Abstract
Objective
Glucosamine hydrochloride (GH) and chondroitin sulfate (CS) are commonly used for the treatment of osteoarthritis (OA). The aim of this study was to assess their effects, alone and in combination, on preventing aggrecan degradation and inflammation in an in vitro model of OA.
Design
To test the effects of GH and/or CS as a preventative treatment, cartilage explants were pretreated with the compound(s) using concentrations that showed no detrimental effect on chondrocyte viability. Interleukin-1α (IL-1α) was added to induce cartilage degradation, supernatant and explants were analyzed for proteoglycan degradation products, aggrecanase mRNA expression and activity, and for the release of inflammatory markers.
Results
Following treatment with IL-1α, 2 mg/mL dose of GH pretreatment was associated with a reduction of glycosaminoglycan release, reduced generation of the pathological interglobular domain aggrecan catabolites, decreased mRNA levels of ADAMTS-4 and -5 and reduced activity of ADAMTS-4. In contrast, CS alone did not have a significant effect on IL-1α-induced cartilage degradation and the addition of 0.4 mg/mL CS to 2 mg/mL GH did not further inhibit IL-1α-induced activity. Pretreatment with 2 mg/mL GH also reduced the release of inflammatory markers, prostaglandin E2 and nitric oxide induced by IL-1α while CS did not have a significant effect.
Conclusions
The results suggest that GH prevents cartilage degradation mediated by aggrecanases ADAMTS-4 and -5, and may also reduce inflammation. This could be part of the mechanisms by which GH is effective in maintaining joint integrity and function, and preventing or delaying early symptoms of OA.
Keywords: glucosamine, chondroitin, osteoarthritis, aggrecanase
Introduction
Osteoarthritis (OA) is a degenerative disease of the joints characterized by a loss of articular cartilage and remodeling of subchondral bone. More than 8.75 million people are affected in the United Kingdom and about 27 million people in the United States.1,2 Conventional treatments, which mainly include analgesics and nonsteroidal anti-inflammatory drugs, can cause serious side-effects. With no cure for OA, agents that not only reduce the pain but also prevent or at least slow down the progression of the disease are desirable and in this study we are interested in testing the 2 commonly used nutraceuticals, glucosamine and chondroitin, to prevent or delay the pathological changes in cartilage that manifest during the early stages of the disease.
Aggrecan consists of a core protein with 100 to 150 glycosaminoglycan (GAG) chains attached to it. Along with type-II collagen, aggrecan forms a major structural component of articular cartilage. The loss of aggrecan from articular cartilage is an early event in the development of OA and continued loss of aggrecan leads to the susceptibility of the collagen network to proteolysis and irreversible tissue damage.3-5 Metalloproteinases, namely ADAMTS-4 and ADAMTS-5 (A Disintegrin And Metalloproteinase with Thrombospondin motifs) also referred to as aggrecanase 1 and 2, are the key enzymes involved in the degradation of aggrecan in normal and osteoarthritic cartilage.6-8 Prevention of early loss of aggrecan during the disease process is likely to contribute to a slowing in the progression of the disease and therefore to improved joint health.
Glucosamine, an amino monosaccharide, and chondroitin, a polysaccharide macromolecule, are both present naturally in cartilage. In clinical trials, consumption of either glucosamine or chondroitin sulfate (CS) has been associated with a reduction in joint pain, improvement of joint function and reduction of joint space narrowing in patients with OA.9-22 However, there is conflicting evidence as to the effectiveness of such treatments.23-30 In vitro studies using glucosamine alone have found positive effects such as decreased interleukin-1 (IL-1) induced expression of matrix metalloproteinases, MMP-3 and MMP-13, cyclooxygenase-2 (COX-2), and nitric oxide synthase, reduced aggrecan degradation, and increased synthesis of aggrecan core protein31-36 while others have found a decrease in prostaglandin E2 (PGE2) production induced by IL-1.36,37 Studies using CS alone have shown that CS has anti-inflammatory and chondroprotective actions.38-40
The aim of this research was to determine whether glucosamine hydrochloride (GH) and CS, individually and in combination, could inhibit the cytokine-induced catabolism of aggrecan in vitro and to elucidate mechanisms specific to each compound or to the combination. While clinical trials and most in vitro studies have focused on the effects of glucosamine and CS after the appearance of the symptoms or after the induction of OA-like pathology in vitro, the present study focuses on the preventive effects.
Methods
Viability of Chondrocyte Monolayer Cell Cultures
Chondrocytes from the metacarpophalangeal joint of 18 month-old oxen showing no visible signs of cartilage degeneration were isolated as previously described.41 Chondrocyte monolayer cultures were established in sterile 12-well plates (Corning Incorporated) at 1 × 106 cells per well in 2 mL basal medium: Dulbecco’s modified Eagle medium (DMEM) containing 50 μg/mL gentamicin and 1% Insulin-Transferrin-Selenium-X supplement (ITS, Invitrogen). Cells were incubated overnight and medium was then replaced with 2 mL basal medium with GH or CS in a range of 0 to 4 mg/mL, or with a combination of 2 mg/mL GH and 0.4 mg/mL CS. This dose combination of GH and CS was based on the formulation of an emulsified product available on the market (VeryWise Nutrition) where the proportion of the 2 compounds was kept consistent (GH:CS, 5:1, w/w). GH and CS (from bovine cartilage) were supplied by Obsidian Research Ltd. Cultures were maintained and tested for cell viability using the thiazolyl blue tetrazolium bromide (MTT) assay.43 Chondrocyte monolayer cultures were generated from articular cartilage collected and pooled from 3 different joints. Each experimental condition was replicated 3 times (n = 3).
Articular Cartilage Explants and Treatments
Articular cartilage was dissected from the metacarpophalangeal joints of 18-month-old oxen showing no visible signs of cartilage degeneration. Cartilage explants (15-60 mg wet weight) were maintained in DMEM containing 10% (v/v) fetal calf serum and 50 μg/mL gentamicin for 3 days.44 Cartilage explants were then maintained individually in 1 mL of basal medium or 1 mL of basal medium with test concentrations of either GH or CS, or a combination of both. Following 24-hour incubation, human IL-1α (PeproTech Inc.) was added to appropriate wells at 10 ng/mL final concentration such that the following experimental conditions were obtained and maintained for a further 24 or 72 hours:
CS and GH individually: (a) basal medium (control); (b) basal medium + IL-1; (c) basal medium + 0.2 mg/mL CS or GH; (d) basal medium + 0.2 mg/mL CS or GH + IL-1; (e) basal medium + 2 mg/mL CS or GH; (f) basal medium + 2 mg/mL CS or GH + IL-1
GH and CS combination: (a) basal medium (control); (b) basal medium + IL-1; (c) basal medium + 2 mg/mL GH; (d) basal medium + 2 mg/mL GH + IL-1; (e) basal medium + 0.4 mg/mL CS; (f) basal medium + 0.4 mg/mL CS + IL-1; (g) basal medium + 2 mg/mL GH + 0.4 mg/mL CS; (h) basal medium + 2 mg/mL GH + 0.4 mg/mL CS + IL-1.
A series of explants were treated for each condition, using cartilage taken from 3 different joints (n = 3). Explants and culture supernatant were harvested and stored at −80°C prior to analysis.
Measurement of Sulfated-Glycosaminoglycans
Sulfated-glycosaminoglycan (s-GAG) release from cartilage explants into the culture supernatant was measured at 72 hours after the addition of IL-1α, using the 1,9-dimethylmethylene blue (DMMB) assay.45 Where appropriate, s-GAG released into the culture supernatant was calculated by subtracting the s-GAG measured in basal media containing CS at the appropriate concentration. This calculation allowed for the subtraction of s-GAG in the CS added to the basal medium prior to incubation with the cartilage explant. The effect of treatments on the release of s-GAG was expressed as micrograms (µg) GAG per milligram (mg) wet weight of cartilage explant.
Western Blot Analysis of Interglobular Domain (IGD) Aggrecan Catabolite ARGSVIL
Western blotting with monoclonal antibody BC-3 recognizing the new N-terminal neoepitope ARGSVIL on aggrecanase-generated interglobular domain (IGD) fragments was used to assess the effect of GH and CS on the generation of the pathological aggrecan catabolic products after IL-1α stimulation.46 BC-3 antibody42 was provided in-house (Cardiff University). Samples of culture supernatants (equivalent of 2 mg wet weight of tissue) collected 72 hours after the addition of IL-1α were deglycosylated, dialyzed, and lyophilized as previously described.41,47 Samples were reconstituted and separated on 4% to 12% Novex Tris-glycine sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels (Invitrogen). After transfer to nitrocellulose membranes the BC-3 antibody was used to recognize the N-terminal ARGSVIL generated by aggrecanase cleavage of the bovine aggrecan core protein at the Glu373-Ala374 bond.41,42 Membranes were developed using alkaline phosphatase (AP) substrate. The density of the bands was measured and expressed as the area under the signal intensity curve (AUC, in arbitrary units) using the ImageJ software (NIH Image).
Measurement of mRNA Expression of ADAMTS-4 and ADAMTS-5
Total RNA was isolated from the cartilage explants collected 24 hours after the addition of IL-1α. Explants were snap-frozen in liquid nitrogen and reduced to a fine powder using a Mikro-Dismembrator (Braun Biotech International). RNA was extracted using Tri reagent and was then isolated using Qiagen RNeasy mini-columns and reagents according to the manufacturer’s protocol. RNA was eluted in sterile RNAase free water and samples were stored at −80°C. Complementary DNA (cDNA) was synthesized from 500 ng RNA by reverse transcription using Moloney Murine Leukemia Virus (MuLV) reverse transcriptase (New England BioLabs). Quantitative real-time polymerase chain reaction (PCR) amplification was performed using an Mx qPCR System (Agilent Technologies). Sequence of specific oligonucleotide primers corresponding to the genes of interest, ADAMTS-4 and ADAMTS-5, are listed in Table 1. Serial dilutions from 1 × 107 copies/μL to 1 × 101 copies/μL of a plasmid containing the corresponding genes were used as standards to quantify the expression of ADAMTS-4 and ADAMTS-5. Brilliant SYBR Green qPCR Master Mix (Stratagene) was used for the qPCR reactions with 1 μL cDNA or plasmid. The amplification cycle used was as follows: initial denaturation for 30 seconds at 95°C (1 cycle), 30 seconds at 95°C followed by 45 seconds at annealing temperature and 1 minute, elongation at 72°C (40 cycles), and a final extension for 5 minutes at 72°C (1 cycle). Melting curves were evaluated for each gene and the fold change in gene expression relative to the control (no treatment, no IL-1α) was expressed using the copy number calculated from the plasmid standards.
Table 1.
Primers Used for Quantitative Real-Time Polymerase Chain Reaction (PCR).
| Target Gene | PCR Primer Sequence (5′-3′) | Annealing Temperature (°C) | Product Size (bp) |
|---|---|---|---|
| ADAMTS-4 | AAGTTCGACAAGTGCATGGTG | 57 | 215 |
| TATTCACCGTTGAGGGCATAG | |||
| ADAMTS-5 | CAAATGTGGCGTCTGTGGAGG | 57 | 254 |
| TCCCGTTGATGTCGATGATGG |
Measurement of ADAMTS-4 Activity
ADAMTS-4 activity was measured in culture supernatant collected 72 hours after IL-1α stimulation, using the SensoLyte 520 Aggrecanase-1 fluorimetric assay kit from AnaSpec. Results were expressed as relative fluorescence normalized to tissue wet weight (in milligrams).
Measurement of Prostaglandin E2
PGE2 was measured in tissue culture supernatant collected at 72 hours after the addition of IL-α, using a competitive ELISA kit (R&D Systems) with standards of PGE2 (0-2500 pg/mL). Results were expressed as picograms of PGE2 per milligram tissue wet weight.
Nitric Oxide Assay
Nitric oxide (NO) was measured in tissue culture supernatant collected at 72 hours after the addition of IL-α, using the Griess reaction and standards of sodium nitrite (0-100 μM) from the Griess Reagent System (Promega) following the manufacturer’s protocol. Absorbance was measured at 540 nm and results were expressed as nanomoles NO per milligram tissue wet weight.
Statistical Analyses
Each condition was carried out using cartilage explants (n = 3) from 3 individual joints. Analyses were carried out on the 3 replicate samples (cartilage explant or media supernatant) from each of the individual joints. Results were expressed as mean (± standard deviation [SD] or standard error of the mean [SEM]) of the 3 samples from the 3 joints. SD was applied to the results from cell viability experiments and SEM was applied to the results of all experiments using explants. One-sample Student t test was used to compare means. Differences were considered significant at P < 0.05.
Results
Effects of Chondroitin Sulfate and Glucosamine Hydrochloride on Chondrocyte Viability
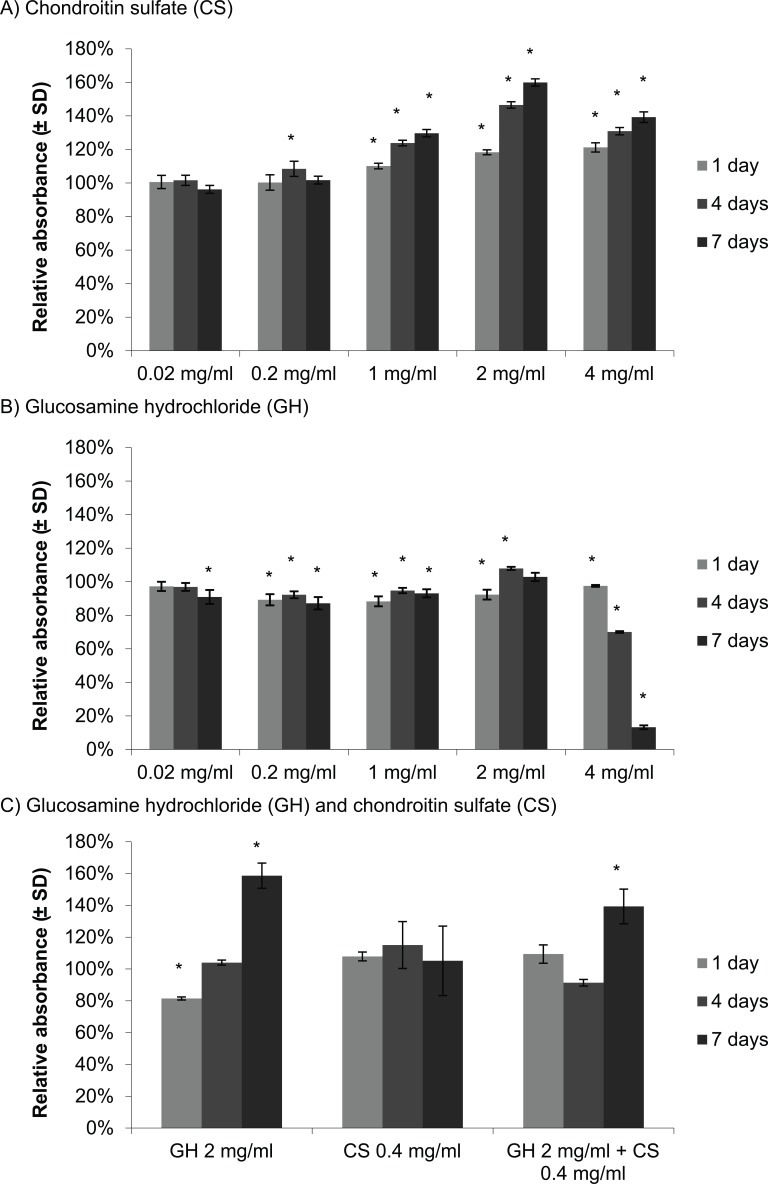
At concentrations of 0.02 to 4 mg/mL, CS had little effect or increased the metabolic activity of the chondrocytes above that seen in controls (Fig. 1A). GH in a range of 0.02 to 2 mg/mL resulted in no major change in cellular metabolic activity except at 4 mg/mL there was a large decrease in metabolic activity, with 70% and 13% average relative absorbance after 4 and 7 days in culture, respectively (Fig. 1B), suggesting that high concentrations of GH have toxic effects on chondrocyte viability and/or metabolism. The combination 2 mg/mL GH and 0.4 mg/mL CS showed no detrimental effect on chondrocyte viability (Fig. 1C). Unlike the first experiment (Fig. 1B), GH at 2 mg/mL showed an increase in chondrocyte cellular metabolic activity after 7 days in culture (Fig. 1C). As expected, CS at 0.4 mg/mL showed no substantial change in metabolic activity (Fig. 1C). Interestingly, the combination of GH and CS resulted in an increase in cellular metabolic activity following 7 days in culture, with 139% relative absorbance (Fig. 1C). Concentrations of GH and CS that did not appear detrimental to chondrocyte viability over a period of 7 days were used to test their potential effects in preventing cartilage degradation induced by IL-1α.
Figure 1.
Cellular metabolic activity of chondrocytes with chondroitin sulfate (A) and glucosamine hydrochloride (B), assessed individually and in combination (C) using MTT assay. Values represent the mean relative absorbance to the control without treatment ± SD, n = 3. One-sample Student t test was used. * indicates P < 0.05 for a comparison with the control without glucosamine hydrochloride (GH) and/or chondroitin sulfate (CS).
Glucosamine Hydrochloride Alone or with Chondroitin Sulfate Suppresses Proteoglycan Degradation Induced by IL-1α
Stimulation of cartilage explants in culture with 10 ng/mL IL-1α significantly induced the release of s-GAG from the extracellular matrix (P < 0.05, Fig. 2). CS 0.2 mg/mL decreased s-GAG release induced by IL-1α significantly (P < 0.001, Fig. 2A) while CS at 2 mg/mL increased s-GAG release further when compared with control with IL-1α (P < 0.05, Fig. 2A). GH 0.2 mg/mL had no significant effect on s-GAG release induced by IL-1α (Fig. 2B), while GH at 2 mg/mL prevented s-GAG release induced by IL-1α significantly (P < 0.05, Fig. 2B). Consistently, GH at 2 mg/mL as well as GH at 2 mg/mL + CS 0.4 mg/mL significantly inhibited the release of endogenous aggrecan loss from the matrix of articular cartilage explants stimulated with 10 ng/mL IL-1α compared with control with IL-1α (P = 0.002 for both, Fig. 2C). However, 0.4 mg/mL CS alone did not inhibit this loss of aggrecan (Fig. 2C).
Figure 2.
Sulfated glycosaminoglycan (s-GAG) release measured using DMMB assay in tissue culture supernatant at 72 hours post-stimulation with interleukin-1α (IL-1α), in the presence of chondroitin sulfate (A) and glucosamine hydrochloride (B) individually, and with a combination (C). Values represent the mean GAG release in μg per mg of tissue ± SEM, n = 3. One-sample Student t test was used. §Indicates P < 0.05 for a comparison with the control without IL-1α stimulation. # indicates P < 0.05 for a comparison with the control with IL-1α stimulation.
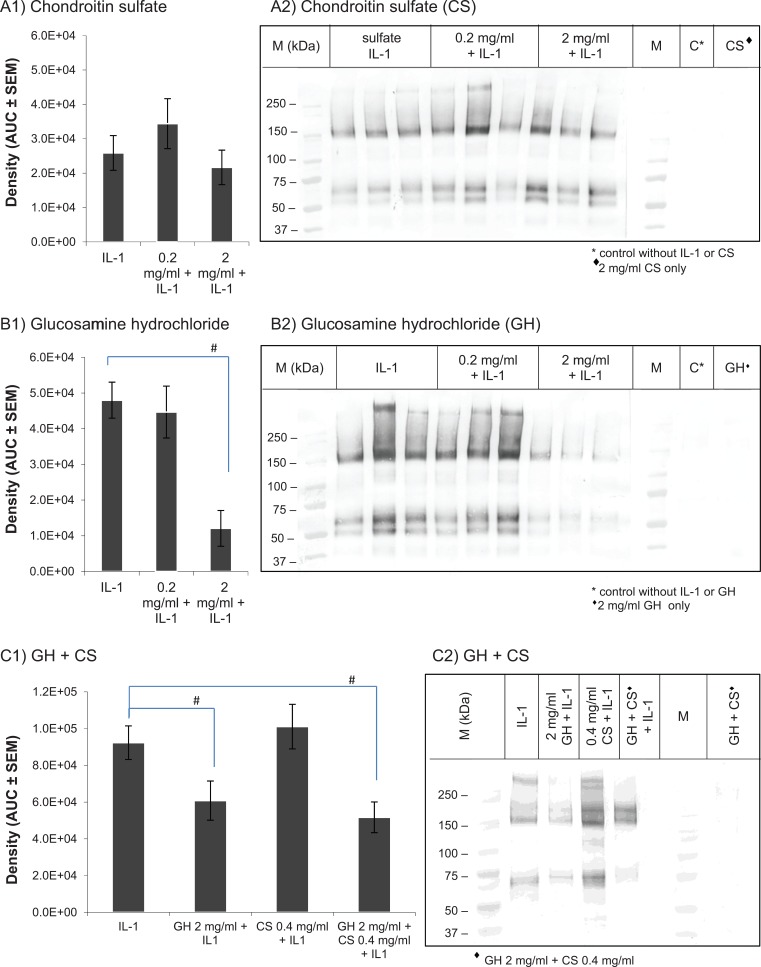
IL-1α-treated cartilage explants released BC-3 positive interglobular domain (IGD) fragments into the culture media, typically generating molecular mass fragments of around 50, 60, and 150 kDa (Fig. 3A2-3C2; IL-1). Reduction in staining for BC-3 positive catabolites was seen in cultures pretreated with GH at 2 mg/mL (Fig. 3B2 and 3C2) and GH at 2 mg/mL + CS 0.4 mg/mL (Fig. 3C2), without further decrease with the addition of CS at 0.4 mg/mL and there was no reduction in staining for BC-3 positive bands in cultures pretreated with CS alone (Fig. 3A2). Densitometric analysis of samples from 3 separate experiments showed a significant reduction of aggrecan loss induced by IL-1α in GH 2 mg/mL only or GH 2 mg/mL + CS 0.4 mg/mL pretreated cultures (P < 0.05, Fig. 3B1 and 3C1).
Figure 3.
Western bolt analysis of tissue culture supernatant using BC-3 antibody to assess the effect of chondroitin sulfate (A) and glucosamine hydrochloride (B) individually and in combination (C) on the level of aggrecanases products at 72 hours post-stimulation with interleukin-1α (IL-1α). (1) Densitometry was used to measure the overall density of the bands. Values represent the mean area under the curve (AUC) in arbitrary units ± SEM, n = 9. # indicates P < 0.05 for a 1-sample Student t test comparison with the control with IL-1α stimulation. (2) Representative BC-3 Western blots. M: Molecular mass marker.
Glucosamine Hydrochloride Suppresses IL-1α-Induced Gene Expression of ADAMTS-4 and ADAMTS-5
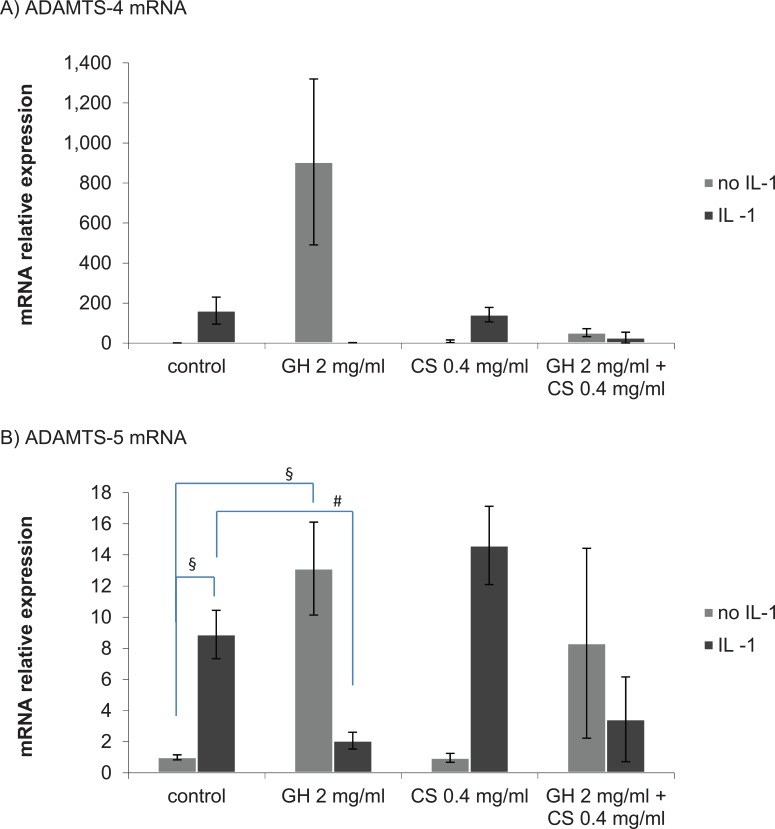
In control explants without treatment, the mRNA expression of ADAMTS-4 was low and stimulation with IL-1α increased the expression of ADAMTS-4 mRNA, but the increase was not statistically significant (P = 0.076, Fig. 4A). An increase in ADAMTS-4 mRNA expression was also seen in cultures treated with GH 2 mg/mL alone but was not statistically significant (P = 0.095, Fig. 4A), and this effect was negated with the addition of CS 0.4 mg/mL (Fig. 4A). In cultures stimulated with IL-1α, in the presence of GH 2 mg/mL or GH 2 mg/mL + CS 0.4 mg/mL, a reduction of ADAMTS-4 mRNA expression was seen compared with the control with IL-1α but was not statistically significant (P = 0.077 and P = 0.138, respectively, Fig. 4A). Pretreatment with CS 0.4 mg/mL only had no significant effect on the expression of ADAMTS-4 mRNA (Fig. 4A).
Figure 4.
ADAMTS-4 mRNA expression (A) and ADAMTS-5 mRNA expression (B) at 24 hours post-stimulation with interleukin-1α (IL-1α), with chondroitin sulfate (CS) and glucosamine hydrochloride (GH) pretreatment. Values represent the mean fold change in expression relative to the control without IL-1α ± SEM, n = 3. One-sample Student t test. § indicates P < 0.05 for a comparison with the control without IL-1α stimulation. # indicates P < 0.05 for a comparison with the control with IL-1α stimulation.
ADAMTS-5 mRNA was constitutively expressed in control cartilage explants and stimulation with IL-1α significantly increased expression of ADAMTS-5 (P = 0.007, Fig. 4B). As observed for ADAMTS-4, the mRNA expression of ADAMTS-5 was also significantly increased in cultures containing GH 2 mg/mL alone (P = 0.015, Fig. 4B), and this effect was lessened with the addition of CS 0.4 mg/mL (Fig. 4B). Pretreatment with GH 2 mg/mL or GH 2 mg/mL + CS 0.4 mg/mL suppressed the expression of ADAMTS-5 mRNA induced by IL-1α. The effect was significant with GH 2 mg/mL alone but not with GH 2 mg/mL + CS 0.4 mg/mL compared with control with IL-1α (P = 0.014 and P = 0.157, respectively, Fig. 4B). As observed for ADAMTS-4, pretreatment with CS 0.4 mg/mL had no significant effect on the expression levels of ADAMTS-5 mRNA (Fig. 4B).
Glucosamine Hydrochloride Only or with Chondroitin Sulfate Suppresses IL-1α-Induced Activity of ADAMTS-4
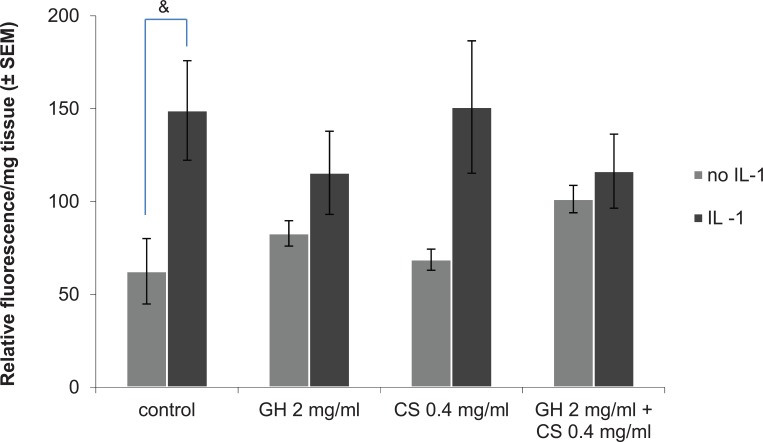
The mean relative activity of ADAMTS-4 in controls was significantly increased with IL-1α stimulation (P = 0.054, Fig. 5). With GH 2 mg/mL or GH 2 mg/mL + CS 0.4 mg/mL and IL-1α stimulation, the mean ADAMTS-4 activity was decreased compared with that of the control with IL-1α stimulation, but this was not statistically significant (Fig. 5). When compared with the samples without IL-1α stimulation, GH 2 mg/mL or GH 2 mg/mL + CS 0.4 mg/mL prevented a significant increase of the relative activity of ADAMTS-4 induced by IL-1α (P = 0.236 and P = 0.518, respectively, Fig. 5).
Figure 5.
Effect of glucosamine hydrochloride (GH) and chondroitin sulfate (CS) on ADAMTS-4 activity at 72 hours post-stimulation with interleukin-1α (IL-1α). Values represent the mean relative fluorescence per mg tissue ± SEM, n = 3. One-sample Student t test was used. & indicates P < 0.05 for comparison between no-IL-1α and IL-1α control samples.
Glucosamine Hydrochloride Only or with Chondroitin Sulfate Decreases IL-1α-Induced Release of Prostaglandin E2 and Nitric Oxide
In control cultures or with CS 0.4 mg/mL, the level of PGE2 was significantly increased by IL-1α (P = 0.002 and P = 0.034, respectively, Fig. 6A). In the presence of GH 2 mg/mL or the combination GH 2 mg/mL + CS 0.4 mg/mL, PGE2 average concentration was reduced when compared with the IL-1α-treated control, significantly with GH alone (P = 0.005, Fig. 6A).
Figure 6.
Effect of glucosamine hydrochloride (GH) and chondroitin sulfate (CS) on prostaglandin E2 (PGE2) production (A) and on nitric oxide (NO) production (B) at 72 hours post-stimulation with interleukin-1α (IL-1α). (A) Values represent the mean PGE2 level expressed in pg per mg tissue ± SEM, n = 3. (B) Values represent the mean NO level expressed in nanomoles per mg tissue ± SEM, n = 3. One-sample Student t test was used & indicates P < 0.05 for comparison between no-IL-1α and IL-1α samples with the same treatment. # indicates P < 0.05 for a comparison with the control with IL-1α stimulation.
A significant increase in NO concentration was seen in all cultures stimulated with IL-1α (P < 0.01, Fig. 6B). In GH 2 mg/mL and GH 2 mg/mL + CS 0.4 mg/mL pretreated cultures, the increase in NO generated by stimulation with IL-1α was significantly suppressed when compared with control with IL-1α (P = 0.009 and P = 0.010, respectively, Fig. 6B).
Discussion
Our results support the hypothesis that GH can partially prevent the degradative and pro-inflammatory effects of IL-1α in a dose-dependent manner, minimizing cartilage degradation by down-regulating aggrecanase activity and partially preventing inflammation by reducing the release of mediators of inflammation (PGE2, NO).
The reduction of aggrecan degradation was associated with the higher concentration of GH (2 mg/mL) but not the lower (0.2 mg/mL), suggesting that the effect is dose dependent. No significant reduction of IL-1α-induced aggrecan loss was observed in explant cultures with CS and the addition of CS to GH did not further decrease the loss of aggrecan observed with GH. Our results are in agreement with other studies where IL-1 induced GAG release in culture media of porcine cartilage explants was decreased in the presence of 20 to 80 mM GH in a dose-dependent manner.35 Also, the release of aggrecanase products was reduced in the presence of 1.5 to 15 mM d-glucosamine in IL-1-induced rat chondrosarcoma cells and bovine cartilage explants.31 In the study of Dechant et al.,48 only the combination of glucosamine and CS at 250 µg/mL (1:1 ratio) reduced IL-1-induced GAG release in equine cartilage explants, while the individual compounds did not have a significant effect.
mRNA expression and activity of catabolic enzymes such as ADAMTS-4 and ADAMTS-5 are reported to be significantly increased in OA.48 The ability of 2 mg/mL GH (with or without 0.4 mg/mL CS) to inhibit cartilage degradation and prevent aggrecan release in the presence of IL-1 may be partly attributed to the downregulation of the mRNA for these enzymes. In line with the previous results, 2 mg/mL GH (with or without 0.4 mg/mL CS) reduced the increase of ADAMTS-4 and ADAMTS-5 mRNA levels induced by IL-1α. More specifically, although trends were similar for both enzyme genes, a significant (P = 0.014) decrease in IL-1 induced mRNA expression was only obtained in the presence of GH for ADAMTS-5, suggesting that ADAMTS-5 may have a role in the degradation of aggrecan and that GH may prevent its activity through a reduction in production of the enzyme that is measurable by a reduction in mRNA. However, in the case of ADAMTS-4, we were able to demonstrate that the decreased IL-1-induced mRNA expression in the presence of GH correlated with a decrease in ADAMTS-4 activity, suggesting that ADAMTS-4 has a role in the degradation of aggrecan in this culture system. Surprisingly, mRNA expression for ADAMTS-4 and ADAMTS-5 was upregulated by 2 mg/mL GH alone. These results did not correlate with ADAMTS-4 activity or aggrecan degradation suggesting that there is another level of control downstream of mRNA expression.
It has been previously shown that ADAMTS-5 is secreted as active enzyme through the removal of a prodomain by furin cleavage so undergoing post-translational modification to become active.49 Indeed, a similar concentration of glucosamine as used in the present study impaired furin glycosylation compromising its conversion to active furin, which secondarily leads to failed activation of ADAMTS-5 zymogen.49 This supports our speculation that ADAMTS-5 activity is regulated post-translationally and this process is downregulated by glucosamine. In our study, glucosamine was shown for the first time to decrease levels of ADAMTS-4 and ADAMTS-5 aggrecan degradative products, together with its effects on mRNA expression and enzymatic activity of ADAMTS-4. The correlation between levels of degradative products with the enzymatic activity of ADAMTS-4 but not mRNA expression together with the findings of McCulloch et al.,49 allow us to speculate that glucosamine affects mRNA expression of ADAMTS-4 but downregulates its activity at a post-translation stage. In line with our results, 5 mM GH or glucosamine-3-sulfate significantly downregulated the mRNA expression of ADAMTS-4 and ADAMTS-5 in human osteoarthritic explants.50 Also consistent with our findings, in equine chondrocytes, 10 µg/mL glucosamine reduced IL-1-induced mRNA expression of ADAMTS-4 and ADAMTS-5 while 5 to 50 µg/mL CS had no significant effect on the expression of these genes.34 In bovine cartilage explants, 5 µg/mL GH and 20 µg/mL CS individually or in combination suppressed IL-1-induced mRNA expression of ADAMTS-4 and ADAMTS-5.33,51,52 However, the effect of glucosamine alone on ADAMTS-5 mRNA expression and the increase in expression has only been previously shown by McCulloch et al.,49 making this a new finding and adding to the understanding of ADAMTS-4 and ADAMTS-5 regulation in OA and by glucosamine.
To complement our investigation, we tested the effects of GH and CS on inflammation by measuring levels of PGE2 and NO. PGE2 is one of the most characteristic prostaglandins found in joints with OA.53 Limiting its synthesis may possibly lessen inflammation and pain involved in OA. NO synthesis is also associated with cartilage degradation and a reduction of proteoglycan synthesis. Therefore, limiting NO synthesis may be critical to delay the progression of OA and may also indicate a preventive effect of treatments. In our experiments, 2 mg/mL GH alone and with 0.4 mg/mL CS reduced the increase of both PGE2 and NO synthesis caused by IL-1α. These results show that, in addition to preventing aggrecanase activity and cartilage degradation, GH could also prevent inflammation processes involved in OA. Our results are in agreement with studies where glucosamine or a combination with CS inhibited the release of PGE2 in cartilage explant induced by IL-1 or lipopolysaccharide.36,51,52 However, results of the 2 compounds on NO release or of CS alone are mixed.51,52,54 As IL-1α promotes the phosphorylation of signaling proteins leading to the activation of transcription factors such as nuclear factor-κB (NF-κB), and NF-κB stimulates the expression of iNOS and COX-2 involved in the synthesis of NO and PGE2 respectively, it is possible that in our study, the regulation of PGE2 and NO production stimulated by IL-1α and down-regulated by GH may have occurred via regulation of NF-κB.
Although this study has found some positive effects of GH in an in vitro model of OA, the validation of the effectiveness of this neutraceutical would require confirmation using normal human cartilage. In addition, future studies would require different treatment periods with GH and CS as well as prolonged time points of evaluation for the metrics tested, that is cartilage catabolism at the pathological IGD site and the release of inflammatory mediators. A significant drawback within the current type of in vitro studies is the assessment of bioavailabilty of either GH or CS after oral ingestion of the compounds either individually or combined. It is possible that CS may be broken down into smaller molecules, including GH, which from our results could be advantageous. Although, the absorption and bioavailability of such dietary supplements have not been fully clarified, ingested chondroitin and glucosamine are partially absorbed and it has been documented that some reach the synovial fluid and cartilage.38,55-57
In conclusion, the results of this study, using an established model system, demonstrate that GH can partially prevent cartilage degradation generated by aggrecanases and reduce the release of inflammatory molecules. In contrast, and consistent with other studies, CS did not suppress the catabolism of aggrecan and the release of inflammatory factors following treatment with IL-1.34,54 However, the beneficial effects of CS may be principally related to anabolic processes, such as its role in promoting collagen and proteoglycan synthesis,58 rather than the prevention of catabolic processes.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research project was supported by the Knowledge Transfer Partnership (Grant 6676), funded by the Technology Strategy Board (UK) and Obsidian Research Ltd. The work was carried out at Cardiff University.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Cardiff University and some of the authors (Bruce Caterson and Clare E. Hughes) received royalties from commercial sale of the monoclonal antibody BC-3 used in this article.
Ethical Approval: This study does not need an approval from the institutional review board.
References
- 1. Arthritis Research UK. Data and statistics. 2015. http://www.arthritisresearchuk.org/arthritis-information/data-and-statistics.
- 2. Arthritis Foundation. Osteoarthritis. 2015. http://www.arthritis.org/conditions-treatments/disease-center/osteoarthritis/.
- 3. Hardingham T. Extracellular matrix and pathogenic mechanisms in osteoarthritis. Curr Rheumatol Rep. 2008;10:30-6. [DOI] [PubMed] [Google Scholar]
- 4. Wang M, Shen J, Jin H, Im HJ, Sandy J, Chen D. Recent progress in understanding molecular mechanisms of cartilage degeneration during osteoarthritis. Ann N Y Acad Sci. 2011;1240:61-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldring MB. Articular cartilage degradation in osteoarthritis. HSS J. 2012;8:7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith RL. Degradative enzymes in osteoarthritis. Front Biosci. 1999;15:D704-12. [DOI] [PubMed] [Google Scholar]
- 7. Nagase H, Kashiwagi M. Aggrecanases and cartilage matrix degradation. Arthritis Res Ther. 2003;5:94-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verma P, Dalal K. ADAMTS-4 and ADAMTS-5: key enzymes in osteoarthritis. J Cell Biochem. 2011;112:3507-14. [DOI] [PubMed] [Google Scholar]
- 9. Noack W, Fischer M, Forster KK, Rovati LC, Setnikar I. Glucosamine sulfate in osteoarthritis of the knee. Osteoarthritis Cartilage. 1994;2:51-9. [DOI] [PubMed] [Google Scholar]
- 10. Houpt JB, McMillan R, Paget-Dellio D, Russel A, Gahunia HK. Effect of treatment of glucosamine hydrochloride in the treatment of pain of osteoarthritic of the knee. J Rheumatology. 1999;26:2423-30. [PubMed] [Google Scholar]
- 11. Reginster JY, Deroisy R, Rovati LC, Lee RL, Lejeune E, Bruyere O, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357:251-6. [DOI] [PubMed] [Google Scholar]
- 12. Pavelka K, Gatterova J, Olejarova M, Machacek S, Giacovelli G, Rovati LC. Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3-year, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2002;162:2113-23. [DOI] [PubMed] [Google Scholar]
- 13. Michel BA, Stucki G, Frey D, De Vathaire F, Vignon E, Bruehlmann P, et al. Chondroitins 4 and 6 sulfate in osteoarthritis of the knee: a randomized, controlled trial. Arthritis Rheum. 2005;52:779-86. [DOI] [PubMed] [Google Scholar]
- 14. Mazières B, Hucher M, Zaim M, Garnero P. Effect of chondroitin sulphate in symptomatic knee osteoarthritis: a multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2007;66:639-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kahan A, Uebelhart D, De Vathaire F, Delmas PD, Reginster JY. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60:524-33. [DOI] [PubMed] [Google Scholar]
- 16. Kulkarni C, Leena A, Lohit K, Mishra D, Saji MJ. A randomized comparative study of safety and efficacy of immediate release glucosamine HCL and glucosamine HCL sustained release formulation in the treatment of knee osteoarthritis: a proof of concept study. J Pharmacol Pharmacother. 2012;3:48-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qiu GX, Weng XS, Zhang K, Zhou YX, Lou SQ, Wang YP, et al. A multi-central, randomized, controlled clinical trial of glucosamine hydrochloride/sulfate in the treatment of knee osteoarthritis. Zhonghua Yi Xue Za Zhi. 2005;85:3067-70. [PubMed] [Google Scholar]
- 18. Zhang WB, Zhuang CY, Li JM, Yang ZP, Chen XL. Efficacy and safety evaluation of glucosamine hydrochloride in the treatment of osteoarthritis. Zhonghua Wai Ke Za Zhi. 2007;45:998-1001. [PubMed] [Google Scholar]
- 19. Henrotin Y, Marty M, Mobasheri A. What is the current status of chondroitin sulfate and glucosamine for the treatment of knee osteoarthritis? Maturitas. 2014;78:184-7. [DOI] [PubMed] [Google Scholar]
- 20. Bottegoni C, Muzzarelli RA, Giovannini F, Busilacchi A, Gigante A. Oral chondroprotection with nutraceuticals made of chondroitin sulphate plus glucosamine sulphate in osteoarthritis. Carbohydr Polym. 2014;109:126-38. [DOI] [PubMed] [Google Scholar]
- 21. Fransen M, Agaliotis M, Nairn L, Votrube M, Bridgett L, Su S, et al. Glucosamine and chondroitin for knee osteoarthritis: a double-blind randomised placebo-controlled clinical trial evaluating single and combination regimens. Ann Rheum Dis. 2015;74:851-8. [DOI] [PubMed] [Google Scholar]
- 22. Hochberg MC, Martel-Pelletier J, Monfort J, Möller I, Castillo JR, Arden N, et al. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: a multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann Rheum Dis. Epub 2015. January 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clegg DO, Reda DJ, Harris CL, Klein MA, O’Dell JR, Hooper MM, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808. [DOI] [PubMed] [Google Scholar]
- 24. Bruyere O, Reginster JY. Glucosamine and chondroitin sulfate as therapeutic agents for knee and hip osteoarthritis. Drugs Aging. 2007;24:573-80. [DOI] [PubMed] [Google Scholar]
- 25. Sawitzke AD, Shi H, Finco MF, Dunlop DD, Bingham CO, 3rd, Harris CL, et al. The effect of glucosamine and/or chondroitin sulfate on the progression of knee osteoarthritis: a report from the glucosamine/chondroitin arthritis intervention trial. Arthritis Rheum. 2008;58:3183-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sawitzke AD, Shi H, Finco MF, Dunlop DD, Harris CL, Singer NG, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wandel S, Jüni P, Tendal B, Nüesch E, Villiger PM, Welton NJ, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fox BA, Stephens MM. Glucosamine hydrochloride for the treatment of osteoarthritis symptoms. Clin Interv Aging. 2007;2:599-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Block JA, Oegema TR, Sandy JD, Plaas A. The effects of oral glucosamine on joint health: is a change in research approach needed? Osteoarthritis Cartilage. 2010;18:5-11. [DOI] [PubMed] [Google Scholar]
- 30. Yang S, Eaton CB, McAlindon TE, Lapane KL. Effects of glucosamine and chondroitin supplementation on knee osteoarthritis: an analysis with marginal structural models. Arthritis Rheumatol. 2015;67:714-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sandy JD, Gamett D, Thompson V, Verscharen C. Chondrocyte-mediated catabolism of aggrecan: aggrecanase-dependent cleavage induced by interleukin-1 or retinoic acid can be inhibited by glucosamine. Biochem J. 1998;335:59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dodge GR, Jimenez SA. Glucosamine sulfate modulates the levels of aggrecan and matrix metalloproteinase-3 synthesized by cultured human osteoarthritis articular chondrocytes. Osteoarthritis Cartilage. 2003;11:424-32. [DOI] [PubMed] [Google Scholar]
- 33. Chan PS, Caron JP, Orth MW. Effect of glucosamine and chondroitin sulfate on regulation of gene expression of proteolytic enzymes and their inhibitors in interleukin-1-challenged bovine articular cartilage explants. Am J Vet Res. 2005;66:1870-6. [DOI] [PubMed] [Google Scholar]
- 34. Neil KM, Orth MW, Coussens PM, Chan PS, Caron JP. Effects of glucosamine and chondroitin sulfate on mediators of osteoarthritis in cultured equine chondrocytes stimulated by use of recombinant equine interleukin-1β. Am J Vet Res. 2005;66:1861-9. [DOI] [PubMed] [Google Scholar]
- 35. Phitak T, Pothacharoen P, Kongtawelert P. Comparison of glucose derivatives effects on cartilage degradation. BMC Musculoskelet Disord. 2010;15:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kapoor M, Mineau F, Fahmi H, Pelletier JP, Martel-Pelletier J. Glucosamine sulfate reduces prostaglandin E(2) production in osteoarthritic chondrocytes through inhibition of microsomal PGE synthase-1. J Rheumatol. 2012;39:635-44. [DOI] [PubMed] [Google Scholar]
- 37. Byron CR, Stewart MC, Stewart AA, Pondenis HC. Effects of clinically relevant concentrations of glucosamine on equine chondrocytes and synoviocytes in vitro. Am J Vet Res. 2008;69:1129-34. [DOI] [PubMed] [Google Scholar]
- 38. Ronca F, Palmieri L, Panicucci P, Ronca G. Anti-inflammatory activity of chondroitin sulfate. Osteoarthritis Cartilage. 1998;6:14-21. [DOI] [PubMed] [Google Scholar]
- 39. Lippiello L. Glucosamine and chondroitin sulfate: biological response modifiers of chondrocytes under simulated conditions of joint stress. Osteoarthritis Cartilage. 2003;11:335-42. [DOI] [PubMed] [Google Scholar]
- 40. Yang SR, Peng S, Ko CY, Chu IM. The effects of different molecular weight chondroitin-4-sulfates in chondrocyte pellet culture. Cytotechnology. Epub 2014. October 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hughes CE, Little CB, Büttner FH, Bartnik E, Caterson B. Differential expression of aggrecanase and matrix metalloproteinase activity in chondrocytes isolated from bovine and porcine articular cartilage. J Biol Chem. 1998;273:30576-82. [DOI] [PubMed] [Google Scholar]
- 42. Hughes CE, Caterson B, Fosang AJ, Roughley PJ, Mort JS. Monoclonal antibodies that specifically recognize neoepitope sequences generated by ‘aggrecanase’ and matrix metalloproteinase cleavage of aggrecan: application to catabolism in situ and in vitro. Biochem J. 1995;305:799-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55-63. [DOI] [PubMed] [Google Scholar]
- 44. Powell AJ, Little CB, Hughes CE. Low molecular weight isoforms of the aggrecanases are responsible for the cytokine-induced proteolysis of aggrecan in a porcine chondrocyte culture system. Arthritis Rheum. 2007;56:9:3010-9. [DOI] [PubMed] [Google Scholar]
- 45. Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulfated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173-7. [DOI] [PubMed] [Google Scholar]
- 46. Caterson B, Flannery CR, Hughes CE, Little CB. Mechanisms involved in cartilage proteoglycan catabolism. Matrix Biol. 2000;19:333-44. [DOI] [PubMed] [Google Scholar]
- 47. Arner EC, Hughes CE, Decicco CP, Caterson B, Tortorella MD. Cytokine-induced cartilage proteoglycan degradation is mediated by aggrecanase. Osteoarthritis Cartilage. 1998;6:214-28. [DOI] [PubMed] [Google Scholar]
- 48. Dechant JE, Baxter GM, Frisbie DD, Trotter GW, McIlwraith CW. Effects of glucosamine hydrochloride and chondroitin sulphate, alone and in combination, on normal and interleukin-1 conditioned equine articular cartilage explant metabolism. Equine Vet J. 2005;37:2227-31. [DOI] [PubMed] [Google Scholar]
- 49. McCulloch DR, Wylie JD, Longpre JM, Leduc R, Apte SS. 10 mM glucosamine prevents activation of proADAMTS5 (aggrcanase-2) in transfected cells by interference with post-translational modification of furin. Osteoarthritis Cartilage. 2010;18:455-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Uitterlinden EJ, Jahr H, Koevoet JL, Jenniskens YM, Bierma-Zeinstra SM, Degroot J, et al. Glucosamine decreases expression of anabolic and catabolic genes in human osteoarthritic cartilage explants. Osteoarthritis Cartilage. 2006;14:250-7. [DOI] [PubMed] [Google Scholar]
- 51. Chan PS, Caron JP, Orth MW. Short-term gene expression changes in cartilage explants stimulated with interleukin beta plus glucosamine and chondroitin sulfate. J Rheumatol. 2006;33:1329-40. [PubMed] [Google Scholar]
- 52. Chan PS, Caron JP, Orth MW. Effects of glucosamine and chondroitin sulfate on bovine cartilage explants under long-term culture conditions. Am J Vet Res. 2007;68:709-15. [DOI] [PubMed] [Google Scholar]
- 53. Attur M, Krasnokutsky-Samuels S, Samuels J, Abramson SB. Prognostic biomarkers in osteoarthritis. Curr Opin Rheumatol. 2013;25:136-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Orth MW, Peters TL, Hawkins JN. Inhibition of articular cartilage degradation by glucosamine-HCl and chondroitin sulphate. Equine Vet J Suppl. 2002;224-9. [DOI] [PubMed] [Google Scholar]
- 55. Conte A, Volpi N, Palmieri L, Bahous I, Ronca G. Biochemical and pharmacokinetic aspects of oral treatment with chondroitin sulfate. Arzneimittelforschung. 1995;45:918-25. [PubMed] [Google Scholar]
- 56. Persiani S, Rotini R, Trisolino G, Rovati LC, Locatelli M, Paganini D, et al. Synovial and plasma glucosamine concentrations in osteoarthritic patients following oral crystalline glucosamine sulphate at therapeutic dose. Osteoarthritis Cartilage. 2007;15:764-72. [DOI] [PubMed] [Google Scholar]
- 57. Henrotin Y, Chevalier X, Herrero-Beaumont G, McAlindon T, Mobasheri A, Pavelka K, et al. Physiological effects of oral glucosamine on joint health: current status and consensus on future research priorities. BMC Res Notes. 2013;6:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jerosch J. Effects of glucosamine and chondroitin sulfate on cartilage metabolism in OA: outlook on other nutrient partners especially omega-3 fatty acids. Int J Rheumatol. 2011;2011:969012. [DOI] [PMC free article] [PubMed] [Google Scholar]