Abstract
Purpose:
The aim of this study was to compare the effectiveness of 2 different meniscal scaffolds in treating patients with irreparable partial medial meniscal tear and patients complaining of pain in the medial compartment of the knee due to a previous partial medial meniscectomy. Based on previous studies, we hypothesized that both the scaffolds are effective in improving clinical outcomes in these patient populations.
Material and Methods:
Twenty-eight patients underwent collagen-based medial meniscus implantation (CMI-Menaflex) and 25 with a second-generation scaffold (Actifit). All patients were assessed with Lysholm, Tegner scale, and MRI evaluation—preoperatively, at 6 months, at 12 moths, and followed-up for a minimum of 2 years. Second look arthroscopy and concomitant biopsy were performed in 7 and 12 patients of CMI and Actifit groups, respectively.
Results:
The CMI group at final follow-up showed improvement in Lysholm score from 58.4 ± 17.3 to 94.5 ± 6.0, while the Actifit group showed improvement from 67.0 ± 15.7 to 90.3 ± 13.1; the improvement was statistically significant in both the groups but intergroup difference was not statistically significant (P = 0.1061). Tegner Activity Scale score improved in both the groups, but intergroup difference was not statistically significant (P = 0.5918). MRI evaluation showed in situ scaffold and no progression of degenerative arthritis in both the groups at final follow-up. Histological evaluation showed more fibrous tissue with blood vessels in the CMI group and the Actift group showed avascular cartilaginous features.
Conclusion:
Both the scaffolds are effective in improving patients’ symptoms and joint function at short-term follow-up.
Keywords: scaffolds, meniscus, polarized light microscopy, meniscal injury, knee
Introduction
It is well known that deficiency of meniscal tissue can result in alteration of joint homeostasis and degenerative changes overtime.1-4 In the past few years, meniscal scaffolds designed with tissue engineering techniques have been proposed to treat the meniscus-deficient knee to improve joint function and possibly delay arthritis. These scaffolds are structures designed with porous gaps of specific size and orientation, and its biomechanical characteristics and stiffness protect excess loading during normal joint function and also promote tissue regeneration.5-8 Different types of scaffolds are currently under investigation, but to date only 2 meniscus implants are used to treat partial meniscus deficiencies: CMI-Menaflex (Ivy Sports Medicine, Montvale, NJ), composed of collagen and glycosaminoglycan; and Actifit (Orteq, London, UK), composed of polycaprolactone-polyurethane.
The CMI-Menaflex was proposed in 19929 and has been available for clinical use since 2000. It is composed of type I collagen isolated and purified from bovine Achilles tendon with added glycosaminoglycans and has a shape similar to the normal human meniscus. The CMI scaffold is arthroscopically implantable, biocompatible, and bioresorbable; ultrastructurally this scaffold is very porous. These features facilitate induction, proliferation, and differentiation of cellular elements within the scaffold, with consequent production of extracellular matrix to reproduce a meniscal-like tissue while the scaffold is gradually absorbed. In vivo studies in both animal and human models confirmed that CMI encourages proliferation of fibrochondrocytes and production of extracellular matrix.9,10 In the recent years, several studies on collagen meniscus implants have showed significant clinical improvement and no progression of degenerative articular changes in most cases at mid-term and long-term follow-up.11-15
The Actifit is a synthetic and biodegradable scaffold composed of polycaprolactone-polyurethane and was introduced for clinical purposes for meniscal regeneration more recently. This structure seems to have better mechanical properties, as it is more resistant to surgical procedures, particularly to sutures, and to the loads during normal joint function. The increased absorption rate also allows full tissue regeneration. The scaffold’s ultrastructure is characterized by 80% porosity and 20% low reabsorption rate polymer. Within the polymer there are softer polycaprolactone segments that constitute 80% of the polymer, and the rest of the 20% is a more rigid urethane. Degradation starts with hydrolysis of polycaprolactone segments that lasts up to 5 years, and polyurethane segments are removed by macrophages and giants cells or it may get integrated into the surrounding tissues.15,16 A multicenter study showed that 81% of patients treated with the biodegradable polyurethane scaffold showed tissue ingrowth 3 months postoperatively, and biopsies at 12 months showed tissue infiltration with no sign of cell death or necrosis in 97% of the cases.17 Several other clinical studies have also shown significant improvement of clinical scores without degenerative changes and scaffold-related adverse events at 2-year follow-up.17-22
The aim of this study was to compare the effectiveness of these different meniscal scaffolds in treating patients with irreparable partial medial meniscal lesions or in patients complaining painful medial compartment of the knee due to a previous partial medial meniscectomy. Based on previous individual studies, we hypothesized that both the scaffolds could be effective in improving symptoms and function in the above-mentioned patient groups.
Materials and Methods
Twenty-eight patients underwent collagen-based medial meniscus implantation (CMI-Menaflex), and 25 patients were operated using Actifit at our institution from 2001 to 2012. All patients were evaluated pre- and postoperatively with a minimum 2-year follow-up. Clinical evaluation was assessed with Lysholm score, Tegner scale preoperatively at 6 to 12 and 24 months after surgery, and radiological evaluation with MRI was done preoperatively and 2 years after surgery. Second look arthroscopy and concomitant biopsies were performed in 7 patients of the CMI group and 12 of the Actifit group at 4 to 45 months.
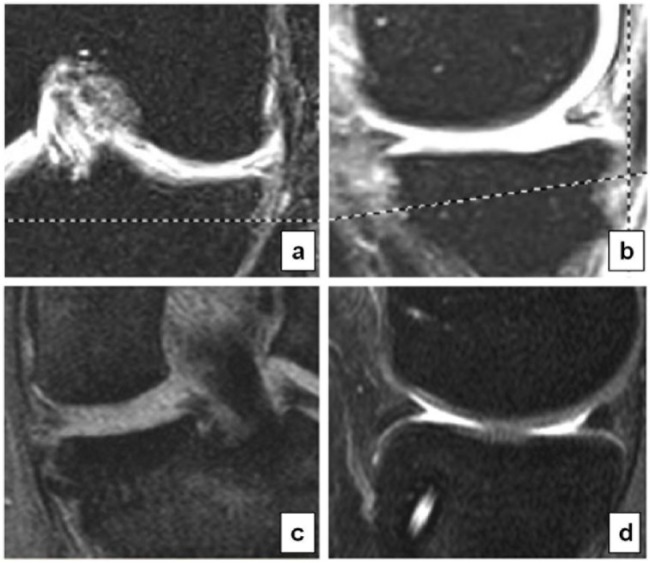
Our inclusion criteria were the following: skeletally mature male or female patients with irreparable medial meniscal tear or partial meniscus loss with intact rim and anterior and posterior horn (Fig. 1) who had normal axial alignment (mechanical tibiofemoral angle ≤3°) and stable joint. Patients with malalignment requiring osteotomy as well as patients requiring ligament repair for instability were been included and treated concomitantly.
Figure 1.
(a) Painful medial compartment, a chronic sequelae after previous medial partial meniscectomy. (b) Young patient with an irreparable bucket handle tear of the medial menisci.
The exclusion criteria were the following: advanced knee joint osteoarthritis, inflammatory arthropathy, and patients not willing to give consent for our follow-up and rehabilitation protocol.
CMI patients were treated between 2001 and 2002, and these patients were included in a previous study,11 while Actifit surgeries were performed from 2009 to 2012 and all the patients were prospectively followed-up for a minimum of 2 years. All patients signed an informed consent to be involved in the study. The study has been approved by the Ethics Committee of the Ospedale di Circolo Fondazione Macchi, Varese, Italy (June 18, 2013; Protocol Number 0023851; Registered Clinical Trials 54/2013).
MRI Evaluation
Twenty-six patients in the CMI group and 21 patients in the Actifit group had a knee MRI preoperatively and 2 years after surgery. MRI evaluation was done in different centers according to a codified protocol as described in a previous study.11
The morphology and the signal intensity of the implant-regenerated tissue complex of the 2 scaffolds were evaluated with modified Genovese score.23 Three patterns were identified and were classified from 1 to 3, with higher scores reflecting patterns more closely resembling those of the normal meniscus. The scaffold/residual meniscus complex was classified as grade 1, scaffold was totally reabsorbed; grade 2, scaffold appeared small with irregular-regular morphology; and grade 3, shape and size were identical to the ones of the normal meniscus. Regarding signal intensity, a markedly hyperintense scaffold was considered as grade 1, a slightly hyperintense was considered as grade 2, and if the scaffold signal intensity was isointense to the normal meniscus it was considered as grade 3. With regard to signal intensity, we divided the grade 2 of the Genovese classification into 2 subgroups: 2A and 2B depending on the degree (greater or lesser) of signal hyperintensity. The appearance of joint cartilage of the index compartment was evaluated using Yulish score, which was also used in other studies for evaluating the same.11,14 According to this classification, cartilage lesions are defined as follows: grade 1, cartilage with normal contour ± abnormal signal; grade 2, superficial fraying and erosion or ulceration of <50% of thickness as demonstrated on MRI; grade 3, presence of partial thickness defect of >50% but <100%; grade 4, full thickness cartilage loss. Normal cartilage was classified as grade 0.
Surgical Technique
Standard knee arthroscopy was performed through anterolateral and anteromedial portals. The native meniscus was trimmed back to the vascularized zone and the resultant defect was measured with a dedicated device. We provided extra blood supply by making puncture holes in the peripheral rim with a Steadman awl. Implant was prepared to the appropriate size and placed into the joint space; finally, it was firmly sutured to the native meniscus with inside-out technique or with a hybrid technique as needed. Few differences are present in the surgical technique of the implantation performed in different time period: in the first technique, CMI was introduced wet into the articulation using suture inside-out for the posterior horn with a posteromedial incision; in a second technique Actifit was introduced dry into the articulation using an all-inside suture (Fast-fix) for the posterior region.
Second Look Arthroscopy and Histological Evaluation
Seven cases of the CMI group and 11 cases of the Actifit group were subjected to second look. The biopsy was performed on the residual scaffold new tissue complex, and the center of the inner free edge of the scaffold was taken for histological analysis as suggested by Verdonk et al.17 The histological evaluations of the CMI biopsies and the procedure to carry out the light microscopy analysis have been reported previously by Reguzzoni et al.,7 and we adopted similar principles during our evaluation of these 2 scaffolds. All the specimens were dehydrated in ascending grades of ethanol and then embedded in paraffin. They were sectioned to 5-µm thickness with a Reichert Ultracut S ultratome (Leica, Vienna, Austria) and then stained with hematoxylin and eosin. Histological evaluation was performed with light microscopy (Nikon Eclipse E600 microscope, Nikon, Tokyo, Japan).7 Furthermore, all the biopsy samples were also evaluated for any foreign body reaction in the synovium and in the pores of the implant, and scored according to an ordinal scale (grade 0 to grade 4) proposed by Van Tienen et al.24
Rehabilitation Protocol
Physical therapy was started from the first postoperative day: a knee brace was applied locked in full extension immediately after the surgery, and it was worn by the patient for 6 weeks. The patients were allowed to remove the brace 3 to 4 times a day to perform assisted passive motion exercises 0° to 30° for the first 2 weeks, 0° to 60° for the third week, 0° to 90° for the subsequent 2 weeks, and then were allowed free range of motion. Weight bearing was not allowed for the first 6 weeks; ambulation was permitted only with the aid of crutches. After 6 weeks, progressive weight bearing was started, eventually reaching complete weight bearing around the 10th week. Muscle strengthening was started from the second postoperative day with isometric exercises. All patients followed our rehabilitation protocol for 6 months until they returned to full unrestricted activity as tolerated. High-impact sports were allowed from the ninth month.
Statistical Analysis
The statistical analysis was performed using MedCalc (MedCalc software, Acacialaan 22, Ostend, Belgium). A priori power analysis was performed to establish the adequate sample size; a minimum of 23 patients for each group were required in order to detect a significant difference between CMI and Actifit of 10 ± 12 points at Lysholm score, with an effect size of 0.76, a statistical power of 0.80, and a probability level of 0.05.
Considering a 15% dropout rate, 28 patients for each group were included in the study.
Statistical comparison between the preoperative and postoperative follow-up parametric scores of both groups was performed using paired Student’s t-test. The population study was tested for normal distribution before the t-test was applied. For differences between time points in Tegner level, the non parametric Wilcoxon test was used. For differences in categorical variables, the Pearson chi-square test was utilized. Comparison between the 2 groups and subgroups was performed using independent Student’s t-test, Pearson chi-square test, or Mann–Whitney test depending on the variables.
Multiple regression analyses were performed to evaluate the whole case series using postoperative Lysholm score and Tegner Activity Scale as main outcomes. The regression analyses were performed in a backward fashion; in both models, independent variables considered were CMI or Actifit scaffold, acute or chronic pattern, age at surgery, isolated or combined procedure, male or female sex, and presence of chondral damage based on preoperative Yulish score I to IV. A logistic regression analysis was performed on the whole case series as well. Yulish score of grade I to IV was used as main outcome, while CMI or Actifit scaffold, acute or chronic pattern, age at surgery, isolated or combined procedure, and male or female sex were used as independent variables. Results are expressed using mean values ± standard deviation (SD) for parametric values, median ± interquartile range (IQR) for nonparametric values, and odds ratios and 95% confidence intervals (CIs) for logistic regression. Results were considered statistically significant at P < 0.05.
Results
Clinical evaluation
All 28 patients who underwent CMI were available at final follow-up (100%), while in the Actifit group 3 patients (11%) were lost at final follow-up. Both groups were comparable except for Tegner values, as the patients in the Actifit group, which included more chronic patients, were subjected to concomitant surgical procedure (Table 1).
Table 1.
Demographic Data of the 2 Groups.
| Article I. | CMI (n = 28) | Actifit (n = 25) | P Value |
|---|---|---|---|
| Agea | 38.7 ± 9.7 | 34.4 ± 11.4 | P = 0.1569 |
| Sex (male/female) | 19/9 | 20/5 | P = 0.4909 |
| Knee involved (right/left) | 20/8 | 13/12 | P = 0.2409 |
| Concomitant procedure | 13 | 21 | P = 0.0105 |
| Meniscal lesion (acute/chronic) | 22/6 | 7/18 | P = 0.0006* |
| Surgical timea (minutes) | 84±27 | 92±34 | P = 0.3254 |
Values expressed are mean ± standard deviation.
Statistically significant.
The mean duration for scaffold implantation after meniscectomy in the Actifit group was 7.29 years (1-18 years), and for CMI group it was 8 years (2-16 years). The mean scaffold size during implantation in the Actifit group was 4.30 cm (1.8-6.5 cm), and in the CMI group it was 4.50 cm (2.1-6 cm).
The location of meniscectomy and details on concomitant procedures of both groups are presented in Tables 3 and 2, respectively.
Table 3.
Concomitant Procedures in Both Groups.
| Concomitant Procedures | Actifit Groupa | CMI Groupa |
|---|---|---|
| Tibial osteotomy | 11 | 3 |
| ACL reconstruction | 9 | 11 |
| Microfracture | 3 | 1 |
| Healing response | 1 | — |
| Suture | 1 | — |
Few patients have undergone >1 concomitant procedure.
Table 2.
Type and Location of Meniscal Lesions in Both Groups.
| Actifit Group | CMI Group | |
|---|---|---|
| Chronic lesions | 18 | 6 |
| Acute lesion | 7 | 22 |
| Posterior horn | 5 | 8 |
| Body | — | — |
| Body + Posterior horn | — | 7 |
| Bucket handle | 2 | 7 |
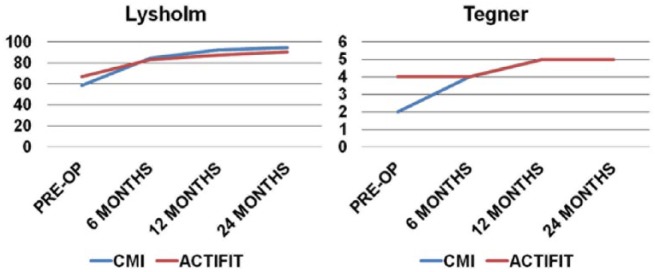
The patients treated with CMI were older than those in the Actifit group; however, the difference was not statistically significant (CMI = 38.7 ± 9.7; Actifit = 34.4 ± 11.4; P = 0.1569). Though preoperative Lysholm and Tegner scored have higher values in the Actifit group, statistically significant difference was found only with Tegner scale, but these observations are completely random and probably are related to the relatively younger patients in the Actifit group compared with those in the CMI group. However, the difference in the Lysholm score and Tegner scale improvement, between the scaffolds, from preoperative to 6, 12, and 24 months (P = 0.5918, 0.4916, 0.5918) after surgery are not statistically significant (Fig. 2).
Figure 2.
Linear graph representing Lysholm and Tegner scale improvement from preoperative up to 12 and 24 months after surgery in both groups of patients.
Analysis of individual groups showed statistically significant improvement in Lysholm and Tegner scores from preoperative to 12 months after surgery and at final follow-up (Table 4). The Lysholm score in the CMI group improved from 58.4 ± 17.3 to 94.5 ± 6.0 and in the Actifit group from 67.0 ± 15.7 to 90.3 ± 13.1 at final follow-up. The Tegner activity scale in the CMI group improved from 2 to 5 and in Actifit group from 4 to 5 at final follow-up. An intergroup analysis of Lysholm score (P = 0.281 and 0.106) and Tegner activity (P = 0.491 and 0.591) showed no significant statistical difference at 12 months and final follow-up, respectively (Table 5).
Table 4.
Clinical Outcome Scores of the 2 Groups.
| Group | Functional Outcome Scores | Preoperative Score | 1-Year Follow-Up Score | Final Follow-Up Score | P Value; Preoperative vs. 1-Year Follow-Up | P Value; 1-Year Follow-Up vs. Final Follow-Up |
|---|---|---|---|---|---|---|
| CMI | Lysholma | 58.4 ± 17.3 | 92.5 ± 8.5 | 94.5 ± 6.0 | P < 0.0001* | P = 0.1310 |
| Tegner | 2 | 5 | 5 | P < 0.0001* | P = 0.4015 | |
| Actifit | Lysholma | 67.0 ± 15.7 | 87.4 ± 13.0 | 90.3 ± 13.1 | P < 0.0001* | P = 0.9460 |
| Tegner | 4 | 4 | 5 | P < 0.0001* | P = 0.1309 |
Values expressed are mean ± standard deviation.
Statistically significant.
Table 5.
Intergroup Analysis of Clinical Outcome scores.
| Functional Outcomes | CMI Group |
Actifit Group |
CMI Group vs. Actifit Group |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Preoperative | 1-Year Postoperative Follow-Up | Final Follow-up | Preoperative | 1-Year Postoperative Follow-Up | Final Follow-up | P Value; Preoperative Score | P Value; 1-Year Follow-Up | P Value; Final Follow-Up | |
| Lysholma | 58.4 ± 17.3 | 92.5 ± 8.5 | 94.5 ± 6.0 | 67.0 ± 15.7 | 87.4 ± 13.0 | 90.3 ± 13.1 | P = 0.0775 | P = 0.2814 | P = 0.2395 |
| Tegner | 2 | 5 | 5 | 4 | 4 | 5 | P < 0.0001* | P = 0.3927 | P = 0.9341 |
Values expressed are mean ± standard deviation.
Statistically significant.
Multiple regression analysis showed that the type of implant did not affect Lysholm and Tegner values, but females and patients with concomitant procedure showed less improvement in Lysholm scores by 11 and 5.5 points, respectively, at final follow-up; chronic patients showed a decrease of Tegner score by 0.73 points at final follow-up.
Complications
Three complications were recorded in CMI group: neuro apraxia of infrapatellar branch of the saphenous nerve, which resolved after neurolysis; persistent synovitis; and superficial infection, which resolved after appropriate antibiotic therapy. In the Actifit group, 5 complications were recorded: joint stiffness, which resolved with arthroscopic release and manipulation under anesthesia, and 4 cases of synovitis, all of which resolved with anti-inflammatory therapy.
MRI Evaluation
MRI showed that the scaffolds in both groups were present in situ and were filled with new tissue but showed irregularity in shape and dimension of the scaffolds, while the signal intensity was higher in both the groups at final follow-up (Fig. 3). Except one CMI scaffold, which underwent complete resorption, all the remaining meniscal implants were partially resorbed in both the groups. The size of the remaining intact scaffold was reduced in 61% of CMI patients and 79% of Actifit patients. Scaffolds identical to native meniscus were observed in 39% and 21% of the patients in the CMI and Actifit groups, respectively.
Figure 3.
MRI of the 2 scaffolds with 2-year follow-up (CMI a-b, Actifit c-d); both the scaffolds are well positioned and well integrated. Both the scaffolds have irregular shape and different signals than the native meniscus.
Regarding signal intensity, 54% and 68% of the patients showed grade 2A; 46% and 38% showed grade 2B signal intensity in the CMI and Actifit groups, respectively. However, we must underline that the composition of the 2 scaffolds is different and might affect MRI images.
Analysis of Yulish scale showed a greater degree of joint degeneration in the Actifit group both preoperatively and at final follow-up (P = 0.0009 and P = 0.006, respectively); however, no evolution of degenerative joint disease has been observed with the time (Actifit P = 0.708 and P = 0.892 CMI) (Fig. 4). Through logistic regression it was observed that chronic lesion patterns were associated with 4.312 increased risk of chondropathy (Yulish 1-4) at 2 years of follow-up (confidence interval = 1.096 to 16.955).
Figure 4.
Bar graph representing Yulish scale in the CMI and Actifit groups both preoperatively and at final follow-up.
Second Look Arthroscopy
At arthroscopic evaluation an intact bare scaffold that was well integrated with surrounding tissue without any synovial tissue covering was seen in all except one case from the CMI group (Fig. 5). One patient from the CMI group who had persistent synovitis had complete scaffold resorption. The size of the scaffolds appeared to shrink over time and the margins appear frayed and irregular in the majority of patients of both the groups. The Actifit scaffold displayed a yellowish color that progressively disappeared during further evaluations after 40 months. The articular cartilage appeared intact without signs of progression of already existing articular injury in majority of the patients in both the groups.
Figure 5.
Second look arthroscopic evaluation: a, b, c—CMI at 7, 18, and 20 months, respectively; c, d, e—Actifit at 4, 18, and 27 months, respectively, after surgery showing intact, stable, and well-integrated scaffold.
Histological Evaluation
Histological analysis with light microscopy revealed that implant was present and was more compact filled with new tissue and extracellular matrix deposited in a heterogeneous way in both the groups. The biopsy samples from the CMI group showed more fibrous tissue that was rich in spindle and rounded fibroblast-like cells and blood vessels (Fig. 6). The Actifit biopsy samples appeared completely avascular with more cartilaginous-like appearance consisting plenty of chondroblast-like roundish, large, and active cells, and over time there was a greater percentage of smaller cells that had completely differentiated into chondrocytes, surrounded by a capsule and inserted in gaps, with few displaying a typical columnar arrangement (Fig. 7). All the biopsy samples showed vital cell and matrix structures with no evidence of necrosis. In 3 of the 11 Actifit patients who underwent second look arthroscopy, histological evaluation showed presence of plasma cells, macrophages, and rare lymphocytes, which could be a result of foreign body reaction; histological details of these patients are described in Table 6. However, we can consider this as grade 1, which is a low-grade foreign body reaction based on the classification suggested by Van Tienen et al.24
Figure 6.

CMI histological evaluation at 7 months: Light microscopy of the implant stained with hematoxylin and eosin. The CMI scaffold is clearly evident. Connective tissue inside the lacunae and new vessels are evident. The new tissue appears fibrous, rich of spindle and round cell and with blood vessels present up to 2 years after implantation. Reguzzoni et al.7
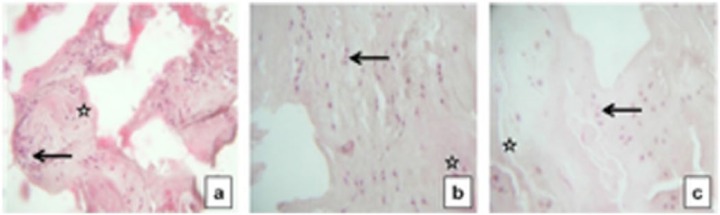
Figure 7.
Actifit histological evaluation at 4, 18, and 27 months (a, b, and c) with hematoxylin and eosin stains: the implant is filled by new tissue cartilaginous like and avascular. Arrows indicating at new tissue regenerate; stars indicating at native meniscal tissue.
Table 6.
Histological Findings After Second Look Arthroscopy and Concomitant Biopsy of the 2 Groups.
| Sr. No. | Patient Initials | Group | a) Reason for Second Look Arthroscopy | Duration at Biopsy (months) | Histological Findings |
|---|---|---|---|---|---|
| 1 | AL | CMI | Chondrocyte implantation | 7 | b) Fibrous tissue, spindle and rounded fibroblast-like cells and blood vessels |
| 2 | DG | CMI | Debridement | 3 | c) Fibrous tissue, spindle and rounded fibroblast-like cells and blood vessels |
| 3 | GM | CMI | Microfracture | 36 | d) Fibrous tissue, spindle and rounded fibroblast-like cells and blood vessels |
| 4 | BA | CMI | Implant removal | 12 | e) Fibrous tissue, spindle and rounded fibroblast-like cells and blood vessels |
| 5 | TL | CMI | Chondrocyte implantation | 8 | f) Fibrous tissue, spindle and rounded fibroblast-like cells and blood vessels |
| 6 | GS | CMI | Persistent pain | 6 | g) Fibrous tissue, spindle and rounded fibroblast-like cells and blood vessels |
| 7 | IG | CMI | Implant removal | 14 | h) Fibrous tissue, spindle and rounded fibroblast-like cells and blood vessels |
| 8 | DF | Actifit | Joint stiffness | 4 | i) Avascular, cartilaginous-like appearance consisting plenty of chondroblast-like roundish, large, and active cells |
| 9 | GD | Actifit | Implant removal | 11 | j) Cartilaginous-like appearance consisting of plenty of chondroblast-like roundish, large, and active cells |
| 10 | BA | Actifit | Implant removal | 18 | k) Avascular, cartilaginous-like appearance consisting of plenty of chondroblast-like roundish, large, and active cells |
| 11 | DM | Actifit | Implant removal | 22 | l) Avascular, cartilaginous-like appearance consisting of plenty of chondroblast-like roundish, large, and active cells |
| 12 | ML | Actifit | Implant removal | 27 | m) Avascular, cartilaginous-like appearance consisting of plenty of chondroblast-like roundish, large, and active cells |
| 13 | CF | Actifit | Implant removal | 34 | n) Avascular, cartilaginous-like appearance consisting of plenty of chondroblast-like roundish, large, and active cells |
| 14 | DV | Actifit | Implant removal | 41 | o) Avascular, cartilaginous-like appearance consisting of plenty of chondroblast-like roundish, large, and active cells |
| 15 | CM | Actifit | Revision ACL reconstruction | 45 | p) Avascular, cartilaginous-like appearance consisting of plenty of chondroblast-like roundish, large, and active cells |
| 16 | MW | Actifit | Implant removal | 14 | q) Avascular, cartilaginous-like appearance consisting of plenty of chondroblast-like roundish, large, and active cells; with plasma cells, macrophages, and rare lymphocytes |
| 17 | GD | Actifit | Implant removal | 15 | r) Avascular, cartilaginous-like appearance consisting of plenty of chondroblast-like roundish; with plasma cells, macrophages, and rare lymphocytes large and active cells |
| 18 | CC | Actifit | Implant removal | 20 | s) Avascular, cartilaginous-like appearance consisting of plenty of chondroblast-like roundish, large, and active cells; with plasma cells, macrophages, and rare lymphocytes |
Discussion
Our data showed clinical improvement in both groups of patients at 12 months after surgery and at final follow-up, which was statistically significant, and an intergroup statistical analysis showed no significant difference in improvement between the 2 groups. To our knowledge few studies compared the effectiveness of different scaffolds in treating irreparable partial medial meniscal lesions.
The patients treated with CMI were older than those in the Actifit group; however, these data are not significant (CMI: 38.7 ± 9.7; Actifit: 34.4 ± 11.4; P = 0.1569). Lysholm scale has higher values in Actifit patients but it is not significant (CMI: 58.4 ± 17.3; Actifit: 67.0 ± 15.7; P = 0.0775). The higher Tegner baseline values in the Actifit group are the only ones statistically significant (CMI: 2ds 2-3; Actifit: 4ds 3-5) but they are completely random and may be related to the relatively younger patients compared with those treated with CMI. However, there are no difference in improvements between the scaffolds from preoperative to 6, 12, and 24 months after surgery (0.5918, 0.4916, 0.5918, respectively).
Spencer et al.,21 in a similar study, presented similar results in a smaller number of patients with shorter follow-up; however, even in this study the type of scaffold did not influence the outcomes at final follow-up. Our final results confirmed similar clinical improvement at short- and mid-term follow-up as described in previous studies.11-14,17-22,25 Meniscal implantation along with concomitant procedure like ACL reconstruction has been reported to have less satisfactory results26; however, few other studies have reported that meniscal implantation along with a concomitant procedure does not affect the final clinical outcome.11-13 In our study, we observed worse results when meniscal implantation was associated with a concomitant procedure. The preoperative status of articular cartilage also negatively influences the results of meniscal transplantation irrespective of the type of scaffold used, and it has also been proved in several previous studies that concluded that associated cartilage damage should not exceed ICRS grade 2 to obtain predictable results after meniscal implantation.17,18,20,22 Similar to previous studies we have observed that a chronic knee injury pattern is accompanied by poor clinical and MRI results regardless of the type of scaffold implanted.13,14 Two complications were seen in the CMI group, a case of neuroapraxia of the saphenous nerve, which can be attributed to its injury during a posteromedial incision made for inside-out sutures fixation, and a case of synovitis, which was related to poor patient compliance to our rehabilitation protocol. In the Actifit group, 4 cases of intra-articular effusion and pain were documented at different time points (26, 30, 36, and 42 months), which probably is related to implant reaction during the resorption process.
During MRI evaluation, morphology and signal intensity appeared different with both scaffolds when compared with normal meniscus. In fact, both products are porous structures and this justifies the hyperintense signal detected after implantation and even up to 2 years when the implant was still present with newly formed tissue still maturing. The signal intensity from the newly regenerated tissue seen within the scaffold gradually becomes hypointense over time, but the signal intensity never reduces to the level of a native menisci. Other studies showed similar results when these scaffolds beneficial effects were evaluated separately.11-14,17-25 With regard to the signal intensity, we divided grade 2 of the Genovese classification into 2 subgroups, 2A and 2B, depending on the degree (greater or lesser) of signal hyperintensity. By doing this, considering the extreme subjectivity of the evaluation, we were able to differentiate the amount of hyperintensity among different patients that would have otherwise gone unnoticed. Moreover, this subdivision looks even more important while assessing the evolution of MRI signal with progression of time in the same subject. We did not find evidence of evolution of the degenerative processes at 2-year follow-up, and this could be related to a possible chondroprotective effect of these scaffolds; however, at the moment we are not aware of any clinical study focused on this possible effect.
During second look arthroscopy and concomitant biopsy, we found the Actifit scaffold to be of yellowish color, which could be due to the oxidation of polyurethane by the fatty acids of the joint environment. Histological evaluation of both scaffolds showed presence of new tissue without signs of cell death or necrosis. The biopsy from the CMI group showed more fibrous tissue rich in vessels, while Actifit showed more cartilaginous-like avascular tissue; in both cases the tissue was more compact with cells and extracellular matrix deposited in a heterogeneous way due to the compressive forces and due to varying distribution of the applied loads. Our findings are similar to that reported in the literature.11,14,18,22 Regarding the Actifit histological appearance, Verdonk et al.22 reported a trilaminar structure based on cells, extracellular matrix, and the blood vessels, which was not observed during our histological analysis. The authors also observed the presence of multiple fibrocartilaginous cells, which was also seen in our samples, but excluded presence of monocyte-macrophage cells and inflammatory reaction.22 We also found 3 patients with intra-articular inflammatory cells, 2 of whom were completely asymptomatic, and only 1 presenting with significant effusion and pain during clinical evaluation. In our study, there are several limitations. First this is not a prospective randomized study; second is the extreme heterogeneity of the 2 groups with a greater number of chronic patients or those simultaneously treated with additional surgical procedures in the Actifit group. This important bias has to be seen positively since the Actifit scaffold was introduced later into clinical practice, after learning from the experience gained after years of use and related publications on the CMI scaffold. The best results in chronic patients were also taken into account and led to modifications in the indications of this procedure. Currently, the main indications for meniscal scaffold implantation are compartmental pain as a result of previous meniscectomy (chronic) and the irreparable meniscal tears (acute) just in case we assume the rapid evolution of degenerative disease (instability, misalignment that must be corrected). It has been emphasized by Kon et al.19 that these scaffolds can also be used in complex knee lesions, where multiple comorbidities need to be properly addressed, and they have shown good clinical and MRI results at short-term evaluation.
Though we have presented only 2-year follow-up results and these patients have been treated at different time periods, there are only few studies in the literature comparing 2 different types of meniscal scaffolds treated in the same institution.
Conclusion
This study showed that both the meniscal implants are effective in improving the symptoms and joint function at short-term evaluation. MRI evaluation showed the presence of the scaffolds at 2 years, however with difference in shape, size, and intensity of the residual scaffold when compared with the native meniscus. We also noticed lack of progression of degenerative processes of the knee joint, suggesting a possible protective effect on articular cartilage.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The study has been approved by the Ethics Committee of the Ospedale di Circolo Fondazione Macchi, Varese, Italy (June 18,2013; Protocol Number 0023851; Registered Clinical Trials 54/2013).
References
- 1. Allen PR, Denham RA, Swan AV. Late degenerative changes after meniscectomy: factors affecting the knee after operation. J Bone Joint Surg Am. 1984;66(5):666-71. [DOI] [PubMed] [Google Scholar]
- 2. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30B(4):664-70. [PubMed] [Google Scholar]
- 3. Krause WR, Pope MH, Johnson RJ, Wilder DG. Mechanical changes in the knee after meniscectomy. J Bone Joint Surg Am. 1976;58(5):599-604. [PubMed] [Google Scholar]
- 4. Tapper EM, Hoover NW. Late results after meniscectomy. J Bone Joint Surg Am. 1969;1(3):517-26. [PubMed] [Google Scholar]
- 5. Buma P, Ramrattan NN, van Tienen TG, Veth RP. Tissue engineering of the meniscus. Biomaterials. 2004;25(9):1523-32. [DOI] [PubMed] [Google Scholar]
- 6. Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure, function, pathophysiology, current repair. Bio-materials. 2011;3(30):7411-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reguzzoni M, Manelli A, Ronga M, Raspanti M, Grassi FA. Histology and ultrastructure of a tissue engineered collagen meniscus before and after implantation. J Biomed Mater Res B Appl Biomater. 2005;74(2):808-16. [DOI] [PubMed] [Google Scholar]
- 8. Stone KR, Steadman JR, Rodkey WG, Li ST. Regeneration of meniscal cartilage with use of a collagen scaffold. Analysis of preliminary data. J Bone Joint Surg Am. 1997;79(12):1770-7. [DOI] [PubMed] [Google Scholar]
- 9. Stone KR, Rodkey WG, Webber R, McKinney L, Steadman JR. Meniscal regeneration with copolymeric collagen scaffolds: in vitro and in vivo studies evaluated clinically, histologically and biochemically. Am J Sports Med. 1992;20(2):104-11. [DOI] [PubMed] [Google Scholar]
- 10. Rodkey WG, Steadman JR. Clinical study of collagen meniscus implants to restore the injured meniscus. Clin Orthop Rel Res. 1999;(367 Suppl):S281-92. [DOI] [PubMed] [Google Scholar]
- 11. Bulgheroni P, Murena L, Ratti C, Bulgheroni E, Ronga M, Cherubino P. Follow-up of collagen meniscus implant patients: clinical, radiological and magnetic resonance imaging results at 5 years. Knee. 2010;17(3):224-9. [DOI] [PubMed] [Google Scholar]
- 12. Monllau JC, Gelber PE, Abat F, Pelfort X, Abad R, Hinarejos P, et al. Outcome after partial medial meniscus substitution with the collagen meniscal implant at a minimum of 10 years’ follow-up. Arthroscopy. 2011;27(7):933-43. [DOI] [PubMed] [Google Scholar]
- 13. Rodkey WG, DeHaven KE, Montgomery WH, 3rd, Baker CL, Jr, Beck CL, Jr, Hormel SE, et al. Comparison of the collagen meniscus implant with partial meniscectomy. A prospective randomized trial. J Bone Joint Surg Am. 2008;90(7):1413-26. [DOI] [PubMed] [Google Scholar]
- 14. Zaffagnini S, Marcheggiani Muccioli GM, Lopomo N, Bruni D, Giordano G, Ravazzolo G, et al. Prospective long term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy: a minimum 10 years follow up-study. Am J Sport Med. 2011;39(5): 977-85. [DOI] [PubMed] [Google Scholar]
- 15. Zuidema J, van Minnen B, Span MM, Hissink CE, van Kooten TG, Bos RR. In vitro degradation of a biodegradable polyurethane foam, based on 1,4-butanediisocyanate: a three years study of physiological and elevated temperature. J Biomed Mater Res A. 2009;90(3):920-30. [DOI] [PubMed] [Google Scholar]
- 16. van Minnen B, van Leeuwen MB, Kors G, Zuidema J, van Kooten TG, Bos RR. In vivo resorbtion of a biodegradable polyurethane foam, based on 1,4-butanediisocyanate: a three years subcutaneous implantation study. J Biomed Mater Res A. 2008;85(4):972-82. [DOI] [PubMed] [Google Scholar]
- 17. Verdonk R, Verdonk P, Huysse W, Forsyth R, Heinrichs EL. Tissue ingrowth after implantation of a novel, biodegradable polyurethane scaffold for treatment of partial meniscal lesions. Am J Sports Med. 2011;39(4):774-82. [DOI] [PubMed] [Google Scholar]
- 18. Efe T, Getgood A, Schofer MD, Fuchs-Winkelmann S, Mann D, Paletta JR, et al. The safety and short-term efficacy of a novel polyurethane meniscal scaffold for the treatment of segmental medial meniscus deficiency. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1822-30. [DOI] [PubMed] [Google Scholar]
- 19. Kon E, Filardo G, Zaffagnini S, Di Martino A, Di Matteo B, Marcheggiani Muccioli GM, et al. Biodegradable polyurethane meniscal scaffold for isolated partial lesions or as combined procedure for knees with multiple comorbidities: clinical results at 2 years. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):128-34. [DOI] [PubMed] [Google Scholar]
- 20. Schüttler KF, Pöttgen S, Getgood A, Rominger MB, Fuchs-Winkelmann S, Roessler PP, et al. Improvement in outcomes after implantation of a novel polyurethane meniscal scaffold for the treatment of medial meniscus deficiency. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):1929-35. [DOI] [PubMed] [Google Scholar]
- 21. Spencer SJ, Saithna A, Carmont MR, Dhillon MS, Thompson P, Spalding T. Meniscal scaffolds: early experience and review of the literature. Knee. 2012;19(6):760-5. [DOI] [PubMed] [Google Scholar]
- 22. Verdonk P, Beaufils P, Bellemans J, Djian P, Heinrichs EL, Huysse W, et al. Successful treatment of painful irreparable partial meniscal defects with a polyurethane scaffold: two-year safety and clinical outcomes. Am J Sports Med. 2012;40(4):844-53. [DOI] [PubMed] [Google Scholar]
- 23. Genovese E, Angeretti MG, Ronga M, Leonardi A, Novario R, Callegari L, et al. Follow-up of collagen meniscus implants by MRI. Radiol Med. 2007;112(7):1036-48. [DOI] [PubMed] [Google Scholar]
- 24. Van Tienen TG, Heijkants RG, Buma P, de Groot JH, Pennings AJ, Veth RP. Tissue ingrowth and degradation of 2 biodegradable porous polymers with different porosities and pore sizes. Biomaterials. 2002;23(8):1731-8. [DOI] [PubMed] [Google Scholar]
- 25. Zaffagnini S, Giordano G, Vascellari A, Bruni D, Neri MP, Iacono F, et al. Arthroscopic collagen meniscus implant results at 6 to 8 years follow up. Knee Surg Sports Traumatol Arthrosc. 2007;15(2):175-83. [DOI] [PubMed] [Google Scholar]
- 26. Hirschmann MT, Keller L, Hirschmann A, Schenk L, Berbig R, Lüthi U, et al. One-year clinical and MR imaging outcome after partial meniscal replacement in stabilized knees using a collagen meniscus implant. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):740-7. [DOI] [PubMed] [Google Scholar]