Abstract
Type I IFNs are needed for the production of antiviral antibodies in mice; whether they also stimulate primary antibody responses in vivo during human viral infections is unknown. This was assessed in patients acutely infected with HIV-1 and treated with IFN-α2b. Patients with acute HIV-1 infection were randomized to receive anti-retroviral therapy alone (Group A, n=60) or combined for 14 weeks with pegylated-IFN-α2b (Group B, n=30). Emergence of anti-HIV antibodies was monitored during 32 weeks by Western blot (WB) analyses of serum samples. IFN-α2b treatment stimulated the production of anti-HIV antibodies. On Week 32, 19 weeks after the last IFN-α2b administration, there were 8.5 (6.5–10.0) HIV WB bands (median, interquartile range) in Group B and 7.0 (5.0–10.0) bands in Group A (P=0.054), and band intensities were stronger in Group B (P<0.05 for p18, p24, p34, p40, and p55 HIV antigens). IFN-α2b treatment also increased circulating concentrations of the B cell-activating factor of the TNF family (P<0.001) and ex vivo production of IL-12 (P<0.05), reflecting its effect on innate immune cells. Withdrawal of antiretroviral treatment on Week 36 resulted in a lower rebound of HIV replication in Group B than in Group A (P<0.05). Therefore, type I IFNs stimulate the emerging anti-HIV immune response in patients with acute HIV-1 infection, resulting in an improved control of HIV replication. Type I IFNs are thus critical in the development of efficient antiviral immune responses in humans, including the production of antiviral antibodies.
INTRODUCTION
The cytokine family of type I IFNs is composed of several IFN-α proteins and of IFN-β, which all act on the same receptor. Viruses stimulate production of type I IFNs, which in turn, have a dual effect on viral replication [1, 2]. Type I IFNs stimulate infected cells to produce various antiviral proteins. They also stimulate the antiviral immune response, acting on NK cells and B and CD8+ T lymphocytes. Type I IFNs activate these effector cells directly [3–6] or indirectly through the production of cytokines [2, 7, 8], the development of Th lymphocytes [9, 10], the induction of dendritic cell (DC) maturation [2, 11–13], and improved antigen presentation [14]. It is not clear whether control of viral replication by type I IFNs is mostly a result of their antiviral properties or of their ability to stimulate antiviral immune responses. IFN-α treatment of hepatitis C virus infection is more efficient when administered during the acute phase of the disease than when administered later [15], suggesting that early stages of viral infections are more sensitive to type I IFNs.
In vivo stimulation of antibody production by type I IFNs has been demonstrated in several murine models [3, 11, 16 –19]. In humans, type I IFNs enhance the production of autoantibodies [20, 21]. It is yet unknown whether they can stimulate a normal antibody response induced by an infection. This information may highlight the contribution of virus-induced type I IFN in the development of protective, antiviral immunity.
Earlier, we had conducted an open and uncontrolled trial to evaluate the effect of pegylated-IFN-α2b added to highly active antiretroviral treatment (HAART) in patients with acute primary HIV infection (PHI) [22]. This pilot study suggested a beneficial effect of IFN-α2b treatment on viral replication, leading us to undertake a larger and controlled trial, the Interprim (ANRS 112) study. In this study, PHI patients were randomized to receive or not IFN-α2b during the first 14 weeks of HAART. This enabled us to assess the impact of type I IFNs on the primary anti-HIV immune response. We show that transient administration of IFN-α2b stimulated the emergence of anti-HIV antibodies. After HAART withdrawal, the rebound of HIV viremia was lower in the group of patients who had previously received IFN-α2b. Our results show for the first time that type I IFNs stimulate a primary in vivo antibody response in humans. Further, IFN-α2b has a beneficial and persisting effect on HIV replication, highlighting its potency to stably improve antiviral immunity.
MATERIALS AND METHODS
Study design
Patients with PHI were enrolled if they tested positive for HIV viral load and had a negative or incomplete HIV-1 Western blot (WB), defined as less than or equal to three bands within 15 days. The ethics committee of the Kremlin-Bicêtre Hospital (France) approved the study protocol. All patients gave written, informed consent before entering the study. There was a central randomization system, and all biological evaluations were performed blindly. The clinicaltrials.gov study ID is NCT00196638.
Antibody analyses
Serum levels of IgG against HIV-1 antigens were determined by WB analysis (NEW LAV-Blot I, Bio-Rad, Marnes la Coquette, France). Anti-HIV antibodies were quantified by scanning and digitizing positive bands as described [23]. All values are expressed as arbitrary units (AU). Tetanus toxoid (TT)-specific IgG was measured by an indirect ELISA (Tetanus ELISA IgG, Testkit Virotech, Rüsselsheim, Germany). Rubella virus-specific IgG was determined by a chemiluminescent immunoassay (Access® Immunoassay System Rubella IgG, Beckman Coulter, Fullerton, CA, USA). Circulating Ig levels were determined by nephelometry (BN II nephenolometer, Dade Behring, Marburg, Germany). Avidity determination of HIV-1-specific IgG was carried out as described [23]. Briefly, each serum sample was tested by WB analysis with two strips. After incubation with the serum sample, the first strip was washed with 4 M urea to remove antibodies with low avidity, and the second strip was washed as usual. Both strips were scanned, and avidity percentage was calculated as the ratio of OD values with and without urea treatment. Quantification of anti-HIV memory B lymphocytes (mBL) was performed as described previously by stimulating PBMC with CD40 and cytokines and measuring by ELISPOT assay the number of HIV-specific, spot-forming cells [24].
Cytokine studies
Concentrations of B cell-activating factor of the TNF family (BAFF) were determined by ELISA (Quantikine, R&D Systems, Lille, France). Production of IL-12 p70 and type I IFN was determined as reported previously [25, 26]. Briefly, PBMCs (2×105 cells/well) were cultured for 18 h in the presence or absence of HSV at a multiplicity of infection of 0.25 for type I IFN detection. Secretion of IL-12 was evaluated 24 h after stimulation of PBMCs (1×106 cells/well) with LPS from Escherichia coli (Sigma, Steinheim, Germany, 0.1 μg/mL) and IFN-γ (Roche, Mannheim, Germany, 20 ng/mL).
Statistical analyses
Intergroup differences were assessed by using the Wilcoxon test for continuous variables and the χ2 or Fisher Exact test for categorical variables. To test the variability of parameters, we used a Spearman correlation. Multivariate analysis of rebound of HIV replication upon HAART withdrawal was performed by stepwise logistic regression, entering into the model the parameters associated with rebound of HIV replication with a P value of <0.25. All analyses were performed using SAS® 9.1.3 Service Pack 2 (The SAS Institute, Cary, NC, USA).
RESULTS
Characteristics of patients and treatment
Twenty-seven clinical centers in France enrolled 90 patients with acute HIV-1 infection in an open-label, randomized, and controlled trial between May 2002 and May 2004. Patients were randomly assigned in a 2:1 ratio to two parallel groups of treatment. Follow-up reported in this study ended 38 weeks after enrollment. HAART alone was administered in Group A (n=60). In Group B, 30 patients were treated with HAART up to Week 36 and received pegylated-IFN-α2b (1 μg/kg per injection) once a week from enrollment (Week 0) to Week 13. All patients had an incomplete or negative HIV WB, in accordance with enrollment criteria. Their median [interquartile range (IQR)] age was 34 [27–39] years old, the delay between infection and screning was 36 [31–41] days, the HIV plasma load was 5.70 (5.10–5.86) log10 copies/mL, and the circulating CD4+ cell count was 386 (288–561) cells/μL. The two groups did not differ for any or these parameters or for the combination of antiretroviral drugs used (data not shown). Seventy-seven of the 90 patients were treated with a combination of a protease inhibitor and two nucleoside RT inhibitors. Most patients started HAART on the day of enrollment. All biological evaluations were carried out blinded to a randomization group.
Treatment with IFN-α2b stimulates the primary anti-HIV antibody response
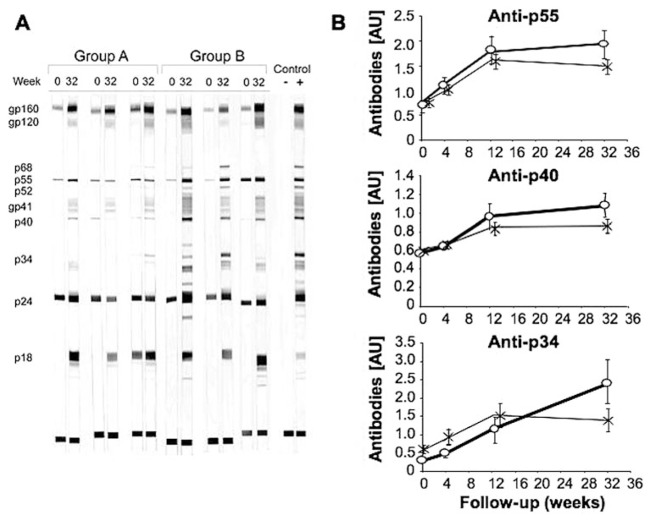
We monitored the progression of the anti-HIV antibody response by comparing the number and the intensity of bands detected by HIV-1 WB analysis of serum samples. In Group A, the intensity of bands present at enrollment increased over time, and additional bands appeared. In Group B, the anti-HIV antibody response was in the same range as in Group A at enrollment. On Week 32, more bands were detected in Group B than in control [8.5 (6.5–10.0) vs. 7.0 (5.0–10.0)] bands, respectively (median IQR, P=0.054), and their intensities were stronger. Progression of the anti-HIV antibody response in typical patients is shown in Figure 1A.
Fig. 1.
Progression of antibodies against HIV antigens. Presence of antibodies against HIV antigens was evaluated by WB analysis. (A) Results from typical patients (n=3 for Groups A and B) at enrollment and on Week 32. The positive control is from a chronically HIV-infected patient and the negative control from a HIV-uninfected individual. (B) Kinetics of antibodies against p55, p40, and p34. *, Group A, n = 30; ○, Group B, n = 15. Results are expressed as log10 [antibody concentration (AU)+1;mean±SEM].
We used scan analysis to quantify the intensity of bands detected by WB analysis. This confirmed the presence of a stronger anti-HIV antibody response in patients from Group B. WB bands against all HIV antigens were more intense than in Group A on Week 32; this difference was significant for most antigens (Table 1). We determined the kinetics of the anti-HIV antibody response in both groups. In Group A patients, the amount of anti-HIV antibodies gradually increased up to Week 12 and then remained stable or decreased up to Week 32. The kinetics of antibody response in Group B was similar to that of Group A during the first weeks; however, the amount of antibodies continued to increase up to Week 32, i.e., 19 weeks after the last IFN-α2b injection (Fig. 1B). Therefore, transient administration of IFN-α2b stimulates the production of anti-HIV antibodies, an effect lasting several months after the end of cytokine administration.
TABLE 1.
IFN-α2b Treatment Stimulates the Primary Anti-HIV Antibody Response
| HIV antigen | Group | Week 0 | Week 32 | Pa |
|---|---|---|---|---|
| p18 | A | <1.1 (<1.1–1.5) | 2.2 (<1.1–3.5) | 0.04 |
| B | <1.1 (<1.1–<1.1) | 3.3 (1.7–4.1) | ||
| p24 | A | 3.1 (2.1–3.5) | 3.5 (1.6–5.6) | 0.02 |
| B | 2.8 (2.1–3.6) | 5.0 (3.2–6.4) | ||
| p34 | A | <0.3 (<0.3–<0.3) | <0.3 (<0.3–1.0) | <0.01 |
| B | <0.3 (<0.3–<0.3) | 2.0 (<0.3–3.2) | ||
| p40 | A | <0.6 (<0.6–1.0) | 1.0 (<0.6–3.6) | <0.01 |
| B | <0.6 (<0.6–0.9) | 2.4 (1.2–5.4) | ||
| gp41 | A | <0.6 (<0.6–<0.6) | 0.9 (<0.6–1.6) | 0.05 |
| B | <0.6 (<0.6–<0.6) | 1.4 (0.7–2.3) | ||
| p55 | A | 1.7 (0.8–2.5) | 2.2 (1.4–4.0) | <0.01 |
| B | 1.7 (1.0–2.6) | 3.9 (2.4–5.5) | ||
| gp120 | A | <0.5 (<0.5–<0.5) | 2.6 (<0.5–4.5) | 0.22 |
| B | <0.5 (<0.5–<0.5) | 3.4 (1.2–5.4) | ||
| gp160 | A | 2.5 (1.2–3.8) | 6.0 (4.2–6.8) | 0.25 |
| B | 3.1 (1.6–4.1) | 6.3 (4.9–7.2) |
The intensity of bands in HIV-1 WB analysis was measured in samples from Weeks 0 and 32. Results are expressed as log10 [antibody concentration (Au)+1], median (IQR).
Comparisons between Week 32 samples from Groups A (n = 60) and B (n = 30).
Treatment with IFN-α2b and maturation of anti-HIV antibody avidity
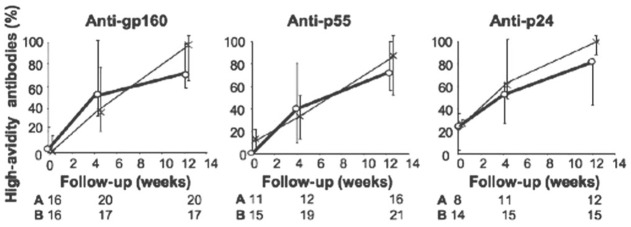
We investigated whether qualitative modifications of anti-HIV antibodies were associated with their increased production in IFN-α-treated patients by comparing maturation of anti-HIV antibody avidity in the two groups of patients. This analysis included only patients with a less-mature anti-HIV antibody response at enrollment, defined by the absence of a WB band or the presence of less than 40% of high-avidity antibodies for the HIV antigen considered. Antibody avidity rapidly increased in Group A, with an enrichment of high-avidity antibodies as early as 4 weeks after enrollment. Avidity maturation did not occur faster in Group B than in Group A (Fig. 2).
Fig. 2.
High-avidity antibodies for HIV antigens and IFN-α2b treatment. The fraction (median, IQR) of high-avidity antibodies among IgG antibodies against p24, p55, and gp160 antigens was determined. Numbers for Groups A and B correspond to patients considered for the analysis (low avidity at enrollment and detectable antibodies thereafter). *, Group A; ○, Group B; P > 0.1 for all comparisons between Groups A and B.
Effect of IFN-α2b treatment on the frequency of anti-HIV mBL
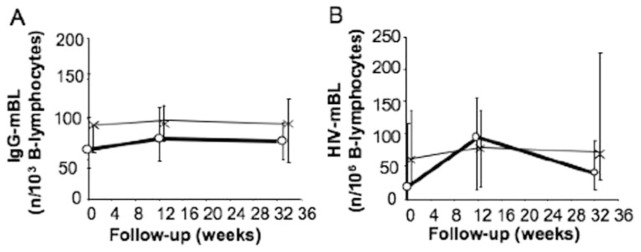
We evaluated the impact of IFN-α2b treatment on the development of anti-HIV mBL. To this end, we stimulated purified B lymphocytes with CD40 ligand and cytokines. Six days later, we quantified by ELISPOT assay IgG-producing mBL and determined what fraction of these cells produced anti-HIV IgG. The fraction of IgG-producing mBL remained relatively stable throughout the follow-up in both groups (Fig. 3A). The number of anti-HIV mBL also remained stable, corresponding to slightly less than 1/1 × 103 of total mBL, with no significant difference at any time between Groups A and B (Fig. 3B). Therefore, stimulation of the primary anti-HIV antibody response by IFN-α2b treatment was not associated with expansion of the circulating anti-HIV mBL compartment.
Fig. 3.
Anti-HIV mBL and IFN-α2b treatment. Numbers (median, IQR) of circulating, IgG-producing mBL (IgG-mBL; A) and HIV-specific mBL (HIV-mBL; B) were determined; (A and B) n = 30. The numbers of IgG- and HIV-mBL were 105 (97–152)/1 × 103 B lymphocytes and <1/1 × 106 B lymphocytes, respectively, in healthy individuals (n=9).
Effect of IFN-α2b treatment on antibodies other than anti-HIV antibodies
The stronger anti-HIV antibody production in PHI patients treated with IFN-α2b may be a generalized effect of this cytokine on the B lymphocyte compartment or an effect restricted to B lymphocytes recently engaged in the anti-HIV immune response. We determined circulating concentrations of Ig to investigate this issue. The concentration of IgG in Group A decreased between enrollment and Week 32 (P<0.001). In contrast, the IgG concentration in Group B remained stable (P>0.5), resulting in a higher IgG concentration than that in Group A on Week 32 (P<0.05). Progression of IgM and IgA levels was similar in the two groups (Table 2). We also measured the impact of IFN-α2b treatment on the concentration of circulating antibodies recognizing Rubella virus and TT antigens. These concentrations did not differ between the two groups at enrollment and on Week 32 (Table 2). Therefore, IFN-α2b treatment did not affect the concentration of antibodies recognizing antigens encountered before PHI.
TABLE 2.
Progression of Circulating Levels of Ig and of Antibodies Recognizing HIV-Unrelated Antigens
| Group | Week 0 | Week 32 | Pa | |
|---|---|---|---|---|
| IgG (g/L) | A | 9.0 (6.6–12.4) | 8.0 (5.8–10.4) | |
| B | 10.0 (7.3–13.1) | 9.9 (8.2–12.3) | 0.02 | |
| IgM (g/L) | A | 1.3 (0.8–1.7) | 0.9 (0.6–1.2) | |
| B | 1.1 (0.9–1.9) | 1.0 (0.7–1.3) | >0.1 | |
| IgA (g/L) | A | 1.9 (1.2–2.4) | 1.9 (1.2–2.5) | |
| B | 1.6 (1.4–2.6) | 1.9 (1.5–2.7) | >0.1 | |
| Rubella virus (IU/mL) | A | 105 (42–174) | 90 (36–156) | |
| B | 113 (75–188) | 115 (63–167) | >0.1 | |
| TT (IU/mL) | A | 3.9 (1.7–9.0) | 3.2 (1.6–6.0) | |
| B | 3.9 (1.9–5.9) | 3.1 (1.5–8.0) | >0.1 |
Concentrations (medians, IQR) of circulating Ig and of antibodies against HIV-unrelated antigens were determined at enrollment and on Week 32; n = 57 for A, and n = 28 for B.
Significances of differences between Groups A and B on Week 32. IU, International units.
Stimulation of the primary anti-HIV antibody response by IFN-α2b treatment is not explained by an effect on HIV viremia or on Th lymphocytes
We investigated whether IFN-α2b treatment affected HIV viremia and CD4+ T lymphocytes, two parameters influencing the intensity of the primary anti-HIV antibody response. The decrease of HIV viremia in all patients from enrollment to Week 12 correlated inversely with the concentration of anti-p55 antibodies on Week 32 (P=0.05; data not shown), confirming in HAART-treated patients the relationship between HIV replication and production of anti-HIV antibodies previously demonstrated by comparing treated and untreated PHI patients [22, 42, 43]. Importantly, the decrease in HIV replication was similar in Groups A and B (data not shown), suggesting that the effect of IFN-α2b treatment on an anti-HIV antibody response was independent of HIV viremia. Recovery of circulating CD4+ T lymphocyte numbers was delayed in Group B, as compared with Group A, but the two groups did not differ any more for this parameter on Week 24 after IFN-α2b withdrawal. The response to p24 antigen stimulation, measured by proliferation or IFN-γ-release assays, did not differ at any time between the two groups (data not shown). Therefore, stronger production of anti-HIV antibodies in patients treated with IFN-α2b is not explained by a higher viral load or by an accelerated or stronger recovery of CD4+ T lymphocyte numbers and function.
IFN-α2b treatment increases the production of IL-12p70 and BAFF
To evaluate whether modulation of DC functions could be involved in IFN-α2b-mediated enhancement of antibody response, we determined ex vivo productions of IL-12p70 and IFN-α by PBMC. Production of IL-12 in Group A gradually decreased up to Week 32 (P<0.01 for Weeks 12 and 32, as compared with enrollment). In contrast, IL-12 production remained stable in Group B up to Week 12, with a higher production of IL-12 at this time than in Group A (P<0.05). IL-12 production in Group B decreased after Week 12 and reached a level similar to that in Group A by Week 32 (Table 3). Production of IFN-α at enrollment was substantially lower than in healthy individuals. It remained extremely low up to Week 32, with no difference at any time between the two groups (Table 3).
TABLE 3.
IFN-α2b Effects on Cytokine Production
| PHI patients
|
Healthy controlsa | |||||
|---|---|---|---|---|---|---|
| Group | Week 0 | Week 4 | Week 12 | Week 32 | ||
| IL-12b | A | 171 (50–282) | 78 (26–196) | 57 (7–140) | 13 (<0.5–116) | 64 |
| Production | B | 140 (66–180) | 118 (13–152) | 153 (79–206)c | 15 (<0.5–176) | (44–111) |
| IFN-αb | A | 70 (4–300) | 100 (6–400) | 16 (<2–135) | 12 (<2–120) | 875 |
| Production | B | 8 (<2–163) | 25 (<2–75) | 22 (<2–50) | 17 (<2–110) | (600–1575) |
| Circulating | A | 1.08 (0.85–1.52) | 0.78 (0.62–1.02) | 0.67 (0.49–0.83) | 0.70 (0.56–0.98) | 0.73 |
| BAFFd | B | 1.27 (0.84–1.87) | 1.64 (1.18–2.09)e | 1.32 (1.06–1.76)e | 0.84 (0.46–1.08) | (0.66–0.82) |
n = 8.
Cytokine concentration in supernatants (pg/mL for IL-12 and IU/mL for IFN-α); n = 28 for Group A, and n = 14 for Group B.
P < 0.05.
Concentrations (ng/mL) of circulating BAFF; n = 60 for Group A, and n = 30 for Group B. BAFF concentration in 10 control, untreated, HIV-infected patients 12 months after infection was 1.50 ng/mL (1.26–1.80 ng/mL). All results are expressed as medians (IQR).
P < 0.001, as compared with Group A.
We measured the serum concentration of the BAFF. At enrollment, it was higher in both groups than in healthy controls. BAFF concentration gradually decreased in Group A (P<0.01 for Weeks 4 and 12, as compared with enrollment), reaching normal values by Week 12. In contrast, BAFF concentration increased in Group B between Weeks 0 and 4 (P<0.01), leading to a higher BAFF concentration than that in Group A on Weeks 4 and 12 (P<0.001). BAFF concentration decreased after Week 12, reaching normal values by Week 32 (Table 3). Therefore, IFN-α2b treatment increased the ex vivo production of IL-12 and the concentration of circulating BAFF. These effects lasted as long as IFN-α2b was administered.
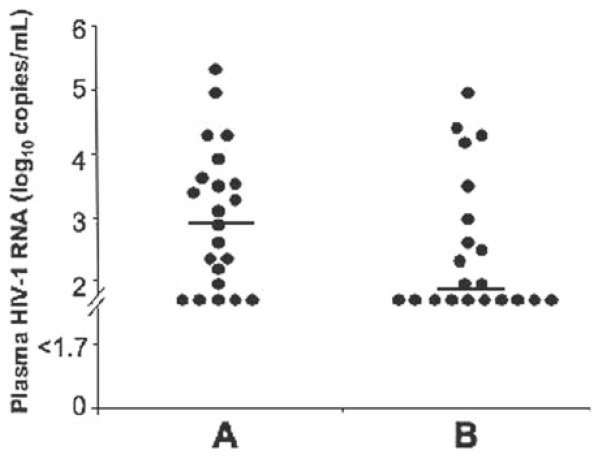
IFN-α2b treatment and rebound of HIV viremia after HAART withdrawal
HAART was withdrawn on Week 36 in 30 patients from Group A and in all from Group B. We determined whether transient treatment with IFN-α2b durably influenced the host-virus equilibrium. Plasma HIV concentration was assessed 14 days after HAART withdrawal in 22 patients from Group A and in 21 patients from Group B (Fig. 4). Nine (41%) patients in Group A and 15 (71%) in Group B (P<0.05) had a plasma HIV load below 400 copies/mL. We asked whether the concentration of anti-HIV antibodies before withdrawal of HAART explains the lower viral rebound in Group B. There was no correlation (P>0.5) between the concentration of anti-p55 antibodies on Week 32 and plasma HIV load on Week 38. We obtained similar findings for the concentration of anti-p34, -p40, -gp120, and -gp160 antibodies and for the number of bands in WB assays (P>0.15 for all comparisons). Therefore, transient IFN-α2b treatment in patients with acute PHI partially prevented the rebound of HIV replication at HAART interruption 23 weeks after the last administration of IFN-α2b. However, the higher concentrations of anti-HIV antibodies in IFN-α2b-treated patients played little if any role in this limitation of viral replication rebound.
Fig. 4.
IFN-α2b effect on rebound of HIV replication after HAART withdrawal. HAART was withdrawn on Week 36 and HIV viremia determined 14 days later. Horizontal bars represent medians.
DISCUSSION
In this sub-study of the ANRS 112 Interprim trial, we analyzed in patients recently infected with HIV and treated with HAART the impact of a transient IFN-α2b treatment on the emergence of the anti-HIV antibody response and on the control of HIV replication. We show that IFN-α2b treatment stimulates anti-HIV antibody production. Higher concentrations of antibodies against the various HIV antigens were present on Week 32 in the circulation of IFN-α2b-treated patients than in controls. The difference was significant for antibodies against all nonglycosylated HIV antigens but limited (P=0.05 for gp41) or nonsignificant (for gp120 and gp160) for antibodies against glycosylated HIV antigens. This may reflect a different sensitivity to type I IFN of B lymphocytes recognizing glycosylated antigens. The stimulating effect of type I IFN on anti-HIV antibody production was rather selective, as IFN-α2b treatment influenced neither the rate of avidity maturation of anti-HIV antibodies nor the size of the anti-HIV mBL compartment. This suggests that IFN-α2b treatment affects the decision of B lymphocytes to differentiate into antibody-producing cells or that it targets B lymphocytes already committed to this differentiation pathway. Plasma cells are divided into short- and long-lived populations [44]. As the high anti-HIV antibody concentration persisted 19 weeks after the end of IFN-α2b treatment, IFN-α2b treatment presumably increased the size of the long-lived anti-HIV plasma cell compartment.
HIV infection, including acute infection, drives B cell hyperactivity and increases circulating IgG levels. This polyclonal activation rapidly decreases with HAART [27]. This decrease was prevented in patients receiving IFN-α2b. In contrast, IFN-α2b treatment did not influence circulating levels of IgM and IgA and antibodies recognizing TT and Rubella virus, two nonpersisting antigens. Thus, IFN-α2b treatment had no effect on long-lived plasma cells already established when HIV infection occurs. The higher concentrations of total IgG in Group B possibly reflects an effect of IFN-α2b treatment on cells producing IgG against HIV and other recently encountered antigens.
In mice, type I IFNs are mandatory for the in vivo development and persistence of a primary antibody response [3, 11, 16, 18] or for the production of pathogenic autoantibodies [17, 19]. A combined effect of IFN on DCs and B and T lymphocytes explains increased antibody production [11, 16]. Bellardelli and coworkers [28, 29] used a model in which human cells were transferred to immunodeficient mice to show that type I IFNs stimulate the development of an anti-HIV human immune response, which involved HIV-specific CTL and antibodies. The action of IFNs on DCs appeared critical in this induction of anti-HIV immunity, which prevented HIV replication in mice [28, 29]. Autoantibodies may develop in patients treated with recombinant IFN-α [20, 21], suggesting that as in mice, type I IFNs stimulate antibody production in humans. We here extend these studies in a more physiological setting, a human primary antibody response to a viral infection.
We investigated the mechanism used by IFN-α2b treatment to stimulate the primary anti-HIV antibody response. This effect was not explained by differences between groups in HIV plasma load or anti-HIV Th lymphocyte numbers and functions. IFN-α stimulates BAFF production by human DCs in vitro, resulting in enhanced B lymphocyte differentiation [7]. The numbers of long-lived plasma cells are reduced in mice with an inactivated BAFF receptor gene [30], showing that BAFF improves the development or survival of Ig-secreting cells. BAFF also improves the ex vivo survival of human Ig-secreting cells [31]. We show that IFN-α2b treatment strongly increased circulating levels of BAFF, making BAFF a good candidate to explain the IFN-α2b effect on anti-HIV antibody response. IFN-α2b treatment also increased the ex vivo production of IL-12, a cytokine involved in B lymphocyte differentiation, directly or through T lymphocytes [32]. These findings suggest that stimulation of anti-HIV antibody production by IFN-α2b was at least partially mediated by an action of the cytokine on innate immune cells, in agreement with previous reports in mice models [11, 16, 18, 28]. This hypothesis does not rule out that a direct effect of type I IFNs on antibody-producing B lymphocytes also contributed to the stronger anti-HIV antibody response.
Another major finding of our study was the effect of transient IFN-α2b treatment on the host-HIV relationship. This was the rationale for undertaking the Interprim study. We show that 23 weeks after the last IFN-α2b injection, HIV replication rebounded following HAART withdrawal but to a lesser extent than in control patients treated with HAART alone. In general, the contributions of the antiviral and immune-stimulating properties of type I IFNs in control of virus spreading have not been defined clearly. Our findings unambiguously show that in HIV infection, early administration of type I IFNs durably change the host-virus relationship, resulting in improved immune control of the infection. However, this control was partial. After Week 38, patients underwent sequential HAART interruptions, with or without IFN-α2b treatment. Monitoring of these patients should show whether the short-term and partial effect observed here on HIV replication translates into a long-term benefit.
We investigated whether the enhanced anti-HIV antibody response contributed to the control of HIV replication in patients treated with IFN-α2b. Anti-HIV antibodies may indeed limit HIV (or SIV) replication [33–41, 45]. We found no correlation between anti-HIV antibody concentrations before HAART withdrawal and viral rebound levels afterwards (data not shown). Antibodies may limit HIV replication by a combination of effects associating neutralization, opsonization, antibody-dependent cellular cytotoxicity, and complement activation [34, 36, 38, 46 – 49]. Although unlikely, it is possible that IFN-α2b treatment selectively strengthens one of these mechanisms, an effect not reflected by quantification of total anti-HIV antibodies. Nevertheless, our finding argues that the anti-HIV antibodies produced by patients treated efficiently and early during PHI are little-involved, if at all, in the control of HIV replication. The arms of the anti-HIV immune response potentiated by IFN-α2b and limiting HIV replication remain to be determined. They may involve DCs or anti-HIV T lymphocytes, cells contributing to the control of HIV replication and potentially activated by type I IFNs.
This study shows that in acute HIV-1 infection treated with HAART, addition of transient IFN-α2b treatment increases the production of anti-HIV antibodies, an effect still detected 19 weeks after the last administration of IFN-α2b. This is the first demonstration that systemic levels of type I IFNs modulate the intensity of a primary antibody response in humans. Therefore, beyond the specific case of patients with PHI reported in this study, our observation highlights the role of virus-induced type I IFNs in the development of a potent, antiviral antibody response. IFN-α2b did not improve all aspects of the anti-HIV B lymphocyte response but seemed to rather target antibody-producing cells, especially their long-lived compartment. We also show that such transient IFN-α2b treatment durably improves the host-virus relationship, resulting in a decreased HIV replication rebound after HAART withdrawal. Consistent with a previous report [25], we observed a severe defect in type I IFN production in PHI. Our findings suggest that this defect may impair anti-HIV antibody production in patients with PHI. The improved control of HIV replication we found in IFN-α2b-treated patients indicates that this IFN type I defect may also contribute to the poor control of HIV infection. Anti-HIV antibodies did not significantly account for the effect of IFN-α2b treatment on HIV replication. Thus, immune stimulation by IFN-α2b in PHI must extend beyond antibody production, and these other arms of the antiviral immune response may explain the limitation of HIV replication by type I IFNs.
Acknowledgments
This work was supported by the Agence Nationale de Recherches sur le SIDA et les Hépatites Virales. L. A-P. received fellowships from CONACYT (No. 116731) and from “Sidaction.” We acknowledge P. Galanaud for constant support, N. Misra for English editing of the manuscript, J. P. Vendrell and L. Grangeot-Keros for helpful technical advice, C. Roussillon and V. Boilet for coordinating the monitoring and data management, C. Goujard and M. Sinet for critical review of the manuscript, and A. M. Delavalle, M. V. Prejean, and S. Louis for technical assistance. The authors thank the Data Management and Safety Board of the Interprim study (D. Costagliola, V. Calvez, and J. L. Pellegrin)
Footnotes
The authors declare no competing financial interest.
References
- 1.Garcia-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: a lesson in detente. Science. 2006;312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- 2.Stetson DB, Medzhitov R. Antiviral defense: interferons and beyond. J Exp Med. 2006;203:1837–1841. doi: 10.1084/jem.20061377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coro ES, Chang WL, Baumgarth N. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J Immunol. 2006;176:4343–4351. doi: 10.4049/jimmunol.176.7.4343. [DOI] [PubMed] [Google Scholar]
- 4.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 5.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 6.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD4-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 9.Krug A, Veeraswamy R, Pekosz A, Kanagawa O, Unanue ER, Colonna M, Cella M. Interferon-producing cells fail to induce proliferation of naive T cells but can promote expansion and T helper 1 differentiation of antigen-experienced unpolarized T cells. J Exp Med. 2003;197:899–906. doi: 10.1084/jem.20021091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen KB, Watford WT, Salomon R, Hofmann SR, Pien GC, Morinobu A, Gadina M, O’Shea JJ, Biron CA. Critical role for STAT4 activation by type 1 interferons in the interferon-γ response to viral infection. Science. 2002;297:2063–2066. doi: 10.1126/science.1074900. [DOI] [PubMed] [Google Scholar]
- 11.Le Bon A, Schiavoni G, D’Agostino G, Gresser I, Belardelli F, Tough DF. Type 1 interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–470. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 12.Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, Borrow P, Tough DF. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–1015. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 13.Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P, Tough DF. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99:3263–3271. doi: 10.1182/blood.v99.9.3263. [DOI] [PubMed] [Google Scholar]
- 14.Shin EC, Seifert U, Takanobu K, Rice CM, Feinstone SM, Kloetzel PM, Rehermann B. Virus-induced type I IFN stimulates generation of immunoproteasomes at the site of infection. J Clin Invest. 2006;116:3006–3014. doi: 10.1172/JCI29832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lampertico P, Rumi M, Romeo R, Craxi A, Soffredini R, Biassoni D, Colombo M. A multicenter randomized controlled trial of recombinant interferon-α 2b in patients with acute transfusion-associated hepatitis C. Hepatology. 1994;19:19–22. [PubMed] [Google Scholar]
- 16.Le Bon A, Thompson C, Kamphuis E, Durand V, Rossmann C, Kalinke U, Tough DF. Enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J Immunol. 2006;176:2074–2078. doi: 10.4049/jimmunol.176.4.2074. [DOI] [PubMed] [Google Scholar]
- 17.Mathian A, Weinberg A, Gallegos M, Banchereau J, Koutouzov S. IFN-α induces early lethal lupus in preautoimmune (New Zealand Black × New Zealand White) F1 but not in BALB/c mice. J Immunol. 2005;174:2499–2506. doi: 10.4049/jimmunol.174.5.2499. [DOI] [PubMed] [Google Scholar]
- 18.Proietti E, Bracci L, Puzelli S, Di Pucchio T, Sestili P, De Vincenzi E, Venditti M, Capone I, Seif I, De Maeyer E, Tough D, Donatelli I, Belardelli F. Type I IFN as a natural adjuvant for a protective immune response: lessons from the influenza vaccine model. J Immunol. 2002;169:375–383. doi: 10.4049/jimmunol.169.1.375. [DOI] [PubMed] [Google Scholar]
- 19.Santiago-Raber ML, Baccala R, Haraldsson KM, Choubey D, Stewart TA, Kono DH, Theofilopoulos AN. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gogas H, Ioannovich J, Dafni U, Stavropoulou-Giokas C, Frangia K, Tsoutsos D, Panagiotou P, Polyzos A, Papadopoulos O, Stratigos A, Markopoulos C, Bafaloukos D, Pectasides D, Fountzilas G, Kirkwood JM. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354:709–718. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 21.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (α/β) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 22.Emilie D, Burgard M, Lascoux-Combe C, Laughlin M, Krzysiek R, Pignon C, Rudent A, Molina JM, Livrozet JM, Souala F, Chene G, Grangeot-Keros L, Galanaud P, Sereni D, Rouzioux C Primoferon A Study Group. Early control of HIV replication in primary HIV-1 infection treated with antiretroviral drugs and pegylated IFN α: results from the Primoferon A (ANRS 086) Study. AIDS. 2001;15:1435–1437. doi: 10.1097/00002030-200107270-00014. [DOI] [PubMed] [Google Scholar]
- 23.Adalid-Peralta L, Grangeot-Keros L, Rudent A, Ngo-Giang-Huong N, Krzyiek R, Goujard C, Deveau C, Le Gall M, Meyer L, Emilie D, Rouzioux C. Impact of highly active antiretroviral therapy on the maturation of anti-HIV-1 antibodies during primary HIV-1 infection. HIV Med. 2006;7:514–519. doi: 10.1111/j.1468-1293.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 24.Fondere JM, Huguet MF, Yssel H, Baillat V, Reynes J, van de Perre P, Vendrell JP. Detection of peripheral HIV-1-specific memory B cells in patients untreated or receiving highly active antiretroviral therapy. AIDS. 2003;17:2323–2330. doi: 10.1097/00002030-200311070-00006. [DOI] [PubMed] [Google Scholar]
- 25.Kamga I, Kahi S, Develioglu L, Lichtner M, Maranon C, Deveau C, Meyer L, Goujard C, Lebon P, Sinet M, Hosmalin A. Type I interferon production is profoundly and transiently impaired in primary HIV-1 infection. J Infect Dis. 2005;192:303–310. doi: 10.1086/430931. [DOI] [PubMed] [Google Scholar]
- 26.Servet C, Zitvogel L, Hosmalin A. Dendritic cells in innate immune responses against HIV. Curr Mol Med. 2002;2:739–756. doi: 10.2174/1566524023361907. [DOI] [PubMed] [Google Scholar]
- 27.Titanji K, Chiodi F, Bellocco R, Schepis D, Osorio L, Tassandin C, Tambussi G, Grutzmeier S, Lopalco L, De Milito A. Primary HIV-1 infection sets the stage for important B lymphocyte dysfunctions. AIDS. 2005;19:1947–1955. doi: 10.1097/01.aids.0000191231.54170.89. [DOI] [PubMed] [Google Scholar]
- 28.Lapenta C, Santini SM, Logozzi M, Spada M, Andreotti M, Di Pucchio T, Parlato S, Belardelli F. Potent immune response against HIV-1 and protection from virus challenge in hu-PBL-SCID mice immunized with inactivated virus-pulsed dendritic cells generated in the presence of IFN-α. J Exp Med. 2003;198:361–367. doi: 10.1084/jem.20021924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T, Belardelli F. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–1788. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, Lin LL, Mantchev GT, Bram RJ, Noelle RJ. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199:91–97. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avery DT, Kalled SL, Ellyard JI, Ambrose C, Bixter SA, Thien M, Brink R, Mackay F, Hodgkin PD, Tangye SG. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. 2003;112:286–297. doi: 10.1172/JCI18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 33.Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou TC, Ruprecht RM. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 34.Frost SD, Wrin T, Smith DM, Kosakovsky Pond SL, Liu Y, Paxinos E, Chappey C, Galovich J, Beauchaine J, Petropoulos CJ, Little SJ, Richman DD. Neutralizing antibody responses drive the evolution of human immunodeficiency virus type 1 envelope during recent HIV infection. Proc Natl Acad Sci USA. 2005;102:18514–18519. doi: 10.1073/pnas.0504658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gauduin MC, Parren PW, Weir R, Barbas CF, Burton DR, Koup RA. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat Med. 1997;3:1389–1393. doi: 10.1038/nm1297-1389. [DOI] [PubMed] [Google Scholar]
- 36.Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, Steenbeke TD, Venturi M, Chaiken I, Fung M, Katinger H, Parren PW, Robinson J, Van Ryk D, Wang L, Burton DR, Freire E, Wyatt R, Sodroski J, Hendrickson WA, Arthos J. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420:678–682. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- 37.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 38.Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci USA. 2003;100:4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho MW, Martin MA. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 40.Trkola A, Kuster H, Rusert P, Joos B, Fischer M, Leemann C, Manrique A, Huber M, Rehr M, Oxenius A, Weber R, Stiegler G, Vcelar B, Katinger H, Aceto L, Gunthard HF. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med. 2005;11:615–622. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- 41.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, Ketas T, Marx PA, Klasse PJ, Burton DR, Moore JP. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 42.Lafeuillade A, Poggi C, Tamalet C, Profizi N, Tourres C, Costes O. Effects of a combination of zidovudine, didanosine, and lamivudine on primary human immunodeficiency virus type 1 infection. J Infect Dis. 1997;175:1051–1055. doi: 10.1086/516442. [DOI] [PubMed] [Google Scholar]
- 43.Markowitz M, Vesanen M, Tenner-Racz K, Cao Y, Binley JM, Talal A, Hurley A, Jin X, Chaudhry MR, Yaman M, Frankel S, Heath-Chiozzi M, Leonard JM, Moore JP, Racz P, Nixon DF, Ho DD. The effect of commencing combination antiretroviral therapy soon after human immunodeficiency virus type 1 infection on viral replication and antiviral immune responses. J Infect Dis. 1999;179:527–537. doi: 10.1086/314628. [DOI] [PubMed] [Google Scholar]
- 44.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KGC, Dörner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 45.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Dwong PD, Shaw GM. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 46.Aasa-Chapman MM, Holuigue S, Aubin K, Wong M, Jones NA, Cornforth D, Pellegrino P, Newton P, Williams I, Borrow P, McKnight A. Detection of antibody-dependent complement-mediated inactivation of both autologous and heterologous virus in primary human immunodeficiency virus type 1 infection. J Virol. 2005;79:2823–2830. doi: 10.1128/JVI.79.5.2823-2830.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Connick E, Marr DG, Zhang XQ, Clark SJ, Saag MS, Schooley RT, Curiel TJ. HIV-specific cellular and humoral immune responses in primary HIV infection. AIDS Res Hum Retroviruses. 1996;12:1129–1140. doi: 10.1089/aid.1996.12.1129. [DOI] [PubMed] [Google Scholar]
- 48.Forthal DN, Landucci G, Daar ES. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J Virol. 2001;75:6953–6961. doi: 10.1128/JVI.75.15.6953-6961.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huber M, Fischer M, Misselwitz B, Manrique A, Kuster H, Niederöst B, Weber R, Von Wyl V, Günthard F, Trkola A. Complement lysis activity in autologous plasma is associated with lower viral loads during the acute phase of HIV-1 infection. PLoS Med. 2006;3:e441. doi: 10.1371/journal.pmed.0030441. [DOI] [PMC free article] [PubMed] [Google Scholar]