Abstract
In honeybees, two olfactory conditioning protocols allow the study of appetitive and aversive Pavlovian associations. Appetitive conditioning of the proboscis extension response (PER) involves associating an odor, the conditioned stimulus (CS) with a sucrose solution, the unconditioned stimulus (US). Conversely, aversive conditioning of the sting extension response (SER) involves associating the odor CS with an electric or thermal shock US. Each protocol is based on the measure of a different behavioral response (proboscis versus sting) and both only provide binary responses (extension or not of the proboscis or sting). These limitations render the measure of the acquired valence of an odor CS difficult without testing the animals in a freely moving situation. Here, we studied the effects of both olfactory conditioning protocols on the movements of the antennae, which are crucial sensory organs for bees. As bees’ antennae are highly mobile, we asked whether their movements in response to an odorant change following appetitive or aversive conditioning and if so, do odor-evoked antennal movements contain information about the acquired valence of the CS? We implemented a tracking system for harnessed bees’ antennal movements based on a motion capture principle at a high frequency rate. We observed that differential appetitive conditioning had a strong effect on antennal movements. Bees responded to the reinforced odorant with a marked forward motion of the antennae and a strong velocity increase. Conversely, differential aversive conditioning had no associative effect on antennal movements. Rather than revealing the acquired valence of an odorant, antennal movements may represent a novel conditioned response taking place during appetitive conditioning and may provide a possible advantage to bees when foraging in natural situations.
In order to survive, animals must detect and integrate environmental signals to adapt their behavior when facing potentially positive (food, sex-mate) or negative (danger, predator) situations (Alcock 1997). These adaptive behaviors are for the most part acquired through experience. Through associative learning, animals learn associations between a particular behavioral response and its consequence (operant learning; Skinner 1936) or between initially neutral environmental (color, sound, odor) stimuli and other meaningful (food, danger, etc.) stimuli (classical or Pavlovian learning; Pavlov 1927).
Classical conditioning has been intensively studied in many species from mammals to invertebrates (Rescorla 1988; Crow 2004; Busto et al. 2010). Among invertebrates, the honeybee Apis mellifera represents an influential and biologically relevant model for studying associative learning. Learning is an essential part of their daily behavior, especially while foraging when they must learn and memorize floral odors or colors (Giurfa 2007; Menzel 2012). Pavlovian learning can be effectively studied in the laboratory thanks to the development of two main olfactory conditioning assays performed on restrained individuals. The most prominent learning assay developed for honeybees is the olfactory conditioning of the proboscis extension response (PER), in which bees learn to associate an initially neutral odor (conditioned stimulus—CS) with a sucrose reward (unconditioned stimulus—US) applied to the antennae and then to the proboscis (Bitterman et al. 1983; Giurfa and Sandoz 2012). Following conditioning, bees extend their proboscis in response to the odor alone (Takeda 1961; Bitterman et al. 1983). The odorant thus acquires a positive valence and becomes attractive to bees so that in a free-moving situation, they will orient toward this stimulus (Sandoz et al. 2000; Chaffiol et al. 2005; Carcaud et al. 2009). Another important classical conditioning procedure, the olfactory conditioning of the sting extension response (SER) was developed only recently (Vergoz et al. 2007). In this procedure, the odor CS is associated with an aversive US (electric shock: Vergoz et al. 2007; thermal shock: Junca et al. 2014). Once the association has been made, bees extend their sting to the aversively reinforced odor alone. The odor CS thereby acquires a negative valence and bees clearly avoid it in a freely moving test (Carcaud et al. 2009). Both types of conditioning allow the use of invasive techniques such as electrophysiology, optical imaging, and pharmacology enabling us to understand the behavioral, cellular, and molecular basis of appetitive and aversive learning, respectively (Giurfa and Sandoz 2012; Menzel 2012; Tedjakumala and Giurfa 2013).
In standard PER and SER conditioning procedures, responses are stereotyped and operate in a binary “all or nothing” fashion (extension or not of the proboscis or sting) (Bitterman et al. 1983; Vergoz et al. 2007). Therefore, they do not allow a graded measure of learning success or a precise measure of the acquired valence of an odorant at the individual level. For this reason, studies using PER or SER conditioning usually discuss individual performances from response proportions in groups of bees, which has been criticized (Pamir et al. 2011). Moreover, when using restrained animals, positive and negative valences have to be studied based on totally different behavioral responses (PER or SER), thereby inducing a potential bias. Therefore, we asked whether the movements of other body parts may indeed reveal and integrate both the positive and the negative acquired values of odorants. We focused on honeybee's antennae, which are highly mobile sensory structures displaying a wide range of possible movements around the bee’s head.
Many insects use antennal movements to acquire crucial sensory information about their surroundings. As for other insects, the honeybee antenna is a prominent interface between the individual and its environment as it contains complex sensory equipment tuned to different sensory modalities (olfactory, gustatory, thermosensory, mechanosensory, etc.) (Lacher and Schneider 1963; Lacher 1964; Vareschi 1971; Esslen and Kaissling 1976; Whitehead and Larsen 1976; Dreller and Kirchner 1993). Honeybees use their antennae in a great variety of behavioral tasks and contexts. Inside the hive, the bees’ antennae allow them to probe food, wax, or other substrates (Martin and Lindauer 1966; Winston 1987; Nagari and Bloch 2012) and to communicate with conspecifics, during food exchanges (Free 1956; Montagner and Pain 1971; Galliot and Azoeuf 1979; Galliot et al. 1982; Korst and Velthuis 1982; Crailsheim 1998) or the waggle dance (von Frisch 1967; Rohrseitz and Tautz, 1999; Gil and De Marco, 2010). Outside of the hive, bees use their antennae during foraging, allowing them to detect and learn multisensory cues from flowers (olfactory, tactile, gustatory) (Kevan and Lane 1985; Menzel 1990; Wright and Schiestl 2009). Therefore, the honeybee antennae are crucial, highly mobile sensory organs, whose movements are essential to their sensory ecology and behavior. One may thus ask whether bees’ antennal movements are affected by previous associative experience, and if so, if these movements contain information about the acquired appetitive or aversive value of an odorant.
Previous work used electrophysiological recordings or photodiodes to study honeybees’ antennal movements in response to visual, olfactory, or tactile stimuli (Suzuki 1975; Erber and Schildberger 1980; Erber et al. 1993). Typically, bees exhibit an antennal scanning behavior in response to sugar stimulation or to odorants, characterized by sweeping movements from the front to the back of the head (Erber et al. 1993). The advent of video capture provided more precise spatial information about antennal movements. The first such study, using marked antenna tips, demonstrated that antennal movements can be operantly conditioned, by rewarding contacts of the antenna with an object with sucrose solution (Erber et al. 1997; see also Erber et al. 1998, 2000; Kisch and Erber 1999; Haupt 2007). Several studies since then used video means to measure antennal movements but they mostly concentrated on the technical aspects of such recordings (Lambin et al. 2005; Mujagić et al. 2012) or aimed to monitor bees’ sleep state (Sauer et al. 2003, 2004; Hussaini et al. 2009). Until now, no in-depth study has addressed the possible plasticity of antennal movements following olfactory Pavlovian conditioning.
In the present study, we thus aimed to determine the influence of an appetitive or an aversive olfactory learning procedure, assigning a positive or a negative valence to an odorant, on bees’ antennal responses. We thus implemented an original antenna tracking system based on a motion capture principle (Erber et al. 1997) enabling us to record the antennal movements from harnessed bees, at a high frequency rate (90 Hz). We show that olfactory learning can indeed strongly modify antennal movements to odorants.
Results
Measure of antennal response to odorants
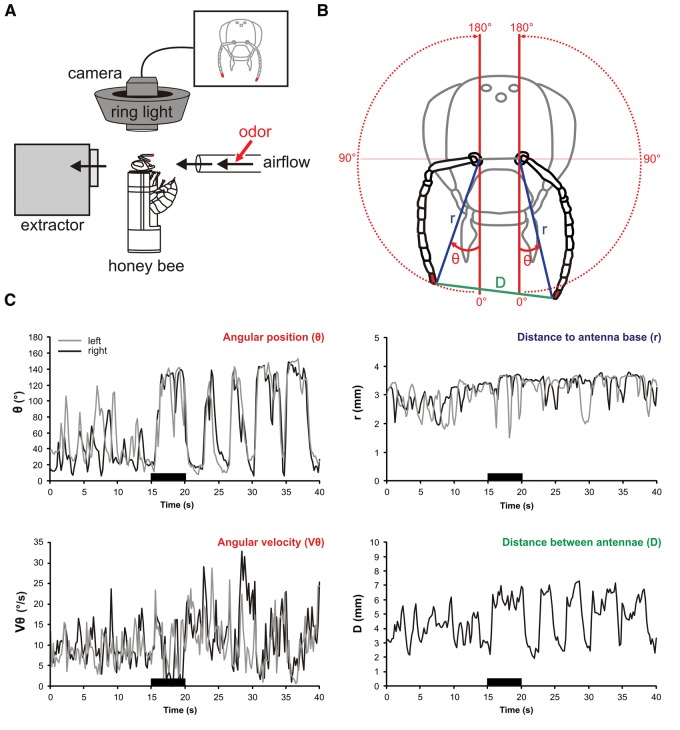
To monitor antennal movements in harnessed honeybees, a camera-based tracking system using a motion capture principle was placed above the bee's head (Fig. 1A). The upper sides of the bees’ antenna tips were marked with small dots of red acrylic paint. The system was tuned to this red color and was able to track the location of both antenna tips at a frequency of 90 Hz. Bees’ antennae are highly mobile and can move around their socket (henceforth termed “antenna base”) from the front of their head to the rear on each side (traveling an ∼180° angle). Therefore, the position of each antenna tip was best described using polar coordinates, i.e., by a radius (r) and an angle (θ) with the center being the antenna base (Fig. 1B). The radius r was defined as the distance between antenna tip and base while the angle θ was measured from the front (0°) to the back of the bee (180°) via the ipsilateral side (90°). From these values, the angular velocity (Vθ) as well as the distance between both antenna tips (D) could be calculated. An odor-stimulation trial lasted 40 s. After 15 sec of an odorless airflow, a 5-sec odorant stimulation was applied. Figure 1C presents the recording of the four variables during an odorant stimulation trial in a naïve bee (for average values on groups of bees see Figs. 4C,D, 7C,D; Supplemental Fig. S1, S2). Typically, bees’ antennal movements displayed stronger variations in angle than in radius, their antennae oscillating between the front (∼10°) and a position at the back of their head (here ∼140°). The presentation of a pure odorant usually induced a slight backward motion of the antennae, as shown by an increase in the angle (θ) and in the distance between both antennae (D) during odor delivery.
Figure 1.
Antennal movement recording. (A) Apparatus for recording antennal movements. Harnessed bees were placed in a dark room, under a cold light ring encircling a camera which recorded the coordinates of both antennal tips at a rate of 90 Hz. Olfactory stimulation was delivered to the bee from the front and an air extractor placed behind the animal prevented odorant accumulation. (B) Representation of the variables measured from antennal tip positions: (blue) distance to antenna base (r); (red) angular position (θ); (green) distance between both antennal tips (D). (C) Recordings taken before conditioning in response to 1-hexanol (black bar) for an individual bee. The same variables as in B are shown for this bee's two antennae (black line, right antenna; gray line, left antenna), with the addition of the angular velocity (Vθ) calculated from the angular position (θ).
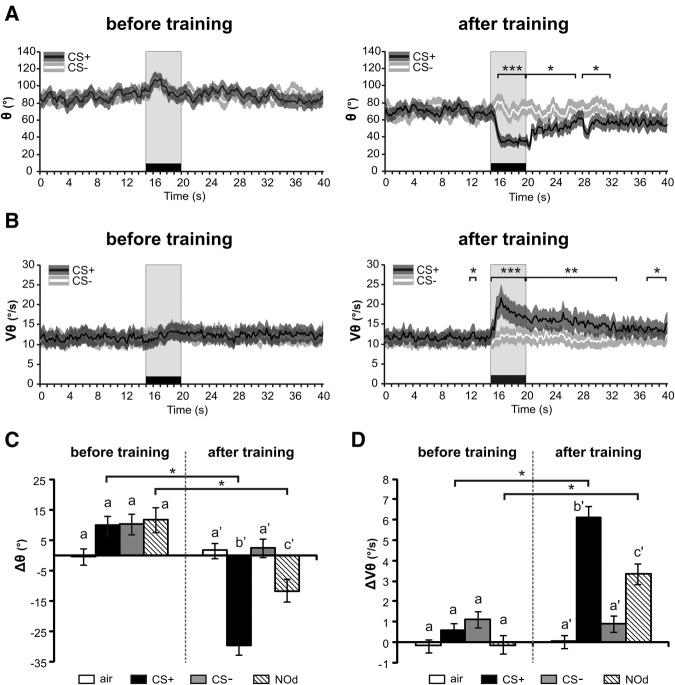
Figure 4.
Effect of appetitive conditioning on antennal responses to odors. (A,B) Temporal variation curves (averaged every 200 msec) before and after training for (A) antenna angular position (θ) and (B) angular velocity (Vθ). After training appetitive conditioning induced a forward motion of the antennae with an antenna acceleration. Stars indicate significant differences between CS+ and CS− in paired t-tests performed at every second of the recordings ((*) P < 0.05; (**) P < 0.01; (***) P < 0.001). (C,D) Histograms showing the change in (C) angular position (Δθ) or (D) angular velocity (ΔVθ) during odor presentation (during–before odor) for the air control (white), the CS+ (black), the CS− (light gray) and the novel odorant (NOd, stripes), before and after conditioning. Before conditioning, any olfactory stimulation led to a backward motion of the antennae, whereas after conditioning the CS+ but not the CS− induced a forward motion of the antennae (C, Δθ). Conditioning also induced an increase in antenna velocity for the CS+ but no for the CS− (D, ΔVθ). Both effects generalized to the novel odorant (NOd) but on a smaller scale. Stars and different letters in C and D indicate significant differences in paired t-tests including a threshold correction for multiple comparisons (P < αcorr1 = 0.0125, N = 44).
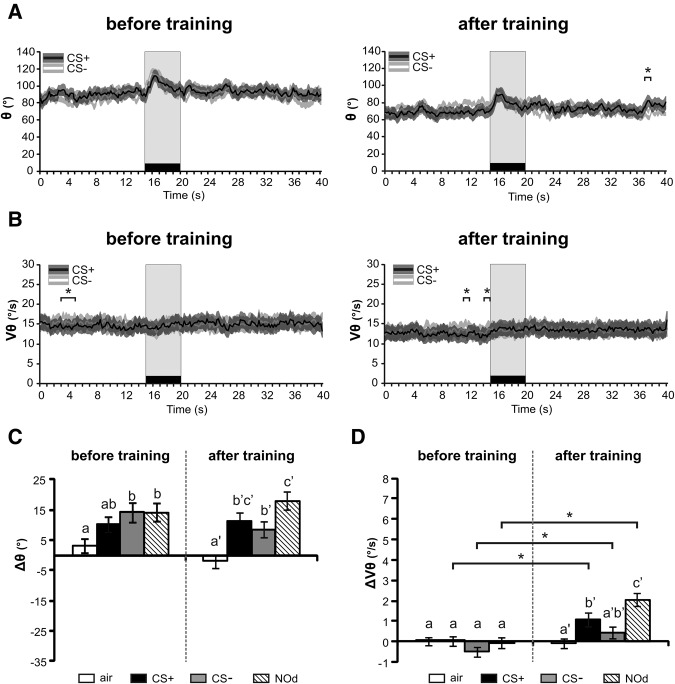
Figure 7.
Effect of aversive conditioning on antennal responses to odors. (A,B) Temporal variation curves (averaged every 200 msec) before and after training for (A) antenna except in very few instances either before or long after the stimulus angular position (θ) and (B) angular velocity (Vθ). No difference appeared between CS+ and CS− (stars, paired t-tests at every second of the recordings (* P < 0.05)). (C,D) Histograms showing the change in C angular position (Δθ) or D angular velocity (ΔVθ) during odor presentation (during–before odor) for the air control (white), the CS+ (black), the CS− (light gray) and the novel odorant (NOd, stripes), before and after conditioning. All odorants induced a backward antenna motion both before and after training and antenna velocity increased after training for all odorants. No associative (i.e., CS+ specific) effect of aversive conditioning was observed. Different letters indicate significant differences in paired t-tests performed either before or after conditioning (P < 0.05). Stars and different letters indicate significant differences in paired t-tests (P < αcorr1 = 0.0125, N = 68).
Olfactory learning performances
To assess how olfactory learning with different reinforcements impacts antennal movements to odorants, bees were subjected either to an appetitive (PER) or to an aversive (SER) differential conditioning procedure. In both cases, bees had to differentiate between a reinforced odorant (CS+) and a nonreinforced odorant (CS−). Bees received 6 CS+ and 6 CS− trials in a pseudorandomized order with 10 min inter-trial intervals. Antennal movements in response to a panel of stimuli were measured 1 h before and 1 h after conditioning. During each of these test sessions, the responses to the CS+, to the CS−, to a novel odorant and to an air control were measured in a randomized order (see Materials and Methods).
PER conditioning
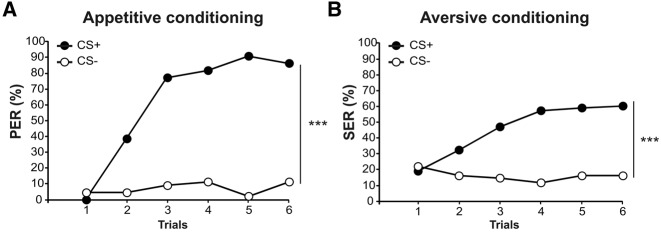
Differential conditioning of the PER was performed to evaluate the effect of appetitive learning on bees’ antennal movements (Fig. 2A, N = 44). In this procedure, bees learned to differentiate between the odorant paired with sucrose reward (CS+) and the nonreinforced odorant (CS−) in the course of training (RM-ANOVA: trial × stimulus interaction, F(5,215) = 33.5, P < 0.001). Responses to the CS+ increased significantly, from 0% at the first trial to 86% at the sixth trial (RM-ANOVA, “trial” effect, F(5,215) = 46.3, P < 0.001), whereas responses to the CS− remained stable, between 5% and 11% (RM-ANOVA, trial effect, F(5,215) = 1.47, NS). Overall, 75% of the bees (33 out of 44) responded only to the CS+ and not to the CS− at the sixth trial.
Figure 2.
Appetitive and aversive conditioning performances. Acquisition curves are shown for bees trained in (A) an appetitive or (B) an aversive differential conditioning protocol. The curves show the percentage of individuals eliciting a behavioral response (proboscis extension in A, sting extension in B) to the reinforced odorant (CS+) or the nonreinforced one (CS−) along the trials. All bees learned to discriminate the reinforced odorant from the nonreinforced one, both in appetitive and aversive conditioning ((***) P < 0.001; appetitive: N = 44; aversive: N = 68).
SER conditioning
Differential conditioning of the SER was performed to evaluate the impact of aversive learning on bees’ antennal movements (Fig. 2B, N = 68). In this procedure, bees learned to discriminate the odorant paired with a thermal shock (CS+) from the nonreinforced odorant (CS−) (RM-ANOVA, trial × stimulus interaction, F(5,335) = 15.2, P < 0.001). The percentage of SER to the CS+ increased significantly, from 19% at the first trial to 60% at the sixth trial (RM-ANOVA, trial effect, F(5,335) = 18.9, P < 0.001), whereas responses to the CS− did not change and remained between 12% and 22% (RM-ANOVA, trial effect, F(5,335) = 0.95, NS). Overall, 44% of bees (30 out of 68) performed correctly at the sixth trial, responding only to the CS+ and not to the CS−.
Bees thus learned to discriminate the reinforced from the nonreinforced odorant in appetitive and aversive conditioning tasks. As observed in previous studies (Vergoz et al. 2007, Carcaud et al. 2009), performances were lower in SER than in PER conditioning.
Effect of appetitive learning on antennal movements
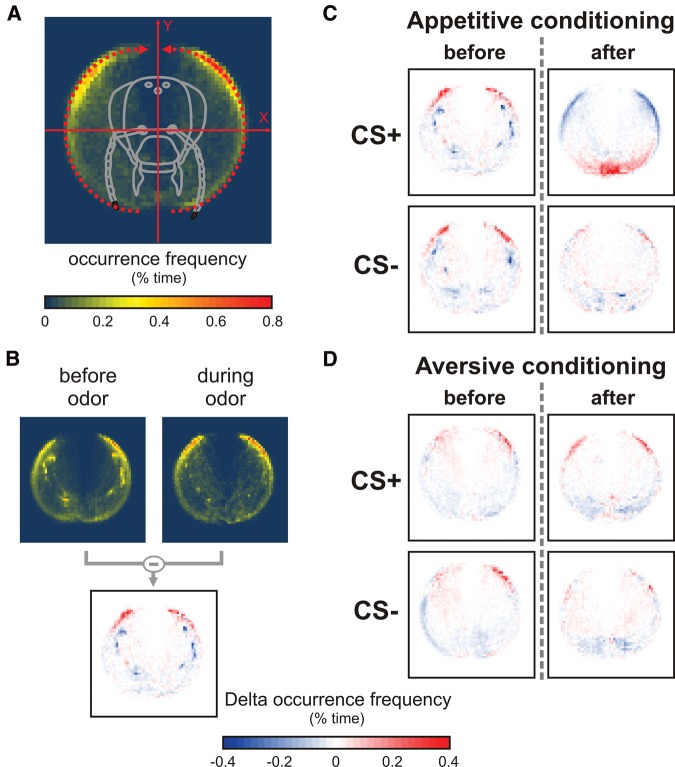
To reveal the effect of olfactory learning on antennal movements, we first computed maps of antennal tip occurrence before and during odor presentations (Fig. 3). In such maps, a color scale from blue to red indicates how often (in % of total time) bees’ antenna tips were positioned at each location (Fig. 3A). As the recordings of all tested bees were calculated in the same coordinate system, all the maps obtained for a group of bees could be overlaid. As shown in the map in Figure 3A, the field of space covered by antennal movements generally formed two crescents on each side of the bee’s head. To observe how antenna tips moved during odor presentations, the map obtained “before” odor presentation was subtracted from the map during odor presentation (Fig. 3B). In the resulting maps, red color showed locations where antenna tips were present more often during odor presentation, while blue color coded locations where antenna tips were present less often. Figure 3C shows such maps for the CS+ and CS− in the recordings performed before and after appetitive conditioning. Before conditioning, the antennae were mostly moving to the rear of the head during odor delivery (for both CS+ and CS−). After appetitive conditioning, a drastic change was observed in the response to the CS+: the bees’ antennae were now moving mostly to the front. Such a strong change in antenna location was not discernible for the CS−, although antenna location seemed slightly more evenly distributed after conditioning (Fig. 3C).
Figure 3.
Heatmap of antennal tip occurrence before and after conditioning. (A) The space explored by bees’ antenna oscillations during odor presentation was calculated by counting the number of times each antennal tip was found at each location. The occurrence frequency at each location is expressed as a percentage of all the recorded occurrences, and displayed following a color scale from dark blue to red. (B) Maps of antenna location change are computed by subtracting the map obtained “before” from the map “during” odor. Such maps are color coded, with blue showing a reduction and red showing an increase in frequency, respectively. (C,D) Heatmaps showing the change in occurrence rate of antennal tips during CS+ and CS− presentation, either 1 h before or 1 h after C an appetitive (N = 44 bees) or D an aversive conditioning (N = 68 bees). The space explored during CS+ presentation after appetitive conditioning differed clearly from the one observed before conditioning, high occurrence areas being located mostly forward. Such a modification was not discernible for the CS−, and no clear change was observed for aversive conditioning.
This strong modification in antennal movements was also striking when observing the mean angular position (Fig. 4A) and velocity (Fig. 4B) throughout a CS+ or CS− recording (N = 44 bees). Before appetitive training, odor presentations induced a slight increase in the angle, i.e., a slight backward motion of the antennae (Fig. 4A, left) with almost no change in antenna velocity (Fig. 4B, left). After training, antenna angle decreased strongly when the CS+ was presented. Conversely, almost no change was observed when the CS− was presented (Fig. 4A, right). This differential effect of CS+ and CS− was significant from 1 s after odor onset until 12 sec after odor offset (paired t-test, t > 2.59, P < 0.05; except the eighth second, t = 1.88, P = 0.07). In addition, antenna velocity strongly increased in response to the CS+, but not to the CS− (Fig. 4B, right). This difference in velocity between CS+ and CS− started on the first second after odor onset until 13 sec after odor offset (paired t-test, t > 3.19, P < 0.01).
To analyze these effects more systematically, we computed Δθ and ΔVθ, defined as the difference in average angular position and velocity between 5 sec during and 15 sec before odorant presentation, for the CS+, the CS−, the novel odorant (NOd) and the air control (Fig. 4CD, N = 44 bees). The change in antennal angular position (Δθ) before and after conditioning was significantly affected by the type of stimuli (Fig. 4C, RM-ANOVA, stimulus × recording interaction, F(3,129) = 16.5, P < 0.001). Before conditioning, the three odorants induced a slight backward motion of the antennae (a positive Δθ) which, compared with the air control, fell just short of significance considering the corrected threshold (paired t-tests, t > 2.46, 0.05 > P > αcorr1 = 0.0125). After conditioning, antennal response to the CS+ was characterized by a 29° forward movement as opposed to a 10° backward movement before conditioning (paired t-test, t = 8.65, P < 0.001). In contrast, the CS− still induced a slight backward movement after conditioning (3°, t = 1.78, NS). Interestingly, the angular response to the CS+ generalized to a novel odorant (NOd) but on a smaller scale. NOd led to a 11° forward movement after conditioning compared with a 12° backward movement before conditioning (paired t-test, t = 4.56, P < 0.001). The angular response to the CS+ after conditioning was significantly different from those to the CS− (paired t-test, t = 7.39, P < 0.001) and NOd (paired t-test, t = 4.07, P < 0.001).
Angular velocity variation (ΔVθ) followed a similar pattern as angular position variation (Δθ), with a differential change for the different odorants between before and after conditioning (Fig. 4D, RM-ANOVA, stimulus × recording interaction, F(3,129) = 21.0, P < 0.001). Before conditioning, odorants did not induce any significant change in angular velocity compared with the air control (paired t-test, t < 2.51, P > 0.0125). Angular velocity variation (ΔVθ) during CS+ stimulation increased from 0.57°/sec before conditioning to 6.09°/sec after conditioning (paired t-test, t = 7.85, P < 0.001). In contrast, velocity variation was stable for the CS− from 1.11°/sec to 0.90°/sec (paired t-test, t = 0.35, NS). The acceleration effect observed for the CS+ generalized to the novel odorant, with a ΔVθ of −0.13°/sec before conditioning and 3.36°/sec after conditioning (paired t-test, t = 5.58, P < 0.001). The velocity increase for the NOd was however significantly smaller than that observed for the CS+ (paired t-test, t = 3.72, P < 0.001).
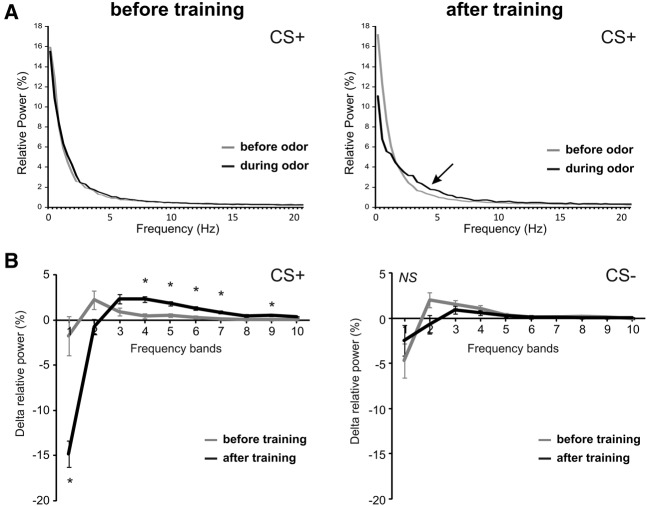
The data above have shown that appetitive differential conditioning modified the angular position and the angular velocity of the antennae. As antennal movements are characterized by back-and-forth oscillations (see angular position graph in Fig. 1C), we next used a frequency analysis, based on a fast Fourier transform (FFT), to explore movement frequency modifications with learning (Fig. 5). When used on the angular position data (θ), this analysis extracts the oscillating power at different frequencies (integrating both number and angular amplitude of oscillations). Figure 5A presents the average frequency spectrum obtained for the CS+ before and during odor presentation (2.84 sec each, see Materials and Methods), before appetitive conditioning (left panel) or after conditioning (right panel). First, these graphs show that antenna oscillatory movements are best described between 0 and 10 Hz, with most of the oscillating power in this frequency range. Second, they show that while odor presentation did not modify the frequency spectrum before conditioning, a strong change was observed after conditioning, with a relative decrease of movements at low frequency and an increase of movements at higher frequencies during odor presentation (see arrow in Fig. 5A). To study this effect statistically, we next compared the change in the power of antennal movements (Delta relative power: during–before odor, in percentage) at 10 frequency bands from 0.35–1.41 Hz (band 1) to 9.84–10.90 Hz (band 10). Note that the exact frequency values for each band are dependent on the recording frequency, in our case 90 Hz (see Materials and methods). Figure 5B presents the Delta power of antennal movements for the CS+ and for the CS−. The frequency spectrum in response to the CS+ was significantly modified after conditioning, with a dissimilar effect at the different frequency bands (RM-ANOVA, recording × band interaction, F(9,387) = 21.3, P < 0.001). Thus, after conditioning, antennal movements were significantly reduced at band 1 (paired t-test, t = 5.01, P < 0.001) and increased at bands 4–7 and 9 (t > 3.61, P < αcorr2 = 0.005). In contrast, appetitive learning did not modify antenna oscillation frequency for the CS− (RM-ANOVA, recording × bands interaction, F(9,387) = 1.65, NS).
Figure 5.
Effect of appetitive conditioning on antennae oscillating frequency. (A) Frequency spectrum of antennal movements to the CS+ obtained with a fast Fourier transform (FFT) on angular position (θ), before (gray line) and during (black line) odor presentation, before (left) and after (right) conditioning. After conditioning, the frequency of antenna oscillations changed toward higher frequencies (arrow). (B) Change in oscillation frequency (Delta relative power) between during and before odor presentation for the CS+ (left) and CS− (right), before (gray line) and after (black line) conditioning. For statistical analysis, frequencies are grouped in 10 bands from 0.35–1.41 Hz (band 1) to 9.84–10.90 Hz (band 10). Oscillation frequency changed significantly for the CS+ but not for the CS−. In response to CS+, antennal movements at low frequency were reduced (band 1) while movements at higher frequencies (bands 4–7 and 9) were increased ((*) P < αcorr2 = 0.005, N = 44).
Antennal movements being mostly symmetrical, a forward movement as the one observed above for Δθ (Fig. 4A,C) brings both antennae significantly closer to each other during CS+ presentations. Accordingly, variations in the distance between antennae (ΔD) followed the same pattern as the angular position (Δθ) (Supplemental Fig. S1A, RM-ANOVA, interaction stimulus × recording, F(3,129) = 18.7, P < 0.001). In contrast, as the bees’ antennae are mostly extended throughout the experiment, appetitive conditioning had no effect on the variation of the distance to the antenna base (Δr, Supplemental Fig. S1B, RM-ANOVA, F(3,129) = 0.95, NS).
Co-occurrence of PER and forward antennal movements
Bees show a forward-oriented antenna response to the CS+ and, in some cases, to a novel odorant after appetitive training. This pattern of responses is very similar to that observed with PER (Fig. 6A). We may therefore ask whether the two responses co-occur. To answer this question, we aimed to compare antennal responses of bees responding or not to an odorant with a PER. Appetitive learning was very effective so that 89% of the bees were learners, responding with a PER to the CS+ and not to the CS− during the tests after conditioning (Fig. 5A). The sample sizes for comparing antennal responses of bees responding or not to the CS+ were too unbalanced for proper statistical comparison (Fig. 6A, n = 1 versus n = 43, respectively). However, in learner bees, roughly half of them responded to the novel odorant (NOd) (59%, 23 out of 39, Fig. 5A). This provided a good opportunity to evaluate the possible co-occurrence of PER and antenna response on two similarly sized groups of animals (Fig. 6B, “PER generalizers,” n = 23 versus “PER nongeneralizers,” n = 16). If PER and antenna responses co-occur, these two groups should show the same antennal behavior for the CS+, the CS− and the air, but not for the NOd. This is exactly what we observed for the angular position. In both subgroups, Δθ strongly decreased for the CS+, but not for the CS− or the air control, without any difference between subgroups for these stimuli after conditioning (t-test, t < 0.92, NS). In contrast, the PER generalizers showed a strong decrease in Δθ for the NOd, while the PER nongeneralizers did not. Accordingly, Δθ for the NOd was different between subgroups after conditioning (t-test, t = 2.85, P < 0.01). A different pattern was however observed when considering the change in angular velocity (ΔVθ, Fig. 6C). As described above, no difference between groups was found in the velocity responses to the CS+, CS−, and air (t-test, t < 1.60, NS). Yet, the velocity response was also not significantly different between subgroups for the NOd (t-test, t = 1.15, NS). Indeed, a significant velocity increase to the NOd with conditioning was observed for PER generalizers (paired t-test, t = 4.56, P < 0.001) and nongeneralizers alike (paired t-test, t = 3.06, P < 0.01). We conclude that the acquired forward motion of the antennae to an odorant, but not the acquired velocity increase, co-occur with conditioned PER.
Figure 6.
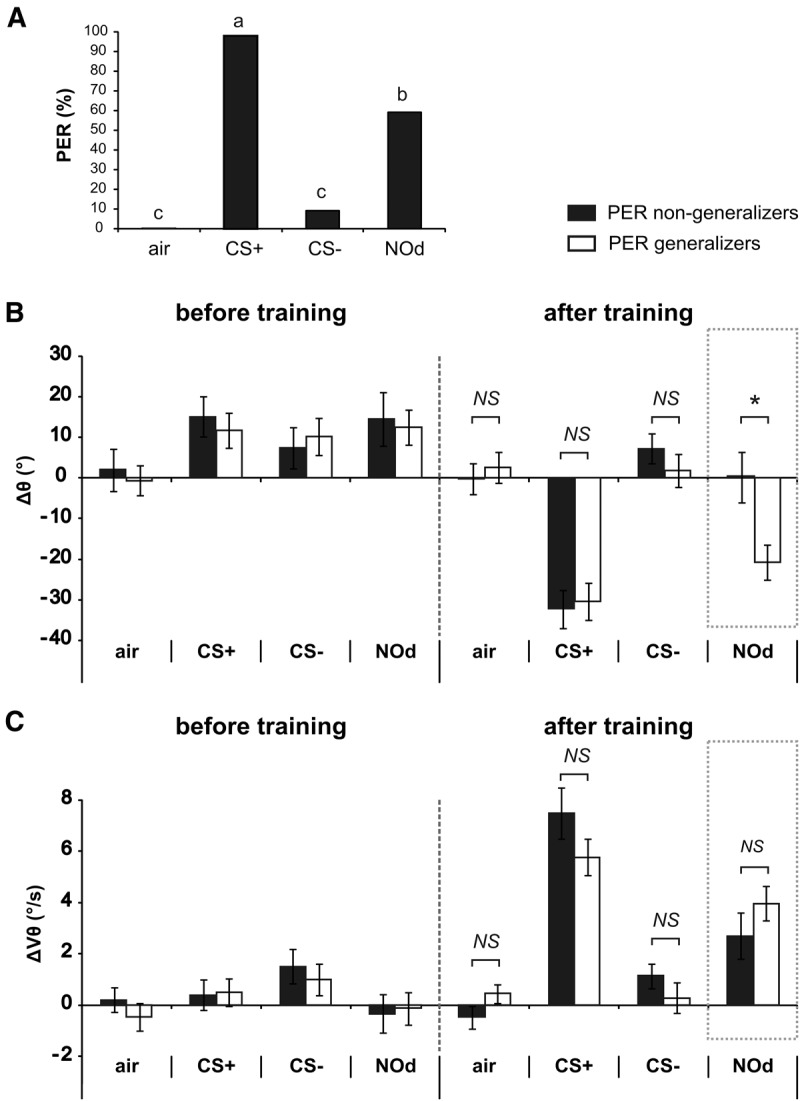
Antennal movement variation as a function of PER generalization to the novel odorant after appetitive conditioning. (A) Proportion of PER recorded to the air control, the CS+, the CS−, and the novel odorant (NOd) in the recording session following training. According to learner bees’ responses to the NOd, two subgroups were made: generalizers and nongeneralizers. (B,C) Histogram showing the change in B angular position (Δθ), and C angular velocity (ΔVθ) during odor presentation (during–before odor) for the air control, the CS+, the CS−, and the novel odorant (NOd) in individuals that extended their proboscis in response to NOd (generalizers, white, N = 23) and the ones that did not (nongeneralizers, black, N = 16). A difference in the angular position response appeared between subgroups only for the NOd (t-test, P < 0.05), not for the CS+, the CS− or the air control. No difference appeared between subgroups for the angular velocity.
Effect of aversive learning on antennal movements
The general effect of aversive olfactory learning on antennal movements can be observed on the maps showing the changes in antennal tip location for presentations of the CS+ and CS−, before and after conditioning (Fig. 3D). As observed previously (Fig. 3C), before conditioning, the antennae were mostly located at the rear of the head during odor delivery (for both CS+ and CS−). In contrast to appetitive conditioning, no drastic change was observed in the response to the CS+ or CS− after aversive conditioning: the bees’ antennae remained at the rear of the head, although for both odorants antenna tips appeared slightly more evenly distributed than before conditioning (Fig. 3D).
These observations were confirmed by the measure of the mean angular position (Fig. 7A) and velocity (Fig. 7B) throughout a CS+ or CS− trial (N = 68 bees). Before aversive conditioning, the odorant stimulation induced an increase in the angular position (Fig. 7A, left), as observed before appetitive conditioning (Fig. 4A, left). After aversive conditioning, the same change in angle as before conditioning was observed, for both the CS+ and CS− (Fig. 7A, right). Antenna angular velocity did not appear to change before conditioning, and only a slight increase during odor presentation was seen after conditioning (Fig. 7B, right). No significant difference in angular position or velocity appeared between CS+ and CS−, neither during odor presentation (paired t-test, angular position: t < 0.63, NS; angular velocity: t < 0.86, NS), nor in the first 10 sec after odor offset (paired t-test, angular position: t < 1.37, NS; angular velocity: t < 0.50, NS). Some transient differences between CS+ and CS− appeared, but they were long after stimulus offset for angular position, (18th s after odor offset, t = 2.45, P < 0.05) or even before odorant onset for angular velocity (t > 2.22, P < 0.05). These differences which sometimes also appeared before conditioning (Fig. 7B) can be attributed to random fluctuations of antenna movements.
According to these observations, the variation in angular position (Δθ, Fig. 7C) did not show any deviation between stimuli following aversive conditioning (RM-ANOVA, stimulus × recording interaction, F(3,201) = 1.96, NS). Indeed, the difference between airflow and odorant stimulations which was observed prior to conditioning (it reached significance for NOd and CS−, paired t-tests, t > 2.08, P < 0.0125) was also prevalent after conditioning (for all odorants, paired t-tests, t > 2.59, P < 0.0125). No change was observed for any of the odorants between before and after conditioning (paired t-test, t < 1.33, NS).
On the other hand, variation in angular velocity (ΔVθ) changed during conditioning (RM-ANOVA, recording effect, F(1,67) = 19.5, P < 0.001) with a different effect for the various stimuli (RM-ANOVA, stimulus × recording interaction, F(3,201) = 8.59, P < 0.001). Before conditioning, none of the odorants induced any velocity change compared with the air control (paired t-test, t < 1.83, NS). The three odorant stimuli displayed an increase in the velocity response following conditioning compared with before conditioning (CS+, CS−, and NOd, paired t-test, t > 2.69, P < 0.0125). However, no difference appeared between the velocity response to the CS+ and to the CS− after conditioning (paired t-test, t = 1.63, NS). The stimulus × recording interaction was thus attributed to a stronger velocity change for the novel odor compared with the CS− (NOd versus CS−: paired t-test, t = 4.70, P < 0.001). We therefore interpret this effect as a slight nonassociative velocity increase after conditioning (see Discussion).
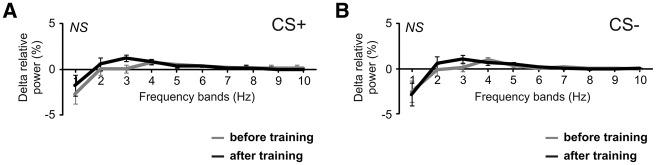
We performed a frequency analysis (FFT) on the angular position curves (θ), but again, there was no associative effect of aversive learning on bees’ antennal responses. The antennal movement frequency response (Delta relative power, see above) to the CS+ and CS− were similar before and after conditioning (Fig. 8). Consequently, no interaction was observed between frequency band and recording period, neither for the CS+ (Fig. 8A, RM-ANOVA recording × band interaction, F(9,603) = 0.63, NS), nor for the CS− (Fig. 8B, F(9,603) = 0.42, NS).
Figure 8.
Effect of aversive conditioning on antennae oscillating frequency. (A) Change in oscillation frequency (Delta relative power) between during and before odor presentation for the CS+ (A) and CS− (B), before (gray line) and after (black line) training. For statistical analysis, frequencies are grouped in 10 bands from 0.35–1.41 Hz (band 1) to 9.84–10.90 Hz (band 10). Oscillation frequency was neither modified for the CS+ nor for the CS− (NS: nonsignificant, band × recording RM-ANOVA, N = 68).
Variations in the distance between antennae (ΔD) in response to olfactory stimuli showed a differential change throughout conditioning (Supplemental Fig. S2A, RM-ANOVA, stimulus × recording interaction, F(3,201) = 3.48, P < 0.05). However, detailed analysis showed that this effect occurred for the NOd and the CS− (paired t-test, t > 3.51, P < 0.001), but not for the CS+ (t = 1.66, NS) and again no significant difference appeared between CS+ and CS− (t = 1.23, NS). On the other hand, variations in the distance to the antenna base (Δr) did not show any differential change with conditioning (Supplemental Fig. S2B, RM-ANOVA, stimulus × recording interaction, F(3,201) = 1.14, NS).
Is an effect of aversive conditioning on the antennal response hidden by nonlearners?
In contrast to PER conditioning, SER conditioning was moderately effective, with 44% of the bees responding to the CS+ and not to the CS− at the end of training (“learners,” Fig. 2B). We thus wanted to verify that a learning effect was not present in learners, which would be hidden by the data of nonlearners when analyzed as a whole group. We thus entered the learning success as a variable in our analyses, categorizing bees as learners (N = 30) or nonlearners (N = 38) based on their performances at the last CS+ and CS− trial of the conditioning phase (see Fig. 2B). We found that this variable had no effect on the results. For the variation in angular position (Δθ), we found no effect of learning success, nor any interaction with the other variables (learning success × stimulus × recording RM-ANOVA, learning success effect: F = 0, NS, all interactions F < 2.3, NS). Likewise, for the change in angular velocity (ΔVθ), no effect of learning success and no significant interaction with other variables were found (learning success × stimulus × recording RM-ANOVA, learning success effect: F = 2.24, NS, all interactions F < 1.05, NS). We also verified that learner bees when analyzed alone, did not exhibit a learning-induced change in antennal responses. In this subgroup, response to the CS+ was still not different from that to the CS− after conditioning, neither in terms of angular position (Δθ, t = 1.48, NS), nor in terms of angular velocity (ΔVθ, t = 0.36, NS). In addition, no significant change was observed for any of the tested odorants between before and after conditioning, neither for the angular position (t < 1.79, NS), nor for the angular velocity (t < 2.62, P > αcorr1 = 0.0125). Thus, no difference in the angular position or in the angular velocity appeared depending on bees’ learning success in the aversive conditioning task. We thus conclude, as above, that aversive conditioning did not have any associative effect on bees’ antennal responses.
Discussion
Using an original motion capture system for recording antenna positions, this study demonstrates important changes in bees’ antenna position and velocity following appetitive conditioning. These changes appeared only in response to the reinforced odorant but not in response to the unreinforced one. An intermediate effect was also observed for a novel odorant. In contrast, no clear associative changes were observed following aversive conditioning.
A motion capture principle to measure antennal movements
Our apparatus, based on a motion capture principle, allows recording the position of antenna tips with a very high success rate and at a high frequency (up to 120 Hz). This technique allowed us to monitor the high speed movements of antenna tips, with high temporal resolution. Based on the location of each antenna tip, a number of complementary variables can be calculated, such as its distance from the antenna base, its angular position, and its angular velocity, etc. This provides a precise and complete description of antennal movements, which was not achieved in previous studies (Erber et al. 1993; Erber 2012; Lambin et al. 2005; Hussaini et al. 2009; Mujagić et al. 2012). As the BipCam system is commercially available (Brain Vision Systems), the implementation of our motion capture system by other researchers should be relatively easy.
A minute drop of paint at the end of each antenna is required for our motion capture system. For optimal monitoring, the drop was placed on the dorsal side at the distal end of each antenna, as in a previous study (Erber et al. 1997). One may ask whether such marking affects bees’ olfactory or gustatory perceptual capacities. It should be noted that olfactory sensilla are located throughout the flagellum (Esslen and Kaissling 1976; Letzkus et al. 2006) and that gustatory sensilla are mostly located on the ventral side of the antenna tip, which was not covered (Esslen and Kaissling 1976; Haupt 2004; de Brito Sanchez 2011). During our experiments, no deleterious effects on the bees’ vitality or their behavioral responses were observed as a result of this marking. In particular, marked bees showed olfactory learning performances that are fully consistent with standard performances, both for appetitive (Bitterman et al. 1983; Giurfa and Sandoz 2012) and aversive learning (Vergoz et al. 2007; Junca et al. 2014). Two previous studies in which both appetitive and aversive conditioning were performed, using the same odorants, found highly similar performances to those described in the present work (Vergoz et al. 2007; Carcaud et al. 2009). It can thus be concluded that antenna marking did not affect the detection of or the responses to odorants, sucrose, and temperature.
It should be noted that our system, like all formerly described systems (Erber et al. 1997; Lambin et al. 2005; Mujagić et al. 2012), can only measure movement variations in two dimensions, here in the frontal plane of the honeybee head. Even if a three-dimensional tracking system would procure finer measurements, close observations show that most of the bees’ antennal movements take place in this plane (Fig. 2). We are therefore confident that the changes in antennal movement observed in the present study represent a prominent part of the bees’ antennal behavior during learning. In the future, however our system may be upgraded into a three-dimensional recording system by using two or more motion capture systems placed around the bee’s head and by temporally synchronizing their dataflows.
Odor response before conditioning
Bees exhibit specific antennal responses to sensory stimuli (Erber et al. 1993). Two previous studies, which were based on a less precise monitoring of antennal movements, suggested that bees tend to orient the antennae toward an odorant upon olfactory stimulation (Suzuki 1975; Erber et al. 1993). In our experiments, odorants had little influence on angular position before conditioning, and even induced a slight—often nonsignificant—backward movement (Figs. 4, 7). Such differences could be attributed to different previous experiences with these odorants and/or to differences in the innate values of the tested odorants for bees. Suzuki (1975) described odor responses only qualitatively, providing photographs of a bee responding to an odorant (ethyl methyl ketone, also called 2-butanone). On these photographs, the bee's proboscis is partly extended during odor delivery, suggesting that the odorant might have acquired an appetitive value for this bee before the observation. The behavior of this bee corresponds well to the behavior of our bees after appetitive conditioning. In the later study by Erber et al. (1993), bees exhibited forward antennal movements to three out of four odorants, but all tested odorants had a strong innate value for bees. Bees oriented their antennae toward geraniol and citral, two main components of the bees’ aggregation pheromone (Pickett et al. 1980; Boch 1962a) and to caprylic acid (also called octanoic acid), the major royal jelly volatile (Boch et al. 1979; Nazzi et al. 2009). In contrast, they did not respond to isopentyl acetate (also known as iso-amyl acetate), the major component of the alarm pheromone (Boch 1962b). Therefore, all odorants that produced a forward antennal movement already had a strong positive value for bees (aggregation or royal jelly). We thus believe that these previous observations may not represent the general case, and that, as recognized by Erber et al. (1993), different odorants may induce different antennal responses. Future work should thus compare antennal responses to a range of pheromonal and nonpheromonal odorants systematically, in naïve bees where prior exposure to test odorants has been carefully controlled. Our recording system is adequate to accomplish this task.
Influence of conditioning on antennal movements—the valence hypothesis
The aim of this study was to compare the effect of two conditioning procedures which convey either an appetitive or an aversive value to an odorant, on odor-evoked antennal movements. Our initial hypothesis posited that these two types of conditioning would induce opposite antennal movement modifications. This idea originated from a study in cockroaches where two odorants with opposite innate values (positive or negative) were tested. Antennal movements were, respectively, increased by the appetitive odorant and decreased by the aversive odorant (Nishiyama et al. 2007). Our results only partly confirmed our initial hypothesis. Appetitive conditioning indeed had a strong effect on antennal movements to the reinforced odorant. A strong forward motion of the antennae (Figs. 3, 4) and a velocity increase (Fig. 4) associated with a higher scanning activity (antenna oscillation frequency, Fig. 5) were observed. On the contrary, no clear associative effect of aversive conditioning was found on antennal responses (Figs. 7, 8). Therefore, our data suggest that there is a correlation between an odor's acquired positive valence and an increase in the scanning frequency in the direction of the odorant. One possibility is that only appetitively associated odorants can induce such an antennal response. Conversely, our experimental conditions may not have been optimal for measuring a specific response to aversively associated odorants. In particular, we must note that bees tended to place their antennae to the rear of their head during odor delivery, i.e., away from the odorant, before conditioning. Therefore, if a specific antennal response change to aversive conditioning included moving the antennae away from the learned odorant, our conditions may not have been optimal for measuring such response change. However, if such a response existed, we believe that we should have observed it, as the backward motion of the antennae before conditioning covered a small angle (∼10°) and was short-lived (a few seconds), whereas the acquired response seen in the appetitively conditioned group covered a much wider angle (∼29°) and lasted longer (until about 10 sec after odor delivery). In any case, future experiments should confirm this result. When the systematic study of bees’ innate antennal response to a range of odorants is performed, as mentioned above, it will be possible to choose as CSs odorants (or odorant concentrations), which do not induce a backward antennal response prior to conditioning. Use of such an odor in aversive conditioning could clarify whether the absence of any change in antennal response to odorants with a negative acquired valence is a genuine observation, or whether possible backward movements were masked in our study.
Influence of conditioning on antennal movements—a Pavlovian mechanism?
The plasticity of antennal responses we observed after appetitive conditioning can be explained in the context of “classical conditioning.” In this context, the unconditioned response (UR) would be a forward antenna motion with increased scanning activity. This hypothesis is substantiated by previous work demonstrating that a high-concentration sucrose stimulus applied to the bee antennae induces an increased scanning activity and touching frequency of the presented solution (Haupt 2004). This process is thought to involve increased activity of an antenna muscle, the pedicel fast flexor muscle (Pribbenow and Erber 1996; Erber et al. 2000; Haupt 2007). Through repeated pairing of the odor CS with the sucrose US, the CS would gain control not only over the PER (Takeda 1961; Bitterman et al. 1983), but also over this antennal scanning response (ASR). Thus, appetitively conditioned bees would exhibit a double conditioned response upon CS+ presentation: the PER and the ASR. Like PER, the ASR is not produced for the CS−, but generalization can take place to a novel odor (see Figs. 4, 6).
Double conditioned responses such as this may be an important adaptive advantage under natural conditions. Antennal movements often occur during tasks which involve proboscis extension, for instance during foraging or during trophallactic contacts (Free 1956; Montagner and Pain 1971; Galliot and Azoeuf 1979; Galliot et al. 1982; Korst and Velthuis 1982; Crailsheim 1998; Wright et al. 2012). One may thus wonder if both responses are part of a common motor pattern and are therefore always co-occurring. In this study, we addressed this question by comparing antennal responses in bees that exhibited or not a PER generalization to a novel odorant (Fig. 6). If the two responses were part of a common motor pattern, one would expect ASR generalization to be found only in bees that showed a PER generalization. Our data only partly substantiated this prediction. While antennal angular position clearly correlated with PER responses, antennal angular velocity did not. Bee that did not generalize with a PER to the novel odor still showed an antenna acceleration to this odor (i.e., they generalized this antennal acceleration to the novel odor). This suggests that the two conditioned responses may be in part triggered by the same neural substrate, deciding or not to generalize to a novel odorant and inducing both PER and a forward antenna movement. In addition, an antenna speed increase could still appear, even if bees do not extend their PER, probably because of a higher response threshold for the latter than for the former.
Influence of conditioning on antennal movements—an operant contribution?
Intensive previous work has shown that antennal movements can be subjected to “operant conditioning” (Erber et al. 1997, 1998, 2000; Kisch and Erber 1999; Haupt 2007). This applies, for instance, to studies that carried out motor learning by reinforcing high scanning activity (monitoring either antennal contact frequency or muscle activity) with sucrose (Kisch and Erber 1999; Erber et al. 2000; Haupt 2007). In our case, the magnitude of the ASR may have been strengthened through an operant process. The bees could have associated their active scanning behavior, caused by the sucrose stimulation applied to the antennae (Haupt 2004) with the subsequent sucrose reward applied to the proboscis. However, even if ASR magnitude was enhanced by operant processes, the core of the response plasticity we found has a Pavlovian nature. It is the quality of the presented odorant that triggers the ASR (CS+) or not (CS−), just as in free-flying conditioning experiments, in which visual stimuli trigger or not an operant-approach behavior (Menzel 1999).
Lack of aversive conditioning effect
We did not observe associative effects of aversive conditioning on antennal movements (Figs. 3D, 7). However, we did observe a small increase in angular velocity for all odor stimuli after conditioning (Fig. 7B,D). This velocity increase was not significantly different between the CS+ and the CS− (Fig. 7D) suggesting that it may correspond to a nonassociative effect of the procedure. Possibly, after aversive training, bees may be in a sensitized state (related to the six thermal shocks received) or may display increased attention to external stimuli. A similar effect could also exist in the case of appetitive conditioning with sucrose stimulations, but it would be difficult to observe because of the strong associative effect on antenna velocity. Further experiments comparing bees that received only thermal shocks, only sucrose stimulations or remained naïve throughout the experiment may help examining this possibility.
Lastly, we used two standard protocols for conditioning bees appetitively (Bitterman et al. 1983) or aversively (Junca et al. 2014). However, it is important to bear in mind that there are differences concerning the application of the US, between the two protocols. In PER conditioning, the US was a compound applied to the antennae and then to the proboscis (Bitterman et al. 1983). In SER conditioning, the US was a heated probe applied to the mouthparts (Junca et al. 2014). The two protocols thus differ in the mode of delivery and their respective contact with the antennae. It will thus be necessary to consider whether a thermal stimulation on the antennae would induce such classical or operant processes similar to those observed for appetitive conditioning. Following the Pavlovian hypothesis detailed above, bees could show an antennal unconditioned response to such a thermal US, which may then be classically conditioned. Future experiments will test this hypothesis.
Conclusion
In this study, we observed a striking difference in the effects of appetitive and aversive conditioning on odor-induced antennal movements, the former inducing a strong forward-oriented scanning response while the latter had little influence. Our current interpretation of this phenomenon is that the ASR following appetitive conditioning could be linked to a classical conditioning process rather than relating to the positive acquired valence of the odorant.
Materials and Methods
Insects
Honeybee workers (A. mellifera) were caught at the entrance of outdoor hives on the CNRS campus of Gif-sur-Yvette, from March to May 2014. The bees were caught in the morning, were fed, and then chilled on ice until they stopped moving. They were then harnessed individually in metal holders, leaving their antennae, abdomen, and mouthparts free. The honeybees were positioned with their back toward the front of the tube, allowing both SER and PER conditioning under the same conditions (Fig. 1A; Junca et al. 2014).
Antenna monitoring apparatus
The recording apparatus was composed of a camera positioned above the bee holder and an olfactory stimulation apparatus (Fig. 1A). The camera included an integrated processing card allowing adaptive detection (using a motion prediction algorithm) of the two color dots, up to a rate of 120 Hz (BIPcam, Brain Vision Systems). The camera managed to follow and record the coordinates of the two color dots on the antenna tips, in real time at a rate of 90 Hz (90 frames per second). In order to optimize the detection of the color dots, the apparatus was placed in a room with low light conditions (controlled and kept constant). A cold light illumination ring was placed around the lens of the camera, diffusing homogeneous white light on the bee's head (Leica CLS 150XE, Leica, Jena, Germany). The intensity of the light source was tuned precisely and kept constant for the duration of the experiments.
The olfactory stimulation apparatus was connected to a pump, enabling the constant circulation of an air flow of 52.5 mL/sec. This flow, composed of a principal air flow of 50 mL/sec and a secondary flow of 2.5 mL/sec, was directed to the bee by a glass tube (0.5 cm diameter), at a distance of 2 cm. The secondary air flow could be directed to one of two subcircuits (one containing an odorant source, and another without any odorant) before being reinjected into the main airflow. Most of the time, air flowed through the odorless subcircuit. Olfactory stimulation was applied manually inducing a switch of the secondary flow to the odorant subcircuit for 5 sec. The odorant subcircuit included a Pasteur pipette containing a piece of filter paper (20 × 2 mm) soaked with 5 µL of odorant solution. The other subcircuit included an identical Pasteur pipette without odorant. An air extractor, placed behind the bee prevented odorant accumulation.
Insect preparation
The aim of this study was to determine the influence of appetitive or aversive learning on antennal responses to an olfactory stimulation. To this aim, antennal movements of each individual were recorded before and after either an appetitive PER (Proboscis Extension response) conditioning procedure or an aversive SER (Sting Extension response) conditioning procedure. Once mounted in a metal holder, each individual was fed with sucrose solution (50% w/w). To maintain a good survival rate throughout the experiment, individuals subjected to appetitive conditioning received 5-μL sucrose solution, while individuals assigned to the aversive conditioning received a higher amount (15 μL). This was to compensate for the fact that these individuals do not receive any sucrose solution during conditioning (in contrast with appetitive conditioning, see below). After feeding, bees were prepared for the motion capture system, by marking their antenna tips with paint. Red color dots were applied using water-based paint (Posca PC-5M, Mitsubishi Pencil Co.) on the upper surface of the last two flagelomers of each antenna. Once mounted, fed and marked, individuals were placed in a moist, dark polystyrene box for 30 min, before the start of the experiments.
Antennal movement recordings
Antennal movements were recorded 1 h before the beginning of the conditioning procedure and 1 h after the end of the conditioning phase. Before the recording period, each bee was left to acclimatize to the airflow for 20 sec. Each recording lasted 40 sec: 15 sec of airflow, 5 sec of olfactory stimulation, and 20 sec of airflow. Each bee was recorded four times, three recordings with an olfactory stimulation and one with a constant air flow. These recordings were separated by 1 min and were carried out in a randomized order. Three odorants were used; 1-hexanol (A) and 1-nonanol (B) were used as conditioned stimuli (CSs) and octanal (C) was used as a novel odor (NOd) (all from Sigma Aldrich). These odorants were chosen because they are easily learned and well discriminated by the bees (Guerrieri et al. 2005). In addition, these CSs have been used in several studies comparing SER and PER conditioning (Vergoz et al. 2007; Carcaud et al. 2009). During these antenna movement recordings, proboscis extensions could be clearly seen and recorded by the experimenter. However, due to the position of the bee and the lighting directed only to the bees’ head, sting extensions could not be monitored during these recordings.
Conditioning procedure
Bees were allocated either to an appetitive conditioning group or to an aversive conditioning group.
In both groups, the bees were prepared in an identical manner to avoid any potential bias resulting from their position. The bees were thus fixed to the metal tube with a piece of tape placed below the head to the front, leaving the abdomen and the mouthparts free to move. In this position, both SER and PER could be easily observed. The appetitive conditioning of the proboscis extension response (PER) was carried out according to standard procedures (Bitterman et al. 1983; Matsumoto et al. 2012). For aversive conditioning of the sting extension response (SER), the novel procedure developed by Junca et al. (2014) was used. All bees received a differential conditioning procedure in which one odorant (CS+) was associated with the US (i.e., reinforced) and another odorant (CS−) was presented explicitly without US (i.e., nonreinforced). Such a protocol contains an internal control, as animals that efficiently learned the CS–US association will respond to the CS+ but not to the CS− (Matsumoto et al. 2012). If associative learning modifies antennal responses to odorants, we thus expect to observe these modifications for the CS+ but not for the CS−.
For PER conditioning, the unconditioned stimulus (US) was sucrose (50% w/w) applied to the antennae and the proboscis. For SER conditioning, the aversive US was a thermal stimulation (65°C) applied to the mouthparts by means of a pointed copper cylinder (diameter: 6 mm; length: 13 mm), placed on a soldering iron (HQ-Power, PS1503S). The CSs were 5 µL of pure odorant (1-hexanol or 1-nonanol) applied to pieces of filter paper placed into 20 mL syringes. Odor CSs were delivered manually to the antennae of the bee at a distance of 2 cm in a homogeneous flow throughout the 5 sec of stimulation.
Each day, half of the individuals received 1-hexanol (A) reinforced and 1-nonanol (B) nonreinforced, and vice versa for the other half of the bees. Conditioning consisted of 12 trials (6 CS+, 6 CS−) with an inter-trial interval of 10 min. Odorants were presented in a pseudo-random sequence of six reinforced and six nonreinforced trials (ABBA BAAB ABBA) starting with the odorant A or B in a balanced manner, so that no effect of a particular odorant could influence the results. Each conditioning trial lasted 35 sec (20 sec of airflow, 5 sec of olfactory stimulation, and 10 sec of airflow). Each individual was placed on the stimulation site, under a cold light source, in front of the air extractor to prevent odorant accumulation. In the case of the CS+, the US was applied 3 sec after odorant onset, for 2 sec. In all experiments, PER or SER responses to the CS were measured during the 3 sec in which the bees were exposed to the odor only (before the US).
Antennal movement analysis
The monitoring apparatus recorded at each time point (90 times per second) the location of the two antenna tips of each bee on the camera sensor. First, all the recordings from all bees were recalculated in the same coordinate system (x,y), with the socket of the right antenna as the origin (coordinate 0,0) and the socket of the left antenna as the unit reference on the x-axis (coordinate 1,0). Each recording thus resulted in a series of (x,y) coordinates for each antenna at each time-step (1/90 sec).
This allowed a comparison between the antennal movements of different bees. In addition, heat maps describing the number of times each antenna tip was located at each coordinate could be constructed (Fig. 3). In these heatmaps, the number of occurrences of each data point was normalized with regard to the total number of occurrences on the entire map, to make them comparable in the various conditions. Occurrence frequency is represented on a color scale ranging from dark blue to red. Maps of antenna location change were computed by subtracting the map obtained before odor from that during odor. On these new maps, occurrence frequency reduction and increase are shown with blue and red color, respectively.
Previous studies (Lambin et al. 2005; Hussaini et al. 2009) and our preliminary experiments showed that bees’ antennal movements are best described using circular coordinates (r, θ), as each antenna moves around its socket (Fig. 1B). Thus, each antenna's movements were described in their own coordinate system, with the antenna socket (base) as the origin (0,0).
Angular position (θ): it was defined as the angle between a line connecting the antenna tips to their base (r) and an anteroposterior line passing through the corresponding antenna base. This variable indicates if the antenna is positioned to the front (0°), to the side (90°) or backward (180°). Note that the measured angle is symmetrical for the left or the right antenna so that 90° is on the left for the left antenna and on the right for the right antenna.
Distance to antenna base (r): it was defined as the distance between the antenna base and the antenna tip. This variable thus measures whether the antenna is in a stretched or retracted position.
From these, two other variables were computed:
Angular velocity (Vθ): it was calculated as the angle θ traveled by each antenna during a frame (1/90 sec). It is expressed in degrees per second.
Distance between antenna tips (D): it was the distance in the recording plane between the antennae distal ends. This variable enabled us to detect any variation in terms of the separation or approach of the two antennae.
As explained in the results, θ and Vθ proved to be the most pertinent for measuring changes induced by conditioning and are thus presented in the figures. r and D data are presented in Supplemental Material.
As antennal movements are mainly composed of back-and-forth scanning motions around the socket with amplitude and frequency variations (Erber et al. 1993; Lambin et al. 2005; Hussaini et al. 2009), we used a fast Fourier transform (FFT) to determine the frequency spectrum of these oscillations. Due to mathematical constraints of this analysis (which uses 2n data points), the FFT was performed on an angular position (θ) data using 256 data points (i.e., 2.84 sec) either during odor presentation (starting at the first frame of odor presentation) or before odor presentation (finishing at the last frame before odor presentation). The obtained frequency spectrum represented the repartition of the oscillating power of antennal movements (integrating both the number and angular amplitude of oscillations) according to 128 different frequency bands from 0 to 45 Hz (half the recording frequency). In the figures, the power at each frequency band was represented as a percentage of total power over the whole-frequency range (relative power in %). In order to study the effect of an olfactory stimulation on the antennal movement frequency, the differences between the relative frequency spectrum before and during the olfactory stimulation was calculated (Δpower in %). As shown in the results, antenna oscillations are best described between 0 and 10 Hz, for which reason further analysis concentrated on this frequency range. Δpower values were thus analyzed according to 10 frequency bands from 0.35–1.41 Hz (band 1) to 9.84–10.90 Hz (band 10). FFT analyses were performed using the analysis toolpack in Microsoft Excel 2007.
Statistical analysis
During conditioning, the occurrence of a proboscis or sting extension (depending on conditioning assay), was recorded as 1 and nonextension as 0. The acquisition curves show the percentage of individuals showing a PER or a SER to each presentation of the CS+ or of the CS−. To analyze learning performances, a repeated-measure analysis of variance (RM-ANOVA) was used, with trial (from 1 to 6) and stimulus (CS+/CS−) as within-group factors. Monte Carlo simulations demonstrated that it is permissible to use an ANOVA on dichotomous data under controlled conditions (Lunney 1970). For each conditioning type, the two subgroups receiving 1-hexanol (odorant A; PER n = 21; SER n = 33) and 1-nonanol (odorant B; PER n = 23; SER n = 35) as CS+ were pooled. No effect of these subgroups or interaction with other variables were found (RM-ANOVA, interaction stimuli × odors × trials, PER: F(5,210) = 0.72, P = 0.61; SER: F(5,330) = 1.57, P = 0.17).
Antennal movements to 4 stimuli (CS+, CS−, Nod, and air) were measured before and after conditioning. To analyze possible differences in angular position and angular velocity between the CS+ and the CS− after conditioning, a paired t-test was performed every second throughout the recording. To analyze changes in the different recorded variables (θ, r, Vθ, and D) with odor presentation, we calculated the difference (called Δθ, Δr, ΔVθ, and ΔD) between the average values recorded during the stimulation (5 sec) and the average values recorded “before the stimulation” (15 sec). A RM-ANOVA was used with the recording (before or after conditioning) and the stimulus (CS+, CS−, Nod, or air) as within-group factors. When this analysis was significant, a limited number of planned (a priori) comparisons were carried out, using paired t-tests. Each data point was compared with only four other data points. (1) To compare responses between stimuli within each recording session, the value observed for each stimulus at each recording session (for instance Δθ for the CS+ before conditioning) was compared with the values observed for the three other stimuli within the same recording session (here, Δθ for the CS−, NOd and air before conditioning—three comparisons). (2) To evaluate the change in the response to each stimulus between recording sessions, the value observed for each stimulus at each recording session was compared with the response to the same stimulus in the other recording session (here, Δθ for the CS+ after conditioning—1 comparison). To correct for the multiple use of each data point in these planned contrasts, a Bonferroni correction for multiple comparisons was applied, and the significance threshold for all post hoc comparisons was αcorr1 = 0.05/4 = 0.0125.
The frequency analysis (FFT) concentrated on the change in the frequency spectra of antennal movements observed before and after training for the CS+ and the CS−. A RM-ANOVA was used with the recording (before or after conditioning), and the frequency band (band 1 to band 10) as within-group factors. A comparison between data obtained before and after training at each of the 10 frequency bands were performed using paired t-tests. The significance threshold was corrected for multiple comparisons as αcorr2 = 0.05/10 = 0.005.
Statistical tests were performed with STATISTICA 5.5 (Statsoft) and R 3.0.2 (Foundation for Statistical Computing, Vienna, Austria).
Supplementary Material
Acknowledgments
We are indebted to P. Pirim, L. Josselin, and G. Havard (Brain Vision Systems, Paris) for their help in software development and to S. Famié for initial tests with the motion capture system. This work was supported by the Agence Nationale de la Recherche (ANR), Paris, France (Project BEE-CHANNELS, ANR-13-BSV7-0010). H.C. and P.J. thank the French Ministère de la Recherche et de l'Enseignement Supérieur for PhD funding. We thank the Evolbee Team for fruitful discussions and A. French and two anonymous referees for helpful comments on the manuscript.
Footnotes
[Supplemental material is available for this article.]
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.038448.115.
References
- Alcock J. 1997. Animal behavior: an evolutionary approach. Sinauer Associates, Sunderland. [Google Scholar]
- Bitterman ME, Menzel R, Fietz A, Schäfer S. 1983. Classical conditioning of proboscis extension in honeybees (Apis mellifera). J Comp Psychol 97: 107–119. [PubMed] [Google Scholar]
- Boch R. 1962a. Identification of geraniol as the active component in the Nassanoff pheromone of the honey bee. Nature 194: 704–706. [DOI] [PubMed] [Google Scholar]
- Boch R. 1962b. Identification of iso-amyl acetate as an active component in the sting pheromone of the honey bee. Nature 195: 1018–1020. [DOI] [PubMed] [Google Scholar]
- Boch R, Shearer DA, Shuel RW. 1979. Octanoic and other volatile acids in the mandibular glands of the honeybee and in royal jelly.J Apicult Res 18: 250–252. [Google Scholar]
- Busto GU, Cervantes-Sandoval I, Davis RL. 2010. Olfactory learning in Drosophila. Physiology (Bethesda) 25: 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcaud J, Roussel E, Giurfa M, Sandoz JC. 2009. Odour aversion after olfactory conditioning of the sting extension reflex in honeybees. J Exp Biol 212: 620–626. [DOI] [PubMed] [Google Scholar]
- Chaffiol A, Laloi D, Pham-Delègue MH. 2005. Prior classical olfactory conditioning improves odour-cued flight orientation of honey bees in a wind tunnel. J Exp Biol 208: 3731–3737. [DOI] [PubMed] [Google Scholar]
- Crailsheim K. 1998. Trophallactic interactions in the adult honeybee (Apis mellifera L.). Apidologie 29: 97–112. [Google Scholar]
- Crow T. 2004. Pavlovian conditioning of Hermissenda: current cellular, molecular, and circuit perspectives. Learn Mem 11: 229–238. [DOI] [PubMed] [Google Scholar]
- de Brito Sanchez MG. 2011. Taste perception in honey bees. Chem Senses 36: 675–692. [DOI] [PubMed] [Google Scholar]
- Dreller C, Kirchner WH. 1993. Hearing in honeybees: localization of the auditory sense organ. J Comp Physiol A 173: 275–279. [Google Scholar]
- Erber J. 2012. Tactile antennal learning in the honey bee. In Honeybee neurobiology and behavior (ed. Galizia CG, Eisenhardt D, Giurfa M), pp. 439–455. Springer, The Netherlands. [Google Scholar]
- Erber J, Schildberger K. 1980. Conditioning of an antennal reflex to visual stimuli in bees (Apis mellifera L.). J Comp Physiol 135: 217–225. [Google Scholar]
- Erber J, Pribbenow B, Bauer A, Kloppenburg P. 1993. Antennal reflexes in the honeybee: tools for studying the nervous system. Apidologie 24: 283–296. [Google Scholar]
- Erber J, Pribbenow B, Grandy K, Kierzek S. 1997. Tactile motor learning in the antennal system of the honeybee (Apis mellifera L.). J Comp Physiol A 181: 355–365. [Google Scholar]
- Erber J, Kierzek S, Sander E, Grandy K. 1998. Tactile learning in the honeybee. J Comp Physiol A 183: 737–744. [Google Scholar]
- Erber J, Pribbenow B, Kisch J, Faensen D. 2000. Operant conditioning of antennal muscle activity in the honey bee (Apis mellifera L.). J Comp Physiol A 186: 557–565. [DOI] [PubMed] [Google Scholar]
- Esslen J, Kaissling KE. 1976. Zahl und Verteilung antennaler Sensillen bei der Honigbiene (Apis mellifera L.). Zoomorphol 83: 227–251. [Google Scholar]
- Free JB. 1956. A study of the stimuli which release the food begging and offering responses of worker honeybees. Br J Anim Behav 4: 94–101. [Google Scholar]
- Galliot G, Azoeuf P. 1979. Etude quantitative des transferts de nourriture entre ouvrières d'âge connu chez l'abeille domestique (Apis mellifica mellifica L). Insectes Soc 26: 39–49. [Google Scholar]
- Galliot G, Montagner H, Azoeuf P. 1982. Étude quantitative des transferts de nourriture entre ouvrières et males chez l'abeille domestique (Apis mellifica L.). Insectes Soc 29: 268–279. [Google Scholar]
- Gil M, De Marco RJ. 2010. Decoding information in the honeybee dance: revisiting the tactile hypothesis. Anim Behav 80: 887–894. [Google Scholar]
- Giurfa M. 2007. Behavioral and neural analysis of associative learning in the honeybee: a taste from the magic well. J Comp Physiol A 193: 801–824. [DOI] [PubMed] [Google Scholar]
- Giurfa M, Sandoz JC. 2012. Invertebrate learning and memory: fifty years of olfactory conditioning of the proboscis extension response in honeybees. Learn Mem 19: 54–66. [DOI] [PubMed] [Google Scholar]
- Guerrieri F, Schubert M, Sandoz JC, Giurfa M. 2005. Perceptual and neural olfactory similarity in honeybees. PLoS Biol 3: e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt SS. 2004. Antennal sucrose perception in the honey bee (Apis mellifera L.): behavior and electrophysiology. J Comp Physiol A 190: 735–745. [DOI] [PubMed] [Google Scholar]
- Haupt SS. 2007. Central gustatory projections and side-specificity of operant antennal muscle conditioning in the honeybee. J Comp Physiol A 193: 523–535. [DOI] [PubMed] [Google Scholar]
- Hussaini SA, Bogusch L, Landgraf T, Menzel R. 2009. Sleep deprivation affects extinction but not acquisition memory in honeybees. Learn Mem 16: 698–705. [DOI] [PubMed] [Google Scholar]
- Junca P, Carcaud J, Moulin S, Garnery L, Sandoz JC. 2014. Genotypic influence on aversive conditioning in honeybees, using a novel thermal reinforcement procedure. PLoS One 9: e97333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevan PG, Lane MA. 1985. Flower petal microtexture is a tactile cue for bees. Proc Natl Acad Sci 82: 4750–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisch J, Erber J. 1999. Operant conditioning of antennal movements in the honey bee. Behav Brain Res 99: 93–102. [DOI] [PubMed] [Google Scholar]
- Korst PJAM, Velthuis HHW. 1982. The nature of trophallaxis in honeybees. Insectes Soc 29: 209–221. [Google Scholar]
- Lacher V. 1964. Elektrophysiologische Untersuchungen an einzelnen Rezeptoren für Geruch, Kohlendioxyd, Luftfeuchtigkeit und Tempratur auf den Antennen der Arbeitsbiene und der Drohne (Apis mellifica L.). Z vergl Physiol 48: 587–623. [Google Scholar]
- Lacher V, Schneider D. 1963. Elektrophysiologischer Nachweis der Riechfunktion von Porenplatten (Sensilla placodea) auf den Antennen der Drohne und der Arbeitsbiene (Apis mellifica L.). Z vergl Physiol 47: 274–278. [Google Scholar]
- Lambin M, Déglise P, Gauthier M. 2005. Antennal movements as indicators of odor detection by worker honeybees. Apidologie 36: 119–126. [Google Scholar]
- Letzkus P, Ribi WA, Wood JT, Zhu H, Zhang SW, Srinivasan MV. 2006. Lateralization of olfaction in the honeybee Apis mellifera. Curr Biol 16: 1471–1476. [DOI] [PubMed] [Google Scholar]
- Lunney GH. 1970. Using analysis of variance with a dichotomous dependent variable: an empirical study. J Educ Meas 7: 263–269. [Google Scholar]
- Martin H, Lindauer M. 1966. Sinnesphysiologische Leistungen beim Wabenbau der Honigbiene. Z vergl Physiol 53: 372–404. [Google Scholar]
- Matsumoto Y, Menzel R, Sandoz JC, Giurfa M. 2012. Revisiting olfactory classical conditioning of the proboscis extension response in honey bees: a step toward standardized procedures. J Neurosci Methods 211: 159–167. [DOI] [PubMed] [Google Scholar]
- Menzel R. 1990. Learning, memory, and “cognition” in honey bees. In Neurobiology of comparative cognition (ed. Kesner RP, Olton DS), pp. 237–292. Lawrence Erlbaum Associate, Hillsdale. [Google Scholar]
- Menzel R. 1999. Memory dynamics in the honeybee. J Comp Physiol A 185: 323–340. [Google Scholar]
- Menzel R. 2012. The honeybee as a model for understanding the basis of cognition. Nat Rev Neurosci 13: 758–768. [DOI] [PubMed] [Google Scholar]
- Montagner H, Pain J. 1971. Étude préliminaire des communications entre ouvrières d'abeilles au cours de la trophallaxie. Insectes Soc 18: 177–191. [Google Scholar]
- Mujagić S, Würth SM, Hellbach S, Dürr V. 2012. Tactile conditioning and movement analysis of antennal sampling strategies in honey bees (Apis mellifera. L.). J Vis Exp 70: e50179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagari M, Bloch G. 2012. The involvement of the antennae in mediating the brood influence on circadian rhythms in “nurse” honey bee (Apis mellifera) workers. J Insect Physiol 58: 1096–1103. [DOI] [PubMed] [Google Scholar]
- Nazzi F, Bortolomeazzi R, Della Vedova G, Del Piccolo F, Annoscia D, Milani N. 2009. Octanoic acid confers to royal jelly varroa-repellent properties. Naturwissenschaften 96: 309–314. [DOI] [PubMed] [Google Scholar]
- Nishiyama K, Okada J, Toh Y. 2007. Antennal and locomotor responses to attractive and aversive odors in the searching cockroach. J Comp Physiol A 193: 963–971. [DOI] [PubMed] [Google Scholar]
- Pamir E, Chakroborty NK, Stollhoff N, Gehring KB, Antemann V, Morgenstern L, Felsenberg J, Eisenhardt D, Menzel R, Nawrot MP. 2011. Average group behavior does not represent individual behavior in classical conditioning of the honeybee. Learn Mem 18: 733–741. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. 1927. Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex. Oxford University Press, Oxford. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett JA, Williams IH, Martin AP, Smith MC. 1980. Nasonov pheromone of the honey bee, Apis mellifera L. (Hymenoptera: Apidae). J Chem Ecol 6: 425–434. [DOI] [PubMed] [Google Scholar]
- Pribbenow B, Erber J. 1996. Modulation of antennal scanning in the honeybee by sucrose stimuli, serotonin, and octopamine: behavior and electrophysiology. Neurobiol Learn Mem 66: 109–120. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. 1988. Pavlovian conditioning: it's not what you think it is. Am Psychol 43: 151–160. [DOI] [PubMed] [Google Scholar]
- Rohrseitz K, Tautz J. 1999. Honey bee dance communication: waggle run direction coded in antennal contacts? J Comp Physiol A 184: 463–470. [Google Scholar]
- Sandoz JC, Laloi D, Odoux JF. 2000. Olfactory information transfer in the honeybee: compared efficiency of classical conditioning and early exposure. Anim Behav 59: 1025–1034. [DOI] [PubMed] [Google Scholar]
- Sauer S, Kinkelin M, Herrmann E, Kaiser W. 2003. The dynamics of sleep-like behaviour in honey bees. J Comp Physiol A 189: 599–607. [DOI] [PubMed] [Google Scholar]
- Sauer S, Herrmann E, Kaiser W. 2004. Sleep deprivation in honey bees1. J Sleep Res 13: 145–152. [DOI] [PubMed] [Google Scholar]
- Skinner BF. 1936. The reinforcing effect of a differentiating stimulus. J Gen Psychol 14: 263–278. [Google Scholar]
- Suzuki H. 1975. Antennal movements induced by odour and central projection of the antennal neurones in the honey-bee. J Insect Physiol 21: 831–847. [Google Scholar]
- Takeda K. 1961. Classical conditioned response in the honey bee. J Insect Physiol 6: 168–179. [Google Scholar]
- Tedjakumala SR, Giurfa M. 2013. Rules and mechanisms of punishment learning in honey bees: the aversive conditioning of the sting extension response. J Exp Biol 216: 2985–2997. [DOI] [PubMed] [Google Scholar]
- Vareschi E. 1971. Duftunterscheidung bei der Honigbiene—Einzelzell-Ableitungen und Verhaltensreaktionen. Z vergl Physiol 75: 143–173. [Google Scholar]
- Vergoz V, Roussel E, Sandoz JC, Giurfa M. 2007. Aversive learning in honeybees revealed by the olfactory conditioning of the sting extension reflex. PLoS One 2: e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Frisch K. 1967. The dance language and orientation of bees. Belknap press, Cambridge. [Google Scholar]
- Whitehead AT, Larsen JR. 1976. Ultrastructure of the contact chemoreceptors of Apis mellifera L. (Hymenoptera: Apidae). Int J Insect Morphol Embryol 5: 301–315. [Google Scholar]
- Winston ML. 1987. The biology of the honey bee. Harvard University Press, Cambridge. [Google Scholar]
- Wright GA, Schiestl FP. 2009. The evolution of floral scent: the influence of olfactory learning by insect pollinators on the honest signalling of floral rewards. Funct Ecol 23: 841–851. [Google Scholar]
- Wright GA, Lillvis JL, Bray HJ, Mustard JA. 2012. Physiological state influences the social interactions of two honeybee nest mates. PLoS One 7: e32677. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.