reconstitution of B lymphocytes produces a nonsignificant trend towards even less injury [30], whereas reconstitution with CD3+ T lymphocytes restored susceptibility to injury [30], confirming previous reports of detrimental effect of T lymphocytes [31, 32]. Since the kinetics of B lymphocyte activation after HI brain injury was previously unknown, we first investigated signs of activation with regards to expression of the very early activation antigen CD69, the co-stimulatory molecule CD86 and MHC class II. In WT brains, we found the expected increase in CD86 and MHC II expressing B lymphocytes after HI. In contrast, CD86 expression in B lymphocytes in A1R−/− brains did not increase. The low activation was unexpected with regard to the larger brain damage seen in A1R−/− animals. In addition to their antibody-producing function, B lymphocytes are capable to present antigens and activate T lymphocytes [20]. In our model, this function seemed intact in the A1R−/− mice, as measured by normal MHC II expression compared to WT. Recently, B lymphocytes have also been shown to harbor suppressor activities [20], a regulatory subset which interestingly has been shown to be of importance in an experimental stroke model [33].
If the impaired B lymphocyte response seen in A1R−/− brains represented a reduced regulatory B lymphocyte response, this would fit with the increased infarction size observed. Therefore, we co-stained for B lymphocytes and the immunosuppressive cytokine IL-10. Indeed the majority of brain-infiltrating B lymphocytes expressed IL-10, the hallmark effector cytokine of regulatory B lymphocytes [20]. This finding was confirmed by flow cytometry of brain-infiltrating cells where up to 97 % of the B lymphocytes in the damaged hemisphere expressed IL-10. Except B lymphocytes, a large majority of CD11c+ and Gr-1+ cells in the brain after HI also produced IL-10, whereas only a few percent of CD11b+ and TCRβ+ cells stained positive, establishing a differential expression of IL-10 between these cell types. In addition, densitometric evaluation of IL-10 revealed that brains from A1R−/− mice had overall decreased IL-10 levels after HI. Of note, IL-10 has previously been described as a protective cytokine in the neonatal brain [34]. To address whether the observed effects were due to acute A1R signaling, IL-10 production was investigated in WT and A1R−/− in the presence and absence of A1 agonist and antagonist, as well as selected concentrations of the pan adenosine agonist NECA. Significantly less IL-10+ B lymphocytes were found in A1R−/− compared to WT, paralleled by a similar trend in the presence of the A1R antagonist DPCPX. The same pattern was observed in T lymphocytes, while CD11b+, CD11c+, and Gr-1+ cells were unaffected by A1 receptor blockade alone. Interestingly, all investigated populations significantly increased their IL-10 production in the presence of the A1 agonist CPA and 100 nM concentration of the pan adenosine receptor agonist NECA (a concentration set to mimic the local levels of endogenous adenosine seen during HI). At 20 nM NECA (probably giving a stimulation comparable to adenosine under physiological conditions), the opposite response was observed with significantly decreased IL-10 production in all investigated populations, a significant interaction counteracted by addition of A1R antagonist DPCPX. Thus, A1R antagonist-mediated regulation of IL-10 depends on the level of adenosine receptor agonist present.
There was no change in infiltration of CD4+ T lymphocytes and Foxp3+ regulatory T lymphocytes (data not shown) when comparing WT and A1R−/− brains. However, in light of the increased brain damage in A1R−/− mice and the unaffected CD4+ T lymphocyte compartment, we were surprised to find the infiltration of CD8+ cytotoxic T lymphocytes greatly diminished. Since adenosine A1R signaling protects against neuronal excitotoxicity [4], damaged cells in A1R−/− could be shifted towards necrosis rather than delayed apoptosis [35], thus limiting the need for cytotoxic killing of injured cells. Another possibility is that adenosine A1R-mediated inhibition of chemokine/cytokine function may lead to a dysregulated lymphocyte activation and entry into the brain. The fact that CD86 expression on B cells was negatively correlated with the PCA-derived estimate of injury size even within the A1R−/− group supports this notion, rather than solely an impaired function of B regulatory cells per se. One potential candidate able to mediate this effect would be the chemokine fractalkine (CX3CL1) that has been reported to be neuroprotective in an A1R-dependent manner [36, 37]. Interestingly, the fractalkine receptor (CX3CR1) is found on all of the affected immune populations in our study but not in the unaffected populations [38, 39, 40]. Moreover, vascular expression of A1R has been associated with regulation of cell migration and extravasation of leukocytes [4] which potentially also could contribute to the clinical outcome.
Multicolor flow cytometry is a powerful tool; however, traditional gating methodology is subjected to investigator-dependent bias and increased intra- as well as inter-experimental variability [41]. Here, we have utilized techniques from screening (semi-automated data-driven analysis) and data mining (PCA), combined with traditional hypothesis-based methodology to enable multifactorial hypothesis testing. The data-driven probabilistic analysis provided an unbiased method of evaluating larger data sets consistently, and principal component analysis enabled us to identify and discriminate between variables explaining treatment modality and genotype and limit the need for more extensive experiments.
Intriguingly, there was less difference in inflammatory cell activation between WT and A1R−/− after HI than after sham operation. This is in line with earlier work where A1R signaling was proinflammatory at low adenosine concentrations, but with higher concentrations, as seen after HI, A2 receptor-mediated anti-inflammatory effects prevailed [42], thus limiting the impact of A1R deletion. This could at least partly explain why a partial blockade of A1R with the antagonist DPCPX in vivo had no effect on neonatal brain HI [5, 25]. Theoretically, a rescue of some A1R function via adenosine A3 receptor signaling may occur, since A3 receptors are coupled to Gi as well. However, in no instance has A1R deletion been associated with an upregulation of adenosine A3 receptors [43]. Furthermore, a rescue of function would require A3 receptors expressed in sufficient number in the appropriate locations. This distribution, together with A1 receptors, is still largely uncharted since it seems to differ between cell type, model system, location, and functional status of the cells, in agreement with the dynamic nature of G protein-coupled receptors [4, 44]. Hence, an A3R-mediated compensatory effect would be minor, if present at all.
The overall aim of this study was to understand the net immunological effect of A1 receptor deletion, rather than to investigate single immune populations, although this may be a challenge considering the intricacy and coupled interactions of adenosine signaling and immunity. To assess all the underlying factors of the immunological phenotype in our model, we applied PCA, which allows discrimination of factors with covariates. In the setting of our model, we suggest that the current approach using PCA is a new, valuable, and cost-effective method in order to separate experimentally entangled but uncorrelated factors, such as genotype, lesion size, and immune activation.
The PCA suggested that treatment (sham or HI) was correlated to innate immune cells such as CD11b-expressing cells which is in agreement with the previous literature [45]. Genotype correlated better with post-ischemic adaptive immunity, including B and cytotoxic T lymphocytes suggesting that A1R signaling has a greater impact on these populations in our model.
The correlation of diminished B lymphocyte activation and infiltration to greater brain damage in A1R−/− mice was surprising. However, most brain-infiltrating B lymphocytes express IL-10 and A1R−/− animals displayed decreased levels of IL-10 in the brain and have impaired regulatory B lymphocyte function. IL-10 can diminish macrophage activation [46] and may thus represent a link between the dysfunctional regulatory B lymphocytes and the increased activation of CD11b+ myeloid cells seen in A1R−/− mice after HI.
Conclusion
A1R-deficient mice displayed worse outcome and an altered immune response after neonatal brain HI. We present evidence of A1R-mediated dysfunction of regulatory B lymphocytes and indications of impaired cytotoxic T lymphocyte function in A1R−/−. We postulate that the loss in inhibition contributes to the exaggerated activation of myeloid cells and that this in turn leads to increased lesion size and adverse behavioral function.
Electronic supplementary material
A) Registration body temperature during 60 min of normoxia or hypoxia in adult animals uncovered no significant differences between WT and A1R−/− animals. B) Recording of temperature CO2 production and heart rate was equal A1R−/− compared to WT mice. Error bars indicate +/− SE. n = 7 for WT and n = 4 for A1R−/−. (GIF 6208 kb)
Example of data driven gating approach in brain. Gates were visually confirmed to avoid erroneous results due to artifacts, however no corrections were needed. A) A polygon gate was first applied to filter out obvious debris in SSC and FSC. Density curv-filter was used to find DAPI negative cells that were selected as live cells. B) Since there was no obvious positive population in the TCR receptor expression the negative population was selected (blue) and events with greater signal was set as positive cells (green). C) CD4+ and CD8+ and were interpreted as T-helper or cytotoxic T-cells respectively. (GIF 27 kb)
(DOCX 16 kb)
(DOCX 13 kb)
Acknowledgments
Many thanks to Ruth Detlofsson for technical assistance, Eva Lindgren for genotyping of the animals, and Melinda Verriere for animal housing.
Compliance with ethical standards
Sources of funding
The present study was supported by the following grants: Swedish Medical Research Council (2011-3981; 2553), the regional agreement on medical training and clinical research (ALF 20120450) between Stockholm County Council and Karolinska Institutet, Marianne and Marcus Wallenberg Foundation (MMW 2011.0085), and Swedish Brain Foundation FO2013-0073.
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Ola Winqvist and Ulrika Ådén contributed equally to this work.
Contributor Information
Max Winerdal, Phone: +46 70 7712833, Email: max.winerdal@medsci.se.
Malin E. Winerdal, Email: malin.winerdal@ki.se
Ying-Qing Wang, Email: yqwang001@sina.com.
Bertil B. Fredholm, Email: bertil.fredholm@ki.se
Ola Winqvist, Email: ola.winqvist@karolinska.se.
Ulrika Ådén, Email: ulrika.aden@ki.se.
References
- 1.Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 2.Shalak LF, Laptook AR, Jafri HS, Ramilo O, Perlman JM. Clinical chorioamnionitis, elevated cytokines, and brain injury in term infants. Pediatrics. 2002;110:673–680. doi: 10.1542/peds.110.4.673. [DOI] [PubMed] [Google Scholar]
- 3.Winerdal M, Winerdal ME, Kinn J, Urmaliya V, Winqvist O, Aden U. Long lasting local and systemic inflammation after cerebral hypoxic ischemia in newborn mice. PLoS One. 2012;7:e36422. doi: 10.1371/journal.pone.0036422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 5.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International union of pharmacology. Xxv. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 6.Aden U, Lindstrom K, Bona E, Hagberg H, Fredholm BB. Changes in adenosine receptors in the neonatal rat brain following hypoxic ischemia. Brain Res Mol Brain Res. 1994;23:354–358. doi: 10.1016/0169-328X(94)90247-X. [DOI] [PubMed] [Google Scholar]
- 7.Pimentel VC, Moretto MB, Oliveira MC, Zanini D, Sebastiao AM, Schetinger MR. Neuroinflammation after neonatal hypoxia-ischemia is associated with alterations in the purinergic system: adenosine deaminase 1 isoenzyme is the most predominant after insult. Mol Cell Biochem. 2015;403:169–177. doi: 10.1007/s11010-015-2347-9. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357:1893–1902. doi: 10.1056/NEJMoa073679. [DOI] [PubMed] [Google Scholar]
- 9.Johansson B. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine a1 receptor. Proc Natl Acad Sci. 2001;98:9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 11.Sheldon RA, Sedik C, Ferriero DM. Strain-related brain injury in neonatal mice subjected to hypoxia-ischemia. Brain Res. 1998;810:114–122. doi: 10.1016/S0006-8993(98)00892-0. [DOI] [PubMed] [Google Scholar]
- 12.Aden U, Dahlberg V, Fredholm BB, Lai LJ, Chen Z, Bjelke B. MRI evaluation and functional assessment of brain injury after hypoxic ischemia in neonatal mice. Stroke. 2002;33:1405–1410. doi: 10.1161/01.STR.0000014608.78503.DB. [DOI] [PubMed] [Google Scholar]
- 13.Aghaeepour N, Finak G, Hoos H, Mosmann TR, Brinkman R, Gottardo R, et al. Critical assessment of automated flow cytometry data analysis techniques. Nat Methods. 2013;10:228–238. doi: 10.1038/nmeth.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calvelli T, Denny TN, Paxton H, Gelman R, Kagan J. Guideline for flow cytometric immunophenotyping: a report from the national institute of allergy and infectious diseases, division of aids. Cytometry. 1993;14:702–715. doi: 10.1002/cyto.990140703. [DOI] [PubMed] [Google Scholar]
- 15.Hahne F, LeMeur N, Brinkman RR, Ellis B, Haaland P, Sarkar D, et al. Flowcore: a bioconductor package for high throughput flow cytometry. BMC Bioinformatics. 2009;10:106. doi: 10.1186/1471-2105-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bucky Jones T, Lucin KM, Popovich PG (2007) The immune system of the brain. 7:127–144
- 17.Matsushita T, Tedder TF. Identifying regulatory b cells (b10 cells) that produce IL-10 in mice. Methods Mol Biol. 2011;677:99–111. doi: 10.1007/978-1-60761-869-0_7. [DOI] [PubMed] [Google Scholar]
- 18.Yang JN, Chen JF, Fredholm BB. Physiological roles of a1 and a2a adenosine receptors in regulating heart rate, body temperature, and locomotion as revealed using knockout mice and caffeine. Am J Physiol Heart Circ Physiol. 2009;296:H1141–1149. doi: 10.1152/ajpheart.00754.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeannin P, Delneste Y, Lecoanet-Henchoz S, Gauchat JF, Ellis J, Bonnefoy JY. Cd86 (b7-2) on human b cells. A functional role in proliferation and selective differentiation into ige- and igg4-producing cells. J Biol Chem. 1997;272:15613–15619. doi: 10.1074/jbc.272.25.15613. [DOI] [PubMed] [Google Scholar]
- 20.Mauri C, Bosma A. Immune regulatory function of b cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 21.Phillis JW, Walter GA, O'Regan MH, Stair RE. Increases in cerebral cortical perfusate adenosine and inosine concentrations during hypoxia and ischemia. J Cereb Blood Flow Metab. 1987;7:679–686. doi: 10.1038/jcbfm.1987.122. [DOI] [PubMed] [Google Scholar]
- 22.Lugli E, Pinti M, Nasi M, Troiano L, Ferraresi R, Mussi C, et al. Subject classification obtained by cluster analysis and principal component analysis applied to flow cytometric data. Cytometry A. 2007;71:334–344. doi: 10.1002/cyto.a.20387. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P (2007) Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. CD003311 [DOI] [PubMed]
- 24.Olsson T, Cronberg T, Rytter A, Asztely F, Fredholm BB, Smith ML, et al. Deletion of the adenosine a1 receptor gene does not alter neuronal damage following ischaemia in vivo or in vitro. Eur J Neurosci. 2004;20:1197–1204. doi: 10.1111/j.1460-9568.2004.03564.x. [DOI] [PubMed] [Google Scholar]
- 25.Bona E, Aden U, Gilland E, Fredholm BB, Hagberg H. Neonatal cerebral hypoxia-ischemia: the effect of adenosine receptor antagonists. Neuropharmacology. 1997;36:1327–1338. doi: 10.1016/S0028-3908(97)00139-1. [DOI] [PubMed] [Google Scholar]
- 26.Harukuni I, Bhardwaj A. Mechanisms of brain injury after global cerebral ischemia. Neurol Clin. 2006;24:1–21. doi: 10.1016/j.ncl.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Chopp M, Zhang RL, Bodzin G, Chen Q, Rusche JR, et al. Anti-cd11b monoclonal antibody reduces ischemic cell damage after transient focal cerebral ischemia in rat. Ann Neurol. 1994;35:458–463. doi: 10.1002/ana.410350414. [DOI] [PubMed] [Google Scholar]
- 28.Perego C, Fumagalli S, De Simoni MG. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J Neuroinflammation. 2011;8:174. doi: 10.1186/1742-2094-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, et al. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27:1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleinschnitz C, Schwab N, Kraft P, Hagedorn I, Dreykluft A, Schwarz T, et al. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood. 2010;115:3835–3842. doi: 10.1182/blood-2009-10-249078. [DOI] [PubMed] [Google Scholar]
- 31.Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, et al. Pivotal role of cerebral interleukin-17–producing γδT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- 32.Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 33.Offner H, Hurn PD. A novel hypothesis: regulatory B lymphocytes shape outcome from experimental stroke. Transl Stroke Res. 2012;3:324–330. doi: 10.1007/s12975-012-0187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mesples B, Plaisant F, Gressens P. Effects of interleukin-10 on neonatal excitotoxic brain lesions in mice. Brain Res Dev Brain Res. 2003;141:25–32. doi: 10.1016/S0165-3806(02)00636-3. [DOI] [PubMed] [Google Scholar]
- 35.Fatokun AA, Stone TW, Smith RA. Adenosine receptor ligands protect against a combination of apoptotic and necrotic cell death in cerebellar granule neurons. Exp Brain Res. 2008;186:151–160. doi: 10.1007/s00221-007-1218-3. [DOI] [PubMed] [Google Scholar]
- 36.Lauro C, Di Angelantonio S, Cipriani R, Sobrero F, Antonilli L, Brusadin V, et al. Activity of adenosine receptors type 1 is required for cx3cl1-mediated neuroprotection and neuromodulation in hippocampal neurons. J Immunol. 2008;180:7590–7596. doi: 10.4049/jimmunol.180.11.7590. [DOI] [PubMed] [Google Scholar]
- 37.Cipriani R, Villa P, Chece G, Lauro C, Paladini A, Micotti E, et al. Cx3cl1 is neuroprotective in permanent focal cerebral ischemia in rodents. J Neurosci. 2011;31:16327–16335. doi: 10.1523/JNEUROSCI.3611-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishimura M, Umehara H, Nakayama T, Yoneda O, Hieshima K, Kakizaki M, et al. Dual functions of fractalkine/cx3c ligand 1 in trafficking of perforin+/granzyme b+ cytotoxic effector lymphocytes that are defined by cx3cr1 expression. J Immunol. 2002;168:6173–6180. doi: 10.4049/jimmunol.168.12.6173. [DOI] [PubMed] [Google Scholar]
- 39.Corcione A, Ferretti E, Bertolotto M, Fais F, Raffaghello L, Gregorio A, et al. Cx3cr1 is expressed by human b lymphocytes and mediates [corrected] cx3cl1 driven chemotaxis of tonsil centrocytes. PLoS One. 2009;4:e8485. doi: 10.1371/journal.pone.0008485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu P, Li L, Kuno K, Wu Y, Baba T, Li YY, et al. Protective roles of the fractalkine/cx3cl1-cx3cr1 interactions in alkali-induced corneal neovascularization through enhanced antiangiogenic factor expression. J Immunol. 2008;180:4283–4291. doi: 10.4049/jimmunol.180.6.4283. [DOI] [PubMed] [Google Scholar]
- 41.Lugli E, Roederer M, Cossarizza A. Data analysis in flow cytometry: the future just started. Cytometry A. 2010;77:705–713. doi: 10.1002/cyto.a.20901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cronstein BN. Adenosine, an endogenous anti-inflammatory agent. J Appl Physiol. 1994;76:5–13. doi: 10.1152/jappl.1994.76.1.5. [DOI] [PubMed] [Google Scholar]
- 43.Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Muller CE. International union of basic and clinical pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—an update. Pharmacol Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol. 2012;71:444–457. doi: 10.1002/ana.22620. [DOI] [PubMed] [Google Scholar]
- 46.Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Registration body temperature during 60 min of normoxia or hypoxia in adult animals uncovered no significant differences between WT and A1R−/− animals. B) Recording of temperature CO2 production and heart rate was equal A1R−/− compared to WT mice. Error bars indicate +/− SE. n = 7 for WT and n = 4 for A1R−/−. (GIF 6208 kb)
Example of data driven gating approach in brain. Gates were visually confirmed to avoid erroneous results due to artifacts, however no corrections were needed. A) A polygon gate was first applied to filter out obvious debris in SSC and FSC. Density curv-filter was used to find DAPI negative cells that were selected as live cells. B) Since there was no obvious positive population in the TCR receptor expression the negative population was selected (blue) and events with greater signal was set as positive cells (green). C) CD4+ and CD8+ and were interpreted as T-helper or cytotoxic T-cells respectively. (GIF 27 kb)
(DOCX 16 kb)
(DOCX 13 kb)