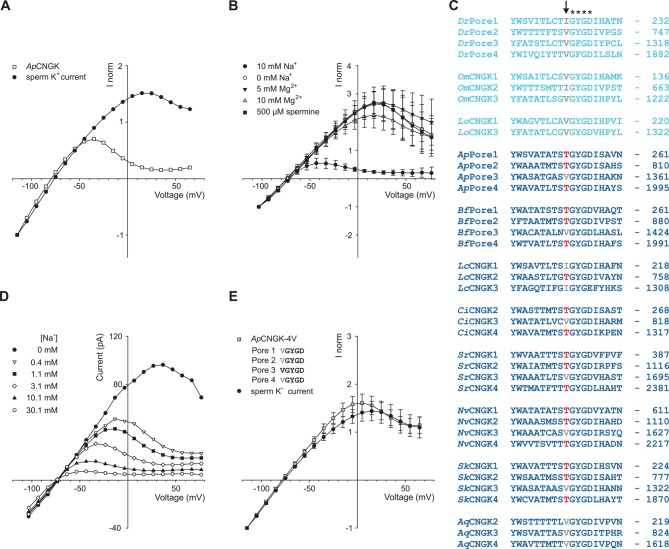
Figure 4. Comparison of sperm K+ current with current from heterologously expressed ApCNGK channels.
(A) Normalized IV relations of whole-cell recordings from zebrafish sperm and ApCNGK channels expressed in HEK293 cells. Pipette solution: standard IS. Bath solution: standard ES. Currents were normalized to -1 at -115 mV. (B) Normalized IV relations (mean current ± sd, n = 6) of inside-out recordings from ApCNGK channels expressed in HEK293 cells. Pipette solution: standard ES, bath solution: NMDG-based IS with the indicated concentrations of Na+, Mg2+, and spermine. Currents were normalized to -1 at -103 mV. (C) Alignment of pore regions from different CNGK channels. Freshwater fishes are highlighted in light blue and seawater species in dark blue. The position of the last amino-acid residue is given on the right. Asterisks indicate the G(Y/F)GD selectivity motif. A key threonine residue that is conserved in three repeats of the ApCNGK channel and other seawater species is highlighted in red (arrow). Hydrophobic amino acids at this position are indicated in gray. (D) IV relations of inside-out recordings of ApCNGK channels expressed in HEK293 cells. Pipette solution: ES; bath solution: NMDG-based IS. Different Na+ concentrations were added to the bath solution. (E) Normalized IV relations (mean current ± sd) of whole-cell recordings from zebrafish sperm (n = 18) and from ApCNGK-4V channels (n = 7) expressed in HEK293 cells. Currents were normalized to -1 at -115 mV. Inset: amino-acid sequence of the pore region of the mutant ApCNGK-4V. ApCNGK channels were activated with 100 µM cGMP.