Abstract
Objective
To study the prognostic role of pregnancy on the progression of human immunodeficiency virus (HIV) infection.
Methods
In a prospective cohort study at the Bordeaux University Hospital, France, 57 women who completed a pregnancy during the course of their HIV infection were compared with 114 HIV-infected women who never conceived. The two groups were matched on CD4 lymphocyte count (CD4), age, and year of HIV diagnosis. The main outcome measures were death, occurrence of a first AIDS-defining event, and drop of the CD4 below 200/mm3.
Results
The mean follow-up period in pregnant women was 61 months from HIV diagnosis (median CD4 at entry 455/mm3) and 54 months from beginning of pregnancy. Nonpregnant women were followed-up for 50 months since HIV diagnosis (median CD4 460/mm3). The proportion of asymptomatic women at entry in the study was 51 of 57 (90%) in pregnant and 87 of 114 (76%) in nonpregnant women. No significant difference was observed between the two groups with regard to the different end points studied, even after adjustment for other prognostic variables. Adjusted hazard ratios (pregnant/nonpregnant) were 0.92 for death (95% confidence interval [CI] 0.40–2.12), 1.02 for occurrence of a first AIDS-defining event (95% CI 0.48–2.18), and 1.20 for drop of the CD4 to less than 200/mm3 (95% CI 0.63–2.27).
Conclusion
In a cohort of HIV-infected women with mild to moderate immunosuppression, pregnancy did not accelerate progression to AIDS or death.
Keywords: Adolescent; Adult; Pregnancy Complications, Infectious; Prognosis; Prospective Studies; Survival Rate; CD4 Lymphocyte Count; Confidence Intervals; Disease-Free Survival; Female; Follow-Up Studies; HIV Seropositivity; Humans; Pregnancy
The literature regarding the prognostic role of pregnancy in the course of human immunodeficiency virus (HIV) infection is contradictory.1 Some studies suggested a harmful effect of pregnancy on disease progression,2–4 whereas others observed no effect.5–11 However, these studies suffer either from the absence of a comparison group or the lack of statistical power because of small sample size or insufficient follow-up.
The goal of this prospective cohort study was to compare the clinical evolution of HIV infection in pregnant and nonpregnant women.
Materials and Methods
The exposed group consisted of HIV-seropositive women who had given birth once after the diagnosis of HIV infection (pregnant women). The control group consisted of HIV-seropositive women who had not conceived during the same period (nonpregnant women). The two groups were chosen within the Cohorte Aquitaine of the Groupe d’Epidémiologie Clinique du SIDA en Aquitaine, a hospital-based cohort of HIV-infected patients implemented in 1985 at the University Hospital of Bordeaux, the capital city of Aquitaine, southwestern France.
Since 1985, patients in 11 hospital departments of the Bordeaux University Hospital (Internal Medicine, Infectious Diseases, Pneumology, and Dermatology) were enrolled in the Cohorte Aquitaine. Since 1992, three medicine departments of general hospitals in the Aquitaine region joined our team. Subjects are notified and included in the Cohorte Aquitaine if they fulfill the following criteria: HIV infection confirmed by a Western blot test (regardless of the clinical stage), age over 13 years, and informed consent of the patient. At every consultation or hospitalization, epidemiologic, clinical, biologic, and therapeutic data are collected by standardized questionnaires. Asymptomatic patients are generally followed-up every 6 months, then every 1–3 months, depending on the clinical and biologic evaluation.12,13
For this study, the group of pregnant women included all HIV-infected women of the Cohorte Aquitaine who gave birth at the maternity department of the Bordeaux University Hospital after being diagnosed with HIV infection between August 1, 1985, and June 1, 1992, and for whom a CD4 lymphocyte count (CD4) was performed at diagnosis of HIV infection. The group of nonpregnant subjects was randomly drawn from all women of childbearing age of the Cohorte Aquitaine who were not pregnant during the study period. Each pregnant woman was matched individually to two nonpregnant women. The following matching criteria were evaluated at diagnosis of HIV infection: age (5-year strata), CD4 (less than 200, 200–500, and more than 500/mm3), and diagnosis period (1985–1988 and 1989–1992). This latter criterion was adopted because of the introduction in France in 1989 of antiretroviral drugs and prophylactic treatments of opportunistic infections at an early stage of HIV infection.14 Antiretroviral drugs were prescribed in both groups when the women became symptomatic or when the CD4 fell to less than 200/mm3; prophylactic treatments were given only for the latter condition. During the study period, zidovudine was not prescribed during pregnancy.
Two main end point criteria were used for analysis, death and progression to AIDS according to the 1987 and 1993 Centers for Disease Control and Prevention (CDC) case definitions.15,16 A drop to less than 200 CD4/mm3 was chosen as a secondary end point for those women who had 200 CD4/mm3 or more at entry. The date of entry in the study was the date of diagnosis of HIV infection; the end point date was April 1, 1994. Within the study period, the rate of CD4 decline was estimated by linear regression of the serial CD4 measurements in calendar time in patients with at least three measurements. It is reported as the mean number of CD4 cells lost per year.
To detect a 2.0 minimum relative risk (RR) of death with a 5% alpha type-one error, an 80% power, and a cumulative mortality of 20% after 5 years of follow-up in nonpregnant women, the group of nonpregnant women had twice as many subjects as the group of pregnant women.
Chi-square test for matched pairs was used to compare the distribution of qualitative variables, and Student t test was used to compare means of quantitative variables. The Kaplan-Meier method17 was used to estimate the cumulative probability of survival or of remaining free of the selected events. Log-rank test and Cox model were used for statistical comparisons of survival curves.18 The following variables were included in the multivariate analysis: CDC group of HIV infection at HIV diagnosis and the matching criteria. Pregnancy may have occurred at any time during follow-up and was therefore taken into account in the model as a time-dependent variable.18
Results
As of April 1, 1994, the Cohorte Aquitaine had enrolled 870 HIV-seropositive women of childbearing age (15–45 years), accounting for 23.4% of all the subjects included. Among them, we were able to include 57 pregnant women and 114 nonpregnant women for the present study.
The mean age for both groups was 26 years (standard deviation [SD] 4), and 142 (83%) of the women enrolled were under 30 years old (Table 1). Overall, HIV infection was diagnosed during 1985–1988 in 109 women (64%) and during 1989–1992 in 62 women (36%). Pregnant women were more frequently intravenous drug users and blood-product recipients than were nonpregnant women: 33 of 57 (58%) versus 51 of 114 (45%) and eight of 57 (14%) versus nine of 114 (8%), respectively. Consequently, heterosexual transmission and transmission from an indeterminate origin were less frequently incriminated in pregnant than in nonpregnant women: 14 of 57 (25%) versus 45 of 114 (40%) and two of 57 (3%) versus nine of 114 (8%), respectively. These differences did not reach statistical significance (χ2 test, P = .1).
Table 1.
Characteristics of Human Immunodeficiency Virus Infection at Entry in the Study in Women With a Completed Pregnancy and in Nonpregnant Women
| Characteristics at entry in the study | Pregnant (n = 57) | Nonpregnant (n = 114) | P |
|---|---|---|---|
| Year of HIV diagnosis | |||
| 1985–1988 | 73 (64%) | 36 (63%) | .72 |
| 1989–1992 | 41 (36%) | 21 (37%) | |
| Mean age at HIV diagnosis (y) | 26.1 | 26.1 | .87 |
| SD | 4.3 | 3.9 | |
| HIV transmission group | |||
| Intravenous drug users | 33 (58%) | 51 (45%) | .09 |
| Blood-product recipients | 8 (14%) | 9 (8%) | |
| Heterosexual | 14 (25%) | 45 (39%) | |
| Indeterminate | 2 (3%) | 9 (8%) | |
| Clinical stage at HIV diagnosis | |||
| Asymptomatic | 51 (90%) | 87 (76%) | .04 |
| Mildly symptomatic | 4 (7%) | 15 (13%) | |
| AIDS | 2 (3%) | 12 (11%) | |
| Median CD4 at HIV diagnosis (/mm3) | 455 | 460 | .57 |
| Interquartile range | 242–670 | 282–728 | |
| Median CD4 at beginning of pregnancy (/mm3) | 360 | ||
| Interquartile range | 234–515 | ||
HIV = human immunodeficiency virus; SD = standard deviation; CD4 = CD4 lymphocyte count.
The median CD4 at the time of HIV infection diagnosis was 455/mm3 (interquartile range 276–681), with no significant difference between the two groups; 27 of the women enrolled (16%) had less than 200 CD4/mm3, 75 of 171 (44%) between 200 and 500/mm3, and 69 of 171 (40%) more than 500/mm3 (Table 1). In pregnant women, the median CD4 at the beginning of pregnancy was 360/mm3 (interquartile range 234–515).
From a clinical point of view, 51 of 57 (90%) pregnant women were asymptomatic at the time of HIV diagnosis (ie, belonged to CDC group A) versus 87 of 114 (76%) of nonpregnant women (χ2 test, P = .04). Among pregnant women, four of 57 (7%) belonged to CDC group B and two of 57 (3%) had AIDS (CDC group C); among nonpregnant women, 15 of 57 (13%) belonged to CDC group B and 12 of 114 (11%) had AIDS (Table 1). The proportion of women treated by zidovudine at any time during follow-up did not differ between the two groups: 33 (57.9%) in pregnant women versus 52 (45.6%) in nonpregnant women (χ2 test, P = .13). The clinical presentation in pregnant women remained unchanged between the time of HIV infection diagnosis and delivery. The median length of this interval was 7 months (interquartile range 0–16).
The mean length of follow-up after the HIV infection diagnosis in pregnant women was 61 months (SD 27; median 58; range 14–106), for a total of 3455 woman-months of follow-up. The mean follow-up since the beginning of pregnancy was 54 months (SD 25; median 51; range 15–91) (ie, a total of 3056 woman-months of follow-up). For nonpregnant women, the mean follow-up since HIV infection diagnosis was 50 months (SD 26; median 48; range 8–103 months), accounting for a total of 5658 woman-months of follow-up. Overall, 26 women (15.2%) were considered lost to follow-up (ie, not seen for more than 1 year at the end of the study period [April 1994]).
Among pregnant women, 36 (63%) had been diagnosed as HIV-infected at least 1 month before pregnancy and 21 (37%) at the time of pregnancy; respectively, 33 of 36 (92%) and 18 of 21 (86%) were asymptomatic at entry in the study (P = .16). Also, the mean CD4 at entry in the study was 557/mm3 (standard error [SE] 286) in those diagnosed for more than 1 month and 453/mm3 (SE 289) for the others (P = .19).
There was no significant difference in the occurrence of the events chosen as end point criteria: death, progression to an AIDS-defining event, or a drop of the CD4 count to less than 200/mm3 (Table 2).
Table 2.
Progression of Human Immunodeficiency Virus Infection in Women With a Completed Pregnancy and in Nonpregnant Women
| Survival | Pregnant (n = 57) (95% CI) | Nonpregnant (n = 114) (95% CI) | Adjusted hazard ratio* (95% CI) |
|---|---|---|---|
| At 1 y | 100% | 99.1% (93.9–99.9) | |
| At 3 y | 96.2% (85.7–99.0) | 85.1% (76.4–90.7) | 0.92 (0.40–2.12) |
| At 5 y | 81.8% (66.4–90.6) | 80.7% (70.7–87.5) |
| Probability of remaining AIDS-free | Pregnant (n = 55) (95% CI) | Nonpregnant (n = 102) (95% CI) | Adjusted hazard ratio* (95% CI) |
|---|---|---|---|
| At 1 y | 98.2% (87.8–99.7) | 98.0% (92.3–99.5) | |
| At 3 y | 92.2% (80.4–97.0) | 87.6% (78.7–93.0) | 1.02 (0.48–2.18) |
| At 5 y | 82.6% (68.0–91.0) | 78.6% (66.9–86.6) |
| Probability of remaining >200 CD4/mm3 | Pregnant (n = 48) (95% CI) | Nonpregnant (n = 96) (95% CI) | Adjusted hazard ratio* (95% CI) |
|---|---|---|---|
| At 1 y | 97.9% (86.1–99.7) | 94.7% (87.8–97.8) | |
| At 3 y | 88.6% (74.6–95.1) | 81.0% (70.8–88.0) | 1.20 (0.63–2.27) |
| At 5 y | 66.7% (48.2–79.9) | 62.1% (49.0 –72.8) |
CI = confidence interval; CD4 = CD4 lymphocyte count.
Adjusted for Centers for Disease Control and Prevention group of human immunodeficiency virus (HIV) infection, CD4, age, and calendar year at the time of HIV diagnosis.
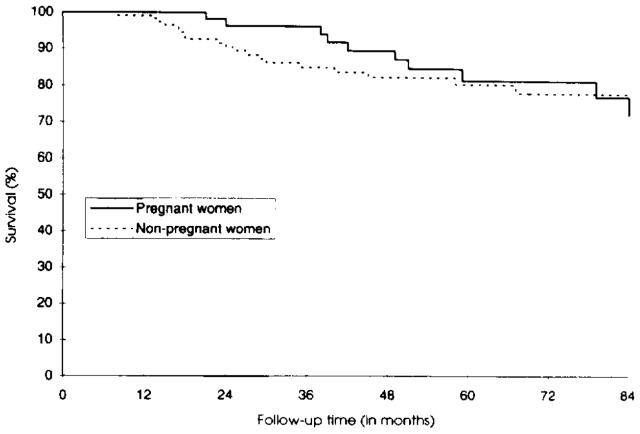
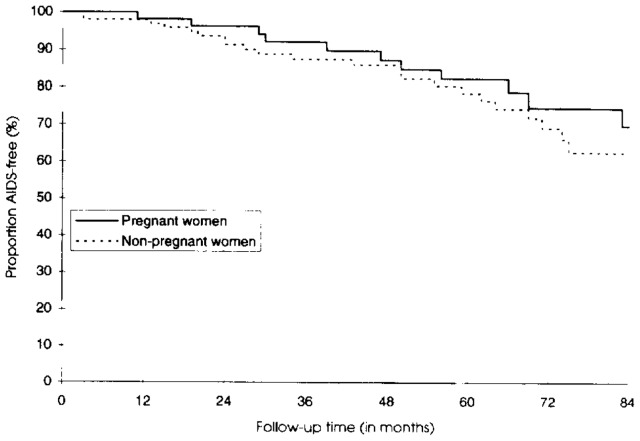
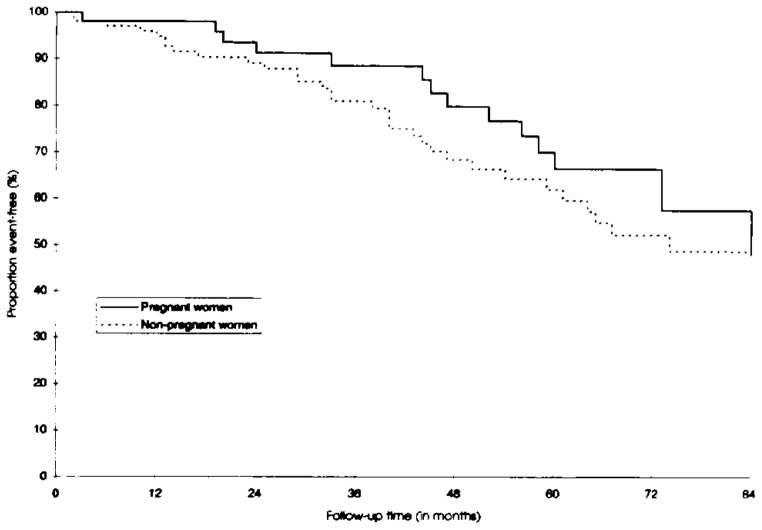
The 5-year cumulative probability of survival was 81.8% in pregnant women (95% confidence interval [CI] 66.4–90.6) and 80.7% in nonpregnant women (95% CI 70.7–87.5) (Figure 1). The difference was not statistically significant (P = .64). In 157 HIV-infected women with mild to moderate immunosuppression at inclusion, the probability of remaining AIDS-free 5 years after inclusion was not significantly different between the two groups (Figure 2): 82.6% in pregnant women (95% CI 68.0–91.0) and 78.6% in nonpregnant women (95% CI 66.9–86.6) (P = .65). Using the 1993 AIDS case definition did not modify the findings. In 144 women presenting with CD4 greater than 200/mm3 at inclusion, the 5-year cumulative probability of the CD4 count remaining greater than 200/mm3 was 66.7% in pregnant women (95% CI 48.2–79.9) and 62.1% in nonpregnant women (95% CI 49.0–72.8) (Figure 3). This difference was not statistically significant (P = .47).
Figure 1.
Probability of survival in pregnant and nonpregnant human immunodeficiency virus–seropositive women.
Figure 2.
AIDS-free survival curve in pregnant and nonpregnant human immunodeficiency virus–seropositive women.
Figure 3.
Probability of not reaching a CD4 count less than 200/mm3 in pregnant and nonpregnant human immunodeficiency virus–seropositive women.
Within the whole study period, the average loss of CD4 was 36.8 cells per year (SE 11) in 55 pregnant women and 52.6 cells per year (SE eight) in 95 nonpregnant women, for which at least three CD4 measurements were available (P = .23). In 21 women with a measurable interval between HIV diagnosis and pregnancy, the mean loss of CD4 was 26 cells per year before pregnancy, and 52 cells per year in the 47 women with at least three measurements between pregnancy and the end of the study period.
A stratified analysis on the initial CD4 count (less than 350/mm3 and at least 350/mm3) was performed to assess whether the effect of pregnancy was not modified by the degree of immunodeficiency. It did not reveal any significant difference of progression between the two groups (Table 3).
Table 3.
Progression of Human Immunodeficiency Virus Infection in Pregnant and Nonpregnant Women After Stratification on Initial CD4 Lymphocyte Count
| Survival | CD4 <350/mm3
|
CD4 ≥350/mm3
|
||
|---|---|---|---|---|
| Pregnant (n = 20) | Nonpregnant (n = 36) | Pregnant (n = 37) | Nonpregnant (n = 78) | |
| At 1 y | 100% | 97.2% | 100% | 100% |
| At 3 y | 89.5% | 56.7% | 100% | 98.5% |
| At 5 y | 57.7% | 47.9% | 96.6% | 96.5% |
| Probability of remaining AIDS-free | CD4 <350/mm3
|
CD4 ≥350/mm3
|
||
|---|---|---|---|---|
| Pregnant (n = 18) | Nonpregnant (n = 28) | Pregnant (n = 37) | Nonpregnant (n = 74) | |
| At 1 y | 94.4% | 92.9% | 100% | 100% |
| At 3 y | 82.6% | 67.0% | 96.9% | 95.3% |
| At 5 y | 75.1% | 55.7% | 86.2% | 87.1% |
| Probability of remaining >200 CD4/mm3 | CD4 <350/mm3
|
CD4 ≥350/mm3
|
||
|---|---|---|---|---|
| Pregnant (n = 11) | Nonpregnant (n = 18) | Pregnant (n = 37) | Nonpregnant (n = 78) | |
| At 1 y | 90.9% | 71.4% | 100% | 100% |
| At 3 y | 58.9% | 51.6% | 97.0% | 87.8% |
| At 5 y | 23.6% | 41.3% | 80.5% | 66.9% |
CD4 = CD4 lymphocyte count.
The prognostic role of pregnancy was not identified for any of the judgment criteria: the hazard ratio (pregnant versus nonpregnant women) was 0.92 (95% CI 0.40–2.12) for survival, 1.02 for the progression to AIDS (95% CI 0.48–2.18), and 1.20 for a drop to less than 200 CD4/mm3 (95% CI 0.63–2.27). The following factors were significantly related to outcome in the multivariate analysis: the initial CD4 count for the progression to death, the initial CD4 count and the year of HIV infection diagnosis for the progression to AIDS, and the initial CD4 count and the year of HIV infection diagnosis (with a significant interaction between these two factors) for a drop to less than 200 CD4/mm3.
During the study period, 28 abortions were recorded in HIV-infected women at the University Hospital of Bordeaux, but none was performed for aggravation of maternal HIV infection. The mean CD4 count at the beginning of pregnancy of these 28 women (458/mm3, SD 192) was not different from the mean CD4 count in the 57 pregnant women (Student t test, P = .65).
Discussion
Some limitations of our study must be emphasized. First, it may have lacked power to detect a difference in survival because the observed progression to AIDS was smaller than expected. However, the survival analysis performed for all three end points shows that superimposable curves and CIs of the estimation of effect of pregnancy include the expected RR (2.0) only at the limit. Moreover, the depiction of the CD4 decline shows similar rates between the two groups. This rate of CD4 decline is also similar to what is currently known in HIV-infected subjects.19 Second, a selection bias may have occurred because we took into consideration only the pregnancies that resulted in live birth. We chose this because if pregnancy had a worsening effect on the course of HIV infection, this would be of particular importance in the group of women exposed to a complete gestation. To rule out selection bias during the study period (eg, abortion because of aggravation of maternal HIV infection), we compared the level of immunosuppression in HIV-positive women who had abortions and found no significant difference. Furthermore, no abortion was performed because of aggravation of HIV infection. Third, to enroll 57 pregnant women with a CD4 count available at study entry, we used the date of HIV diagnosis (rather than the beginning of pregnancy) as the date of inclusion. Furthermore, the median time interval between the diagnosis of HIV infection and delivery was only 7 months, without any event selected as judgment criteria recorded during this period. Moreover, pregnancy was used as a time-dependent covariate. Therefore, we believe that the design chosen was appropriate and that the study results allow us to determine whether pregnancy in an HIV-positive woman alters the process of HIV infection. Finally, we mainly have observed HIV-infected women with mild to moderate immunosuppression, and the results do not allow the extrapolation of our findings to women beginning their pregnancy at an advanced stage of HIV infection. The effect of pregnancy on the clinical course of HIV infection in women with AIDS or severe immunosuppression is unknown.
Both Scott et al2 in 1985 and Minkoff et al3 in 1987 suggested an adverse effect of pregnancy on the progression of HIV infection. In these two studies, 75% of 16 pregnant women and 45% of 34 pregnant women had deteriorated clinically 2 years after delivery. However, it must be stressed that these studies lacked a comparison group and that a selection bias probably occurred, because only mothers whose children revealed HIV infection were enrolled. This bias may have led to an overestimation of the risk of disease progression.
In an abstract presented in 1989, Delfraissy et al also suggested a pejorative progression of HIV infection among women after delivery, based on a prospective study (V International Conference on AIDS. Montreal, June 1989). A progression toward symptomatic HIV disease of 15% at 18 months was reported among 87 women who had delivered and of only 6% among 121 nonpregnant women. The number of women lost to follow-up and the lack of control of confounders did not allow the authors to reach a definite conclusion. Other studies have also suggested a faster deterioration in the gravid group, but the inadequate choice of a comparison group4,20 did not allow for a proper assessment of the prognostic role of pregnancy on the progression of HIV infection. Other authors are also more inclined to predict aggravation, often linked to a Pneumocystis carinii pneumonia.6,21–23
Berrebi et al9 found no difference in the clinical and biologic progression of HIV infection in a cohort of 54 HIV-positive women followed-up after delivery and a cohort of 81 nonpregnant, HIV-positive women matched for age, clinical stage, and duration of follow-up. However, the control group included women who had given birth more than 2 years before inclusion. Moreover, the women were not matched for the initial CD4, and the study suffered from a high rate of loss to follow-up. In Haiti, Deschamps et al11 showed a trend toward earlier manifestation of HIV-related symptoms among the 44 pregnant women and the 96 nonpregnant women followed-up for an average of 44 months. No significant difference was observed in the rate of progression to AIDS or death in the two groups. No CD4 was available for matching or adjustment.
Schoenbaum et al, in another 1989 abstract (V International Conference on AIDS. Montreal, June 1989), reported a progression to symptomatic HIV disease of 65% among 85 HIV-positive pregnant women and 54% among 56 nonpregnant women. The regression analysis showed only two independent prognostic factors: the duration of the symptoms preceeding pregnancy and the initial CD4; on the other hand, pregnancy was not associated with a poor prognosis. Brettle and Leen10 did not observe any prognostic role of one or several pregnancies on the progression of HIV infection in 107 women, including 31 who became pregnant after seroconversion. Other studies have also suggested the absence of aggravation within the gravid group, but the limited number of patients8 and the absence of an adequate comparison group6 did not permit firm conclusions.
In a cohort of HIV-infected women with mild to moderate immunosuppression, pregnancy did not accelerate progression to AIDS or death. Therefore, this risk should not influence the advice given to HIV-positive women willing to have a child. However, counseling must take into account the risk of transmission to an HIV-negative partner when having unprotected sexual intercourse, the risk of maternal-fetal transmission, even with zidovudine,24 and also the parental future.
Acknowledgments
Thanks to Daniele Douard, MD (CISIH, Bordeaux) for providing updated information on medical records.
Dr. Rachid Salmi (Unité INSERM 330) provided statistical advice.
This study was funded in part by the Agence Nationale de Recherches sur le SIDA (ANRS), Paris, France.
References
- 1.Mandelbrot L, Henrion R. Does pregnancy accelerate disease progression in HIV-infected women? In: Johnson MA, Johstone FD, editors. HIV infection in women. Edinburgh: Churchill Livingstone; 1993. pp. 157–71. [Google Scholar]
- 2.Scott GB, Fischl MA, Klimas N, Fletcher MA, Dickinson GM. Mothers of infants with the acquired immunodeficiency syndrome. JAMA. 1985;253:363–6. [PubMed] [Google Scholar]
- 3.Minkoff H, Nanda D, Menez R, Fikrig S. Pregnancies resulting in infants with acquired immunodeficiency syndrome or AIDS-related complex: Follow-up of mothers, children, and subsequently born siblings. Obstet Gynecol. 1987;69:288–91. [PubMed] [Google Scholar]
- 4.Lindgren S, Anzen B, Bohlin AB, Lidman K. HIV and childbearing: Clinical outcome and aspects of mother to infant transmission. AIDS. 1991;5:1111–6. [PubMed] [Google Scholar]
- 5.Schafer A. The effects of pregnancy on the natural evolution of HIV infection. The experience of Berlin. In: Berrebi, editor. HIV infection in mother and child. Paris: Privat; 1988. pp. 127–47. [Google Scholar]
- 6.Chiodo F, Ricchi E, Costigliola P, Michelaci L, Bovicelli L. Effects of HIV infection on the pregnancy. In: Berrebi A, editor. HIV infection in mother and child. Paris: Privat; 1988. pp. 177–89. [Google Scholar]
- 7.Ciraru-Vigneron N, Nguyen Tan Lung R, Brunner C, Boizard B, Deschamps JF. Etude prospective des femmes enceintes HIV positives. A propos de 60 nouveaux cas dépistés entre 1986 et 1987. In: Berrebi A, editor. Infection in mother and child. Paris: Privat; 1988. pp. 221–3. [Google Scholar]
- 8.Selwyn PA, Schoenbaum EE, Davenny K, et al. Prospective study of human immunodeficiency virus infection and pregnancy outcomes in intravenous drug users. JAMA. 1989;261:1289–94. [PubMed] [Google Scholar]
- 9.Berrebi A, Kobuch WE, Puel J, Tricoire J, Herne P. Influence of pregnancy on human immunodeficiency virus disease. Eur J Obstet Gynecol Reprod Biol. 1990;37:211–7. doi: 10.1016/0028-2243(90)90027-x. [DOI] [PubMed] [Google Scholar]
- 10.Brettle RP, Leen CLS. The natural history of HIV and AIDS in women. AIDS. 1991;5:1283–92. doi: 10.1097/00002030-199111000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Deschamps MM, Pape JW, Desvarieux M, et al. A prospective study of HIV-seropositive asymptomatic women of childbearing age in a developing country. J Acquir Immune Defic Syndr. 1993;6:446–51. [PubMed] [Google Scholar]
- 12.Chêne G, Dabis F, Salamon R GECSA group. Hospital-based surveillance of HIV infection: Epidemiological trends, Bordeaux, France, 1983–1989. Eur J Epidemiol. 1992;8:58–66. doi: 10.1007/BF03334973. [DOI] [PubMed] [Google Scholar]
- 13.Morlat P, Parneix P, Douard D, et al. Women and HIV infection: A cohort study of 483 HIV-infected women, Bordeaux, France, 1985–1991. AIDS. 1992;6:1187–93. [PubMed] [Google Scholar]
- 14.Dormont J. Prise en charge des personnes atteintes par le VIH. In: Dormont J, editor. Rapport au Ministre. Paris: Flammarion Médecine-Sciences; 1993. p. 136. [Google Scholar]
- 15.Centers for Disease Control. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR. 1992;41(RR-17):19. [PubMed] [Google Scholar]
- 16.Direction Générale de la Santé. Révision de la définition du SIDA en France. Bull Epidemiol Hebd. 1993:47–8. [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete samples. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 18.Cox DR, Oakes D. Analysis of survival data. London: Chapman & Hall; 1984. [Google Scholar]
- 19.Moss AR, Bacchetti P, Osmond D, et al. Seropositivity for HIV and the development of AIDS or AIDS related conditions: Three year follow up of the San Francisco General Hospital Cohort. BMJ. 1988;296:745–50. doi: 10.1136/bmj.296.6624.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koonin LM, Ellerbrock TV, Atrash HK, Rogers MF, Smith JC. Pregnancy-associated deaths due to AIDS in the United States. JAMA. 1989;261:1306–9. [PubMed] [Google Scholar]
- 21.Landesman SH. Human immunodeficiency virus infection in women: An overview. Semin Perinatol. 1989;13:2–6. [PubMed] [Google Scholar]
- 22.Cohen M, Blanc B. Conduite à tenir chez une femme enceinte séropositive et chez une femme enceinte séronégative dont le partenaire est séropositif. In: Blanc B, editor. Infections à VIH et reproduction. Paris: Arnette; 1991. pp. 148–66. [PubMed] [Google Scholar]
- 23.Gillet JY, Bongain A, Fuzibet JG, Pesce A. Grossesse chez la femme infectée par le VIH. Presse Med. 1992;21:165–70. [PubMed] [Google Scholar]
- 24.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N Engl J Med. 1994;331:1173–80. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]