Abstract
Objective
The efficacy and safety of BST-CarGel, a chitosan-based medical device for cartilage repair, was compared with microfracture alone at 1 year during a multicenter randomized controlled trial (RCT) in the knee. The quality of repair tissue of osteochondral biopsies collected from a subset of patients was compared using blinded histological assessments.
Methods
The international RCT evaluated repair tissue quantity and quality by 3-dimensional quantitative magnetic resonance imaging as co-primary endpoints at 12 months. At an average of 13 months posttreatment, 21/41 BST-CarGel and 17/39 microfracture patients underwent elective second look arthroscopies as a tertiary endpoint, during which ICRS (International Cartilage Repair Society) macroscopic scoring was carried out, and osteochondral biopsies were collected. Stained histological sections were evaluated by blinded readers using ICRS I and II histological scoring systems. Collagen organization was evaluated using a polarized light microscopy score.
Results
BST-CarGel treatment resulted in significantly better ICRS macroscopic scores (P = 0.0002) compared with microfracture alone, indicating better filling, integration, and tissue appearance. Histologically, BST-CarGel resulted in a significant improvement of structural parameters—Surface Architecture (P = 0.007) and Surface/Superficial Assessment (P = 0.042)—as well as cellular parameters—Cell Viability (P = 0.006) and Cell Distribution (P = 0.032). No histological parameters were significantly better for the microfracture group. BST-CarGel treatment also resulted in a more organized repair tissue with collagen stratification more similar to native hyaline cartilage, as measured by polarized light microscopy scoring (P = 0.0003).
Conclusion
Multiple and independent analyses in this biopsy substudy demonstrated that BST-CarGel treatment results in improved structural and cellular characteristics of repair tissue at 1 year posttreatment compared with microfracture alone, supporting previously reported results by quantitative magnetic resonance imaging.
Keywords: cartilage repair, biopsy, histology: scoring systems, polarized light microscopy, collagen organization
Introduction
Hyaline cartilage has a unique composition and structure with 2 major extracellular matrix constituents contributing an important role in its biomechanical properties and durability, zonal type II collagen and proteoglycans. However, adult cartilage has a limited healing capacity in response to injury, mainly due to a lack of vascularization and a dense extracellular matrix imprisoning native chondrocytes.1,2 To date, no surgical cartilage repair treatment has been shown to regenerate new tissue with hyaline composition and structure.
Microfracture (MFx), a bone marrow stimulation technique, which involves piercing the subchondral bone to induce bleeding and initiate wound healing, is the most common first-line treatment for cartilage repair and has become the reference standard-of-care in many clinical trials.3-7 Although MFx provides effective short-term symptomatic improvement, it has been purported to result in a more fibrous repair tissue8 with limited durability and a reoccurrence of clinical symptoms as early as 2 years posttreatment.9-11 It is believed that these poor results of MFx are associated in part with a blood clot of suboptimal volume and integrity residing in the cartilage lesion after the procedure.8,12,13
The BST-CarGel medical device was developed to stabilize the blood clot in the cartilage lesion by dispersing a chitosan scaffold throughout whole blood. Chitosan is a natural polymer that has been studied extensively for several biomedical applications due to its biocompatibility and low toxicity, biodegradability, as well as adhesiveness to tissues.14 When mixed with autologous blood, BST-CarGel impedes blood clot retraction while still permitting normal clotting to occur, and increases clot adhesivity in the lesion through chitosan’s cationic properties.12,13 Consequently, BST-CarGel maintains critical blood components above marrow holes, which enhances early healing processes such as cell recruitment, vascularization of the repair tissue, and subchondral bone remodeling.15 BST-CarGel treatment resulted in consistently greater volume of repair tissue of better quality both in animals,12,13 and in a randomized controlled trial.3
Structural assessments of repair tissue are important since the restitution of native or close-to-native composition and structure of articular cartilage (quality of repair tissue) is expected to provide improved clinical benefit and long-term durability.16-19 Noninvasive magnetic resonance imaging (MRI) is frequently used to study cartilage repair and has been correlated to specific clinical outcomes (reviewed in Blackman et al.20), but histological evaluation of osteochondral biopsies remains the most direct biological analysis of cartilage regenerative processes. However, biopsy analyses have several intrinsic drawbacks including the need for a second invasive procedure, difficulty in standardizing the biopsy location, the small amount of tissue available and the question of how representative the small diameter core is of the entire repair tissue area.
A multicenter randomized controlled trial (RCT) comparing BST-CarGel treatment of symptomatic cartilage lesions of the femoral condyle to MFx alone was conducted in 80 patients. Statistically powered co-primary endpoints of repair tissue structure were evaluated by 3-dimensional MRI quantification and previously described,3 and clinical benefit was measured as the secondary endpoint. As a tertiary and unpowered endpoint of the trial, osteochondral biopsies were also obtained during elective second looks at an average of 13 months posttreatment in 38 patients and evaluated in the current study using validated histological assessments to determine if BST-CarGel treatment would result in better repair tissue quality.
Methods
The full description of the 1-year multicenter RCT (http://www.clinicaltrials.gov; #NCT00314236) has been reported previously,3 including trial design, patient eligibility criteria, descriptions of randomization, surgical treatment and rehabilitation. The trial enrolled 80 patients at 26 clinical sites. Eligible male and female patients were 18 to 55 years old with a single, focal, and contained cartilage lesion on the femoral condyles and moderate knee pain (>4 on a 10-cm visual analogue scale). All patients agreed to follow a 12-week standardized posttreatment rehabilitation program. All subjects who participated in the clinical trial were asked to provide written informed consent prior to study activities to undergo this elective second look and biopsy substudy, which was approved by the institutional review boards at each of the clinical sites prior to initiation of activities. Investigators and patients were not blinded due to differences in incision size related to treatment. The biopsy histological analysis was blinded since the independent third party carrying out the analyses was unaware of patient treatment.
Second-Look Arthroscopy and ICRS Macroscopic Scoring
During the second-look arthroscopy and before biopsy collection, at an average of 13 months posttreatment, the repair tissue present in the treated lesion was visually inspected and scored by the unblinded investigator as per the validated International Cartilage Repair Society (ICRS) macroscopic score.21,22 This score is based on 3 parameters: (1) Degree of Defect Repair, (2) Integration to Border Zone, and (3) Macroscopic Appearance, each scored from 0 to 4. The three scores are then summed to an overall repair assessment representing normal (12), nearly normal (11-8), abnormal (7-4), or severely abnormal (3-1) tissue. Macroscopic scoring was carried out by 25 different investigators at 18 trial sites, resulting in limited potential for site bias.
Osteochondral Biopsies
Osteochondral biopsies were retrieved at approximately 13 months posttreatment from 21 BST-CarGel patients (51.2% of patients in treatment group) and 17 MFx patients (43.6% of patients in control group) who had been treated during the trial. Under arthroscopy, an 11G Jamshidi needle (Cardinal Health, Vaughan, Ontario, Canada) was introduced into the treated knee either through an arthroscopic portal or through the patellar tendon in order to achieve a perpendicular orientation to the articular surface. The geometric center of the treated lesion was visually estimated and the needle was inserted through the repair tissue and bone to a depth of 1 cm (premarked on the needle) and then removed gently. The cylindrical osteochondral biopsy sample (≈2 mm in diameter) was placed immediately into a labeled tube containing 10% neutral buffered formalin (NBF, Fisher Scientific, Pittsburgh, PA, USA) fixative. Standardized shipping kits were provided to the clinical sites and contained a return shipping container, a temperature tracing device, and shipping transfer documentation. The biopsy was packed in the shipping container, the temperature tracing device started, and the biopsy shipped directly to a central laboratory (AccelLab, Boisbriand, Quebec, Canada) for storage, tissue processing and hematoxylin and eosin staining under Good Laboratory Practices. Blinded sections were then transferred to another Good Laboratory Practices–compliant central laboratory for Safranin-O staining and blinded histological scoring (Biomaterials and Cartilage Laboratory, Montreal, Quebec, Canada).
Histological Processing
Histological Processing of Osteochondral Biopsies
On receipt at the central laboratory (AccelLab, Boisbriand, Quebec, Canada), biopsies were transferred into fresh 10% NBF and blinded to patient number and treatment. Biopsies were fixed for a minimum of 24 hours, but no more than 5 days. Biopsies were then washed twice for 30 minutes each in phosphate buffered saline at room temperature. Decalcification in a 0.5 N HCl, 0.1% (v/v) glutaraldehyde solution (Electron Microscopy Sciences, Hatfield, PA, USA) at a ratio of at least 1:15 (sample volume:decalcification solution volume) was carried out for a period of 30 hours at 4°C. Biopsies were then washed for 60 minutes in phosphate buffered saline at 4°C, followed by an overnight postfixation in 10% NBF at 4°C. Biopsies were infiltrated/embedded in paraffin, and serial 5-µm sections were collected from the center of each biopsy to obtain complete and similar serial sections for each specimen.
Hematoxylin and Eosin Staining
Duplicate sections were processed and stained with hematoxylin and eosin simultaneously in an automatic stainer (AccelLab). Paraffin sections were deparaffinized in toluene and rehydrated before being stained with Harris hematoxylin (MAT Laboratories, Québec, Canada) for 14 minutes. Sections were washed, dipped in acid alcohol (1% v/v hydrochloric acid in 70% ethanol), and stained for 40 seconds with a 1% alcoholic eosin Y solution, before being dehydrated and mounted. Control slides (human osteochondral tissue) were stained simultaneously for quality assurance.
Safranin-O/Fast Green/Iron Hematoxylin Staining
Duplicate sections were manually processed and stained with Safranin-O/fast green/iron hematoxylin (Saf-O) at the Biomaterials and Cartilage Laboratory (BCL, École Polytechnique de Montréal, Montreal, Quebec, Canada). Paraffin sections were deparaffinized in toluene during three 5-minute steps, washed in 100% anhydrous ethanol and rehydrated before being stained with Weigert iron hematoxylin (equal volumes of Weigert iron hematoxylin parts A and B; Sigma, St. Louis, MO, USA) for 8 minutes. Sections were rinsed in water, and then dipped in a 10% solution (v/v in deionized water) of Weigert iron hematoxylin part B with a subsequent wash of 3 minutes in water. The sections were stained for 1 minute with a 0.04% solution (w/v in deionized water) of fast green FCF (Sigma, USA), followed by a dip into a 1% acetic acid solution. Sections were then stained in a 0.2% solution (w/v in deionized water) of Safranin-O (Sigma, USA) for 4 minutes, before being dehydrated and mounted. Control slides (human and animal osteochondral tissues) were stained simultaneously for quality assurance for each staining batch.
Preparation of Unstained Sections for Polarized Light Microscopy
Duplicate paraffin sections were deparaffinized, dehydrated in successive baths of anhydrous ethanol and toluene and mounted unstained in Permount.
Digital Slide Scanning
Histological slides were scanned using a Nanozoomer slide scanner (Hamamatsu, model #C9600-02) in the 40× mode, resulting in high-quality digital images (pixel size of 0.23 μm). Scanning system reproducibility was controlled by scanning control slides before and after each batch of scanned slides.
ICRS I and II Histological Scoring
The best of the duplicate sections was selected for scoring based on the absence of histological processing artefacts. The osteochondral biopsies were scored from 0 (poorest repair) to 3 (ideal repair) according to the ICRS I scoring system23 (Table 1). The ICRS II scoring system was also applied according to Mainil-Varlet et al.24 (Table 1), using a 100-mm visual analogue scale (where 100 represents ideal repair), except for some parameters which were modified to facilitate scoring as described in Table 2. Readers were trained to score each parameter of the ICRS I and II systems under in-house standard operating procedures that were first validated by measuring intra- and interobserver variability using normal, degraded, and repair cartilage samples. Each biopsy section was scored independently by 3 trained blinded readers for ICRS I parameters 3, 4, and 6, and ICRS II parameters 3, 4, 7 and 10 using hematoxylin and eosin–stained sections since it better reveals cell morphology, tidemarks, and calcification. All other parameters were scored using Saf-O–stained sections. All scoring was conducted using the digitized histological slides and slide scanner software (NDP-view, version 1.0.6), which permitted imaging up to 400× magnification (equivalent to observation with a microscope equipped with a 40× objective and 10× oculars). Histological slides were also available for direct viewing by light microscopy. All osteochondral biopsies retrieved during the clinical trial were analyzed. Sections were scored for all parameters except when a parameter was impossible to score (missing feature, tear, or crack, etc.). This occurred in only 1.17%, 1.32%, and 1.75%, respectively, for ICRS I and II, and polarized light microscopy (PLM) analyses.
Table 1.
International Cartilage Repair Society (ICRS) I and II Histological Parameters.
| ICRS I Parameters | ICRS II Parameters | |
|---|---|---|
|
Modified ICRS II parameters (see Table 2).
Table 2.
Modified International Cartilage Repair Society (ICRS) II Histological Parameters.
| ICRS II Parameters | Modifications |
|---|---|
| 1. Tissue morphology | Tissue morphology was evaluated using Saf-O histological stain only. A separate polarized light microscopy (PLM) score was developed to specifically evaluate the collagen organization25,26 |
| 3. Cell morphology | Since cartilage cell morphology is zone-dependent, the percentage of cells that had the correct zone-dependent morphology was evaluated. When zones were not obvious, the estimate of 10%, 30% and 60% was applied respectively for the height of the superficial, transitional and deep zones. Acellular zones indicated a lack of cell viability and thus incorrect morphology |
| 5. Surface architecture | In addition to smoothness of the surface, flatness was also taken into account when scoring for surface architecture as an assessment of the quality of the articulating surface |
| 10. Abnormal calcification | Abnormal calcification was evaluated above the highest tidemark only (not specified in the original ICRS II publication24) |
| 12. Surface/superficial assessment | When zones were not obvious, the estimate of 10%, 30%, and 60% was applied respectively for the height of the superficial, transitional, and deep zones |
| 13. Mid/deep assessment |
Polarized Light Microscopy Scoring
Collagen organization in the osteochondral biopsies (best duplicate) was scored on a scale of 0 to 5 using a validated PLM scoring system,25,26 where 0 = no organization, 1 = vertical deep zone (DZ) apparent, 2 = DZ well-developed, 3 = 3 zones present, 4 = zonal proportions of normal articular cartilage, and 5 = hyaline cartilage organization. Tissues that received a score of at least 3 out of 5 contained a vertically oriented DZ and at least 2 additional zones approximating the transitional (TZ) and superficial (SZ) zones. This multizone structure, which is difficult to achieve in cartilage repair, represents a successful repair and is rarely reported in publications that use PLM scoring.24,27-30 PLM readers were trained under in-house standard operating procedures. Unstained sections were scored independently by 3 trained blinded readers directly at a light microscope (Zeiss, Toronto, Ontario, Canada) equipped with polarizing filters. A simple yes/no flowchart of the PLM score was used to assist readers when evaluating histological sections. All osteochondral biopsies retrieved during the clinical trial were analyzed.
Identification of Outliers and Consensus Scoring
A preapproved procedure was established to account for the presence of outliers found during the analysis. For categorical ratings (i.e., ICRS I and PLM), an outlier was identified when there was a difference greater than 1 between any of the 3 readers. For continuous ratings (i.e., ICRS II), an outlier was identified when there was a difference greater than 30%. All outliers were systematically reviewed by a committee composed of the readers and a mediator, and a consensus score was determined and justified. No data were excluded during this process. The incidence of outliers was 7.5%, 11.8%, and 6.1% for ICRS I and II, and PLM, respectively.
Statistical Analysis
All statistical analyses followed a preapproved statistical analysis plan and were performed by a third-party according to the intention-to-treat principle. Normality and homogeneity of variance of the data was tested for all parameters. A general linear model was then used to compare the analysis of covariance between treatment groups, adjusting for cartilage lesion volume as assessed by quantitative MRI, which was found to be a significant covariate. Only parametric results are presented herein because the nonparametric results were consistent with parametric models and the fact that analysis of covariance is robust to deviations from normality.31
Enrollment bias was investigated between patients who consented to the biopsy substudy and those who did not, using the bootstrap method to compare baseline demographic variables (age, gender, body mass index, activity level, smoking status, onset of symptoms, number of physiotherapy sessions after study treatment, pain at screening, lesion area and volume), as well as 1-year MRI outcome variables of lesion %Fill and mean T2 MRI values.
Analyses were performed with Statistical Analysis System Software (SAS, SAS Institute, Inc, Cary, NC, USA). All reported P values are 2-sided and P values of less than 0.05 were considered statistically significant.
Results
Enrollment
There were no significant differences between patients who elected to undergo the biopsy procedure and those who did not for all baseline demographic variables studied, as well as for MRI outcome variables at 1 year (%Fill and T2 relaxation time).
ICRS Macroscopic Scoring
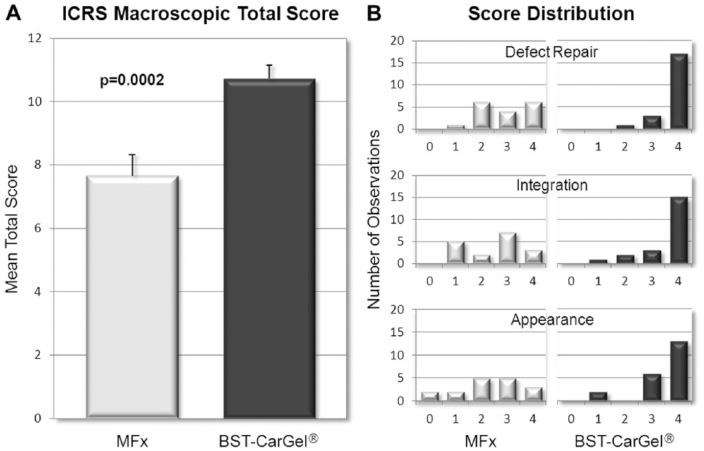
The mean ICRS macroscopic score (out of a possible 12) for the 3 parameters combined was 10.7 ± 2.0 (n = 21) in the BST-CarGel group compared with 7.6 ± 2.7 (n = 17) in the MFx group, a between-group difference that was statistically significant (Fig. 1A; P = 0.0002). The score distribution shows a more consistent and superior repair in the BST-CarGel group compared with MFx (Fig. 1B), with a majority of BST-CarGel patients demonstrating either normal or nearly normal repair for each criterion.
Figure 1.
Macroscopic assessment of cartilage repair during second-look arthroscopies. (A) The total International Cartilage Repair Society (ICRS) macroscopic scores revealed a significant improvement (P = 0.0002) in cartilage repair after BST-CarGel treatment compared with microfracture (MFx) alone (mean ± SE). (B) The score distribution of the individual parameters showed a more consistent and superior repair in the BST-CarGel group (higher scores) compared with MFx. BST-CarGel n = 21; MFx n = 17.
A total of 81.0% (17/21) of BST-CarGel patients received the highest score of 4 for the individual parameter Degree of Defect Repair (i.e., a repair tissue level with surrounding cartilage), compared with only 35.3% (6/17) of patients in the MFx group. For the parameter Integration to Border Zone, 71.4% (15/21) of patients in the BST-CarGel treatment group had complete integration of the repair tissue (score of 4) compared with only 17.6% (3/17) in the MFx group. Finally, the repair tissue of 61.9% (13/21) of patients in the BST-CarGel treatment group received a score of 4 for the parameter Macroscopic Appearance, indicating an intact and smooth surface compared with only 17.6% (3/17) in the MFx group. Conversely, 11.8% (2/17) of patients in the MFx group showed poor Macroscopic Appearance of the defect area (score of 0) compared with none in the BST-CarGel treatment group.
ICRS I and II Histological Scoring
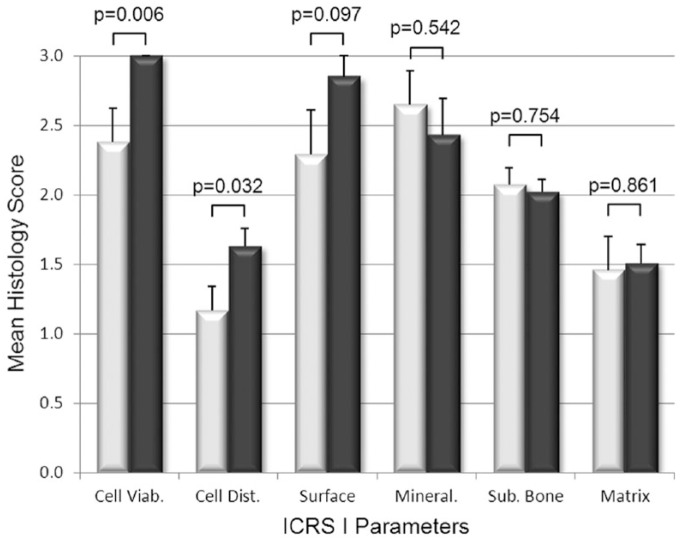
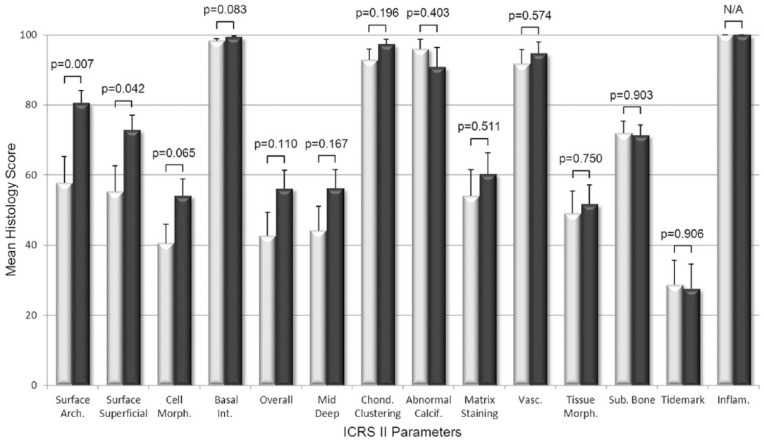
The histological scoring of biopsies found that BST-CarGel treatment resulted in a significant improvement of the structural parameters Surface Architecture (P = 0.007) and Surface/Superficial Assessment (P = 0.042), as well as cellular parameters Cell Viability (P = 0.006) and Cell Distribution (P = 0.032) compared with MFx alone at 13 months posttreatment (Figs. 2 and 3). Other parameters such as Surface, Cell Morphology, Basal Integration, and Overall Assessment were statistically trending toward improvement (0.05 < P < 0.11) following BST-CarGel treatment. The remaining 12/20 ICRS I and II histological parameters were similar for both groups. No histological parameters were better for the MFx group over the BST-CarGel group. All biopsies received perfect scores of 100 for the Inflammation parameter indicating an absence of inflammation at 13 months posttreatment as shown in Figure 3.
Figure 2.
Histological assessment of cartilage repair using the International Cartilage Repair Society (ICRS) I scoring system. White and black bars represent microfracture (MFx) and BST-CarGel, respectively. P values are shown for differences in means (±SE) between BST-CarGel and MFx. Parameters are shown in order of ascending P values from left to right. BST-CarGel treatment resulted in a significant improvement in the cellular parameters Cell Viability and Cell Distribution, and trended (P < 0.11) for the structural parameter Surface. No histological parameters were improved significantly in the MFx group over the BST-CarGel group. BST-CarGel n = 20 except for Cell Viability, Mineralization, and Matrix where n = 21; MFx n = 17.
Figure 3.
Histological assessment of cartilage repair using the International Cartilage Repair Society (ICRS) II scoring system. White and black bars represent microfracture (MFx) and BST-CarGel, respectively. P values are shown for differences in means (±SE) between BST-CarGel and MFx. Parameters are shown in order of ascending P values from left to right. BST-CarGel treatment resulted in a significant improvement of structural parameters Surface Architecture and Surface/Superficial Assessment at 13 months posttreatment, and trended (P < 0.11) for Cell Morphology, Basal Integration, and Overall Assessment. No histological parameters were improved significantly for the MFx group over the BST-CarGel group. All biopsies received scores of 100 for Inflammation, which indicate an absence of inflammation. BST-CarGel n = 20 except for Chondrocyte Clustering, Abnormal Calcification, Matrix Staining, Vascularization, Tissue Morphology, and Inflammation where n = 21; MFx n = 17.
To highlight histologically observable differences, the best, median, and worst biopsies from each group (according to the ICRS II Overall Assessment parameter) were compared (Fig. 4). The best biopsies for both the BST-CarGel and the MFx groups showed complete staining for Saf-O (glycosaminoglycan). However, the best BST-CarGel repair biopsy had a thicker repair tissue and a smoother articulating surface compared with the best MFx biopsy, consistent with the higher ICRS macroscopic score and higher histological scores for structure parameters. The difference between the 2 groups is more apparent with the comparison of the median repair biopsies where the BST-CarGel biopsy demonstrated better Saf-O staining in the DZ, a more organized repair tissue and subchondral bone, and a smoother articulating surface (similar to what was observed by PLM and histological structure parameters), compared with the MFx biopsy, which demonstrated a faint Saf-O stain and a disorganized repair tissue. The worst biopsies in each group were very similar with comparable thicknesses, smooth articulating surfaces and were mainly composed of fibrous tissue.
Figure 4.
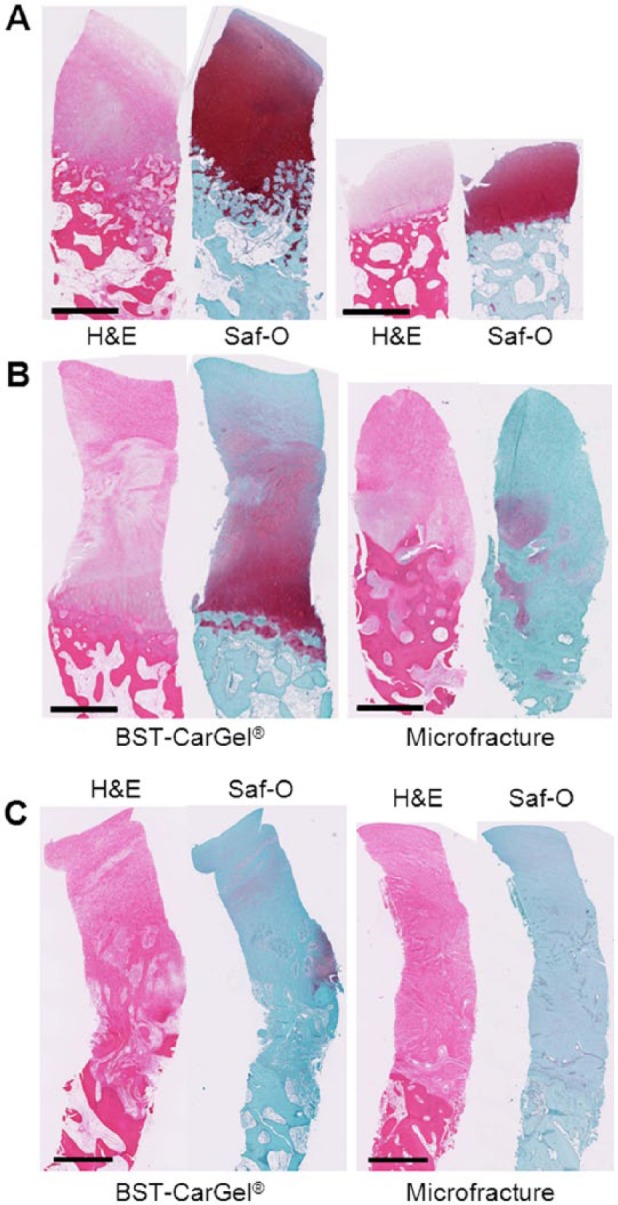
Biopsy histology of the best (A), median (B), and worst (C) repairs of the BST-CarGel and microfracture (MFx) groups at 13 months posttreatment, according to the International Cartilage Repair Society (ICRS) II parameter Overall Assessment. The median repairs represent the 11th of 21 and 9th of 17 highest scores for BST-CarGel and MFx, respectively. The BST-CarGel biopsies show superior tissue quality and organization compared with the MFx biopsies for both the best and median repairs. The worst biopsies in each group were very similar with comparable thicknesses, smooth articulating surfaces, and were mainly composed of fibrous tissue. Scale bars = 1 mm.
Polarized Light Microscopy Scoring
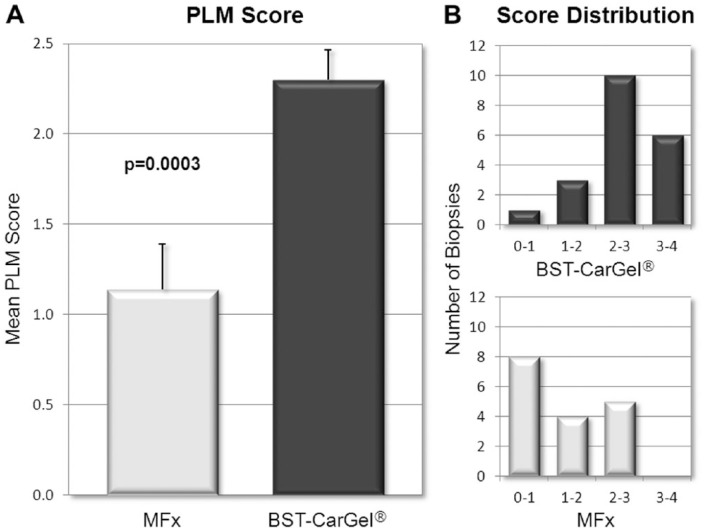
BST-CarGel treatment resulted in a mean PLM score significantly greater than the MFx group (Fig. 5A; P = 0.0003), indicating a more organized repair tissue with collagen stratification more similar to native hyaline cartilage. More BST-CarGel patients had high quality tissue (i.e., scores from 2-4) compared with MFx patients (distribution shown in Fig. 5B), indicating that BST-CarGel treatment consistently resulted in a more organized and stratified repair tissue.
Figure 5.
Polarized light microscopy (PLM) scoring of biopsies at 13 months posttreatment. (A) PLM mean scores demonstrated a significant improvement in collagen organization after BST-CarGel treatment compared with MFx alone (mean ± SE). (B) The PLM score distribution shows that BST-CarGel treatment produced a more consistent and superior collagen organization in the BST-CarGel group compared with MFx alone. BST-CarGel n = 20; MFx n = 17.
Additionally, only 1/20 (5%) biopsies in the BST-CarGel group received a score of 0 from all 3 readers, compared with 6/17 (35%) in the MFx group, an example of which is shown in Figure 6A. Also, 6/20 (30%) of biopsies in the BST-CarGel treatment group, versus none in the MFx group, had 3 organized zones (i.e., score of 3 or more). Figure 6B provides an example of this from the BST-CarGel group and shows a well-organized tissue that received a PLM score of 4. Finally, 19/20 (95%) biopsies in the BST-CarGel treatment group had at least an organized DZ (score of 1 or more) compared with only 9/17 (53%) in the MFx group.
Figure 6.
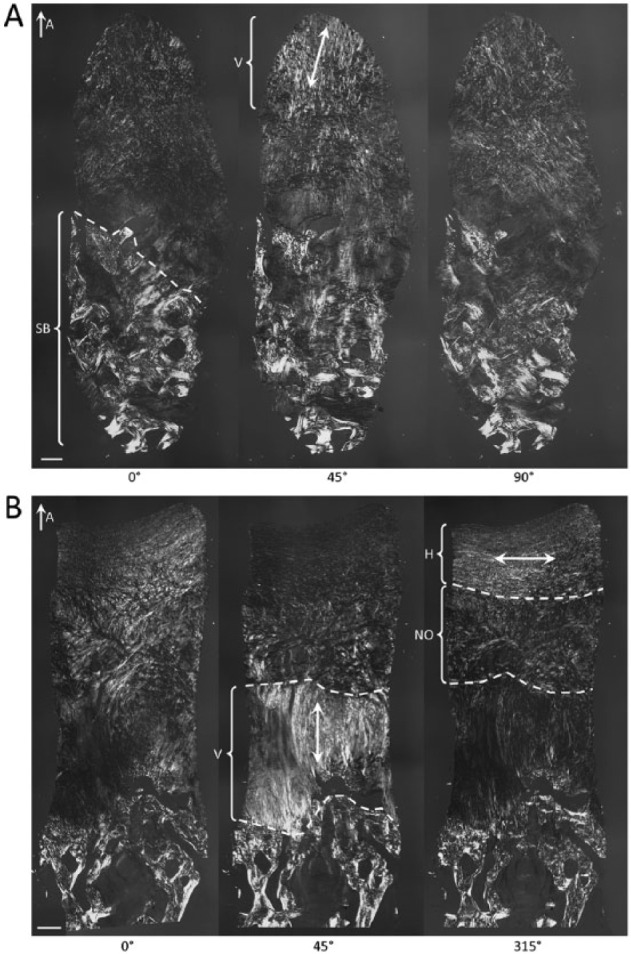
Polarized light microscopy (PLM) images of biopsies at 13 months posttreatment. (A) Disorganized tissue that received a score of 0 out of 5 showing a deep zone (above the subchondral bone [SB]) that lacks organized collagen, which was the case for 35% of the microfracture (MFx) group but only 5% of the BST-CarGel group. Here, some vertically oriented tissue (V) was observed in the superficial zone. (B) Well-organized tissue that received a score of 4 out of 5 demonstrating 3 zones with collagen that is vertically oriented in the deep zone, nonoriented (NO) in the transitional zone, and horizontally oriented (H) in the superficial zone. In the BST-CarGel treatment group, 30% of biopsies had 3 organized zones (score of 3 or more) versus none in the MFx group. Double-headed arrows indicate the orientation of collagen. The images were published previously.26 Scale bars = 250 µm.
Discussion
The use of osteochondral biopsies to assess cartilage repair has been reported in several clinical studies of cartilage repair with level I5-7,32,33 and level II evidence.34,35 Most studies rely on qualitative evaluations of repair tissue quality, reporting only tissue type (e.g., fibrous or hyaline), except for 2 studies where the validated ICRS II histological scoring system24 was applied.5,35 The current study compared BST-CarGel treatment with MFx alone and is the first to apply both ICRS I and II scores, as well as a PLM score in the same analysis of biopsies collected at 13 months posttreatment under Good Clinical Practice guidelines during an RCT with level 1 evidence.3 In this study, BST-CarGel treatment resulted in the improvement of several structural and cellular characteristics of repair tissue over MFx. This was demonstrated by ICRS I and II histological assessments resulting in significant improvement of 4 parameters, Surface Architecture, Surface/Superficial Assessment, Cell Viability, and Cell Distribution, as well as by PLM scoring, despite the fact that the biopsy analysis was a tertiary endpoint of the trial and not statistically powered. A clinical trial comparing characterized chondrocyte implantation with MFx where biopsy analysis5 was the powered endpoint found significant improvement in 6 of 14 ICRS II parameters for characterized chondrocyte implantation (also structural and cellular parameters), but almost 25% of the biopsies (21/86) were excluded from the analysis due to poor staining or incomplete tissue. No biopsies were excluded from the current analysis.
Visual assessment of the repair area during second-look arthroscopies is common practice by orthopedic surgeons. The ICRS developed and validated a macroscopic scoring system to help standardize the practice and to permit quantitative comparisons.21 One study comparing autologous chondrocyte implantation with MFx demonstrated a higher, but nonsignificant ICRS macroscopic score for MFx at 12 months.7 Here, at 13 months posttreatment, BST-CarGel treatment resulted in a highly significant improvement (P = 0.0002) compared with MFx alone in the total macroscopic score, which encompasses the assessment of lesion fill, integration to native cartilage, and surface quality of the repair tissue. This result corresponds well with other findings from the same RCT where repair tissue quantity and quality, blindly assessed by quantitative MRI, were both significantly improved over MFx at 12 months following BST-CarGel treatment.3
It is generally believed in cartilage repair that improved structure is predictive of long-term durability.16-19,36 The smoother cartilage surface more closely resembling articular cartilage detected by the ICRS macroscopic and ICRS I and II analyses following BST-CarGel treatment is an important finding since many cartilage degradation processes are thought to be initiated by surface fibrillation and degradation.37,38 Superior surface properties were previously described to occur due to an alteration by BST-CarGel of the timing, maturation and position of chondrogenic foci,39 which are believed to improve chondral resurfacing and repair tissue organization. Furthermore, the improvements seen for the BST-CarGel group in the cell-based parameters in ICRS I and II suggest greater long-term viability and durability of the repair tissue. Indeed, longer term follow-up in the same BST-CarGel RCT demonstrated that the structural superiority observed at 1 year by quantitative MRI following BST-CarGel treatment over MFx alone is significantly maintained over 5 years.36 ICRS I and II histological parameters related to the matrix or subchondral bone did not show a difference between groups, which could be explained by the fact that maturation of repair tissue and bone remodeling are continuous processes that occur over time and biopsies harvested at 13 months contain an ongoing maturing tissue. Other features were not often observed like abnormal calcification (ICRS I-6, II-10) which was a parameter developed for the first generation of autologous chondrocyte implantation, vascularization (ICRS II-11) present only in fibrous tissue and chondrocyte clustering (ICRS II-4), which is believed to be a hallmark of osteoarthritis.40
The collagen network and its specialized organization in articular cartilage is a primary determinant of cartilage mechanical properties and durability.41,42 In this study, BST-CarGel treatment resulted in superior collagen organization compared with MFx, as demonstrated by improved PLM scores. This finding aligns well with other measures of repair tissue quality, such as the ICRS macroscopic score, and ICRS I and II histological parameters such as Surface Architecture and Surface/Superficial Assessment. Additionally, quantitative MRI from this same RCT3 found that the transverse relaxation time (T2) of the repair tissue, indicative of collagen content, organization and hydration,43-47 was nearer to native cartilage for BST-CarGel–treated patients compared with that of MFx alone. Only a moderate correlation was found between T2 MRI and PLM scoring of biopsies collected at an average of 13 months posttreatment.36 No correlations were found between biopsy parameters and clinical outcomes (WOMAC [Western Ontario and McMaster Universities Osteoarthritis Index] scores), which was not unexpected since the same was observed between the MRI co-primary endpoints (%Fill and T2) and WOMAC scores both at 1 year3 and at 5 years.36
The consistency of repair following BST-CarGel treatment is demonstrated by the improved distribution of macroscopic and PLM scores compared with MFx, and is corroborated by the structural superiority and consistency found by MRI at 1 year3 in this trial. The improved repair tissue quality based on collagen parameters at 13 months posttreatment as measured in biopsies in this study, but also previously by T2 MRI,3 was predictive of longer term improved durability of repair seen at 5 years posttreatment in these same patients.36
This analysis of quality of repair has demonstrated distinct differences in structural outcomes associated with BST-CarGel treatment over microfracture alone. The 3-fold mode of action of BST-CarGel which distinguishes it from MFx has been previously elucidated in rabbits and sheep where the BST-CarGel implant (1) acts as a scaffold to stabilize the blood clot in the cartilage lesion, (2) impedes blood clot retraction while allowing normal clotting, and (3) adheres to cartilage lesion surfaces.12,13 The residency of the BST-CarGel/blood implant, which maintains critical blood components above the marrow holes, leads to increased cell recruitment, vascularization of the provisional repair tissue and intramembranous bone formation and bone remodeling in animal models.15,48 These events are consistent with the results observed in this study where structural and cellular characteristics are improved by BST-CarGel treatment.
This biopsy substudy was performed under the confines of a regulated and standardized Good Clinical Practice randomized trial. A major strength of this study was the use of third parties for biopsy shipping, storage, processing, scoring, and statistical analysis. Other strengths included the scoring, which was performed on every harvested biopsy by trained and blinded readers using validated methodologies, and that ICRS guidelines were followed for histological processing and analyses of osteochondral biopsies.49 On the other hand, biopsies are intrinsically limiting, since they are not representative of the entire repair tissue volume and present patient consent challenges (e.g., only 48% of patients agreed in this trial). Here, the histological results are treated as supplemental to the actual clinical trial primary endpoint of 3-dimensional quantitative MRI, for which the trial was powered.3 And while the 13-month follow-up could be considered short term (biopsies with ongoing maturing tissue) since current regulatory guidance for cartilage repair trials suggests 24 months,50 the quantitative MRI primary endpoint in this trial brought a level of precision and sensitivity that permitted sufficient detection of differences in cartilage structure at 12 months and longitudinally to 5 years.36
In conclusion, previously published data from this pivotal cartilage repair RCT showed by MRI that the BST-CarGel single-step cartilage repair treatment consistently resulted in a greater volume and better quality of repair tissue compared with MFx alone.3 The present biopsy substudy further revealed BST-CarGel treatment improved structural and cellular characteristics of cartilage repair tissue at 13 months posttreatment, compared with MFx alone. The overall agreement between analyses from multiple, independent, and quantitative methods taken together form a convincing body of evidence supporting the beneficial effects of BST-CarGel treatment at 1 year posttreatment compared with MFx alone, which translated to longer term structural superiority at 5 years.36
Footnotes
Acknowledgments and Funding: We are indebted to the BST-CarGel Clinical Trial Group investigators who conducted the trial and retrieved the osteochondral biopsies from their trial patients: in Canada, William Stanish (Halifax), Nicholas Mohtadi (Calgary), Peter MacDonald (Winnipeg), Robert McCormack, Jordan Leith, Patrick Chin, and Mike Gilbart (Vancouver), Stéphane Pelet, Réjean Cloutier, Jean Lamontagne, and Sylvain Belzile (Quebec City), Don Johnson and Allan Liew (Ottawa), Paul Marks (Toronto), Michel Malo, Julio Fernandes, Pierre Ranger, Jacques Desnoyers, Patrick Lavigne, and Sébastien Guimond Simard (Montreal), Paul Zalzal and Tim Deakon (Oakville), and Frank Smith (Hamilton); in Spain, Francisco Forriol, Felipe Lopez-Oliva, Gloria Lopez, Manuel Leyes, Javier Vaquero, Diego Garcia, Santiago Bello, Alonso Moreno, and Patricia Villanueva (Madrid), Francisco Macule (Barcelona), and Antonio Maestro Fernandez (Gijon); and in South Korea, Myung Chul Lee, Sang-Hoon Lee, and Kyoung Ho Yoon (Seoul). Biopsies were received, decalcified, embedded, sectioned, and stained with hematoxylin and eosin at AccelLAB by Robin Turcotte, under the supervision of Jean-Martin Lapointe. Biopsy histology was carried out at the Biomaterials and Cartilage Laboratory at École Polytechnique de Montréal (EPM) by Viorica Lascau-Coman and Geneviève Picard. Special thanks to Anik Chevrier, Claire Jarry, Catherine Marchand and Evgeny Rossomacha for scoring of the biopsies, and Julie Tremblay for quality assurance (EPM). Statistical analysis was performed by Cato Canada (Montreal); additional statistical input and oversight was provided by Alex Yaroshinsky (San Andreas, California). Piramal Life Sciences, Bio-Orthopaedics Division, was the sponsor of the randomized controlled trial comparing BST-CarGel treatment with microfracture alone for the repair of focal cartilage lesions of the condyle and was involved in the study design, the preparation of this manuscript and the decision to submit it for publication. Collection and analysis of the data were conducted by third-party laboratories or companies. This work was partly sponsored by a Natural Sciences and Engineering Council Industrial Chair (MDB; MOP 115186) and the Canadian Institutes of Health Research (CDH; MOP 185810).
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Authors AC and NTK have no financial disclosures or notable competing interests. Authors SM and AR are employees and CDH, WDS, MSS, and MDB consulted for Piramal Life Sciences, Bio-Orthopaedics Division.
Ethical Approval: This international multicenter project was submitted to each countries regulatory authority (Canada: ITA 93444, Spain: AEMPS # expte. 286/06/EC and Korea: KFDA: April 16, 2008–156th) including the subject’s Informed Consent Form (ICF) and received Investigational testing authorization by each countries regulatory authority. All participating research centers obtained, prior to study commencement, individual Research Ethics Board committee approval.
References
- 1. Newman AP. Articular cartilage repair. Am J Sports Med. 1998;26:309-24. [DOI] [PubMed] [Google Scholar]
- 2. Buckwalter JA, Mankin HJ. Instructional Course Lectures, The American Academy of Orthopaedic Surgeons—Articular Cartilage. Part II: degeneration and osteoarthrosis, repair, regeneration, and transplantation. J Bone Joint Surg Am. 1997;79:612-32. [Google Scholar]
- 3. Stanish WD, McCormack R, Forriol F, Mohtadi N, Pelet S, Desnoyers J. et al. Novel scaffold-based BST-CarGel treatment results in superior cartilage repair compared with microfracture in a randomized controlled trial. J Bone Joint Surg Am. 2013;95:1640-50. [DOI] [PubMed] [Google Scholar]
- 4. Saris D, Price A, Widuchowski W, Bertrand-Marchand M, Caron J, Drogset JO, et al. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: two-year follow-up of a prospective randomized trial. Am J Sports Med. 2014;42:1384-94. [DOI] [PubMed] [Google Scholar]
- 5. Saris DB, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36:235-46. [DOI] [PubMed] [Google Scholar]
- 6. Gudas R, Kalesinskas RJ, Kimtys V, Stankevicius E, Toliusis V, Bernotavicius G, et al. A prospective randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy. 2005;21:1066-75. [DOI] [PubMed] [Google Scholar]
- 7. Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grøntvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A:455-64. [DOI] [PubMed] [Google Scholar]
- 8. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37:2053-63. [DOI] [PubMed] [Google Scholar]
- 9. Vanlauwe J, Saris DB, Victor J, Almqvist KF, Bellemans J, Luyten FP, et al. Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med. 2011;39:2566-74. [DOI] [PubMed] [Google Scholar]
- 10. Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14:1119-25. [DOI] [PubMed] [Google Scholar]
- 11. Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87:1911-20. [DOI] [PubMed] [Google Scholar]
- 12. Hoemann CD, Sun J, McKee MD, Chevrier A, Rossomacha E, Rivard GE, et al. Chitosan-glycerol phosphate/blood implants elicit hyaline cartilage repair integrated with porous subchondral bone in microdrilled rabbit defects. Osteoarthritis Cartilage. 2007;15:78-89. [DOI] [PubMed] [Google Scholar]
- 13. Hoemann CD, Hurtig M, Rossomacha E, Sun J, Chevrier A, Shive MS, et al. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J Bone Joint Surg Am. 2005;87:2671-86. [DOI] [PubMed] [Google Scholar]
- 14. Kumar MN, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104:6017-84. [DOI] [PubMed] [Google Scholar]
- 15. Chevrier A, Hoemann CD, Sun J, Buschmann MD. Chitosan-glycerol phosphate/blood implants increase cell recruitment, transient vascularization and subchondral bone remodeling in drilled cartilage defects. Osteoarthritis Cartilage. 2007;15:316-27. [DOI] [PubMed] [Google Scholar]
- 16. Eshed I, Trattnig S, Sharon M, Arbel R, Nierenberg G, Konen E, et al. Assessment of cartilage repair after chondrocyte transplantation with a fibrin-hyaluronan matrix—correlation of morphological MRI, biochemical T2 mapping and clinical outcome. Eur J Radiol. 2012;81:1216-23. [DOI] [PubMed] [Google Scholar]
- 17. Brun P, Dickinson SC, Zavan B, Cortivo R, Hollander AP, Abatangelo G. Characteristics of repair tissue in second-look and third-look biopsies from patients treated with engineered cartilage: relationship to symptomatology and time after implantation. Arthritis Res Ther. 2008;10:R132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krishnan SP, Skinner JA, Jagiello J, Carrington RWJ, Flanagan AM, Briggs TWR, et al. Durability of cartilage repair—does histology matter? J Bone Joint Surg Br. 2008;90-B(Suppl II):323-4. [Google Scholar]
- 19. Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigsen TC, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89:2105-12. [DOI] [PubMed] [Google Scholar]
- 20. Blackman AJ, Smith MV, Flanigan DC, Matava MJ, Wright RW, Brophy RH. Correlation between magnetic resonance imaging and clinical outcomes after cartilage repair surgery in the knee: a systematic review and meta-analysis. Am J Sports Med. 2013;41:1426-34. [DOI] [PubMed] [Google Scholar]
- 21. Peterson L, Minas T, Brittberg M, Nilsson A, Sjögren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;(374):212-34. [DOI] [PubMed] [Google Scholar]
- 22. van den Borne MP, Raijmakers NJ, Vanlauwe J, Victor J, de Jong SN, Bellemans J, et al. International Cartilage Repair Society (ICRS) and Oswestry macroscopic cartilage evaluation scores validated for use in autologous chondrocyte implantation (ACI) and microfracture. Osteoarthritis Cartilage. 2007;15:1397-402. [DOI] [PubMed] [Google Scholar]
- 23. Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, et al. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS). J Bone Joint Surg Am. 2003;85-A(Suppl 2):45-57. [PubMed] [Google Scholar]
- 24. Mainil-Varlet P, Van Damme B, Nesic D, Knutsen G, Kandel R, Roberts S. A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II. Am J Sports Med. 2010;38:880-90. [DOI] [PubMed] [Google Scholar]
- 25. Changoor A, Nelea M, Methot S, Tran-Khanh N, Chevrier A, Restrepo A, et al. Structural characteristics of the collagen network in human normal, degraded and repair articular cartilages observed in polarized light and scanning electron microscopies. Osteoarthritis Cartilage. 2011;19:1458-68. [DOI] [PubMed] [Google Scholar]
- 26. Changoor A, Tran-Khanh N, Methot S, Garon M, Hurtig MB, Shive MS, et al. A polarized light microscopy method for accurate and reliable grading of collagen organization in cartilage repair. Osteoarthritis Cartilage. 2011;19:126-35. [DOI] [PubMed] [Google Scholar]
- 27. Richardson JB, Caterson B, Evans EH, Ashton BA, Roberts S. Repair of human articular cartilage after implantation of autologous chondrocytes. J Bone Joint Surg Br. 1999;81-B:1064-8. [DOI] [PubMed] [Google Scholar]
- 28. Roberts S, McCall IW, Darby AJ, Menage J, Evans H, Harrison PE, et al. Autologous chondrocyte implantation for cartilage repair: monitoring its success by magnetic resonance imaging and histology. Arthritis Res Ther. 2003;5:R60-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roberts S, Menage J, Sandell LJ, Evans EH, Richardson JB. Immunohistochemical study of collagen types I and II and procollagen IIA in human cartilage repair tissue following autologous chondrocyte implantation. Knee. 2009;16:398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vasara AI, Hyttinen MM, Pulliainen O, Lammi MJ, Jurvelin JS, Peterson L, et al. Immature porcine knee cartilage lesions show good healing with or without autologous chondrocyte transplantation. Osteoarthritis Cartilage. 2006;14:1066-74. [DOI] [PubMed] [Google Scholar]
- 31. Olejnik SF, Algina J. Parametric ANCOVA and the rank transform ANCOVA when the data are conditionally non-normal and heteroscedastic. J Educ Stat. 1984;9:129-49. [Google Scholar]
- 32. Bentley G, Biant LC, Carrington RW, Akmal M, Goldberg A, Williams AM, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223-30. [DOI] [PubMed] [Google Scholar]
- 33. Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87:640-5. [DOI] [PubMed] [Google Scholar]
- 34. Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85-A:185-92. [DOI] [PubMed] [Google Scholar]
- 35. Saw KY, Anz A, Siew-Yoke Jee C, Merican S, Ching-Soong Ng R, Roohi SA, et al. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy. 2013;29:684-94. [DOI] [PubMed] [Google Scholar]
- 36. Shive MS, Stanish WD, McCormack R, Forriol F, Mohtadi N, Pelet S, et al. BST-CarGel treatment maintains cartilage repair superiority over microfracture at 5 years in a multicenter randomized controlled trial. Cartilage. 2015;6:62-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hollander AP, Pidoux I, Reiner A, Rorabeck C, Bourne R, Poole AR. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995;96:2859-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu JP, Kirk TB, Zheng MH. Study of the collagen structure in the superficial zone and physiological state of articular cartilage using a 3D confocal imaging technique. J Orthop Surg Res. 2008;3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chevrier A, Hoemann CD, Sun J, Buschmann MD. Temporal and spatial modulation of chondrogenic foci in subchondral microdrill holes by chitosan-glycerol phosphate/blood implants. Osteoarthritis Cartilage. 2011;19:136-44. [DOI] [PubMed] [Google Scholar]
- 40. Maldonado M, Nam J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed Res Int. 2013;2013:284873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shirazi R, Shirazi-Adl A, Hurtig M. Role of cartilage collagen fibrils networks in knee joint biomechanics under compression. J Biomech. 2008;41:3340-8. [DOI] [PubMed] [Google Scholar]
- 42. Korhonen RK, Wong M, Arokoski J, Lindgren R, Helminen HJ, Hunziker EB, et al. Importance of the superficial tissue layer for the indentation stiffness of articular cartilage. Med Eng Phys. 2002;24:99-108. [DOI] [PubMed] [Google Scholar]
- 43. Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8:355-68. [DOI] [PubMed] [Google Scholar]
- 44. Nieminen MT, Rieppo J, Toyras J, Hakumaki JM, Silvennoinen J, Hyttinen MM, et al. T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med. 2001;46:487-93. [DOI] [PubMed] [Google Scholar]
- 45. Trattnig S, Marlovits S, Jurvelin J, Welsch GH, Potter HG. Magnetic resonance imaging of cartilage repair: a review. Cartilage. 2011;2:5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. White LM, Sussman MS, Hurtig M, Probyn L, Tomlinson G, Kandel R. Cartilage T2 assessment: differentiation of normal hyaline cartilage and reparative tissue after arthroscopic cartilage repair in equine subjects. Radiology. 2006;241:407-14. [DOI] [PubMed] [Google Scholar]
- 47. Xia Y, Moody JB, Alhadlaq H. Orientational dependence of T2 relaxation in articular cartilage: a microscopic MRI (microMRI) study. Magn Reson Med. 2002;48:460-9. [DOI] [PubMed] [Google Scholar]
- 48. Chen G, Sun J, Lascau-Coman V, Chevrier A, Marchand C, Hoemann CD. Acute osteoclast activity following subchondral drilling is promoted by chitosan and associated with improved cartilage repair tissue integration. Cartilage. 2010;2:173-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hoemann CD, Kandel R, Roberts S, Saris DB, Creemers L, Mainil-Varlet P, et al. International Cartilage Repair Society (ICRS) recommended guidelines for histological endpoints for cartilage repair studies in animal models and clinical trials. Cartilage. 2011;2:153-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. US Food and Drug Administration. Guidance for industry: preparation of IDEs and INDs for products intended to repair or replace knee cartilage. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm. December 2011.