Abstract
Objective
To identify the collagen type IV (Col4) isoform in articular cartilage and to evaluate the expressions of Col4 and laminin in the pericellular matrix (PCM) in damaged cartilage and during cartilage repair.
Design
The Col4 isoform was determined in chondrocytes isolated from 6 patients cultured up to 6 days and in 21% O2 or 1% O2, and the gene expression of Col4 α-chains was investigated. The distribution of Col4 and laminin in traumatically damaged cartilage (n = 7) and clinically failed cartilage repair (microfracture, TruFit, autologous chondrocyte implantation; n = 11) were investigated using immunohistochemistry. Normal human cartilage was used as control (n = 8). The distribution during clinical cartilage repair procedures was investigated in a minipig model with 6-month follow-up (untreated chondral, untreated osteochondral, microfracture, autologous chondrocyte implantation; n = 10).
Results
The Col4 isoform in articular cartilage was characterized as α1α1α2, which is an isoform containing antiangiogenic domains in the NC1-terminals (arresten and canstatin). In normal cartilage, laminin and Col4 was exclusively found in the PCM. High amounts (>50%) of Col4 in the PCM significantly decreased in damaged cartilage (P = 0.004) and clinically failed repair tissue (P < 0.001). Laminin was only found with high expression (>50%) in 4/8 of the normal samples, which was not statistically significantly different from damaged cartilage (P = 0.15) or failed cartilage repair (P = 0.054).
Conclusions
Col4 in cartilage contain antiangiogenic domains and may play a role in the hypoxic environment in articular cartilage. Col4 and laminin was not found in the PCM of damaged and clinically failed repair.
Keywords: collagen type IV, laminin, articular cartilage, cartilage repair
Introduction
The morphological characteristics of the repair tissue following surgical repair of articular cartilage defects often consist of a mixture of fibrous tissue, fibrocartilage, and hyaline cartilage. Rarely are even small regions displaying the architectural features of articular cartilage found (viz., an arcuate structure of the collagen type II fibrils comprising the hyaline cartilage and a columnar arrangement of the chondrocytes). While many advanced biomaterials and treatment modalities are available for surgical cartilage repair,1,2 very little is known about the course of events during the in vivo repair process that would determine the outcomes of the cartilage repair.
Healthy chondrocytes reside in lacunae surrounded by a layer of proteins termed the pericellular matrix (PCM), initially distinguished from the interterritorial matrix by differences in the fibrillar ultrastructure as revealed by scanning and transmission electron microscopy.3 More recent studies, however, have demonstrated an important difference in the chemical makeup of the PCM and interterritorial extracellular matrix (ECM). While the interterritorial ECM, which contributes to the majority of the dry weight of the tissue is composed mainly of collagen type II and proteoglycans, the PCM consists mainly of collagen type IV and laminin, molecules normally associated with the basement membrane and basal lamina. Collagen type IV and laminin have previously been identified as part of the PCM in articular cartilage and in the intervertebral disc (IVD).4-7 We recently described how collagen type IV and laminin are observed in the PCM of healthy (nondegenerated) cartilage tissues but generally absent in degenerated and fibrotic cartilage tissues.8 Additionally, we showed that the expression of collagen type IV and laminin in mesenchymal stem cells (MSCs) followed a spatiotemporal shift in pattern from a diffused territorial and interterritorial distribution to a defined pericellular localization, as seen in normal articular cartilage, during chondrogenesis in a pellet assay.9 The role of the PCM and its specific molecules has yet to be elucidated but accumulating evidence is suggesting that the homeostatic maintenance of the PCM integrity in articular cartilage, if perturbed may be implicated in osteoarthritis.10-12
While laminin isoforms in articular cartilage have previously been identified as laminin-111 in adult articular cartilage and laminin-332 in embryonic cartilage,7 collagen type IV isoforms remain unknown. Collagen type IV has a triple-helical structure composed of 3 of 6 different α-chains (1-6). Only 3 different isoforms have been identified: α1α1α2(IV), α3α4α5(IV), α5α5α6(IV). In the noncollagenous (NC1) domain in 3 of these 6 different α-chains, anti-angiogenic properties have been detected. These unique protein fragments are named: Arresten (α1[IV]NC1), Canstatin (α2[IV]NC1), and Tumstatin (α3[IV]NC1).13 Since articular cartilage is an avascular structure, collagen type IV isoforms α1α1α2(IV) and α3α4α5(IV) with their unique anti-angiogenic properties might be involved in the temporal control of vascularization during cartilage repair and in cartilage homeostasis.
The aim of the present study was to identify collagen type IV isoforms in articular cartilage, and to evaluate the expression level and tissue distribution of collagen type IV and laminin in the heterogeneous repair tissues arising from different surgical cartilage repair techniques in human subjects and in a large animal model. We hypothesized that collagen type IV and laminin would be found in the PCM in normal articular cartilage and that the expression of these molecules would be different in immature repair tissue and in clinically failed cartilage repair tissue.
Methods
Study Design
To determine the isoforms of collagen type IV in cartilage, gene expression analysis of all collagen type IV α-chains was carried out on chondrocytes isolated from biopsies of 6 patients undergoing anterior cruciate ligament reconstruction. To investigate the distribution of laminin and collagen type IV following articular cartilage damage and during repair, we obtained cartilage tissues from human donors and from a large animal model (Table 1). Traumatically damaged articular cartilage was obtained as the debrided tissue from patients undergoing cartilage repair procedures in the knee using a curette. Cartilage repair tissue at intermediate stages of repair in a minipig model (6 month) and long-term clinically failed cartilage repair (microfracture, autologous chondrocyte implantation [ACI], TruFit) in humans (>2 years) were obtained using a curette or oscillating saw depending on subsequent treatment. Normal cartilage controls were obtained from the intercondylar notch from eight patients undergoing anterior cruciate ligament reconstruction using a biopsy punch.
Table 1.
Tissue Specifications.
| Tissue Type | Species | Time | Treatment | n |
|---|---|---|---|---|
| Normal cartilage | Human | 8 | ||
| Damaged cartilage | Human | At treatment | 7 | |
| Intermediate repair | Minipig | 6 months | Empty chondral | 2 |
| Microfracture | 3 | |||
| Scaffold-supported autologous chondrocyte implantation | 2 | |||
| Empty osteochondral | 3 | |||
| Late failed repair | Human | >2 years | Microfracture | 4 |
| Scaffold-supported autologous chondrocyte implantation | 4 | |||
| TruFit | 3 |
Cell Culture
Cells were isolated as previously described.14 Chondrocytes (passage 2) from 6 patients were seeded in agarose-coated wells at a density of 2 × 105 cells/cm2 for up to 6 days. Culture media consisted of DMEM/F-12 with Glutamax (Gibco-Invitrogen) with 10% fetal calf serum (Invitrogen), 1% streptomycin and penicillin (#P7539, Sigma-Aldrich). To ensure physiological oxygen tension, hypoxic culture at 1% oxygen was used by culturing the cells in a closed and controlled workstation chamber (Xvivo System, BioSpherix), in addition to traditional culture in ambient air.14 Cells were cultured in 37°C with 5% CO2.
Gene Expression Analysis
RNA extraction was performed on days 1, 2, and 6, by Gen-Elute Total RNA Miniprep Kit (Sigma-Aldrich). RNA concentration was assessed spectrophotometrically using Quant-iT RiboGreen RNA Kit (Molecular Probes), and RNA quality was analyzed using Agilent 2100 Bioanalyzer (Agilent Technologies). After treating with DNaseI (Ambion), RNA was converted into cDNA using High Capacity cDNA Archive Kit (Applied Biosystems). All methods were performed according to the manufacturer’s instructions. Real-time quantitative polymerase chain reaction (RT-qPCR) was performed on a 7500 Fast Real-Time PCR system using commercially available TaqMan gene expression assays (all Applied Biosystems): sex determining region Y-box (Sox)5,6,and 9, (Sox5) Hs00753050_s1, (Sox6) Hs00264525_m1, (Sox9) Hs00165814_m1; Collagen type IV alpha (Col4a)1-6, (Col4a1) Hs00266237_m1, (Col4a2) Hs01098873_m1; (Col4a3) Hs01022542_m1, (Col4a4) Hs01011885_m1; (Col4a5) Hs00166712_m1, (Col4a6) Hs00361494_m1. Gene expressions of the target genes were normalized to BestKeeper Index and the relative gene expression was calculated as 2Ct(BestKeeper) − Ct(target gene).15 All samples were run in technical duplicates. Three reference genes were included in the BestKeeper Index: Beta-2-microglobulin (B2m) Hs99999907_m1; Ubiquitin C (Ubc) Hs00824723_m1; Custom TaqMan Human Reverse Transcriptase II (RpII) forward primer sequence GACACAGGACCACTCATGAAGT and reverse primer sequence GTGCGGCTGCTTCCATAAG.
Experimental Animal Model
To investigate expression on collagen type IV and laminin in different cartilage repair tissues during cartilage repair a large animal model was applied. Six skeletally mature Göttingen minipigs received cartilage or osteochondral lesions in the trochlea in both hind legs. The trochlea was exposed by a midline incision through the patella ligament. Only one defect per knee was made. Circular full-thickness chondral defects with a diameter of 6 mm were surgically created using a dermal biopsy punch and a curette. Microfracture was made after debridement of cartilage including calcified cartilage and an awl was used to make four holes per defect. Bleeding from the holes was observed. In the scaffold-supported ACI group, cartilage biopsies were harvested from the proximal trochlea 1 month prior to treatment. Chondrocytes were isolated and cultured as previously described.16 One week prior to surgery 1 × 106 chondrocytes/cm2 were seeded onto the ChondroGide scaffolds. The scaffolds were fixed with fibrin glue. Cylindrical osteochondral defects with a diameter of 6 mm and a depth of 8 mm were created using a curette to remove the cartilage and a 6-mm drill bit was used to create the osseous defect. The number of defects treated with each treatment is found in Table 2. In 2 animals, 1 knee received treatments not relevant in the present study. These were excluded. Treatments were randomized to each knee and no animal received the same treatment in both knees. All animals were observed for 6 months before euthanasia. The animal study was in compliance with the Danish Law on Animal Experimentation and was approved by the Danish Ministry of Justice Ethical Committee, J.nr. 2012-15-2934-00301.
Table 2.
Treatment of Defects in the Minipig Model.
| ID | Knee | Treatment |
|---|---|---|
| 1 | Left | Scaffold-supported autologous chondrocyte implantation |
| 1 | Right | Microfracture |
| 2 | Left | Scaffold-supported autologous chondrocyte implantation |
| 2 | Right | Microfracture |
| 3 | Left | Microfracture |
| 4 | Right | Empty osteochondral |
| 5 | Left | Empty osteochondral |
| 5 | Right | Empty chondral |
| 7 | Left | Empty chondral |
| 7 | Right | Empty osteochondral |
Human Tissues
Human cartilage and osteochondral tissues were obtained from 18 patients undergoing either cartilage repair surgery or cartilage revision surgery at the Cartilage Repair Center, Brigham & Women’s Hospital, Boston, Massachusetts or at the Sports Trauma Clinic at Aarhus University Hospital, Denmark. Mean age was 31.2 years (range 18-46 years) and male:female ratio was 26:74. Note that failed repair leading to revision was determined by clinical symptoms such as persisting pain and not by the appearance of the repair tissue. Damaged cartilage was determined as the tissue being debrided prior to treatment based on assessment by the surgeon. Normal cartilage tissue biopsies were harvested from the non-weightbearing areas in the trochlear notch in 8 patients undergoing anterior cruciate ligament reconstruction. The normality criterion was based on the biopsy coming from a knee with no clinical or radiological signs of degeneration All tissues were full-thickness cartilage tissue. In biopsies from healthy cartilage, orientation was not always distinguishable. Consent was obtained and was approved by the Institutional Review Board (United States) or the Local Ethics Committee under the National Committee on Research Ethics (Denmark). Patient age and gender was noted. The type of prior surgery was obtained from the patient’s records.
Tissue Processing and Preparation
Human tissues were fixed in 10% phosphate-buffered formalin and porcine tissues in 70% ethanol for up to 1 week (2 weeks for large tissues from total knee arthroplasty). Tissue fixation method depended on the approved protocol at the location of tissue collection. The tissues were dehydrated in a graded series of ethanol (70% to 96%), cleared in xylene and embedded in methoxymethylacrylate. The embedded samples were cut in 7-μm thick slices and mounted on microscopic slides. In cases where the anatomical orientation of the biopsy could be defined macroscopically, sections were cut perpendicular to the cartilage surface. Otherwise, sections were cut with a random orientation. The sections were deplastified and rehydrated in a graded series of ethanol and rinsed in water.
Histology and Immunohistochemistry
Tissue morphology was evaluated on hematoxylin and eosin–stained sections. Immunohistochemistry was performed as previously described,8,9 using primary antibodies for polyclonal rabbit antibodies for collagen type IV (Abcam, ab6586) and laminin (Abcam, ab11575). Briefly, protein retrieval was achieved using protease 0.1% (#P6911, Sigma-Aldrich) for 40 minutes. The protocol is based on optimization on protein retrieval using the above fixatives and tissue types. The slides were counter-stained with Mayer’s hematoxylin. Negative controls using 1% bovine serum albumin instead of primary antibody were applied.
Evaluation
Histological evaluation was performed on a Zeiss Axio Imager 2 light microscope (Carl Zeiss). The tissue morphology was evaluated on hematoxylin and eosin– and safranin-O–stained sections. Collagen type IV and laminin expressions for the different tissue types and time points during repair were evaluated by estimation of their distribution in either the PCM or ECM for the tissues. We categorized the distribution of collagen type IV and laminin by assigning the amount of staining found in either PCM or ECM into 3 categories: 1, <5%; 2, 5% to 50%; 3, >50% based on one slide through the center of the defect. These were correlated with the tissue morphology based on previously published criteria (hyaline cartilage, fibrocartilage, fibrous tissue, or bone).17
Statistics
Gene expression data were analyzed using 2-factor analysis of variance (time in culture and oxygen concentration). When no interaction in main effects was observed, the dependent variables were investigated individually. Differences in collagen type IV and laminin protein distribution were analyzed using chi-square test based on the semiquantitative categories above for the different types of tissues. Two-tailed P values less than 0.05 were considered significant in all statistical analyses.
Results
Collagen Type IV Isoform Identification
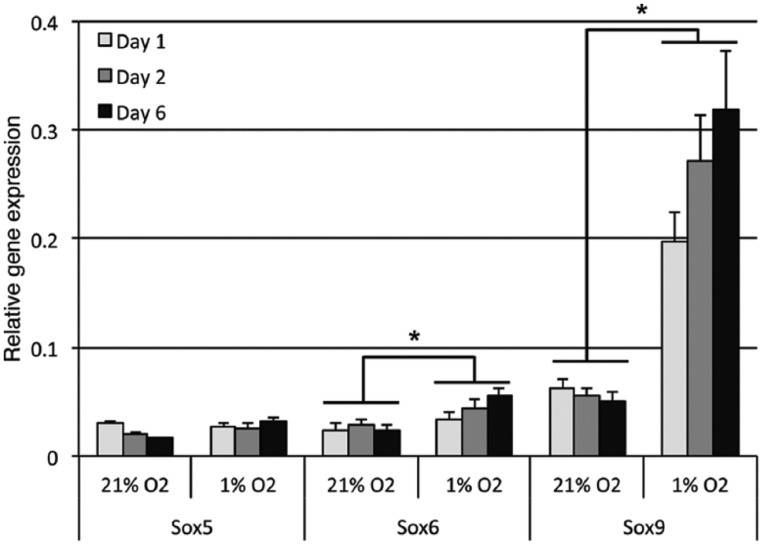
The mean RNA integrity number (RIN) for assigning integrity values to RNA was 9.0 (range 7.0-9.8; ≥7.0 required quality for PCR). The chondrogenic phenotype was validated by the gene expression of the Sox5, Sox6, and Sox9, from day 1 through 6 in culture (Fig. 1). Two-factor analysis of variance revealed a statistically significant effect of oxygen concentration on expression of Sox6 (P = 0.01) and Sox9 (P < 0.0001) in hypoxia compared with normoxia, which was most pronounced for Sox9 after 6 days of culture where there was a 6-fold increase compared with normoxia (Fig. 1). No significant effect of culture time as an independent variable was observed.
Figure 1.
Validation of the chondrogenic phenotype. Relative mRNA expressions of the SOX-Trio genes in chondrocytes cultured in 21% and 1% oxygen for up to 6 days. Error bars are standard error of mean (n = 6). Significant effect (*) of oxygen tension on expressions of Sox6 and Sox9 was observed.
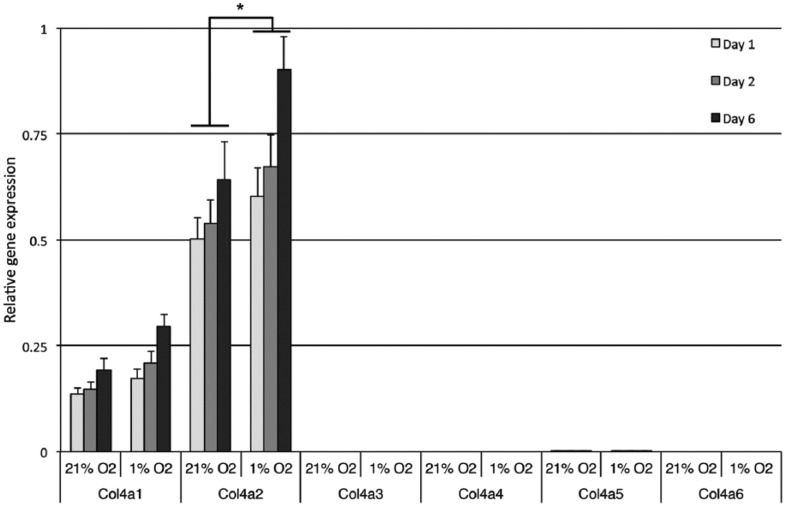
Chondrocytes expressed α1 and α2 chains, but not α3-6 chains neither in ambient air culture nor in hypoxia. The expression Col4a2 (P = 0.002) but not Col4a1 (P = 0.54) was significantly increased in hypoxia (Fig. 2). The ratio of mRNA Col4a1 and Col4a2 expressions is known to vary under different experimental conditions while the ratio of transcribed α-chains in the triple-helical α1α1α2(IV)-structure remains unaffected.18 Hence, the isoform of collagen type IV in normal adult human articular cartilage is expected to be α1α1α2(IV).
Figure 2.
Relative mRNA expressions of the different collagen type IV α-chains. Cells were cultured for up to 6 days in 21% or 1% oxygen. Error bars are standard error of mean; n = 6. The α2-chain was expression 3.5-fold higher than the α1-chain within comparable experimental conditions (i.e., oxygen tension and culture time). Significant effect (*) of oxygen tension was found for Col4a2.
Normal and Damaged Cartilage
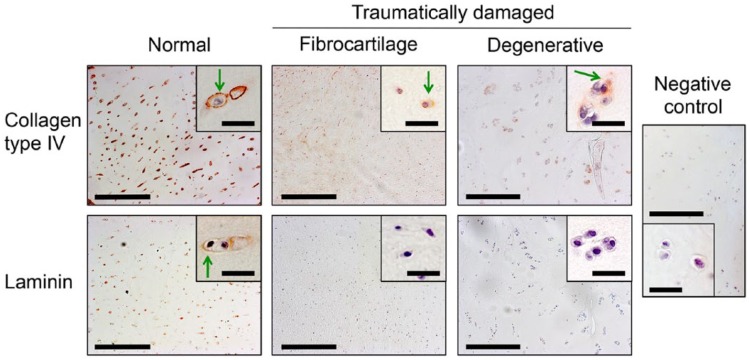
Cartilage biopsies from normal human articular cartilage showed chondrocytes residing in lacunae in hyaline matrix. Collagen type IV and laminin was found in the PCM surrounding all chondrocytes in the biopsies (Fig. 3). Compared with collagen type IV, laminin was found surrounding fewer cells than in our normal cartilage reference tissue. No positive stain was found in the interterritorial matrix. The damaged articular cartilage appeared either fibrocartilaginous or hyaline with degenerative characteristics (Fig. 3). Approximately 80% of the ECM found in these biopsies was hyaline cartilage, but this was generally hypocellular compared with native articular cartilage and showed increased number of chondrocyte clusters.
Figure 3.
Representative immunohistochemical images of normal human articular cartilage and traumatically damage articular cartilage stained with antibodies against collagen type IV (top row) and laminin (bottom row). Collagen type IV and laminin was found in the pericellular matrix (PCM) of normal cartilage. Tissue from patients with traumatically damaged cartilage showed two different morphologies. Fibrocartilage with chondrocytes in fibrous tissue or degenerative hyaline cartilage with hypocellularity and chondrocyte clusters. The PCM in damaged cartilage was generally absent of collagen type IV and laminin, but collagen type IV was found in areas of chondrocyte clusters. Negative control showed for comparison. Green arrows = positive pericellular staining. Low magnification images: Bar = 200 μm. High magnification images: Bar = 30 μm.
Intermediate Cartilage Repair
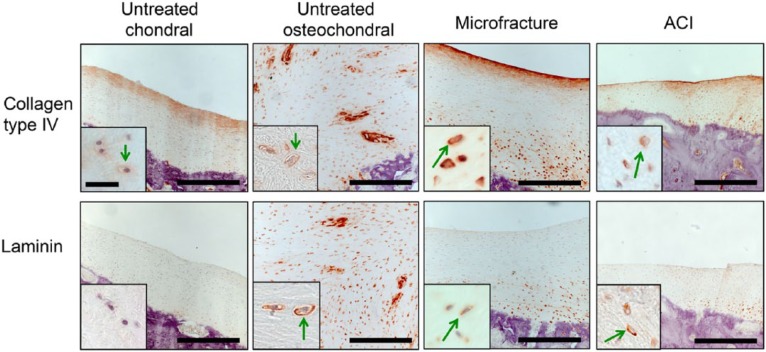
Untreated chondral defects in minipigs showed mainly fibrous tissue with few areas of fibrocartilage (Fig. 4). Collagen type IV was distributed throughout the ECM with increased expression in the superficial layer. In the native tissue, collagen type IV expression was restricted to the PCM, surrounding the chondrocytes. Laminin was absent from the repair tissue but was present in the PCM surrounding the chondrocytes in the native cartilage. Untreated osteochondral defects (Fig. 4) showed predominantly fibrous tissue with fibrocartilage adjacent to the bone at the bottom and at the edges of the defect. Collagen type IV was distributed throughout the ECM in fibrous tissue and fibrocartilage with an increased expression in the PCM of fibrochondrocytes present in the fibrocartilage. Laminin was also found in PCM of the fibrochondrocytes but was absent from the fibrous tissue. Normal articular knee cartilage in the minipig is shown in Figure 5.
Figure 4.
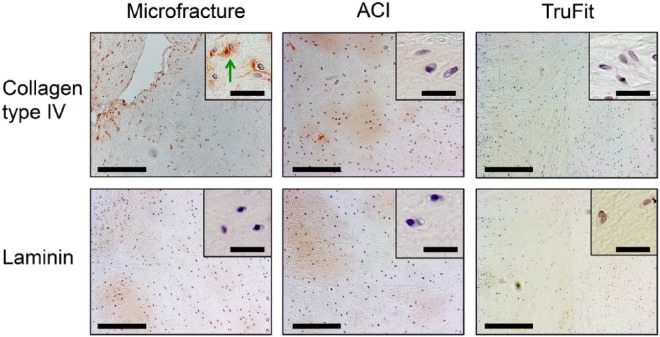
Immunohistochemical images of cartilage repair treatments stained with antibodies against collagen type IV (top row) and laminin (bottom row) in a minipig model with 6-month follow-up. The repair tissue in all treatments except the scaffold-seeded autologous chondrocyte implantation (ACI) was composed mainly of fibrocartilage with collagen type IV and laminin positive pericellular matrix (PCM). Collagen type IV was found in some areas of ECM in fibrous tissue and fibrocartilage. Green arrows = positive pericellular staining. Low magnification images: Bar = 200 μm. High magnification images: Bar = 30 μm.
Figure 5.
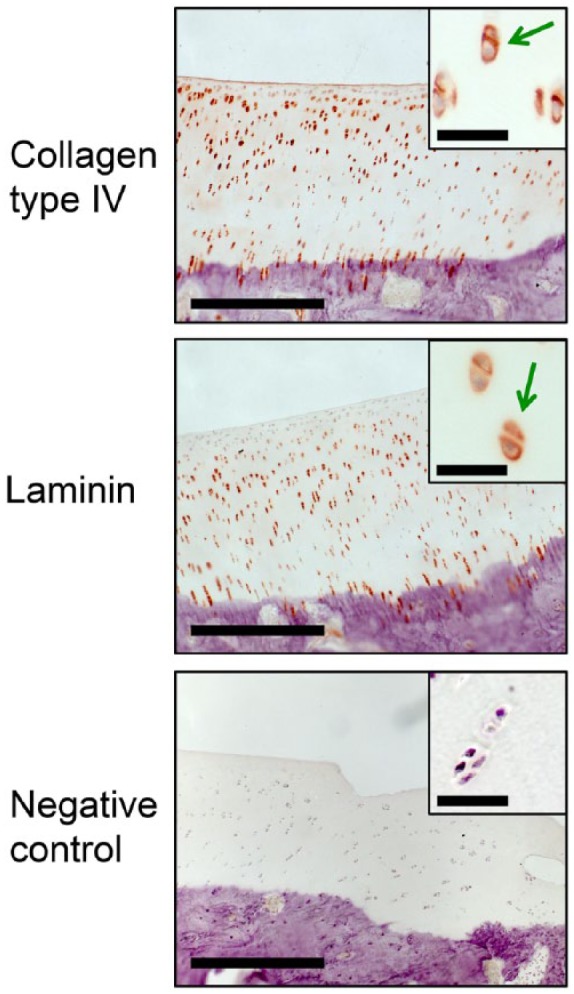
Immunohistochemical images of normal articular knee cartilage from Göttingen minipigs stained with antibodies against collagen type IV and laminin. Negative control showed for comparison. Green arrows = positive pericellular staining. Low magnification images: Bar = 200 μm. High magnification images: Bar = 30 μm.
Fibrocartilage was the predominant repair tissue in the microfracture-treated defects with fibrous tissue in the superficial layer (Fig. 4). Collagen type IV and laminin expressions were restricted to the PCM surrounding the chondrocytes in the hyaline-like tissue while staining of the interterritorial matrix with collagen type IV was observed in the fibrocartilage. Fibrochondrocytes were positive for laminin only in the PCM and not in the fibrocartilage ECM. In the fibrous tissue, collagen type IV was found diffusely distributed in the ECM with no pericellular deposition. Fibrous tissues were absent of laminin.
The repair tissue after scaffold-seeded ACI (Fig. 4) displayed characteristics of hyaline cartilage and fibrocartilage. Collagen type IV and laminin was found solely in the PCM, surrounding the chondrocytes in the hyaline repair tissue. Collagen type IV was distributed throughout the ECM in the fibrocartilage while laminin was generally absent from this type of repair tissue.
Clinically Failed Cartilage Repair
The tissue in biopsies from patients with failed microfracture mainly had morphological characteristics of fibrocartilage (Fig. 6). Biopsies from patients with failed scaffold-seeded ACI consisted of chondrocytes in fibrous and hyaline ECM consistent with the morphological characteristics of fibrocartilage and hyaline cartilage. The tissues from the patients with failed TruFit implantation were mixtures of bone, fibrous tissue, and fibrocartilage. Collagen type IV positive PCM was found in 5% to 50% of the cells in 3/11 patient biopsies and was found positive in the PCM of chondrocytes and not in fibroblasts. Laminin present in the PCM or ECM in <5% of the cells in all patient biopsies. In the interterritorial matrix collagen type IV positive ECM was accounting 5% to 50% of the tissue in 3/11 of the patient biopsies while all patient biopsies had <5% laminin positive ECM. Collagen type IV and laminin was not associated with the bone except the bone marrow in the blood vessels in the Havesian canals. Collagen type IV and laminin positive blood vessel basement membranes were observed in our internal controls in the biopsies such as in the bone.
Figure 6.
Immunohistochemical images of clinically failed cartilage tissues stained with antibodies against collagen type IV (top row) and laminin (bottom row). Tissue despite prior treatment generally consisted of fibrocartilage and fibrous tissue, but hyaline cartilage was also detected in the failed ACI group. Collagen type IV and laminin was generally absent in the PCM. Green arrows = positive pericellular staining. Low magnification images: Bar = 200 μm. High magnification images: Bar = 30 μm.
Comparisons of Expression Patterns
The number of samples containing significant amounts (>50%) of collagen type IV in the PCM significantly decreased in damaged cartilage (P = 0.004) and clinically failed repair (P < 0.001) compared with the healthy cartilage control. Laminin was only found with significant expression (>50%) in 4/8 of the normal samples, which was not statistically significantly different from damaged cartilage (P = 0.15) or failed cartilage repair (P = 0.054). Cartilage repair tissue in the minipig model showed a nonsignificant decrease of these proteins in the PCM compared with normal cartilage. Collagen type IV and laminin were not found in the interterritorial ECM of normal cartilage and traumatically damaged cartilage.
Discussion
In this study, we identified the specific isoform of collagen type IV α1α1α2 as the sole type in articular cartilage. In normal cartilage, this was found in the PCM of the chondrocytes, but was generally depleted in traumatically damaged cartilage and repair tissue following failed cartilage repair procedure. In the minipig model, the expressions of collagen type IV and laminin were analyzed during the course of cartilage repair following different clinical treatments. We found that relatively immature cartilage repair tissue (as seen in the minipig model) was composed mainly of fibrous tissue and fibrocartilage that expressed limited collagen type IV in both interterritorial ECM and PCM. Laminin was found to a lesser extent in the interterritorial ECM and its presence in the PCM was less than that of collagen type IV.
The different α-chains in collagen type IV express homologous structure composed of an NH3-terminal, a long collagenous Gly-Xaa-Yaa triple-repeat, and a noncollagenous COOH-terminal (NC1). In COL4A1 and COL4A2 the NC1 domains are arresten and canstatin, respectively. These are naturally occurring matrix-derived anti-angiogenic proteins. Arresten is a 26-kDa protein fragment previously shown to inhibit proliferation and migration of endothelial cells and to inhibit hypoxia-induced increase in expression and vascular endothelial growth factor (VEGF) and hypoxia-inducible factor 1a (HIF-1α).19 Mutations in the COL4A1gene are associated with small-vessel disease, hemorrhagic stroke, and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps in humans.20,21 The tumor suppressor p53 has recently been shown to inhibit angiogenesis by directly and indirectly inducing the production of arresten.22 Canstatin is a 24-kDa protein fragment originally found to inhibit proliferation and migration and to induce apoptosis in endothelial cells.23 The anti-angiogenic function of arresten is facilitated by binding to α1β1 integrin. Similary, canstatin is facilitated by αVβ3 and αVβ5 integrins, which are all expressed in chondrocytes.19,24,25 The identification of arresten and canstatin in articular cartilage adds to the list of naturally occurring antiangiogenic factors in the PCM, already containing collagen type XVIII/endostatin.26 These proteins collectively emphasize that cartilage tissue hypoxia and anti-angiogenesis may be controlled, at least in part, by endogenous regulation by the PCM.
The present findings of laminin distribution in normal and damaged articular cartilage support our prior observation of depleted laminin expression in osteoarthritic articular cartilage. These findings collectively suggest that normal adult human articular cartilage in normal cartilage homeostasis is characterized by the expression of laminin in the PCM of chondrocytes. However, in cases of immature repair tissue during early cartilage repair or degenerated cartilage in osteoarthritis, the expression of laminin is heavily downregulated.4,8,9
Collagen type IV and laminin in particular was not seen in the PCM of all chondrocytes in our normal cartilage reference tissue in humans, while it was seen in the PCM of all chondrocytes in the native cartilage in the animal model. This may be explained by the nature of our reference tissue, although near normal, was harvested from patients with ACL tears, altered biomechanics, and subsequently some degree of inflammation. This type of cartilage is however, the closest we are able to come to normal, nondegenerative human knee cartilage.
In the histological investigation of cartilage repair, fibrocartilage is a predominant tissue type. The tissue is characterized by the presence of rounded cells in lacunae (i.e., chondrocytes) in a fibrous matrix. Immunohistochemically, the ECM is composed mainly of collagen type I and to a lesser extent collagen type II. In this study we find that collagen type IV and laminin are found in the PCM in some fibrocartilagenous tissues but absent in others. Based on the differential expression of collagen type IV and laminin in the ECM and in the PCM surrounding the chondrocytes, repair tissues may be subdivided into a number of different tissues and the analysis of basement membrane proteins may prove to be a useful indicator for assessing the progressive maturity of the repair tissue toward hyaline cartilage formation.
Also of note in the present study was the significant effect of a hypoxic environment in vitro on the expressions of Sox6, Sox9, and Col4a2. The effect of hypoxia on the gene expression of the transcription factor Sox9 but not Sox6 have previously been demonstrated in differentiated chondrocytes.14 The hypoxia-induced upregulation of Sox6 gene expression has however been found in mesenchymal stem cells and bone marrow pericytes during chondrogenic differentiation.27,28 To date, increase in Col4a2 gene expression in response to hypoxia has not previously been demonstrated in chondrocytes or during chondrogenic differentiation.
While our focus on the cartilage PCM have been on the basement molecules—collagen type IV and laminin—other proteins may be equally or more important in different aspects of cartilage homeostasis and degeneration. Additional molecules of interest identified in the PCM in articular cartilage encounter, collagen types VI, XV, XVI, XVIII (endostatin precursor), and XXVII5,6,26,29-31; COMP32; biglycans (decorin, perlecan, netrin, nidogens)5,7,33,34; and asporin (transforming growth factor-β-binding molecule).35
The present study investigates the spatial distribution, but does not address the quantitative amounts of collagen type IV and laminin during cartilage repair and in failed repair tissue. Stereological analysis of human tissue biopsies as those in the present study is not possible without the risk of selection bias or confounding.17 Additional studies applying quantitative protein analysis during cartilage repair by means of targeted proteomics may provide additional quantitative information of these proteins and their roles in cartilage repair.
In this study, we have described the distribution of the basement membrane molecules—collagen type IV and laminin—in articular cartilage damage and during different types of surgical cartilage repair. We have further identified the collagen type IV isoform α1α1α2(IV) as the sole type in articular cartilage. This isoform contains arresten and canstatin that are anti-angiogenic protein fragments, which may be important regulators of cartilage homeostasis.
Footnotes
Funding: The authors would like to acknowledge their funding sources and technical assistance from the laboratory technicians at the Orthopaedic Research Laboratory, Aarhus University Hospital. The research reported here was supported by the U.S. Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service, and a postdoctoral research grant from the Danish National Research Foundation. A postdoctoral research fellowship for CBF was awarded by an Elite Research Scholarship from the Danish Minister of Science, Technology and Innovation. The funding sources did not play a role in the investigation.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by our institutional review board.
References
- 1. Toh WS, Spector M, Lee EH, Cao T. Biomaterial-mediated delivery of microenvironmental cues for repair and regeneration of articular cartilage. Mol Pharm. 2011;8:994-1001. [DOI] [PubMed] [Google Scholar]
- 2. Toh WS, Foldager CB, Pei M, Hui JH. Advances in mesenchymal stem cell–based strategies for cartilage repair and regeneration. Stem Cell Rev. 2014;10:686-96. [DOI] [PubMed] [Google Scholar]
- 3. Laurie GW, Leblond CP. What is known of the production of basement membrane components. J Histochem Cytochem. 1983;31:159-63. [DOI] [PubMed] [Google Scholar]
- 4. Durr J, Lammi P, Goodman SL, Aigner T, von der Mark K. Identification and immunolocalization of laminin in cartilage. Exp Cell Res. 1996;222:225-33. [DOI] [PubMed] [Google Scholar]
- 5. Kvist AJ, Nystrom A, Hultenby K, Sasaki T, Talts JF, Aspberg A. The major basement membrane components localize to the chondrocyte pericellular matrix—a cartilage basement membrane equivalent? Matrix Biol. 2008;27:22-33. [DOI] [PubMed] [Google Scholar]
- 6. Nerlich AG, Schleicher ED, Boos N. 1997 Volvo Award winner in basic science studies. Immunohistologic markers for age-related changes of human lumbar intervertebral discs. Spine (Phila Pa 1976). 1997;22:2781-95. [DOI] [PubMed] [Google Scholar]
- 7. Kruegel J, Miosge N. Basement membrane components are key players in specialized extracellular matrices. Cell Mol Life Sci. 2010;67:2879-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Foldager CB, Toh WS, Gomoll AH, Olsen BR, Spector M. Basement membrane molecules, laminin, and collagen type IV in healthy and degenerated cartilage tissues. Cartilage. 2014;5:123-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Toh WS, Foldager CB, Olsen BR, Spector M. Basement membrane molecule expression attendant to chondrogenesis by nucleus pulposus cells and mesenchymal stem cells. J Orthop Res 2013;31:1136-43. [DOI] [PubMed] [Google Scholar]
- 10. Alexopoulos LG, Williams GM, Upton ML, Setton LA, Guilak F. Osteoarthritic changes in the biphasic mechanical properties of the chondrocyte pericellular matrix in articular cartilage. J Biomech. 2005;38:509-17. [DOI] [PubMed] [Google Scholar]
- 11. Alexopoulos LG, Setton LA, Guilak F. The biomechanical role of the chondrocyte pericellular matrix in articular cartilage. Acta Biomater. 2005;1:317-25. [DOI] [PubMed] [Google Scholar]
- 12. Wilusz RE, Zauscher S, Guilak F. Micromechanical mapping of early osteoarthritic changes in the pericellular matrix of human articular cartilage. Osteoarthritis Cartilage. 2013;21:1895-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mundel TM, Kalluri R. Type IV collagen-derived angiogenesis inhibitors. Microvasc Res. 2007;74:85-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Foldager CB, Nielsen AB, Munir S, Ulrich-Vinther M, Soballe K, Bunger C, et al. Combined 3D and hypoxic culture improves cartilage-specific gene expression in human chondrocytes. Acta Orthop. 2011;82:234-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26:509-15. [DOI] [PubMed] [Google Scholar]
- 16. Christensen BB, Foldager CB, Hansen OM, Kristiansen AA, Le DQ, Nielsen AD, et al. A novel nano-structured porous polycaprolactone scaffold improves hyaline cartilage repair in a rabbit model compared to a collagen type I/III scaffold: in vitro and in vivo studies. Knee Surg Sports Traumatol Arthrosc. 2012;20:1192-204. [DOI] [PubMed] [Google Scholar]
- 17. Foldager CB, Nyengaard JR, Lind M, Spector M. A stereological method for the quantitative evaluation of cartilage repair tissue. Cartilage. 2015;6:123-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008;71:357-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sudhakar A, Nyberg P, Keshamouni VG, Mannam AP, Li J, Sugimoto H, et al. Human α1 type IV collagen NC1 domain exhibits distinct antiangiogenic activity mediated by α1β1 integrin. J Clin Invest. 2005;115:2801-10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20. Plaisier E, Gribouval O, Alamowitch S, Mougenot B, Prost C, Verpont MC, et al. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N Engl J Med. 2007;357:2687-95. [DOI] [PubMed] [Google Scholar]
- 21. Gould DB, Phalan FC, van Mil SE, Sundberg JP, Vahedi K, Massin P, et al. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl J Med. 2006;354:1489-96. [DOI] [PubMed] [Google Scholar]
- 22. Assadian S, El-Assaad W, Wang XQ, Gannon PO, Barres V, Latour M, et al. p53 inhibits angiogenesis by inducing the production of Arresten. Cancer Res. 2012;72:1270-9. [DOI] [PubMed] [Google Scholar]
- 23. Kamphaus GD, Colorado PC, Panka DJ, Hopfer H, Ramchandran R, Torre A, et al. Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J Biol Chem. 2000;275:1209-15. [DOI] [PubMed] [Google Scholar]
- 24. Magnon C, Galaup A, Mullan B, Rouffiac V, Bouquet C, Bidart JM, et al. Canstatin acts on endothelial and tumor cells via mitochondrial damage initiated through interaction with αvβ3 and αvβ5 integrins. Cancer Res. 2005;65:4353-61. [DOI] [PubMed] [Google Scholar]
- 25. Woods VL, Jr, Schreck PJ, Gesink DS, Pacheco HO, Amiel D, Akeson WH, et al. Integrin expression by human articular chondrocytes. Arthritis Rheum. 1994;37:537-44. [DOI] [PubMed] [Google Scholar]
- 26. Pufe T, Petersen WJ, Miosge N, Goldring MB, Mentlein R, Varoga DJ, et al. Endostatin/collagen XVIII—an inhibitor of angiogenesis—is expressed in cartilage and fibrocartilage. Matrix Biol. 2004;23:267-76. [DOI] [PubMed] [Google Scholar]
- 27. Khan WS, Adesida AB, Tew SR, Lowe ET, Hardingham TE. Bone marrow–derived mesenchymal stem cells express the pericyte marker 3G5 in culture and show enhanced chondrogenesis in hypoxic conditions. J Orthop Res. 2010;28:834-40. [DOI] [PubMed] [Google Scholar]
- 28. Khan WS, Adesida AB, Hardingham TE. Hypoxic conditions increase hypoxia-inducible transcription factor 2α and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res Ther. 2007;9:R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4:30-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pace JM, Corrado M, Missero C, Byers PH. Identification, characterization and expression analysis of a new fibrillar collagen gene, COL27A1. Matrix Biol. 2003;22:3-14. [DOI] [PubMed] [Google Scholar]
- 31. Ronziere MC, Ricard-Blum S, Tiollier J, Hartmann DJ, Garrone R, Herbage D. Comparative analysis of collagens solubilized from human foetal, and normal and osteoarthritic adult articular cartilage, with emphasis on type VI collagen. Biochim Biophys Acta. 1990;1038:222-30. [DOI] [PubMed] [Google Scholar]
- 32. Hedbom E, Antonsson P, Hjerpe A, Aeschlimann D, Paulsson M, Rosa-Pimentel E, et al. Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J Biol Chem. 1992;267:6132-6. [PubMed] [Google Scholar]
- 33. Lorenzo P, Aspberg A, Onnerfjord P, Bayliss MT, Neame PJ, Heinegard D. Identification and characterization of asporin. a novel member of the leucine-rich repeat protein family closely related to decorin and biglycan. J Biol Chem. 2001;276:12201-11. [DOI] [PubMed] [Google Scholar]
- 34. Stanescu V. The small proteoglycans of cartilage matrix. Semin Arthritis Rheum. 1990;20:51-64. [DOI] [PubMed] [Google Scholar]
- 35. Nakajima M, Kizawa H, Saitoh M, Kou I, Miyazono K, Ikegawa S. Mechanisms for asporin function and regulation in articular cartilage. J Biol Chem. 2007;282:32185-92. [DOI] [PubMed] [Google Scholar]